Abstract
Background:
This study investigated blackberry (Persian mulberry) effects on apo A-I, apo B, high-sensitivity-C-reactive protein (hs-CRP), and systolic blood pressure (SBP) and diastolic blood pressure (DBP) in dyslipidemic patients.
Materials and Methods:
In this 8-week randomized clinical trial, 72 dyslipidemic patients were randomly divided into two groups: Intervention (300 mL/day blackberry juice with pulp) and control group (usual diets). Before and after the intervention, fasting blood samples were taken from both groups and serum concentration of lipoprotein, apo A-I and apo B, serum lipids (total cholesterol, low-density lipoprotein, high-density lipoprotein [HDL], and triglyceride), hs-CRP were measured. Blood pressure before and after the study was measured with a mercury manometer.
Results:
At week 8 in the intervention group, apo A-I and HDL increased significantly (P = 0.015, P = 0.001, respectively), apo B and hs-CRP decreased significantly (P = 0.044, P = 0.04, respectively). Mean changes in apo A-I and HDL and apo B/apo A-I ratio were significant between the groups (P = 0.005, P = 0.014, and P = 0.009, respectively). After 8 weeks, there was a significant difference between hs-CRP mean values (P = 0.01) of the groups. At week 8, SBP decreased significantly (P = 0.005) in the intervention group with no significant differences for SBP mean values between the groups. No significant changes were observed in other lipid parameters and DBP in the intervention group and between the groups.
Conclusion:
Blackberry consumption may exert beneficial effects on apolipoproteins, blood pressure, and inflammatory markers in individuals with lipid disorders.
Keywords: Apolipoproteins, blackberry, blood pressure, high-sensitivity-C-reactive protein
INTRODUCTION
Cardiovascular diseases (CVD), the leading cause of death in most countries,[1] are responsible for 35% of deaths in developing countries and 30% of death rate worldwide.[2] In Iran as in other developing countries, these diseases are increasing as a result of nutritional and epidemiological transition which gives rise to mortality rate more than 46%.[3]
In addition to risk factors for atherosclerosis including genetic factors, diet, and lifestyle, lipid profile, and vascular inflammatory responses are of importance.[4] Increased levels of triglyceride (TG) and low-density lipoprotein-cholesterol (LDL-C) are associated with CVD risk. On the other hand, the concentration of high-density lipoprotein-cholesterol (HDL-C), independent of TG and LDL-C levels, is inversely associated with CVD risk.[5,6] Studies conducted in Iran show, the prevalence of lipid disorders, diabetes, hypertension, and obesity is increasing[7] which result from behavioral and nutritional characteristics and physical activity of Iranians.[8] Studies indicate that 10% reduction in serum total cholesterol (TC) reduces coronary heart disease (CHD) incidence to 30%.[1] In this regard, in addition to medical treatments, the role of diet, fruits, and vegetables consumption has been demonstrated in controlling hyperlipidemia.[9] There is an increasing body of epidemiologic evidences showing the positive association between diets rich in fruits and vegetables and CHD risk decrease which can be attributed to both deactivating reactive oxygen species in the initiation and progression of chronic diseases by antioxidants and their phenolic contents.[10,11] Nonnutrient polyphenols such as flavonoids exist in considerable amounts in fruits and vegetables that have powerful antioxidant effects on chemical systems. Both animal studies and clinical trials have shown beneficial effects of polyphenol-rich foods on cardiovascular health.[12,13,14] Among them, berries are rich sources of flavonoids (anthocyanins), some minerals (sodium, potassium, calcium, selenium, zinc, and phosphorus), Vitamins (A, B, C, and E), phenolic acids (gallic, P-coumaric, caffeic, and fluoric), and phenolic polymers (alajik acid).[15] Blackberry (Morus nigra L., Persian mulberry, and black mulberry), a native fruit to Iran, contains high levels of anthocyanins.[16] Interestingly, berries lack interfering compounds such as ethanol, fat and caffeine,[17] and their flavonoids can be effective on cardiovascular health by several mechanisms. For instance, they may inhibit LDL-C oxidation, reduce inflammation associated with atherosclerosis and also affect reverse cholesterol transport by reducing TC and LDL-C.[18] Evidences show adding berries to the diet can positively affect cardiovascular risk factors through inhibition of inflammation, free radical scavenging, regulating eicosanoid metabolism, improving endothelial function, reducing blood pressure, inhibition of platelet aggregation, increasing circulating HDL-C, and increasing resistance of LDL-C oxidation.[19] Studies on the effects of berries on lipid profile and inflammatory markers reveal contradictory results.[20,21,22,23,24,25] In some previous studies, significant changes in lipid profile and some inflammatory markers have been observed whereas other studies did not report any significant changes which may be contributed to limitations including lack of control group,[24] the form of consumed berries (capsules or powder),[25,26] and consuming the combination of different berries,[27] berry consumption along with a special diet,[27] the small sample size, and short intervention period.[26] Therefore, according to the importance of antioxidant role of phenolic compounds in protection of many diseases, it seems that adding berries to diet can affect the heart diseases risk factors, such as lipid profile, blood pressure, and inflammatory markers.
