Abstract
In today's globalized world, rapid urbanization, mechanization of the rural economy, and the activities of trans-national food, drink and tobacco corporations are associated with behavioral changes that increase the risk of chronic non-communicable diseases (NCDs). These changes include less healthy diet, lower physical activity, tobacco smoking and increased alcohol consumption. As a result, population health profiles are rapidly changing. For example, the global burden of type 2 diabetes mellitus is expected to double by 2030, with 80% of adult cases occurring in low and middle-income countries (LMIC). Many LMIC are undergoing rapid changes associated with developing high rates of NCD while concomitantly battling high levels of certain communicable diseases, including HIV, TB and malaria. This has population health, health systems and economic implications for these countries. This critical review synthesizes evidence on the overlap and interactions between established communicable and emerging NCD epidemics in LMIC. The review focuses on HIV, TB and malaria and explores the disease-specific interactions with prevalent NCDs in LMIC including diabetes, cardiovascular disease, chronic obstructive pulmonary disease, chronic renal disease, epilepsy and neurocognitive diseases. We highlight the complexity, bi-directionality and heterogeneity of these interactions and discuss the implications for health systems.
Keywords: Co-morbidity, Communicable disease, Health transition, Infectious disease, Low and middle-income countries, Non-communicable disease
Introduction
The broad classification of diseases into communicable (infectious) and non-communicable diseases (NCDs) is deeply ingrained. However, this classification may be unhelpful for setting public health priorities, particularly in low- and middle-income countries (LMIC).1 For example, using data from Tanzania it has been shown that classifying diseases as acute versus chronic, rather than communicable versus non-communicable, dramatically changes the distribution of disease burden.2 The ‘acute versus chronic’ approach to disease classification demonstrated the equal burden of diseases requiring chronic care versus acute care even though the vast majority of the disease burden was classified as ‘communicable’.2
We focus on interactions likely to be of public health importance in LMIC, but also in marginalized populations in high-income countries. We argue that public health approaches to the prevention and control of these diseases must be fully informed by these interactions and move beyond the communicable/non-communicable divide.
The context of the epidemiologic transition
On a superficial level the original concept of the epidemiologic transition3 can appear to provide a clear rationale for the communicable/non-communicable divide. The transition is seen to consist of falling mortality from communicable diseases, particularly in infancy and childhood, followed by an increasing predominance of deaths from ‘man-made degenerative diseases’.3 However, this was always a limited interpretation. Re-analysis of historical data from Sweden has shown how patterns of falling death rates varied greatly between different regions, with some continuing to experience high rates of communicable disease mortality while this fell dramatically in others.4 It is clear that over the past 50 years many low and middle-income countries have seen emerging epidemics of chronic NCDs while continuing to experience high rates of communicable disease.5
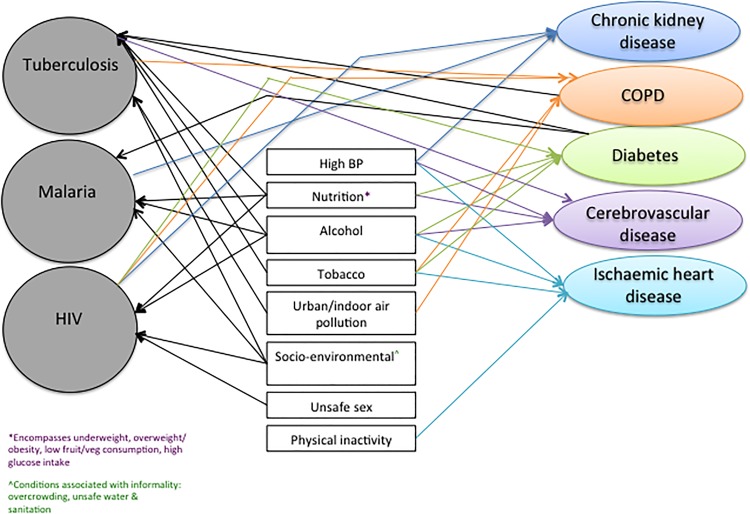
Table 1 illustrates the heterogeneity of conditions contributing to the burden of disease in 12 low and middle-income regions. It highlights the significant contribution of NCDs to disability-adjusted life years lost in most LMIC. In eight of the regions, including Southern Africa, North Africa/Middle East and Latin America, both major communicable diseases and NCDs are within the top 10 conditions contributing to the burden of disease. This highlights the fact that a continued approach to public health along the dichotomous parallel lines of communicable and NCDs is increasingly redundant in these settings. Interactions between communicable and NCDs are complex and often mediated by shared risk factors (Figure 1). This critical review aims to provide an up to date account of interactions between, and co-existence of, NCDs and communicable diseases that lead to increased morbidity and are likely to be of public health importance to LMIC, as well as marginalized populations in high-income settings.
Table 1.
