Abstract
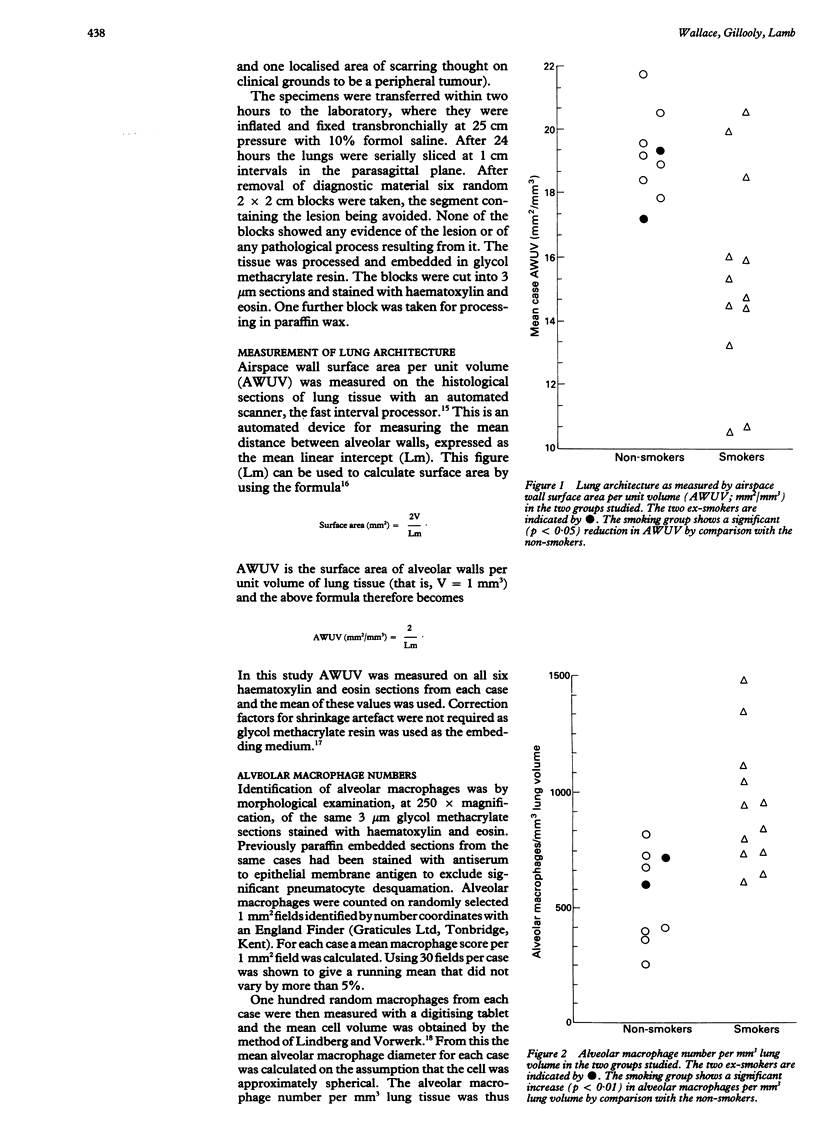
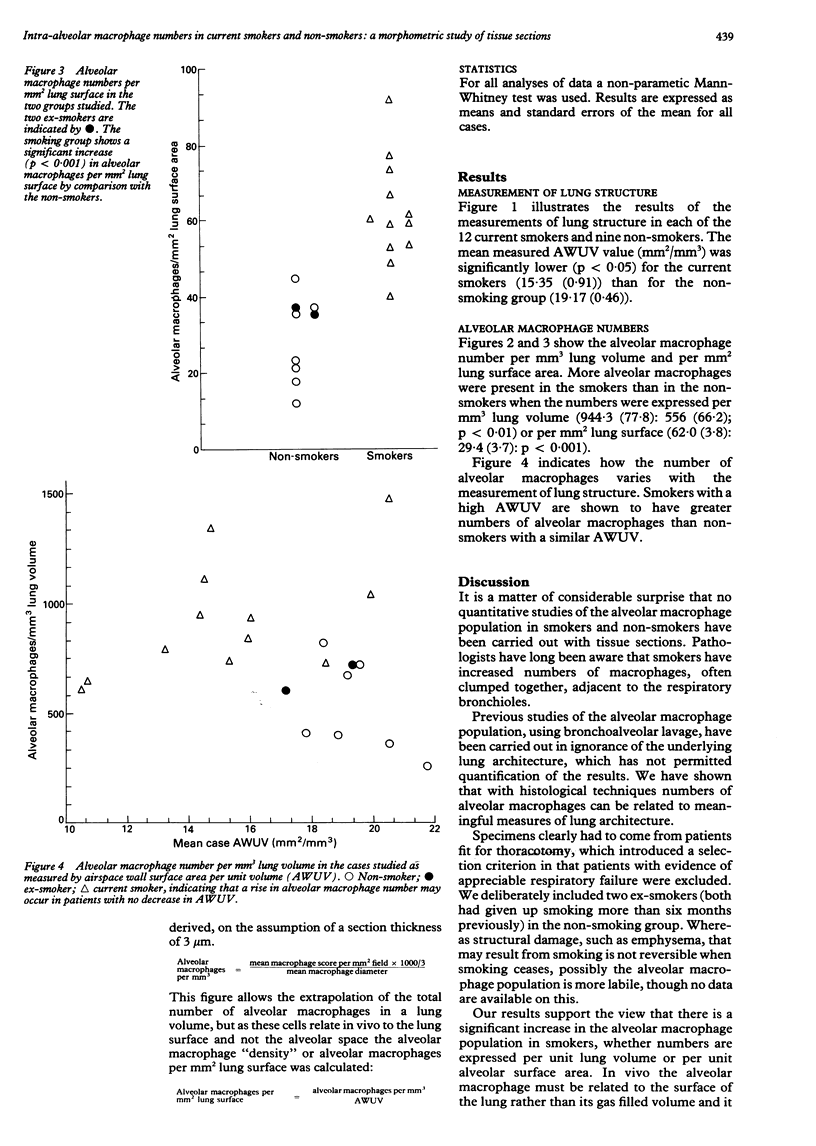
BACKGROUND: The alveolar macrophage is believed to be important in the defence of the lung and possibly in the pathogenesis of lung disease. Cell counts in bronchoalveolar lavage fluid have suggested that smokers have an increased number of alveolar macrophages but have not enabled the number to be related to a measure of lung structure. METHODS: The number of alveolar macrophages was counted in histological sections from lung resection specimens from a group of smokers and non-smokers. The results were related to a measurement of lung structure obtained by means of an automated morphometric technique and expressed in terms of units of lung volume or of lung surface area. RESULTS: The smokers had a significantly increased number of alveolar macrophages per unit lung volume and per unit surface area, through the relative increase was less than has appeared from bronchoalveolar lavage studies. When smokers and non-smokers with similar lung structure were compared the smokers had more alveolar macrophages, indicating that smoking and not loss of lung structure is responsible for the increase. CONCLUSIONS: Smokers had more alveolar macrophages than non-smokers when the number was expressed quantitatively with respect to the underlying architecture. Changes in cell populations postulated to be important in the pathogenesis of disease within the lung should be related to lung architecture because this may vary considerably between individuals.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brain J. D. Lung macrophages: how many kinds are there? What do they do? Am Rev Respir Dis. 1988 Mar;137(3):507–509. doi: 10.1164/ajrccm/137.3.507. [DOI] [PubMed] [Google Scholar]
