Abstract
Background:
Dietary approaches to stop hypertension (DASH) eating plan is a healthy dietary pattern. Our object is to review surveys in the field of major components of DASH diet and different kinds of cancers.
Materials and Methods:
Our search result from PubMed search engine recruited to find related articles.
Results:
Adherence to the DASH diet components was significantly related to lower prevalence of various cancers due to their high content of fiber, nutrients, vitamins, mineral, and antioxidant capacity.
Conclusion:
In this review, positive association of DASH diet components and different cancers were observed. However, the exact association of DASH with cancers should be clarified in future longitudinal studies due to potential interaction among foods and nutrients.
Keywords: Cancer, dietary approaches to stop hypertension, dietary approaches to stop hypertension eating pattern
INTRODUCTION
The most previous studies have been conducted on the relationship between single nutrients, foods, food groups, and cancer risk. Researches in this field are valuable but according to consumption of various dietary groups together and their synergistic and antagonistic effects, evaluation of diet as a healthy dietary pattern can provide a more comprehensive dataset.[1,2,3] Several studies have been demonstrated advantageous influence of dietary approaches to stop hypertension (DASH) diet on cardiovascular disease, metabolic syndrome, diabetes, and mortality.[4,5,6,7,8] Beneficial influence of DASH diet through emphasis on reduction in salt intake and monitoring dietary fat intake on some cancers has been observed. Researchers are mainly focused on the association between DASH diet and colorectal and breast cancer.[9,10,11,12] More surveys require indicating the favorable effect of DASH diet in various cancers. Nevertheless, numerous studies have assessed the effect of DASH component in cancer prevention. In this review, we are going to assess the association between components of DASH diet and different kinds of cancers.
MATERIALS AND METHODS
In order to investigate the association between DASH eating plan component and cancers, PubMed search engine was searched. Our keyword was DASH diet without any limitations.
RESULT
Articles included in our study are demonstrated in Figure 1 and Table 1.
Figure 1.
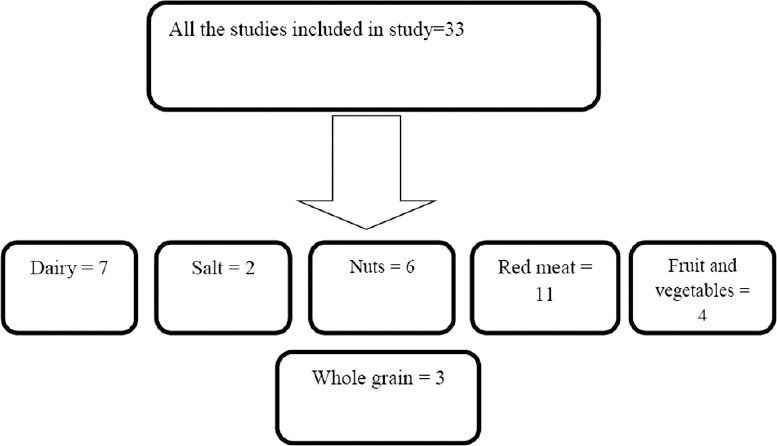
Flowchart of conducted studies in the field of dietary approaches to stop hypertension components
Table 1.
