Abstract
Background:
Endometriosis is a frequent gynecologic disease with a severe impact on the quality of life in the affected women; its pathogenesis is yet to be fully understood, with an altered immunity as a possible key factor. The present study aimed to investigate the serum anti-inflammatory cytokine profile in the patients with endometriosis compared with the healthy controls.
Materials and Methods:
One hundred and sixty women were included, divided into two study groups (Group I — endometriosis; Group 2 — healthy women). We evaluated the serum levels of interleukin-1 receptor antagonist (IL-1Ra), IL-2, IL-2R, IL-4, IL-10, IL-13, and IL-15 with the use of Human multiplex cytokine panels. Statistical analyses (normality distribution analysis, independent t-test, Mann–Whitney U-test) were performed using IBM SPSS software (version 22.0) and GraphPad Prism (version 5.00); receiver operating characteristic curve were used to demonstrate the diagnostic performance of the studied markers.
Results:
The mean serum level of IL-1Ra, IL-4, and IL-10 were significantly higher in women with endometriosis compared to women free of disease from the control group (30.155, 138.459, and 1.489, respectively, compared to 14.109, 84.710, and 0.688, respectively; P < 0.001, P < 0.001, and P = 0.002, respectively.). No significant differences in the mean serum levels of IL-2, IL-13, and IL-15 were observed between the studied groups and IL-2R had a very low detection rate.
Conclusion:
Endometriosis is associated with elevated levels of anti-inflammatory cytokines, IL-1Ra, IL-4, and IL-10, markers that have a potential role as a prognostic factor for endometriosis.
Keywords: Cytokines, endometriosis, immunity, inflammation, interleukin
INTRODUCTION
Endometriosis is a frequent gynecologic disease with a prevalence of about 10-15% of reproductive-aged women, and it represents the existence of endometrial tissue outside the uterine cavity. Most common symptoms are represented by infertility, dysmenorrhea and most frequently by pelvic pain, and at the same time a reduced quality of life. The etiology and pathogenesis of the disease are not clearly understood, tough it is well known that susceptibility to endometriosis depends on complex interactions between genetic, immunologic, hormonal, and environmental factors.[1] A general concept is that endometriosis is a local pelvic inflammatory process with altered function of immune-related cells, thus it was suggested that the serum of women with endometriosis contains an increased number of activated macrophages that secrete products as growth factors and cytokines.[2]
There is a body of evidence which suggests that the inflammation and immune responses play a pivotal role in the pathogenesis of endometriosis, especially a series of cytokines and angiogenic factors.[3] The interleukin-1 (IL-1) system, which comprises 11 cytokines, is believed to play an important role in the pathophysiology of endometriosis, although this finding is controversial.[4] Both IL-1 alpha and IL-1beta (IL-1β) are the most potent pro-inflammatory cytokines, and IL-1 receptor antagonist is a naturally occurring anti-inflammatory cytokine.[5,6] Recent studies have shown decreased levels of IL-1 receptor antagonist (IL-1Ra) in peritoneal fluid (PF) from patients with endometriosis and disease-related dysmenorrhea.[7,8] At the same time, it was seen that the level of IL-4, a typical Th2 cytokine, is increased in endometriotic tissues,[9] with other authors observing that Th2-type immune responses are activated in women with endometriosis.[10,11] It is believed that IL-4 stimulates the proliferation of endometriotic stromal cells (ESCs),[11] suggesting a key role in the development of endometriosis. Being closely related to IL-4, IL-13 has some biological activities in common with IL-4, although their target cells may display variable response,[12] thus with a possible involvement in endometriosis. One of the most studied anti-inflammatory cytokines in endometriosis, IL-10, plays an important role in eliminating unwanted cells and cellular debris in a silent way. A very recent study has suggested that IL-10 may suppress the immunity against endometrial implants, contributing to the development of endometriosis.[13]
The awareness that the immune cell activation is one of most important features of endometriosis and that anti-inflammatory cytokines could have a pivotal role in the development of it, has lead us to investigate the most important anti-inflammatory serum cytokines in the patients with endometriosis. The present study aimed to investigate the serum anti-inflammatory cytokine profile in the patients with endometriosis compared with the healthy controls and the possible role of these markers in the prediction of the disease.