- Demarest G. B., Hudson L. D., Altman L. C. Impaired alveolar macrophage chemotaxis in patients with acute smoke inhalation. Am Rev Respir Dis. 1979 Feb;119(2):279–286. doi: 10.1164/arrd.1979.119.2.279. [DOI] [PubMed] [Google Scholar]
- Gadek J. E., Pacht E. R. The protease-antiprotease balance within the human lung: implications for the pathogenesis of emphysema. Lung. 1990;168 (Suppl):552–564. doi: 10.1007/BF02718178. [DOI] [PubMed] [Google Scholar]
- Gould G. A., MacNee W., McLean A., Warren P. M., Redpath A., Best J. J., Lamb D., Flenley D. C. CT measurements of lung density in life can quantitate distal airspace enlargement--an essential defining feature of human emphysema. Am Rev Respir Dis. 1988 Feb;137(2):380–392. doi: 10.1164/ajrccm/137.2.380. [DOI] [PubMed] [Google Scholar]
- Harris J. O., Olsen G. N., Castle J. R., Maloney A. S. Comparison of proteolytic enzyme activity in pulmonary alveolar macrophages and blood leukocytes in smokers and nonsmokers. Am Rev Respir Dis. 1975 May;111(5):579–586. doi: 10.1164/arrd.1975.111.5.579. [DOI] [PubMed] [Google Scholar]
- Harris J. O., Swenson E. W., Johnson J. E., 3rd Human alveolar macrophages: comparison of phagocytic ability, glucose utilization, and ultrastructure in smokers and nonsmokers. J Clin Invest. 1970 Nov;49(11):2086–2096. doi: 10.1172/JCI106426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb D., Gillooly M., Farrow A. S. Microscopic emphysema and its variations with age, smoking, and site within the lungs. Ann N Y Acad Sci. 1991;624:339–340. doi: 10.1111/j.1749-6632.1991.tb17040.x. [DOI] [PubMed] [Google Scholar]
- Lindberg L. G., Vorwerk P. On calculating volumes of transsected bodies from two-dimensional micrographs. Lab Invest. 1970 Sep;23(3):315–317. [PubMed] [Google Scholar]