Thus, in this study we hypothesised that blackberry consumption can improve apo A-I and B, high-sensitivity-C-reactive protein (hs-CRP), and blood pressure in dyslipidemic patients in a larger sample size and a control group, to determine the effects of the blackberry itself, and in the form of the juice with pulp.
MATERIALS AND METHODS
Study population and design
This study was a randomized clinical trial evaluating the effects of blackberry consumption on serum apo A-I, apo B, hs-CRP, and blood pressure on dyslipidemic patients.
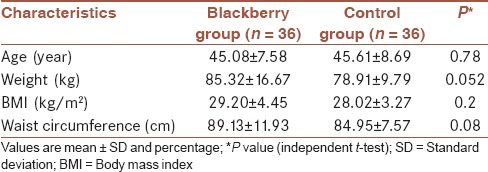
Of patients who referred to social security and Bu-Ali Sina clinics of Qazvin, 72 volunteers aged 25-65 years, were recruited with a diagnosis of hyperlipidemia[28] [Table 1]. Other inclusion criteria were: Body mass index = 20-35 kg/m2, TC >200 mg/dL, and LDL-C >100 mg/dL. Exclusion criteria were: Taking drugs that affect lipid metabolism; smoking and alcohol consumption; pregnancy or lactation; systemic, chronic and inflammatory diseases, liver, and kidney diseases; having allergy to berry families; and changes in diets and physical activities. At the beginning, the objectives of the study were described to participants in detail. It was emphasized that consumption of berries, had no adverse effects, and they could leave the study whenever they wanted. The present study was conducted according to consolidated standards of reporting trials guidelines[29] and it was approved by the Research Ethics Committee of Tehran University of Medical Sciences (90/D/130/28/28) and was registered in Iranian registry of clinical trials (IRCT201205182365N7).
Table 1.
Characteristics of participants at baseline
Treatment juices
Six-hundred kilograms of blackberry were purchased. Afterward, ready berry pulps were mixed with juice and were packed in bottles without preservatives or additives. To eliminate pathogens and increase shelf time, juices were pasteurized which all were done by the Avan Food Company. Prepared bottles were stored at +5°C. Every week, seven bottles were given to the participants in the intervention group, and they were asked to keep them in the fridge.
Study design
As participants were randomly divided into two groups of blackberry and control group using the table of random numbers, there was no confounding effect of sex (two groups of 36 people).
Individuals in the intervention group, took 300 mL of blackberry juice with pulp every day for 8 weeks, and the control group continued their usual diet with no berries. Participants were instructed to have half of the bottle in the morning (at 10:00 am) and the other half in the afternoon snack (5:00 pm) and refrain from eating berries with other meals and from ingesting them immediately after dairy products. Each week, participants were contacted to be sure of taking berries. Participants were asked to deliver full and empty bottles at the end of each week to calculate the compliance.
Data collection
Demographic characteristics of participants, including age, sex, medical history, type, and amount of medication were obtained by interviewing. Height was measured using the height gauge attached to the scale with accurately 0.5 cm without shoes and weight was measured by Seca scale with an accuracy of 100 g, in the fasting state and with light clothes and bare feet. Data of food intake including calorie, macronutrient, and micronutrient and daily servings of fruits and vegetables were obtained by 24 h food recall questionnaires at the beginning and at the end of the study and were analyzed by nutritionist.[4] Data of physical activity were collected using the international physical activity questionnaire at the beginning and at the end of the study. At weeks 0 and 8, fasting blood samples were taken from both groups to measure biochemical markers. Serum was isolated by centrifugation at 3000 rpm and stored at −70°C until the end of the study. TGs, TC, LDL-C, and HDL-C were measured by using colorimetric method (Pars Azmoon commercial kit, Iran). Serum hs-CRP was measured by ELISA (The Binding Site Group Ltd., UK). The concentration of apo A-I and apo B was determined by turbidimetric method (Pars Azmoon commercial kit, Iran). Systolic blood pressure (SBP) and diastolic blood pressure (DBP) were measured once, both at the beginning and at the end of the study, sitting on chair and in nonstress conditions using a standard mercury sphygmomanometer. Fiber in the sample of blackberry was measured according to procedures of American Oil Chemists Society with the device of a single fiber and polyphenols were measured by high-performance liquid chromatography.[26,30]
Measurement of total phenolic content of blackberry
Total phenolic content of blackberry extract was performed employing the literature methods involving Folin-Ciocalteu reagent and gallic acid as standard.[31] The extract samples (0.5 mL of different dilutions) were mixed with 2.5 mL of 0.2 N Folin-Ciocalteau reagent (Sigma-Aldrich) for 5 min and 2.0 mL of 75 g/L sodium carbonate were then added. The mixture was allowed to stand for 2 h at room temperature. The absorbance was measured at 760 nm with a double beam PerkinElmer ultraviolet/visible spectrophotometer (USA). The standard curve was prepared using 50-250 mg/mL solutions of gallic acid in methanol-water (1:1, v/v).