MATERIALS AND METHODS
Study population and design
A case–control study was conducted between June 2013 and June 2014 in “Dominic Stanca” Obstetrics and Gynecology Clinic, Cluj-Napoca, Romania.
The study included 160 patients admitted to the clinic, who were divided into two groups, as follows: Group I (endometriosis group)-80 women with regular menses, and with no history of pelvic infections, autoimmune, and neoplastic diseases, undergoing laparoscopy or laparotomy for suspected endometriosis. The evidence of endometriosis was verified by histopathological analysis. The severity of endometriosis was staged according to the revised American Society for Reproductive Medicine (rASRM) classification; almost all the patients included were staged as III (30-37.50%) or IV (47-58.75%) according to rASRM staging criteria, and only 3 cases (3.75%) were in stage II. Group II (control group)-80 healthy nonpregnant women aged between 18 and 40-year-old, without the clinical and paraclinical evidence of endometriosis.
Exclusion criteria
Previous pelvic surgeries, history of cancer, suspected malignancy, adenomyosis or leiomyoma, presurgical suspicion of evidence of premature ovarian failure, or the use of ovarian suppressive drug such as oral contraceptives, gonadotropin-releasing hormone agonists, progestins, or danazol in the preceding 6 months. None of the patients had taken anti-inflammatory medications or had been diagnosed with an inflammatory or infectious condition for ≥6 months before the study.
The study protocol was approved by the Local Ethics Committee of “Iuliu Haţieganu” University of Medicine and Pharmacy, Cluj-Napoca, Romania, and signed informed consent was received from each woman before the sample collection. The study was conducted under the tenets of Helsinki Declaration.
Data was collected for each subject included in the study in a form containing general and anthropometric data (weight, height), the heredo-collateral history, personal pathological history, and data on the age and onset of symptoms. The body mass index (BMI) was calculated as the ratio between the weight (kg) and the squared height (in meters). Five ml of venous blood was collected from each patient before breakfast, which was centrifuged and the serum obtained was stored at −70°C for future determinations.
Cytokine evaluation
We used multiplex cytokine kits (Invitrogen human cytokine 30-plex panel, LHC6003) in order to measure serum levels of IL-1Ra, IL-2, IL-2R, IL-4, IL-10, IL-13, and IL-15. Dose measurements were performed with the use of a Luminex 200 system (Luminex Corporation, Austin, TX, USA) in accordance with the manufacturer's specifications (Invitrogen Corporation, Carlsbad, CA, USA). The sensitivity of the test was specified by the manufacturer (Invitrogen Corporation, Carlsbad, CA, USA) in the informative material included in the kits.
The average sensitivity of the test for IL-1Ra was <20 pg/mL with an inter-assay variation coefficient of 5.1%. For IL-2, the average sensitivity of the test was <0.5 pg/mL with an inter-assay variation coefficient of 9.6%. The average sensitivity of the test for IL-2R was <10 pg/mL with an inter-assay variation coefficient of 4.2%. In the case of IL-4, the average sensitivity of the test was <0.5 pg/mL with an inter-assay variation coefficient of 8.7%. The sensitivity of the test for IL-10 was <0.5 pg/mL, and the inter-assay variation coefficient of 9.8%. The test for IL-13 revealed an average sensitivity of <5 pg/mL, with inter-assay variation coefficient of 9.6%, and for IL-15, the average sensitivity of the test was <15 pg/mL with an inter-assay variation coefficient of 7.6%.
Statistical analysis
Statistical analyzes were performed usin IBM SPSS Statistics for Windows, Version 22.0. Armonk, NY: IBM Corp. and GraphPad Prism version 5.00 for Windows, GraphPad Software, San Diego, California USA. Data was presented as a mean ± standard deviation for the groups. Independent t-tests and nonparametric Mann–Whitney U-test were used as statistical tests. Normality was tested with the Kolmogorov–Smirnov test with the Lilliefors correction (P > 0.05 was considered for a normal distribution). Receiver operating characteristic (ROC) curve analyzes and graphs were produced in GraphPad. P < 0.05 was regarded as significant.
RESULTS
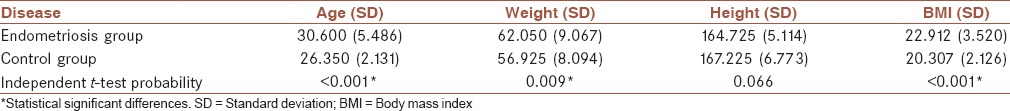
Table 1 presents the biometry data of the patients considered for the study, showing the significant differences in age, weight, and BMI between groups.