Studies examined association of DASH diet component and various cancers
Dairy
Moderate intake of low-fat dairy is suggested by DASH diet. Several studies have assessed the effects of dairy products consumption on various cancers. Meta-analyses could not find supportive independent relationship between the intake of milk or dairy products and the risk of bladder and gastric cancers.[13,14]
Findings of the meta-analysis indicate that increased consumption of total dairy food, but not milk, may be associated with a reduced risk of breast cancer.[15] Meta-analyses carried out on prostate cancer did not strongly support increasing effect of various kinds of dairy products.[16,17] The effects of milk and total dairy products have been indicated in reduction of colorectal cancer risk.[18,19]
Salt
Salt intake is limited in DASH diet. In this regard, meta-analysis on salt intake and risk of various cancers has approved its rising effect significantly.[20,21]
Whole grain
Whole grains that replaced with refined grains in DASH diet recommended increasing in this dietary pattern. An expanded review and meta-analyses on various cancers and whole grain consumption supported the hypothesis of its preventive effect.[22,23,24]
Red meat
Many epidemiological and clinical trials have investigated the relationship between red meat and processed products and different cancers, but their findings are so inconsistent. Findings of a meta-analysis demonstrated high intake of processed meat may related to augmentation of bladder cancer risk (1.22; 95% CI: 1.04-1.43).[25] Increasing association of red and processed meat and lung cancer has been supported by meta-analysis of epidemiological studies.[26] Dose-response analysis indicated every increment of 100 g red meat per day increase stomach cancer risk 17%.[27] Also, esophageal cancer risk has been increased by high consumption of red and processed meat.[28] Meta-analysis suggested that every 50 g increase in processed meat consumption augments risk of pancreatic cancer 19%.[29]
Findings show every 100 g/day increase in red meat consumption, enhance risk of colorectal cancer by 14%.[30] On the other hand, other meta-analyses could not observe significant association between red meat intake and colorectal, ovarian, breast, and prostate cancers.[31,33,34,35] However, frequency of red meat consumption is mostly linked to colorectal cancer risk.[32]
Fruit and vegetable
Increase in fruits and vegetables consumption is recommended in DASH diet due to their fiber, antioxidants, vitamins, and minerals content. This hypothesis has been assessed in meta-analysis on bladder cancer that total fruit and vegetable indicate 17% reduction in cancer risk significantly.[36]
Meta-analysis has been demonstrated significant gastric cancer risk reduction only for fruits, but not vegetables.[37] Moreover, no significant protective effect observed for prostate cancer and fruit and vegetable consumption (vegetable: 0.97; 95% CI: 0.93, 1.01, and fruit: 1.02; 95% CI: 0.98, 1.07).[38] Meta-analysis on nasopharyngeal cancer and fruits and vegetables intake support positively risk reduction.[39]
Nuts
High consumption of nuts and seeds appears to be appropriate for cancer prevention. Epidemiologic studies observed a protective association between the increment in nut consumption and decrement in colorectal cancer, especially in women.[40,41,42] In addition, a large body of data presented that higher consumption of nuts and seeds decrease risk of prostate cancer and mortality.[43,44]
Regards to debates that breast cancer prevention should be started in adolescence, epidemiologic surveys shown that fiber and nuts intake during adolescence might protect against breast cancer in older ages.[45]
DISCUSSION
The evidence from studies approved protective effect of DASH diet components in most of the various cancers. All studies regarding dairy products were meta-analyses that summarized results of papers in that field. The effect of dairy products on cancer prevention refers to its ingredients such as calcium, lactoferrin, fat component, and its bacterial effect. Conjugated linoleic acid is one of the positive health effective parameters of dairy products.[46] Lactic acid bacteria in fermented dairy products can inhibit from Helicobacter pylori growth and its invasion and inflammation thus prevent from gastric cancer.[47,48] Another important component especially in colorectal cancer prevention is calcium with several hypothetical mechanisms.[49,50,51] Salt intake is a component of DASH diet which considered greatly in this healthy dietary pattern.
The potential mechanism could be alteration in mucus viscosity of stomach[52] and increment in H. pylori colonization.[53] Therefore, it causes mucosal injury that result in augmentation of cell proliferation in stomach mucosa.[54,55]
High consumption of whole grains is usually suggested due to beneficial effect of several components such as dietary fibers, antioxidants, vitamins, trace minerals, phytate, phenolic acids, lignans, and phytoestrogens.[42,56,57] Dietary fiber is one of the most important ingredients in colorectal cancer prevention because it can enhance stool bulk, attenuating fecal carcinogens, and decline transit time so decrease contact between carcinogens and colorectal cells.[58] Moreover, bacterial activation in colon results in fiber fermentation and short chain fatty acid output that is effective in cancer inhibition.[56]
High red meat consumers are at risk of different cancers more than low consumers. Modification of dietary pattern and lifestyle should be a priory to prevent of cancers and reduce burden of disease. The effect of red and processed meat in incidence of cancers connected to preservation, cooking or processing that could produce mutagens and carcinogens including N-Nitroso compounds (NOCs), heterocyclic amines, and polycyclic aromatic hydrocarbons.[59,60,61,62,63,64] Furthermore, high heme iron content of meat, especially red meat, could provide free radicals[65] such as stimulation of endogenous NOC production,[66] and also iron is crucial growth factor for H. pylori.[67] Saturated fatty acids (SFAs) are another component that may be related to cause of cancer.[65,68]
Beneficial effects of fruit and vegetables have been investigated in some cancers. Multiple components of fruits and vegetables such as beta-carotene, fiber, vitamins, alpha-tocopherol, retinoids, phytoestrogens and folate can cause their protective effect against cancers[69] through potent mechanism such as prohibition of cell growth, normalize DNA synthesis and methylation, and protection against DNA damage and oxidative stress.