Total phenol values are expressed in terms of gallic acid equivalent (mg/g of dry mass) which is a common reference phenolic compound.
Statistical analysis
The data analyzed using SPSS 16 software (SPSS Inc., Chicago, IL, USA). Data were assessed for normality using Kolmogorov-Smirnov test. For variables with no normal distribution, nonparametric test (Wilcoxon test and Mann-Whitney) was used. At weeks 0 and 8, quantitative variables were compared by independent t-test between the two groups. Paired t-test was used to compare the mean changes between pre- and post-intervention. Values are expressed as (mean ± standard deviation) and P < 0.05 was considered as statistically significant.
RESULTS
According to the findings of the current study, the polyphenols were 316 mg and fiber was 9.87 g/100 g of blackberry.
Of 72 participants, 54 (75%) were men and 18 (25%) were female. Demographic and clinical characteristics of participants at baseline show no significant difference between the groups [Table 1].
Dietary intake and physical activity
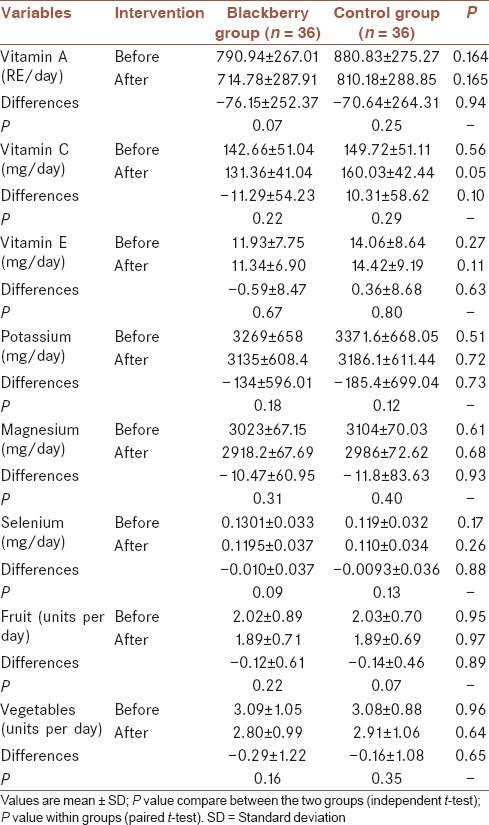
No significant differences were observed for physical activity (P = 0.55, data not shown) and dietary intakes between the groups before and after the study [Tables 2 and 3].
Table 2.
Energy and macronutrient intake in groups before and after of the intervention
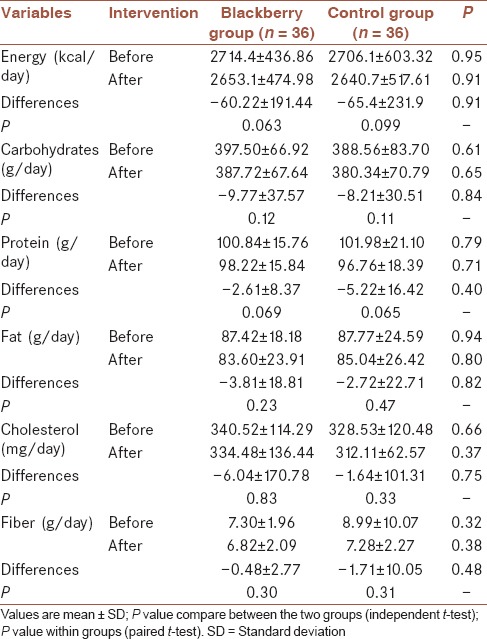
Table 3.