Table 1.
The biometry data of the patients considered for the study
The descriptive statistics of the studied cytokines is presented in Table 2, showing a significantly higher mean serum level of IL-1Ra, IL-4, and IL-10 in the endometriosis group (mean 30.15, 138.45, and 1.48, respectively, compared to 14.10, 84.71, and 0.68, respectively), and no significant differences in the mean serum levels of IL-2, IL-13, and IL-15 between the studied groups. After testing the distributions normality with the Kolmogorov–Smirnov test with Lilliefors correction, we have applied independent t-tests and a nonparametric test Mann–Whitney U-test for independent samples, both test showing the same results as presented in Table 2. The detection rate for IL-1Ra, IL-2, IL-4, IL-10, IL-13, and IL-15, respectively, in the studied groups, was 78.75, 90.00, 95.00, 83.75, 67.50, and 90.00%, respectively. IL-2R was detected in only 6.25% without the possibility of establishing a statistical significance.
Table 2.
Comparative statistics of the studied cytokines in endometriosis and control groups

Assuming the differences observed for IL-1Ra, IL-4, and IL-10 we have built the ROC curves and we have calculated areas under the curve for the studied cytokines, showing an area of 0.957 (0.029) for IL-1Ra, 0.751 (0.070) for IL-4, and 0.777 (0.061), respectively, for IL-10 [Figures 1–3].
Figure 1.
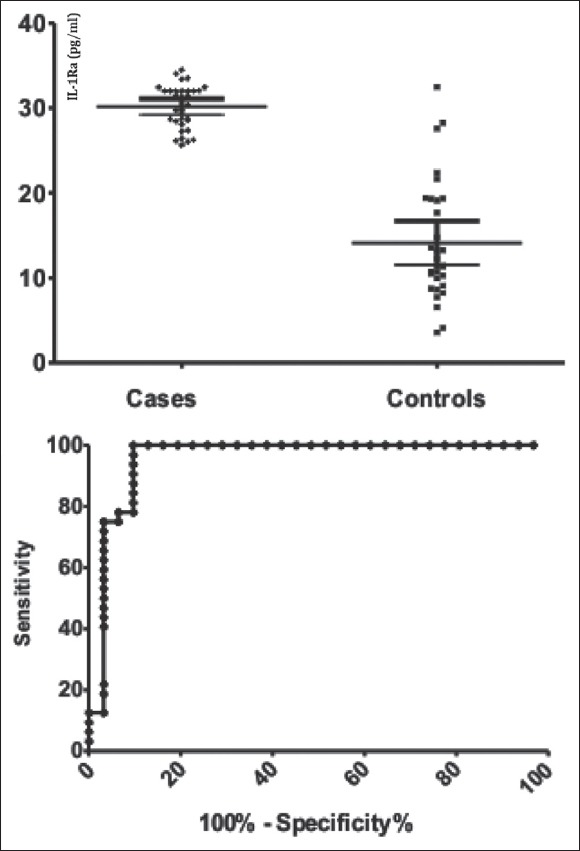
Comparison between endometriosis and control groups interleukin-1 receptor antagonist values (pg/mL) in the left pane and the receiver operating characteristic curve for interleukin-1 receptor antagonist in the right pane
Figure 3.
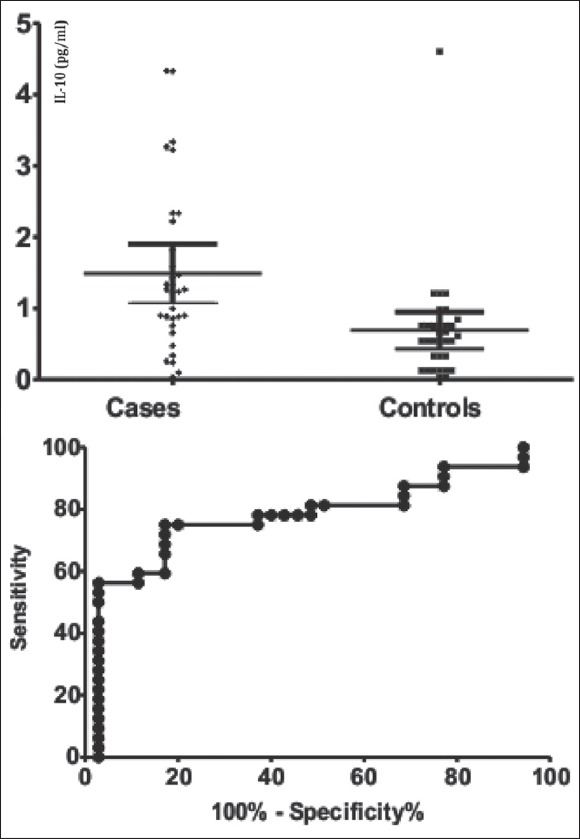
Comparison between endometriosis and control groups interleukin-10 values (pg/mL) in the left pane and the receiver operating characteristic curve for interleukin-10 in the right pane
Figure 2.