Sulforaphane is an isothiocyanate component found in vegetables such as cruciferous vegetables which its protective effect is considered greatly in new epidemiological studies.[70,71,72,73,74]
Nuts are extremely valuable nutritionally due to wide range of nutrients such as proteins, unsaturated fatty acids, vitamins (B6, niacin, folic acid, tocopherol), dietary fiber, copper, magnesium, potassium, zinc, antioxidants (i.e., resveratrol, ellagic acid, and several flavonoids), phytoestrogens, and many phytochemicals (i.e., anacardic acid). Most of these components play important role in cancer prevention through prohibition of cancer cell proliferation, decrease metastasis, inducing cancer cell death and intervention in some other pathways related to cancer cell growth.
This review create new ideas and attract researchers to conduct more surveys in the field of DASH diet because its superior effect to other patterns including emphasis on the amount of salt intake, and restriction in intake of total fat. A limitation was lack of discussion about quality of articles due to different cancer categories of articles. Nevertheless, efforts in our review with covering the major component of DASH diet were to assess their relationship with cancers comprehensively.
CONCLUSION
There are limited investigations regarding the association of DASH eating plan and the risk of different cancers. Although many studies have assessed the association of its component with different cancers, due to potential interaction among foods and nutrients, the exact association of DASH with cancers should be clarified in future longitudinal studies.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
AUTHOR'S CONTRIBUTION
SHO contributed in the conception of the work, drafting the manuscript, FH contributed in the conception of the work, conducting the study, drafting and revising the draft, LA contributed in the conception of the work, conducting the study, revising the draft, approval of the final version of the manuscript, and agreed for all aspects of the work.
Acknowledgments
This research was funded by Isfahan University of Medical Sciences (IUMS), Isfahan, Iran.
REFERENCES
- 1.Hu FB. Dietary pattern analysis: A new direction in nutritional epidemiology. Curr Opin Lipidol. 2002;13:3–9. doi: 10.1097/00041433-200202000-00002. [DOI] [PubMed] [Google Scholar]
- 2.Kant AK. Dietary patterns and health outcomes. J Am Diet Assoc. 2004;104:615–35. doi: 10.1016/j.jada.2004.01.010. [DOI] [PubMed] [Google Scholar]
- 3.Jacobs DR, Jr, Steffen LM. Nutrients, foods, and dietary patterns as exposures in research: A framework for food synergy. Am J Clin Nutr. 2003;78(3 Suppl):508S–13. doi: 10.1093/ajcn/78.3.508S. [DOI] [PubMed] [Google Scholar]
- 4.Salehi-Abargouei A, Maghsoudi Z, Shirani F, Azadbakht L. Effects of Dietary Approaches to Stop Hypertension (DASH)-style diet on fatal or nonfatal cardiovascular diseases – incidence: A systematic review and meta-analysis on observational prospective studies. Nutrition. 2013;29:611–8. doi: 10.1016/j.nut.2012.12.018. [DOI] [PubMed] [Google Scholar]
- 5.Azadbakht L, Mirmiran P, Esmaillzadeh A, Azizi T, Azizi F. Beneficial effects of a Dietary Approaches to Stop Hypertension eating plan on features of the metabolic syndrome. Diabetes Care. 2005;28:2823–31. doi: 10.2337/diacare.28.12.2823. [DOI] [PubMed] [Google Scholar]