Mineral, micronutrient, fruit, and vegetable intake in groups before and after of the intervention
Blood pressure
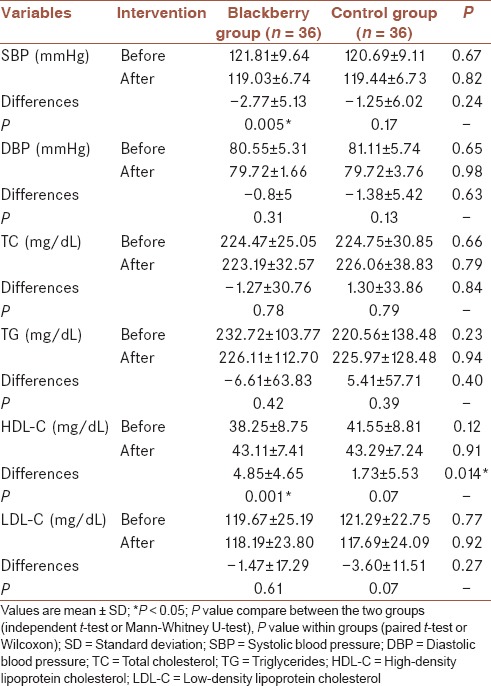
Blackberry consumption reduced mean SBP significantly (P = 0.005) whereas this change was not significant in the control group (P = 0.17). No significant difference was observed in mean changes and mean values of SBP between the groups (P = 0.24 and P = 0.82, respectively) while after the intervention, there was not a significant difference in mean changes and mean values of DBP between the groups (P = 0.63 and P = 0.98, respectively) and within the each group (respectively P = 0.3 [intervention group] and P = 0.13 [control group]) [Table 4].
Table 4.
SBP and DBP and lipid profile before and after of the intervention
Lipid profile and high-sensitivity-C-reactive protein
As shown in Table 4, no significant difference was observed in mean HDL-C between the groups at the end of the study (P = 0.91), however, comparison of mean changes between the groups revealed significant difference (0.014), also the mean concentration of HDL-C levels in the intervention group had a significant increase (0.001). However, we failed to find a significant change in other lipid parameters such as TC, LDL-C, and TG neither within nor between the groups [Table 4]. As shown in Table 5, blackberry consumption increased the mean value of apo A-I significantly (P = 0.015) compared to the baseline. Despite the decrease of mean value of apo A-I in the control group, this reduction was not significant (P = 0.11). However, the differences between the groups in mean changes of apo A-I was significant (P = 0.005). Mean changes of apo B were not significantly different between the groups at the end of study whereas mean changes of apo B decreased significantly in the intervention group (P = 0.044), while no significant differences were observed in the control group (P = 0.07). The mean ratio of apo B to apo A-I in the intervention group was 0.76 ± 0.20 at baseline which declined to 0.69 ± 0.17 at the end of the study and this decrease was statistically significant (P = 0.001) but not in control group (P = 0.77). The statistical tests showed no significant differences between the means of two groups at week 8 (P = 0.47) whereas the differences between the mean change of this ratio was significant between the groups (P = 0.009).
Table 5.
Serum apo A-I and apo B, apo B/apo A-I, and hs-CRP in before and after of the intervention
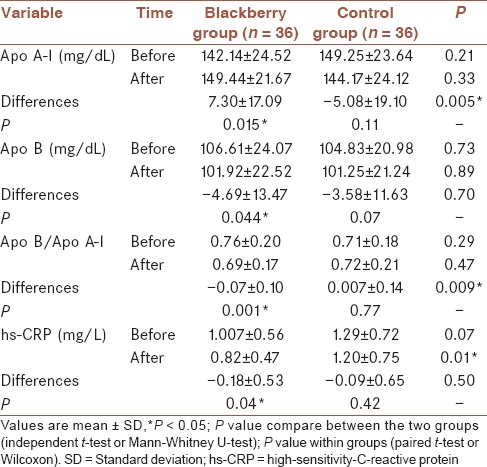
This study showed a significant reduction in mean value of hs-CRP in the intervention group (P = 0.04) and a nonsignificant reduction in the control group (P = 0.42). On the other hand, at the end of the study statistical tests showed a significant difference for mean hs-CRP between the groups (P = 0.01) but we failed to find a significant difference in mean changes of this factor between the groups (P = 0.50) [Table 5].
DISCUSSION
Present study showed that consumption of blackberry juice with pulp for 8 weeks, gave a significant rise to apo A-I and HDL-C and decreased the concentration of apo B, and hs-CRP. Mean changes of HDL-C, apo A-I, and apo B/apo A-I ratio were significant between the groups. Significant differences were observed for mean values of hs-CRP between the groups following blackberry intake. Besides, SBP decreased significantly in the intervention group compared to baseline after blackberry ingestion.