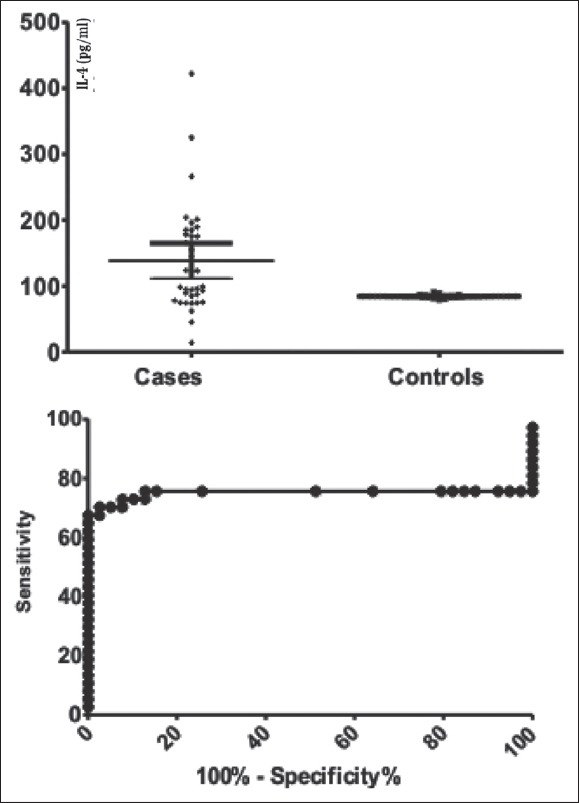
Comparison between endometriosis and control groups interleukin-4 values (pg/mL) in the left pane and the receiver operating characteristic curve for interleukin-4 in the right pane
DISCUSSION
Endometriosis is an inflammatory disease, affecting women of reproductive age, and it is associated with various immune-inflammatory processes and cytokines activation.
In the present study, we have found significantly higher serum level of IL-1Ra, IL-4, and IL-10, compared to healthy controls. We have also showed that there is no significant difference in IL-2, IL-13, and IL-15 serum levels between women with endometriosis and women free of the disease. IL-2R had a very low detection rate in the studied groups, so we were unable to draw any conclusions regarding its implication in the pathogenesis of endometriosis.