- Low R. B., Davis G. S., Giancola M. S. Biochemical analyses of bronchoalveolar lavage fluids of healthy human volunteer smokers and nonsmokers. Am Rev Respir Dis. 1978 Nov;118(5):863–875. doi: 10.1164/arrd.1978.118.5.863. [DOI] [PubMed] [Google Scholar]
- Rennard S. I., Hunninghake G. W., Bitterman P. B., Crystal R. G. Production of fibronectin by the human alveolar macrophage: mechanism for the recruitment of fibroblasts to sites of tissue injury in interstitial lung diseases. Proc Natl Acad Sci U S A. 1981 Nov;78(11):7147–7151. doi: 10.1073/pnas.78.11.7147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds H. Y. Respiratory infections may reflect deficiencies in host defense mechanisms. Dis Mon. 1985 Feb;31(2):1–98. doi: 10.1016/0011-5029(85)90010-0. [DOI] [PubMed] [Google Scholar]
- Rodriguez R. J., White R. R., Senior R. M., Levine E. A. Elastase release from human alveolar macrophages: comparison between smokers and nonsmokers. Science. 1977 Oct 21;198(4314):313–314. doi: 10.1126/science.910131. [DOI] [PubMed] [Google Scholar]
- Sibille Y., Reynolds H. Y. Macrophages and polymorphonuclear neutrophils in lung defense and injury. Am Rev Respir Dis. 1990 Feb;141(2):471–501. doi: 10.1164/ajrccm/141.2.471. [DOI] [PubMed] [Google Scholar]
- Warr G. A., Martin R. R. Chemotactic responsiveness of human alveolar macrophages: effects of cigarette smoking. Infect Immun. 1974 Apr;9(4):769–771. doi: 10.1128/iai.9.4.769-771.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warr G. A., Martin R. R., Sharp P. M., Rossen R. D. Normal human bronchial immunoglobulins and proteins: effects of cigarette smoking. Am Rev Respir Dis. 1977 Jul;116(1):25–30. doi: 10.1164/arrd.1977.116.1.25. [DOI] [PubMed] [Google Scholar]