The finding of serum HDL-C in the present study was similar to previous studies.[21,24,27] However in contrast to our findings, in a study by Lee et al. in 2008 in diabetic patients, there were no significant changes in serum HDL-C after daily consumption of three 500 mg capsules of cranberry extract for 12 weeks.[32] In that study, the extract was in the form of capsules and was administrated for a long time (12 weeks) which reduced participant adherence to the intervention. In that study, since participants were diabetic and according to evidences, lipid abnormalities exist in diabetic patients,[33] this issue may disturb the results and prevent HDL-C from being affected. Similarly, in a study by Basu et al., no significant changes were shown in HDL-C after taking 50 g of dried strawberries for 4 weeks[24] which may be due to the berries consumed in dried powder form or small size and short duration of the intervention compared to this study in which blackberries were consumed in juice form with pulp. Skoczyñska et al. did not detect any significant changes in the HDL-C after consuming 250 mL/day of chokeberry extract during a two 6-week period.[34] Polyphenols administered in that study were approximately 1060 mg but in our study were 948 mg. Regarding equal amounts of polyphenols consumed in these two studies, we believe that HDL-C did not differ because that experiment was conducted on healthy men with mild hyperlipidemia. According to the current study, 8-week consumption of blackberries did not have significant effects on other lipid parameters including TC, LDL-C, and TG, which were in line with previous similar studies.[22,23,24,35,36,37] In contrast, in the study by Skoczyñska et al., daily intake of 250cc of chokeberry extract for two 6-week periods decreased serum TC and LDL-C, significantly[34] which supports the observations by Lee et al. in which LDL-C decreased significantly, and HDL-C did not change significantly following consumption of cranberry (three capsules of 500 mg for 12 weeks), in diabetic patients.[32] These findings could result from longer duration of treatments in both studies in contrast to our study. In Basu's study in 2009, a reduction in TC and LDL-C and no changes in HDL-C, very low-density lipoprotein, and TG were reported following ingesting 50 g of dried strawberries for 4 weeks. This result may be explained by different groups of women suffered from metabolic syndrome, compared with our study.[25] In another study, supplementation with anthocyanins (two capsules of 80 mg for 12 weeks) reduced serum LDL-C whereas no changes in TC and TG were observed.[21] Anthocyanin consumed in that study was 160 mg, however, in our study it was equal to 948 mg of polyphenols thus, the reduction in LDL-C in contrast to our study, may result from a longer period of intervention. Also, in studies by Ruel et al., in 2006 and Burton-Freeman et al., in 2010 that were performed to evaluate the effects of cranberry and strawberry respectively, the only observed reduction was in serum concentration of TG.[20,38]
Further, there were a significant increase in serum apo A-I and a significant decrease of apo B concentration after consumption of blackberry compared to the baseline. In Ruel et al. study, increasing the dose of cranberry extract during 12 weeks (125, 250, and 500 mL) did not alter apo B although it increased apo A-I.[20] In contrast to our findings, in a study by Qin et al. on the effects of anthocyanin supplementation in dyslipidemic people, daily consumption of two capsules of 80 mg anthocyanins for 12 weeks did not affect apo A-I and apo B.[21] This issue could be as a result of lower contents of anthocyanin compared with our study which was 160 mg/day (two capsules of 80 mg) versus intaking 948 mg/day polyphenols. Regarding the fact that apo B and apo A-I are the major apolipoproteins of LDL and HDL-C respectively, the reduction of apo B and increase of apo A-I in the intervention group, could be attributed to a nonsignificant reduction in serum LDL-C and a significant increase in serum HDL-C.[1] By these two significant changes, apo B/apo A-I, an important risk factor for CVD, was significantly decreased in the intervention group. Another finding of this study was a significant reduction in hs-CRP in the intervention group compared to control group and baseline. Contrary to our results, in the study by Skoczyñska et al., daily drinking of 250 mL chokeberry extract caused no significant changes in CRP levels which may be due to the hyperlipidemia in subjects although they were healthy individuals.[34] In another study, 3 weeks of taking two 500 mg capsules of cranberry did not alter CRP significantly[32] which might be as a result of taking cranberries in capsule forms. Additionally, Basu et al. did not report a significant change in concentration of hs-CRP after 4 weeks of consuming 50 g of dried strawberries in 16 women with metabolic syndrome,[25] which a small sample size, short-term intervention, and lack of control group could affect the results. On the other hand, Lehtonen et al. in 2010 and Udani et al. in 2011 failed to find significant changes in serum concentrations of CRP, interleukin, and adiponectin following the consumption of other types of berries.[28,37] This could be due to low dose of strawberries (163 g for 20 weeks) or consuming a combination of berries in the first study and the small sample size and short duration of the intervention (n = 10, 1-month) in the second one.