Cytokines are small proteins involved in intercellular communication, playing an important role in the link between immune and endometriosis tissues. A large number of studies attest a possible implication of pro-inflammatory and anti-inflammatory cytokines in the occurrence and progression of endometriosis.[14,15]
IL-1 is a cytokine that plays an important role in inflammation and immune response. IL-1 family consists of IL-1 alpha, IL-1β, and IL-1 receptor antagonist. IL-1 receptor antagonist is a naturally occurring anti-inflammatory cytokine, with both IL-1 alpha and IL-1β being the most potent pro-inflammatory cytokines.[5,6] IL-1 is a macrophage-derived factor. The effects of IL-1 are inhibited by IL-1 receptor type 2 (IL-1R2), soluble forms of IL-1R2 and IL-1Ra. Elevated serum and PF levels of IL-1α and IL-1Ra were observed in women with endometriosis in comparison with the healthy controls and also higher levels of IL-1Ra were observed in the early stages of the disease.[16] On the other hand, a study that tried to determine the levels of IL-1Ra in PF and serum from the patients with endometriosis, showed no significant difference in serum IL-1Ra between the patients with and without endometriosis, and a lower IL-1Ra concentrations in PF from the patients with endometriosis than in the patients without endometriosis.[7] At the same time, a study that aimed to investigate if IL-1β and IL-1Ra gene polymorphism could be used as markers of susceptibility in endometriosis found no association of endometriosis with IL-1β-511 promoter, IL-1β exon 5, and IL-1Ra gene polymorphisms.[17] A recent study, suggests a possible link between the endometrium, the tissue ectopic endometriosis and endometrioid ovarian cancer with the level expression of IL-1 ligands system (IL-1alpha, IL-1β, and IL-1Ra).[18]
IL-4 and IL-10 family are the main Th2 cytokines, with an anti-inflammatory effect. Several lines of evidence indicate that the Th2 immune response is associated with endometriosis. IL-4 is a cytokine with both stimulatory and inhibitory effects on the inflammatory system such as macrophage inhibition and T-cell activation. Increased concentrations of IL-4, a typical Th2 cytokine, were previously reported in endometriotic tissues, but its role in the pathogenesis of endometriosis is not clear. Previous authors have reported the elevated levels of IL-4 in endometriosis patients, both in plasma and peripheral blood mononuclear cell supernatants and at the same time, no difference in the concentrations of IL-4 in PF between women with and without endometriosis. Moreover, no difference was found between the IL-4 concentrations in women with different stages of endometriosis.[19,20] A very recent study investigating serum and PF immunological markers in adolescent girls with chronic pelvic pain, found that adolescents with endometriosis had significantly higher concentrations of serum and PF IL-4 and lower PF IL-2 compared with the controls, concluding that serum IL-4, peritoneal IL-2, and IL-4 are a good method of discrimination between the subjects with endometriosis and controls.[21] In the same line of ideas, a study that aimed to investigate a possible role of IL-4 in the development of endometriosis suggested that proliferation of endometriotic stromal cells induced by locally produced IL-4 is involved in the development of endometriosis.[15] Same authors have reported that IL-4 induces eotaxin in ectopic endometrial stromal cells (ESCs), which might promote angiogenesis and the subsequent development of endometriosis.[22]
The anti-inflammatory cytokine IL-10 plays an important role in eliminating the unwanted cells and cellular debris in a silent way. IL-10 is an acritical anti-inflammatory cytokine that is known to suppress Th1-like immune responses and promote Th2 responses. The levels of IL-10 in PF are significantly increased in the patients with endometriosis compared with controls, and increased IL-10 production may partially contribute to the disturbed immune regulation in the patients with endometriosis. A study focused on serum level of IL-10 in the patients with endometriosis observed that the serum level of IL-10 in the patients with endometriosis was significantly higher than that in healthy subjects or in control subjects with other gynecological disease and at the same time, IL-10 administration promoted the growth of endometrial lesions in a murine model. Thus, the authors suggested that IL-10 may suppress the immunity against the endometrial implants, contributing to the development of endometriosis.[13] Also, other authors found decreased serum levels of IL-19 and IL-22, but not IL-10, in women with ovarian endometriomas (OE), involving IL-10 family in the pathogenesis of endometriosis.[23] Moreover, it was shown that the antibody-mediated targeted delivery of IL-10 inhibits endometriosis in a syngeneic mouse model.[24] A higher concentration of IL-10 was also observed in the patients with ovarian endometriosis when compared to those without this type of disease, as well as when compared to control group patients in a recent study.[25] On the other hand, a recent meta-analysis, this meta-analysis on IL-10 genetic polymorphisms associated with endometriosis, suggests that the IL-10 -592 A/C polymorphisms conferred susceptibility to endometriosis.[26]
Moreover, different studies investigating IL-2, IL-4, IL-10, and other inflammatory markers have found higher peritoneal concentrations of pro-inflammatory cytokines (IL-1β, tumor necrosis factor-α, and IL-6), but also of IL-10 and lower concentrations of interferon-γ, IL-1Ra, and IL-15 in women with endometriosis. No statistical differences were found in IL-2, IL-4, IL-12, and IL-13 concentrations. Authors concluded that endometriosis development is accompanied by a shift toward Th2 immune response at the systemic and local levels, supporting the hypothesis regarding the immune imbalance and autoimmune nature of endometriosis.[9,10,27]
The values obtained for the areas under the ROC curves showed a good discriminative capacity for IL-1Ra, IL-4, and IL-10, especially IL-1Ra can be retained as a good test for correctly classify those with and without the disease. Our results are in accordance with some of the mentioned studies, but at the same time, the current literature is characterized by contradictory results regarding the involvement of anti-inflammatory Th2 cytokines in the pathogenesis of endometriosis. On the other hand, it is highly improbable that one single cytokine, either pro-inflammatory or anti-inflammatory, could be responsible for the occurrence and progression of endometriosis, and further studies focused especially on an association of inflammatory markers could bring some light in the entangled pathogenesis of this disease.