- 6.Azadbakht L, Fard NR, Karimi M, Baghaei MH, Surkan PJ, Rahimi M, et al. Effects of the Dietary Approaches to Stop Hypertension (DASH) eating plan on cardiovascular risks among type 2 diabetic patients: A randomized crossover clinical trial. Diabetes Care. 2011;34:55–7. doi: 10.2337/dc10-0676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Azadbakht L, Surkan PJ, Esmaillzadeh A, Willett WC. The Dietary Approaches to Stop Hypertension eating plan affects C-reactive protein, coagulation abnormalities, and hepatic function tests among type 2 diabetic patients. J Nutr. 2011;141:1083–8. doi: 10.3945/jn.110.136739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Levitan EB, Lewis CE, Tinker LF, Eaton CB, Ahmed A, Manson JE, et al. Mediterranean and DASH diet scores and mortality in women with heart failure: The Women's Health Initiative. Circ Heart Fail. 2013;6:1116–23. doi: 10.1161/CIRCHEARTFAILURE.113.000495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miller PE, Cross AJ, Subar AF, Krebs-Smith SM, Park Y, Powell-Wiley T, et al. Comparison of 4 established DASH diet indexes: Examining associations of index scores and colorectal cancer. Am J Clin Nutr. 2013;98:794–803. doi: 10.3945/ajcn.113.063602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fung TT, Hu FB, Wu K, Chiuve SE, Fuchs CS, Giovannucci E. The Mediterranean and Dietary Approaches to Stop Hypertension (DASH) diets and colorectal cancer. Am J Clin Nutr. 2010;92:1429–35. doi: 10.3945/ajcn.2010.29242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dixon LB, Subar AF, Peters U, Weissfeld JL, Bresalier RS, Risch A, et al. Adherence to the USDA food guide, DASH eating plan, and Mediterranean dietary pattern reduces risk of colorectal adenoma. J Nutr. 2007;137:2443–50. doi: 10.1093/jn/137.11.2443. [DOI] [PubMed] [Google Scholar]
- 12.Fung TT, Hu FB, Hankinson SE, Willett WC, Holmes MD. Low-carbohydrate diets, dietary approaches to stop hypertension-style diets, and the risk of postmenopausal breast cancer. Am J Epidemiol. 2011;174:652–60. doi: 10.1093/aje/kwr148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li F, An SL, Zhou Y, Liang ZK, Jiao ZJ, Jing YM, et al. Milk and dairy consumption and risk of bladder cancer: A meta-analysis. Urology. 2011;78:1298–305. doi: 10.1016/j.urology.2011.09.002. [DOI] [PubMed] [Google Scholar]
- 14.Sun Y, Lin LJ, Sang LX, Dai C, Jiang M, Zheng CQ. Dairy product consumption and gastric cancer risk: A meta-analysis. World J Gastroenterol. 2014;20:15879–98. doi: 10.3748/wjg.v20.i42.15879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dong JY, Zhang L, He K, Qin LQ. Dairy consumption and risk of breast cancer: A meta-analysis of prospective cohort studies. Breast Cancer Res Treat. 2011;127:23–31. doi: 10.1007/s10549-011-1467-5. [DOI] [PubMed] [Google Scholar]
- 16.Huncharek M, Muscat J, Kupelnick B. Dairy products, dietary calcium and vitamin D intake as risk factors for prostate cancer: A meta-analysis of 26,769 cases from 45 observational studies. Nutr Cancer. 2008;60:421–41. doi: 10.1080/01635580801911779. [DOI] [PubMed] [Google Scholar]
- 17.Aune D, Navarro Rosenblatt DA, Chan DS, Vieira AR, Vieira R, Greenwood DC, et al. Dairy products, calcium, and prostate cancer risk: A systematic review and meta-analysis of cohort studies. Am J Clin Nutr. 2015;101:87–117. doi: 10.3945/ajcn.113.067157. [DOI] [PubMed] [Google Scholar]