The beneficial effects observed can be attributed to phenolic compounds, anthocyanins, and other features existing in blackberry.[39] Anthocyanins represent a wide range of biological activities including antioxidant, anti-inflammatory, antibacterial, and anticancer activities as well as, improving eyesight, induction of apoptosis and neuroprotection. Antioxidant activities of anthocyanins, (clearing free radicals, chelating metal-binding protein), including protecting LDL against oxidation, has been demonstrated in a number of different experimental systems. It has been shown that pelargonidin, cyanidin, defidin, peonidin, and malvidin (common types of anthocyanins) have inhibitory effects on NO production in macrophages.[40] Accordingly, berry anthocyanins may play protective roles in the cardiovascular system through reducing oxidative stress and inflammation, which is mediated by affecting NO activity, modifying dyslipidemia, and modulating the expression of eNO and thus maintaining normal vascular function and blood pressure.[41]
Current study revealed a significant reduction in SBP after consumption of cranberry in the compared to baseline, while no significant changes were observed in DBP although at the end of the study these parameters and their mean changes were not significant between the groups. These results were similar to previous findings.[27] In contrast, Skoczyñska et al.,[34] Basu et al.,[24] and also Lehtonen et al.,[37] revealed significant reductions in both SBP and DBP. Unlike our study, the resulting changes in DBP in these studies could be attributed to the differences in the two studies’ groups as well as longer-term of intervention in the second study. However, contrary to these results, Jenkins et al. and Udani et al. did not detect any significant changes in either SBP or DBP after taking 454 g of strawberry and 200 g, respectively of Acai berry for 1-month.[22,28] In both studies, treatment duration was 1-month, and it can be assumed that the lack of impact on blood pressure in these interventions was due to short-term of the interventions.
According to conducted studies and to our knowledge, this study is the first study on the effects of blackberry fruit, native to Iran, on lipid profile, apolipoproteins, hs-CRP, and blood pressure in patients with lipid disorders. A limitation of the present study was that it was not double blind and had no placebo.
CONCLUSION
Overall, this study showed that blackberry consumption has the potential to improve concentrations of HDL-C, apo A-I, apo B, and hs-CRP which in turn can modify the cardiovascular risk factors. Further research to investigate other inflammatory and oxidative stress markers with regard to longer duration and administrating different doses of blackberry is recommended.
Financial support and sponsorship
Tehran University of Medical Sciences.
Conflicts of interest
There are no conflicts of interest.
AUTHOR's CONTRIBUTIONS
SKA contributed in the conception of the work, designing the study, drafting the draft, approval of the final version of the manuscript, and agreed for all aspects of the work. MV contributed in the conception of the work, conducting the study, revising the draft, approval of the final version of the manuscript, and agreed for all aspects of the work. FS contributed in conducting the study and approval of the final version of the manuscript, and agreed for all aspects of the work. AT contributed in revising the draft, approval of the final version of the manuscript, and agreed for all aspects of the work. MG contributed in analyzing and interpreting the data and approval of the final version of the manuscript, and agreed for all aspects of the work. DK contributed in conducting the work and approval of the final version of the manuscript, and agreed for all aspects of the work. VM contributed in the conducting the work.
Acknowledgments
The present study was funded by Vice Chancellor for Research, Tehran University of Medical Sciences, Tehran, Iran. We thank all the volunteers who participated in this study. We would like to appreciate the cooperation made by the Mr. Khadem Mola the manager of Avan Food Company and staff of Khatam Pathobiology Laboratory of Qazvin.
REFERENCES
- 1.Mahan LK, Raymond JL, Escott-Stump S. Philadelphia Department, PA, USA: Elsevier Health Sciences; 2013. Krause's Food & the Nutrition Care Process. [Google Scholar]
- 2.Writing Group Members. Lloyd-Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, et al. Heart disease and stroke statistics-2010 update: A report from the American Heart Association. Circulation. 2010;121:e46–215. doi: 10.1161/CIRCULATIONAHA.109.192667. [DOI] [PubMed] [Google Scholar]