One main limitation of our study could be the lack of differentiation between the patients with OE and the patients with deep infiltrating endometriosis (DIE). OE and DIE are considered two distinct entities of endometriotic disease, and thus endometriosis can progresses to cystic ovarian disease and pelvic adhesions in some women, and to deeply infiltrating disease in other women, and sometimes to both stages of severe disease in the same woman.[3] Studies on pro-inflammatory cytokines showed differences between the patients with OE and DIE. In consequence, these differences could be present in the relationship with anti-inflammatory cytokines also, and so our results could be influenced by the lack of cleavage of the two forms of the disease in the investigation. Another limitation could be represented by the severity of endometriosis in the study population. Almost all of the patients had advanced endometriosis, and in consequence a differentiation in serum levels of the studied markers between the patients with superficial endometriosis and OE or DIE was not possible. Also, the detection sensitivity of multiplexed immunoassays is debatable. One study has shown that while multiplexed immunoassays have a sensitivity comparable to conventional ELISA, it is possible that the robustness may vary among different multiplex bead arrays.[28]
Due to the nonhomogenous data, the statistical analysis was conducted with the maximum parsimony assuming significance only when both parametric independent t-test for equality of means and the nonparametric Mann–Whitney U-test results were the same.
CONCLUSION
We have evaluated the differences in main anti-inflammatory cytokines serum levels in the patients with endometriosis compared to the healthy controls using a multiplexed cytokine assay. Our study has showed significantly higher serum levels of IL-1Ra, IL-4, and IL-10 in women with endometriosis compared to controls. Also, we showed that there is no difference in the serum levels of IL-2R, IL-13, and IL-15 between women with and without endometriosis. At the same time, our study showed that IL-1Ra, IL-4, and IL-10 could be used as prognostic factors for endometriosis. Further studies are needed to shed light on the possible involvement of anti-inflammatory cytokines in the pathogenesis of endometriosis.
Financial support and sponsorship
This paper was funded by “Iuliu Hatieganu” University of Medicine and Pharmacy internal grant number 1491/7/28.01.2014.
Conflicts of interest
There are no conflicts of interest.
AUTHOR'S CONTRIBUTION
AMM contributed in the conception of the work, study design, conducting the study, participated in analysis and interpretation of data, prepared the article draft, approval of the final version of the manuscript, and agreed for all aspects of the work. TD contributed in the conception of the work, conducting the study, revising the draft, approval of the final version of the manuscript, and agreed for all aspects of the work. RC contributed in the conception of the work, revising the draft, approval of the final version of the manuscript, and agreed for all aspects of the work. RFMH contributed in conducting the study, carried out the analysis and interpretation of data, statistics, revising the draft, approval of the final version of the manuscript, and agreed for all aspects of the work. CB contributed in the acquisition of data, revising the draft, approval of the final version of the manuscript, and agreed for all aspects of the work. PMR contributed with assistance for the acquisition of data, revising the draft, approval of the final version of the manuscript, and agreed for all aspects of the work. MD contributed in the conception and design of the work, drafting and revising the draft, approval of the final version of the manuscript, and agreed for all aspects of the work.
Acknowledgements
The authors would like to acknowledge “Iuliu Hatieganu” University of Medicine and Pharmacy, Cluj-Napoca, for supporting this study with internal grant number 1491/7/28.01.2014.