- 18.Aune D, Lau R, Chan DS, Vieira R, Greenwood DC, Kampman E, et al. Dairy products and colorectal cancer risk: A systematic review and meta-analysis of cohort studies. Ann Oncol. 2012;23:37–45. doi: 10.1093/annonc/mdr269. [DOI] [PubMed] [Google Scholar]
- 19.Huncharek M, Muscat J, Kupelnick B. Colorectal cancer risk and dietary intake of calcium, vitamin D, and dairy products: A meta-analysis of 26,335 cases from 60 observational studies. Nutr Cancer. 2009;61:47–69. doi: 10.1080/01635580802395733. [DOI] [PubMed] [Google Scholar]
- 20.D’Elia L, Rossi G, Ippolito R, Cappuccio FP, Strazzullo P. Habitual salt intake and risk of gastric cancer: A meta-analysis of prospective studies. Clin Nutr. 2012;31:489–98. doi: 10.1016/j.clnu.2012.01.003. [DOI] [PubMed] [Google Scholar]
- 21.Hu J, La Vecchia C, Morrison H, Negri E, Mery L. Canadian Cancer Registries Epidemiology Research Group. Salt, processed meat and the risk of cancer. Eur J Cancer Prev. 2011;20:132–9. doi: 10.1097/CEJ.0b013e3283429e32. [DOI] [PubMed] [Google Scholar]
- 22.Aune D, Chan DS, Lau R, Vieira R, Greenwood DC, Kampman E, et al. Dietary fibre, whole grains, and risk of colorectal cancer: Systematic review and dose-response meta-analysis of prospective studies. BMJ. 2011;343:d6617. doi: 10.1136/bmj.d6617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haas P, Machado MJ, Anton AA, Silva AS, de Francisco A. Effectiveness of whole grain consumption in the prevention of colorectal cancer: Meta-analysis of cohort studies. Int J Food Sci Nutr. 2009;60(Suppl 6):1–13. doi: 10.1080/09637480802183380. [DOI] [PubMed] [Google Scholar]
- 24.Jacobs DR, Jr, Marquart L, Slavin J, Kushi LH. Whole-grain intake and cancer: An expanded review and meta-analysis. Nutr Cancer. 1998;30:85–96. doi: 10.1080/01635589809514647. [DOI] [PubMed] [Google Scholar]
- 25.Li F, An S, Hou L, Chen P, Lei C, Tan W. Red and processed meat intake and risk of bladder cancer: A meta-analysis. Int J Clin Exp Med. 2014;7:2100–10. [PMC free article] [PubMed] [Google Scholar]
- 26.Xue XJ, Gao Q, Qiao JH, Zhang J, Xu CP, Liu J. Red and processed meat consumption and the risk of lung cancer: A dose-response meta-analysis of 33 published studies. Int J Clin Exp Med. 2014;7:1542–53. [PMC free article] [PubMed] [Google Scholar]
- 27.Song P, Lu M, Yin Q, Wu L, Zhang D, Fu B, et al. Red meat consumption and stomach cancer risk: A meta-analysis. J Cancer Res Clin Oncol. 2014;140:979–92. doi: 10.1007/s00432-014-1637-z. [DOI] [PubMed] [Google Scholar]
- 28.Choi Y, Song S, Song Y, Lee JE. Consumption of red and processed meat and esophageal cancer risk: Meta-analysis. World J Gastroenterol. 2013;19:1020–9. doi: 10.3748/wjg.v19.i7.1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Larsson SC, Wolk A. Red and processed meat consumption and risk of pancreatic cancer: Meta-analysis of prospective studies. Br J Cancer. 2012;106:603–7. doi: 10.1038/bjc.2011.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chan DS, Lau R, Aune D, Vieira R, Greenwood DC, Kampman E, et al. Red and processed meat and colorectal cancer incidence: Meta-analysis of prospective studies. PLoS One. 2011;6:e20456. doi: 10.1371/journal.pone.0020456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alexander DD, Weed DL, Cushing CA, Lowe KA. Meta-analysis of prospective studies of red meat consumption and colorectal cancer. Eur J Cancer Prev. 2011;20:293–307. doi: 10.1097/CEJ.0b013e328345f985. [DOI] [PubMed] [Google Scholar]