- 3.Ghassemi H, Harrison G, Mohammad K. An accelerated nutrition transition in Iran. Public Health Nutr. 2002;5:149–55. doi: 10.1079/PHN2001287. [DOI] [PubMed] [Google Scholar]
- 4.Zafra-Stone S, Yasmin T, Bagchi M, Chatterjee A, Vinson JA, Bagchi D. Berry anthocyanins as novel antioxidants in human health and disease prevention. Mol Nutr Food Res. 2007;51:675–83. doi: 10.1002/mnfr.200700002. [DOI] [PubMed] [Google Scholar]
- 5.Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive summary of the third report of the national cholesterol education program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III) JAMA. 2001;285:2486–97. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 6.Assmann G, Schulte H. Relation of high-density lipoprotein cholesterol and triglycerides to incidence of atherosclerotic coronary artery disease (the PROCAM experience) Am J Cardiol. 1992;70:733–7. doi: 10.1016/0002-9149(92)90550-i. [DOI] [PubMed] [Google Scholar]
- 7.Agheli N, Zadeh SA, Rajabi M. The prevalence of cardiovascular risk factors among population aged over 30 years in Rasht and Qazvin. J Qazvin Univ Med Sci. 2005;35:59–65. [Google Scholar]
- 8.Jalali BA, Rafie M, Mozaffari H. Lipoprotein (A) as a strong risk factor for coronary artery disease in Iranian population. Med J Islam Acad Sci. 2000;13:5–9. [Google Scholar]
- 9.Ebrahimzadeh AV, et al. Effect of flaxseed (Linum usitatissimum) on serum lipid profile and malondialdehyde in hyperlipidemic rabbits. Pharm Sci. 2009;15:195–204. [Google Scholar]
- 10.Fernandez-Panchon MS, Villano D, Troncoso AM, Garcia-Parrilla MC. Antioxidant activity of phenolic compounds: From in vitro results to in vivo evidence. Crit Rev Food Sci Nutr. 2008;48:649–71. doi: 10.1080/10408390701761845. [DOI] [PubMed] [Google Scholar]
- 11.Arts IC, Hollman PC. Polyphenols and disease risk in epidemiologic studies. Am J Clin Nutr. 2005;81(1 Suppl):317S–25S. doi: 10.1093/ajcn/81.1.317S. [DOI] [PubMed] [Google Scholar]
- 12.Huntley A. Grape flavonoids and menopausal health. Br Menopause Soc J. 2007;13:165–9. doi: 10.1258/175404507783004177. [DOI] [PubMed] [Google Scholar]
- 13.Baba S, Osakabe N, Kato Y, Natsume M, Yasuda A, Kido T, et al. Continuous intake of polyphenolic compounds containing cocoa powder reduces LDL oxidative susceptibility and has beneficial effects on plasma HDL-cholesterol concentrations in humans. Am J Clin Nutr. 2007;85:709–17. doi: 10.1093/ajcn/85.3.709. [DOI] [PubMed] [Google Scholar]
- 14.Duffy SJ, Keaney JF, Jr, Holbrook M, Gokce N, Swerdloff PL, Frei B, et al. Short- and long-term black tea consumption reverses endothelial dysfunction in patients with coronary artery disease. Circulation. 2001;104:151–6. doi: 10.1161/01.cir.104.2.151. [DOI] [PubMed] [Google Scholar]
- 15.Hassan HA, Abdel-Aziz AF. Evaluation of free radical-scavenging and anti-oxidant properties of black berry against fluoride toxicity in rats. Food Chem Toxicol. 2010;48:1999–2004. doi: 10.1016/j.fct.2010.05.018. [DOI] [PubMed] [Google Scholar]
- 16.Barbour JR, Read RA, Barnes RL, Morus L. Mulberry. The Woody Plant Seed Manual. Agric. 2008;727:728–32. [Google Scholar]
- 17.Hassan HA, Yousef MI. Mitigating effects of antioxidant properties of black berry juice on sodium fluoride induced hepatotoxicity and oxidative stress in rats. Food Chem Toxicol. 2009;47:2332–7. doi: 10.1016/j.fct.2009.06.023. [DOI] [PubMed] [Google Scholar]
- 18.Reed J. Cranberry flavonoids, atherosclerosis and cardiovascular health. Crit Rev Food Sci Nutr. 2002;42(3 Suppl):301–16. doi: 10.1080/10408390209351919. [DOI] [PubMed] [Google Scholar]
- 19.Seeram NP. Berry fruits: Compositional elements, biochemical activities, and the impact of their intake on human health, performance, and disease. J Agric Food Chem. 2008;56:627–9. doi: 10.1021/jf071988k. [DOI] [PubMed] [Google Scholar]
- 20.Ruel G, Pomerleau S, Couture P, Lemieux S, Lamarche B, Couillard C. Favourable impact of low-calorie cranberry juice consumption on plasma HDL-cholesterol concentrations in men. Br J Nutr. 2006;96:357–64. doi: 10.1079/bjn20061814. [DOI] [PubMed] [Google Scholar]
- 21.Qin Y, Xia M, Ma J, Hao Y, Liu J, Mou H, et al. Anthocyanin supplementation improves serum LDL-and HDL-cholesterol concentrations associated with the inhibition of cholesteryl ester transfer protein in dyslipidemic subjects. Am J Clin Nutr. 2009;90:485–92. doi: 10.3945/ajcn.2009.27814. [DOI] [PubMed] [Google Scholar]
- 22.Jenkins DJ, Nguyen TH, Kendall CW, Faulkner DA, Bashyam B, Kim IJ, et al. The effect of strawberries in a cholesterol-lowering dietary portfolio. Metabolism. 2008;57:1636–44. doi: 10.1016/j.metabol.2008.07.018. [DOI] [PubMed] [Google Scholar]