REFERENCES
- 1.Bischoff F, Simpson JL. Genetics of endometriosis: Heritability and candidate genes. Best Pract Res Clin Obstet Gynaecol. 2004;18:219–32. doi: 10.1016/j.bpobgyn.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 2.Sikora J, Anasz-Kondera Z, Mielczarek-Palacz A, Witek A. Monocyte activity after stimulation by serum of women with endometriosis. Ginekol Pol. 2007;78:772–6. [PubMed] [Google Scholar]
- 3.Bulun SE. Endometriosis. N Engl J Med. 2009;360:268–79. doi: 10.1056/NEJMra0804690. [DOI] [PubMed] [Google Scholar]
- 4.Kharfi A, Akoum A. Soluble interleukin-1 receptor type II blocks monocyte chemotactic protein-1 secretion by U937 cells in response to peripheral blood serum of women with endometriosis. Fertil Steril. 2002;78:836–42. doi: 10.1016/s0015-0282(02)03335-6. [DOI] [PubMed] [Google Scholar]
- 5.Rogus J, Beck JD, Offenbacher S, Huttner K, Iacoviello L, Latella MC, et al. IL1B gene promoter haplotype pairs predict clinical levels of interleukin-1beta and C-reactive protein. Hum Genet. 2008;123:387–98. doi: 10.1007/s00439-008-0488-6. [DOI] [PubMed] [Google Scholar]
- 6.Singh H, Sachan R, Goel H, Mittal B. Genetic variants of interleukin-1RN and interleukin-1beta genes and risk of cervical cancer. BJOG. 2008;115:633–8. doi: 10.1111/j.1471-0528.2007.01655.x. [DOI] [PubMed] [Google Scholar]
- 7.Zhang X, Wen J, Deng L, Lin J. Decreased levels of peritoneal interleukin-1 receptor antagonist in patients with endometriosis and disease-related dysmenorrhea. Fertil Steril. 2007;88:594–9. doi: 10.1016/j.fertnstert.2006.11.155. [DOI] [PubMed] [Google Scholar]
- 8.Kondera-Anasz Z, Sikora J, Mielczarek-Palacz A, Jonca M. Concentrations of interleukin (IL)-1alpha, IL-1 soluble receptor type II (IL-1 sRII) and IL-1 receptor antagonist (IL-1 Ra) in the peritoneal fluid and serum of infertile women with endometriosis. Eur J Obstet Gynecol Reprod Biol. 2005;123:198–203. doi: 10.1016/j.ejogrb.2005.04.019. [DOI] [PubMed] [Google Scholar]
- 9.Antsiferova YS, Sotnikova NY, Posiseeva LV, Shor AL. Changes in the T-helper cytokine profile and in lymphocyte activation at the systemic and local levels in women with endometriosis. Fertil Steril. 2005;84:1705–11. doi: 10.1016/j.fertnstert.2005.05.066. [DOI] [PubMed] [Google Scholar]
- 10.Podgaec S, Abrao MS, Dias JA, Jr, Rizzo LV, de Oliveira RM, Baracat EC. Endometriosis: An inflammatory disease with a Th2 immune response component. Hum Reprod. 2007;22:1373–9. doi: 10.1093/humrep/del516. [DOI] [PubMed] [Google Scholar]
- 11.Sinaii N, Cleary SD, Ballweg ML, Nieman LK, Stratton P. High rates of autoimmune and endocrine disorders, fibromyalgia, chronic fatigue syndrome and atopic diseases among women with endometriosis: A survey analysis. Hum Reprod. 2002;17:2715–24. doi: 10.1093/humrep/17.10.2715. [DOI] [PubMed] [Google Scholar]
- 12.Hershey GK. IL-13 receptors and signaling pathways: An evolving web. J Allergy Clin Immunol. 2003;111:677–90. doi: 10.1067/mai.2003.1333. [DOI] [PubMed] [Google Scholar]
- 13.Wu MY, Ho HN. The role of cytokines in endometriosis. Am J Reprod Immunol. 2003;49:285–96. doi: 10.1034/j.1600-0897.2003.01207.x. [DOI] [PubMed] [Google Scholar]
- 14.OuYang Z, Hirota Y, Osuga Y, Hamasaki K, Hasegawa A, Tajima T, et al. Interleukin-4 stimulates proliferation of endometriotic stromal cells. Am J Pathol. 2008;173:463–9. doi: 10.2353/ajpath.2008.071044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rogus J, Beck JD, Offenbacher S, Huttner K, Iacoviello L, Latella MC, et al. IL1B gene promoter haplotype pairs predict clinical levels of interleukin-1beta and C-reactive protein. Hum Genet. 2008;123:387–98. doi: 10.1007/s00439-008-0488-6. [DOI] [PubMed] [Google Scholar]