- 32.Smolinska K, Paluszkiewicz P. Risk of colorectal cancer in relation to frequency and total amount of red meat consumption. Systematic review and meta-analysis. Arch Med Sci. 2010;6:605–10. doi: 10.5114/aoms.2010.14475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wallin A, Orsini N, Wolk A. Red and processed meat consumption and risk of ovarian cancer: A dose-response meta-analysis of prospective studies. Br J Cancer. 2011;104:1196–201. doi: 10.1038/bjc.2011.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Alexander DD, Morimoto LM, Mink PJ, Cushing CA. A review and meta-analysis of red and processed meat consumption and breast cancer. Nutr Res Rev. 2010;23:349–65. doi: 10.1017/S0954422410000235. [DOI] [PubMed] [Google Scholar]
- 35.Alexander DD, Mink PJ, Cushing CA, Sceurman B. A review and meta-analysis of prospective studies of red and processed meat intake and prostate cancer. Nutr J. 2010;9:50. doi: 10.1186/1475-2891-9-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yao B, Yan Y, Ye X, Fang H, Xu H, Liu Y, et al. Intake of fruit and vegetables and risk of bladder cancer: A dose-response meta-analysis of observational studies. Cancer Causes Control. 2014;25:1645–58. doi: 10.1007/s10552-014-0469-0. [DOI] [PubMed] [Google Scholar]
- 37.Wang Q, Chen Y, Wang X, Gong G, Li G, Li C. Consumption of fruit, but not vegetables, may reduce risk of gastric cancer: Results from a meta-analysis of cohort studies. Eur J Cancer. 2014;50:1498–509. doi: 10.1016/j.ejca.2014.02.009. [DOI] [PubMed] [Google Scholar]
- 38.Meng H, Hu W, Chen Z, Shen Y. Fruit and vegetable intake and prostate cancer risk: A meta-analysis. Asia Pac J Clin Oncol. 2014;10:133–40. doi: 10.1111/ajco.12067. [DOI] [PubMed] [Google Scholar]
- 39.Jin J, Ouyang Z, Wang Z. Association of fruit and vegetables with the risk of nasopharyngeal cancer: Evidence from a meta-analysis. Sci Rep. 2014;4:5229. doi: 10.1038/srep05229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jenab M, Ferrari P, Slimani N, Norat T, Casagrande C, Overad K, et al. Association of nut and seed intake with colorectal cancer risk in the European Prospective Investigation into Cancer and Nutrition. Cancer Epidemiol Biomarkers Prev. 2004;13:1595–603. [PubMed] [Google Scholar]
- 41.Yeh CC, You SL, Chen CJ, Sung FC. Peanut consumption and reduced risk of colorectal cancer in women: A prospective study in Taiwan. World J Gastroenterol. 2006;12:222–7. doi: 10.3748/wjg.v12.i2.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cotterchio M, Boucher BA, Manno M, Gallinger S, Okey A, Harper P. Dietary phytoestrogen intake is associated with reduced colorectal cancer risk. J Nutr. 2006;136:3046–53. doi: 10.1093/jn/136.12.3046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jain MG, Hislop GT, Howe GR, Ghadirian P. Plant foods, antioxidants, and prostate cancer risk: Findings from case-control studies in Canada. Nutr Cancer. 1999;34:173–84. doi: 10.1207/S15327914NC3402_8. [DOI] [PubMed] [Google Scholar]
- 44.Hebert JR, Hurley TG, Olendzki BC, Teas J, Ma Y, Hampl JS. Nutritional and socioeconomic factors in relation to prostate cancer mortality: A cross-national study. J Natl Cancer Inst. 1998;90:1637–47. doi: 10.1093/jnci/90.21.1637. [DOI] [PubMed] [Google Scholar]