- 23.Duthie SJ, Jenkinson AM, Crozier A, Mullen W, Pirie L, Kyle J, et al. The effects of cranberry juice consumption on antioxidant status and biomarkers relating to heart disease and cancer in healthy human volunteers. Eur J Nutr. 2006;45:113–22. doi: 10.1007/s00394-005-0572-9. [DOI] [PubMed] [Google Scholar]
- 24.Basu A, Du M, Leyva MJ, Sanchez K, Betts NM, Wu M, et al. Blueberries decrease cardiovascular risk factors in obese men and women with metabolic syndrome. J Nutr. 2010;140:1582–7. doi: 10.3945/jn.110.124701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Basu A, Wilkinson M, Penugonda K, Simmons B, Betts NM, Lyons TJ. Freeze-dried strawberry powder improves lipid profile and lipid peroxidation in women with metabolic syndrome: Baseline and post intervention effects. Nutr J. 2009;8:43. doi: 10.1186/1475-2891-8-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Määttä KR, Kamal-Eldin A, Törrönen AR. High-performance liquid chromatography (HPLC) analysis of phenolic compounds in berries with diode array and electrospray ionization mass spectrometric (MS) detection: Ribes species. J Agric Food Chem. 2003;51:6736–44. doi: 10.1021/jf0347517. [DOI] [PubMed] [Google Scholar]
- 27.Erlund I, Koli R, Alfthan G, Marniemi J, Puukka P, Mustonen P, et al. Favorable effects of berry consumption on platelet function, blood pressure, and HDL cholesterol. Am J Clin Nutr. 2008;87:323–31. doi: 10.1093/ajcn/87.2.323. [DOI] [PubMed] [Google Scholar]
- 28.Udani JK, Singh BB, Singh VJ, Barrett ML. Effects of Açai (Euterpe oleracea Mart.) berry preparation on metabolic parameters in a healthy overweight population: A pilot study. Nutr J. 2011;10:45. doi: 10.1186/1475-2891-10-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schulz KF, Altman DG, Moher D. CONSORT Group. CONSORT 2010 Statement: Updated guidelines for reporting parallel group randomised trials. BMC Med. 2010;8:18. doi: 10.1186/1741-7015-8-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Firestone D. Official Methods and Recommended Practices of the AOCS. American Oil Chemists’ Society. 2009 [Google Scholar]
- 31.Ebrahimzadeh MA, Pourmorad F, Bekhradnia AR. Iron chelating activity, phenol and flavonoid content of some medicinal plants from Iran. Afr J Biotechnol. 2008;7:3188–92. [Google Scholar]
- 32.Lee IT, Chan YC, Lin CW, Lee WJ, Sheu WH. Effect of cranberry extracts on lipid profiles in subjects with Type 2 diabetes. Diabet Med. 2008;25:1473–7. doi: 10.1111/j.1464-5491.2008.02588.x. [DOI] [PubMed] [Google Scholar]
- 33.Forbes JM, Cooper ME. Mechanisms of diabetic complications. Physiol Rev. 2013;93:137–88. doi: 10.1152/physrev.00045.2011. [DOI] [PubMed] [Google Scholar]
- 34.Skoczyñska A, Jêdrychowska I, Porêba R, Affelska-Jercha A, Turczyn B, Wojakowska A, et al. Influence of chokeberry juice on arterial blood pressure and lipid parameters in men with mild hypercholesterolemia. Pharmacol Rep. 2007;59(Suppl 1):177–82. [Google Scholar]
- 35.Kay CD, Holub BJ. The effect of wild blueberry (Vaccinium angustifolium) consumption on postprandial serum antioxidant status in human subjects. Br J Nutr. 2002;88:389–98. doi: 10.1079/BJN2002665. [DOI] [PubMed] [Google Scholar]
- 36.Ruel G, Pomerleau S, Couture P, Lamarche B, Couillard C. Changes in plasma antioxidant capacity and oxidized low-density lipoprotein levels in men after short-term cranberry juice consumption. Metabolism. 2005;54:856–61. doi: 10.1016/j.metabol.2005.01.031. [DOI] [PubMed] [Google Scholar]
- 37.Lehtonen HM, Suomela JP, Tahvonen R, Vaarno J, Venojärvi M, Viikari J, et al. Berry meals and risk factors associated with metabolic syndrome. Eur J Clin Nutr. 2010;64:614–21. doi: 10.1038/ejcn.2010.27. [DOI] [PubMed] [Google Scholar]
- 38.Burton-Freeman B, Linares A, Hyson D, Kappagoda T. Strawberry modulates LDL oxidation and postprandial lipemia in response to high-fat meal in overweight hyperlipidemic men and women. J Am Coll Nutr. 2010;29:46–54. doi: 10.1080/07315724.2010.10719816. [DOI] [PubMed] [Google Scholar]
- 39.Szajdek A, Borowska EJ. Bioactive compounds and health-promoting properties of berry fruits: A review. Plant Foods Hum Nutr. 2008;63:147–56. doi: 10.1007/s11130-008-0097-5. [DOI] [PubMed] [Google Scholar]
- 40.Mazza GJ. Anthocyanins and heart health. Ann Ist Super Sanita. 2007;43:369–74. [PubMed] [Google Scholar]
- 41.Basu A, Rhone M, Lyons TJ. Berries: Emerging impact on cardiovascular health. Nutr Rev. 2010;68:168–77. doi: 10.1111/j.1753-4887.2010.00273.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