- 16.Chun S, Kim H, Ku SY, Suh CS, Kim SH, Kim JG. The association between endometriosis and polymorphisms in the interleukin-1 family genes in Korean women. Am J Reprod Immunol. 2012;68:154–63. doi: 10.1111/j.1600-0897.2012.01136.x. [DOI] [PubMed] [Google Scholar]
- 17.Hsieh YY, Chang CC, Tsai FJ, Wu JY, Shi YR, Tsai HD, et al. Polymorphisms for interleukin-1 beta (IL-1 beta)-511 promoter, IL-1 beta exon 5, and IL-1 receptor antagonist: Nonassociation with endometriosis. J Assist Reprod Genet. 2001;18:506–11. doi: 10.1023/A:1016653127262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Keita M, Bessette P, Pelmus M, Ainmelk Y, Aris A. Expression of interleukin-1 (IL-1) ligands system in the most common endometriosis-associated ovarian cancer subtypes. J Ovarian Res. 2010;3:3. doi: 10.1186/1757-2215-3-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yin H, Wang S, Dai S. Study on interferon gamma and interleukin-4 contents of plasma and cultured monoclear cell supernatants in patients with endometriosis. Zhonghua Fu Chan Ke Za Zhi. 2000;35:327–8. [PubMed] [Google Scholar]
- 20.Gazvani MR, Bates MD, Vince GS, Christmas SE, Lewis-Jones DI, Kingsland CR. Peritoneal fluid concentrations of interleukin-4 in relation to the presence of endometriosis, its stage and the phase of the menstrual cycle. Acta Obstet Gynecol Scand. 2001;80:361–3. [PubMed] [Google Scholar]
- 21.Drosdzol-Cop A, Skrzypulec-Plinta V, Stojko R. Serum and peritoneal fluid immunological markers in adolescent girls with chronic pelvic pain. Obstet Gynecol Surv. 2012;67:374–81. doi: 10.1097/OGX.0b013e31825cb12b. [DOI] [PubMed] [Google Scholar]
- 22.Ouyang Z, Osuga Y, Hirota Y, Hirata T, Yoshino O, Koga K, et al. Interleukin-4 induces expression of eotaxin in endometriotic stromal cells. Fertil Steril. 2010;94:58–62. doi: 10.1016/j.fertnstert.2009.01.129. [DOI] [PubMed] [Google Scholar]
- 23.Santulli P, Borghese B, Chouzenoux S, Streuli I, Borderie D, de Ziegler D, et al. Interleukin-19 and interleukin-22 serum levels are decreased in patients with ovarian endometrioma. Fertil Steril. 2013;99:219–26. doi: 10.1016/j.fertnstert.2012.08.055. [DOI] [PubMed] [Google Scholar]
- 24.Schwager K, Bootz F, Imesch P, Kaspar M, Trachsel E, Neri D. The antibody-mediated targeted delivery of interleukin-10 inhibits endometriosis in a syngeneic mouse model. Hum Reprod. 2011;26:2344–52. doi: 10.1093/humrep/der195. [DOI] [PubMed] [Google Scholar]
- 25.Podgaec S, Dias Junior JA, Chapron C, Oliveira RM, Baracat EC, Abrão MS. Th1 and Th2 ummune responses related to pelvic endometriosis. Rev Assoc Med Bras. 2010;56:92–8. doi: 10.1590/s0104-42302010000100022. [DOI] [PubMed] [Google Scholar]
- 26.Fan W, Li S, Chen Q, Huang Z, Ma Q, Xiao Z. Association between interleukin-10 promoter polymorphisms and endometriosis: A meta-analysis. Gene. 2013;515:49–55. doi: 10.1016/j.gene.2012.11.037. [DOI] [PubMed] [Google Scholar]
- 27.Mier-Cabrera J, Jiménez-Zamudio L, García-Latorre E, Cruz-Orozco O, Hernández-Guerrero C. Quantitative and qualitative peritoneal immune profiles, T-cell apoptosis and oxidative stress-associated characteristics in women with minimal and mild endometriosis. BJOG. 2011;118:6–16. doi: 10.1111/j.1471-0528.2010.02777.x. [DOI] [PubMed] [Google Scholar]
- 28.Elshal MF, McCoy JP. Multiplex bead array assays: Performance evaluation and comparison of sensitivity to ELISA. Methods. 2006;38:317–23. doi: 10.1016/j.ymeth.2005.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]