- 45.Su X, Tamimi RM, Collins LC, Baer HJ, Cho E, Sampson L, et al. Intake of fiber and nuts during adolescence and incidence of proliferative benign breast disease. Cancer Causes Control. 2010;21:1033–46. doi: 10.1007/s10552-010-9532-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Haug A, Christophersen OA, Høstmark AT, Harstad OM. Milk and health. Tidsskr Nor Laegeforen. 2007;127:2542–5. [PubMed] [Google Scholar]
- 47.Ljungh A, Wadström T. Lactic acid bacteria as probiotics. Curr Issues Intest Microbiol. 2006;7:73–89. [PubMed] [Google Scholar]
- 48.El-Adawi H, El-Sheekh M, Khalil M, El-Deeb N, Hussein M. Lactic acid bacterial extracts as anti-Helicobacter pylori: A molecular approach. Ir J Med Sci. 2013;182:439–52. doi: 10.1007/s11845-013-0909-y. [DOI] [PubMed] [Google Scholar]
- 49.Newmark HL, Wargovich MJ, Bruce WR. Colon cancer and dietary fat, phosphate, and calcium: A hypothesis. J Natl Cancer Inst. 1984;72:1323–5. [PubMed] [Google Scholar]
- 50.Lamprecht SA, Lipkin M. Cellular mechanisms of calcium and vitamin D in the inhibition of colorectal carcinogenesis. Ann N Y Acad Sci. 2001;952:73–87. doi: 10.1111/j.1749-6632.2001.tb02729.x. [DOI] [PubMed] [Google Scholar]
- 51.Llor X, Jacoby RF, Teng BB, Davidson NO, Sitrin MD, Brasitus TA. K-ras mutations in 1,2-dimethylhydrazine-induced colonic tumors: Effects of supplemental dietary calcium and vitamin D deficiency. Cancer Res. 1991;51:4305–9. [PubMed] [Google Scholar]
- 52.Tatematsu M, Takahashi M, Fukushima S, Hananouchi M, Shirai T. Effects in rats of sodium chloride on experimental gastric cancers induced by N-methyl-N-nitro-N-nitrosoguanidine or 4-nitroquinoline-1-oxide. J Natl Cancer Inst. 1975;55:101–6. doi: 10.1093/jnci/55.1.101. [DOI] [PubMed] [Google Scholar]
- 53.Fox JG, Dangler CA, Taylor NS, King A, Koh TJ, Wang TC. High-salt diet induces gastric epithelial hyperplasia and parietal cell loss, and enhances Helicobacter pylori colonization in C57BL/6 mice. Cancer Res. 1999;59:4823–8. [PubMed] [Google Scholar]
- 54.Furihata C, Ohta H, Katsuyama T. Cause and effect between concentration-dependent tissue damage and temporary cell proliferation in rat stomach mucosa by NaCl, a stomach tumor promoter. Carcinogenesis. 1996;17:401–6. doi: 10.1093/carcin/17.3.401. [DOI] [PubMed] [Google Scholar]
- 55.Charnley G, Tannenbaum SR. Flow cytometric analysis of the effect of sodium chloride on gastric cancer risk in the rat. Cancer Res. 1985;45(11 Pt 2):5608–16. [PubMed] [Google Scholar]
- 56.Slavin JL. Mechanisms for the impact of whole grain foods on cancer risk. J Am Coll Nutr. 2000;19(3 Suppl):300S–7. doi: 10.1080/07315724.2000.10718964. [DOI] [PubMed] [Google Scholar]
- 57.Webb AL, McCullough ML. Dietary lignans: Potential role in cancer prevention. Nutr Cancer. 2005;51:117–31. doi: 10.1207/s15327914nc5102_1. [DOI] [PubMed] [Google Scholar]
- 58.Lipkin M, Reddy B, Newmark H, Lamprecht SA. Dietary factors in human colorectal cancer. Annu Rev Nutr. 1999;19:545–86. doi: 10.1146/annurev.nutr.19.1.545. [DOI] [PubMed] [Google Scholar]
- 59.Skog KI, Johansson MA, Jägerstad MI. Carcinogenic heterocyclic amines in model systems and cooked foods: A review on formation, occurrence and intake. Food Chem Toxicol. 1998;36:879–96. doi: 10.1016/s0278-6915(98)00061-1. [DOI] [PubMed] [Google Scholar]
- 60.Tricker AR, Preussmann R. Carcinogenic N-nitrosamines in the diet: Occurrence, formation, mechanisms and carcinogenic potential. Mutat Res. 1991;259:277–89. doi: 10.1016/0165-1218(91)90123-4. [DOI] [PubMed] [Google Scholar]
- 61.Bingham SA. High-meat diets and cancer risk. Proc Nutr Soc. 1999;58:243–8. doi: 10.1017/s0029665199000336. [DOI] [PubMed] [Google Scholar]
- 62.Sugimura T. Nutrition and dietary carcinogens. Carcinogenesis. 2000;21:387–95. doi: 10.1093/carcin/21.3.387. [DOI] [PubMed] [Google Scholar]
- 63.Jakszyn P, Gonzalez CA. Nitrosamine and related food intake and gastric and oesophageal cancer risk: A systematic review of the epidemiological evidence. World J Gastroenterol. 2006;12:4296–303. doi: 10.3748/wjg.v12.i27.4296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cross AJ, Sinha R. Meat-related mutagens/carcinogens in the etiology of colorectal cancer. Environ Mol Mutagen. 2004;44:44–55. doi: 10.1002/em.20030. [DOI] [PubMed] [Google Scholar]
- 65.Yang WS, Wong MY, Vogtmann E, Tang RQ, Xie L, Yang YS, et al. Meat consumption and risk of lung cancer: Evidence from observational studies. Ann Oncol. 2012;23:3163–70. doi: 10.1093/annonc/mds207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cross AJ, Pollock JR, Bingham SA. Haem, not protein or inorganic iron, is responsible for endogenous intestinal N-nitrosation arising from red meat. Cancer Res. 2003;63:2358–60. [PubMed] [Google Scholar]
- 67.Pérez-Pérez GI, Israel DA. Role of iron in Helicobacter pylori: Its influence in outer membrane protein expression and in pathogenicity. Eur J Gastroenterol Hepatol. 2000;12:1263–5. doi: 10.1097/00042737-200012120-00001. [DOI] [PubMed] [Google Scholar]
- 68.Hill MJ, Goddard P, Williams RE. Gut bacteria and aetiology of cancer of the breast. Lancet. 1971;2:472–3. doi: 10.1016/s0140-6736(71)92634-1. [DOI] [PubMed] [Google Scholar]
- 69.Chang ET, Adami HO. The enigmatic epidemiology of nasopharyngeal carcinoma. Cancer Epidemiol Biomarkers Prev. 2006;15:1765–77. doi: 10.1158/1055-9965.EPI-06-0353. [DOI] [PubMed] [Google Scholar]
- 70.Gamet-Payrastre L. Signaling pathways and intracellular targets of sulforaphane mediating cell cycle arrest and apoptosis. Curr Cancer Drug Targets. 2006;6:135–45. doi: 10.2174/156800906776056509. [DOI] [PubMed] [Google Scholar]
- 71.Clarke JD, Dashwood RH, Ho E. Multi-targeted prevention of cancer by sulforaphane. Cancer Lett. 2008;269:291–304. doi: 10.1016/j.canlet.2008.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jo GH, Kim GY, Kim WJ, Park KY, Choi YH. Sulforaphane induces apoptosis in T24 human urinary bladder cancer cells through a reactive oxygen species-mediated mitochondrial pathway: The involvement of endoplasmic reticulum stress and the Nrf2 signaling pathway. Int J Oncol. 2014;45:1497–506. doi: 10.3892/ijo.2014.2536. [DOI] [PubMed] [Google Scholar]
- 73.Hussain A, Mohsin J, Prabhu SA, Begum S, Nusri Qel-A, Harish G, et al. Sulforaphane inhibits growth of human breast cancer cells and augments the therapeutic index of the chemotherapeutic drug, gemcitabine. Asian Pac J Cancer Prev. 2013;14:5855–60. doi: 10.7314/apjcp.2013.14.10.5855. [DOI] [PubMed] [Google Scholar]
- 74.Forster T, Rausch V, Zhang Y, Isayev O, Heilmann K, Schoensiegel F, et al. Sulforaphane counteracts aggressiveness of pancreatic cancer driven by dysregulated Cx43-mediated gap junctional intercellular communication. Oncotarget. 2014;5:1621–34. doi: 10.18632/oncotarget.1764. [DOI] [PMC free article] [PubMed] [Google Scholar]