Rankings of diseases according to their contribution to disability adjusted life years (DALYs) in different global regions
| Disease rankings | Southern Africa | East Africa | Central Africa | Western Africa | N. Africa/ Middle East | Southern Latin America | Tropical Latin America | Central Latin America | South East Asia | South Asia | East Asia | Central Asia |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ischemic heart disease | 14 | 21 | 19 | 20 | 1 | 1 | 1 | 2 | 3 | 4 | 2 | 1 |
| Lower respiratory infections | 2 | 3 | 4 | 2 | 5 | 6 | 7 | 6 | 4 | 1 | 15 | 2 |
| Cerebrovascular disease | 7 | 16 | 14 | 16 | 4 | 3 | 4 | 11 | 1 | 12 | 1 | 3 |
| Diarrheal disease | 3 | 4 | 2 | 3 | 11 | 44 | 26 | 14 | 8 | 3 | 49 | 18 |
| HIV/AIDS | 1 | 1 | 5 | 4 | 58 | 34 | 12 | 13 | 13 | 17 | 38 | 31 |
| Malaria | 20 | 2 | 1 | 1 | 66 | 166 | 145 | 154 | 22 | 44 | 169 | 162 |
| COPD | 9 | 20 | 20 | 22 | 13 | 7 | 10 | 16 | 9 | 5 | 3 | 11 |
| Major depressive disorder | 10 | 13 | 17 | 19 | 3 | 4 | 6 | 5 | 6 | 14 | 8 | 6 |
| TB | 4 | 7 | 7 | 12 | 33 | 65 | 46 | 44 | 2 | 8 | 37 | 15 |
| Diabetes | 8 | 29 | 28 | 26 | 9 | 9 | 8 | 3 | 10 | 16 | 10 | 12 |
COPD: chronic obstructive pulmonary disease.
Adapted from Murray CJ, Vos T, Lozano R et al. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012;380:2197–223.
Figure 1.
Interaction between TB, Malaria and HIV, and risk factors/disease precursors and non-communicable diseases. BP: blood pressure; COPD: chronic obstructive pulmonary disease. This figure is available in black and white in print and in color at International Health online.
Methods
The literature search was carried out between October 2013 and December 2014 and included literature published until December 2014. Our aim was to conduct a ‘critical review’.6 Unlike a systematic review, this does not aim at a comprehensive assessment of original research but rather seeks to identify the conceptual contribution of existing literature to the field of study.6 We searched for literature (English language) using the PubMed and EMBASE databases and the following combination terms (as MESH terms and key words): (HIV OR tuberculosis OR malaria) AND (diabetes mellitus OR chronic obstructive pulmonary disease OR chronic kidney disease OR cardiovascular disease OR cardiomyopathy OR metabolic syndrome OR neurocognitive disease OR dementia OR epilepsy). From the 24 864 articles identified by this search strategy, we honed down on original research and review articles with titles and abstracts that were clearly pertinent to co-morbidity and interactions between one or more of the three communicable diseases and NCDs. Articles were selected to ensure comprehensive representation of interactions reported in the literature. Both authors identified and reviewed papers and 80 articles were eventually included in the review. Of note, there was a lot of information about the NCDs and TB or HIV interactions but far fewer studies on malaria and NCDs.
Results
Tuberculosis
As Table 1 shows, TB remains a leading cause of disability-adjusted life years in many regions of the world, particularly in poor populations in LMIC. In 2012 it is estimated that there were 8.6 million new cases and 1.3 million deaths from TB.7 Tuberculosis is preventable and curable, and therefore the goal of much lower incidence and mortality is appropriate.8 In 2014 the World Health Assembly adopted ambitious new targets for TB: a 90% reduction in incidence and a 95% reduction in the number of deaths between 2015 and 2035.9 Achieving a 90% reduction in incidence by 2035 will require a marked improvement in the rate of decline, from around 2% per year at present, to 10% per year by 2025.8 Achieving a 95% decrease in mortality will require more than halving the case fatality from 15% to 6.5% by 2025.8 Tackling this challenge will require improvements in diagnostic and treatment services and identification and reduction of risk factors that increase TB susceptibility. Diseases and risk factors that impair immune function, such as malnutrition, alcoholism or HIV co-infection, can increase the likelihood of infection or reactivation of latent TB. A study of the effect of multiple exposures to these risk factors reported that tobacco use, alcohol, type 2 diabetes mellitus (T2DM) and low body mass index (BMI) were significant individual risk factors and associated with triple or quadruple the risk of TB with multiple exposures.10
Tuberculosis and diabetes
Type 2 diabetes mellitus is a risk factor for TB. Two systematic reviews have demonstrated that T2DM increases the risk of incident TB by around threefold.11,12 Together these reviews included 15 studies, the vast majority of which were from high-income countries. Since they were published more data have accumulated from low and middle-income settings, essentially confirming the increased risk of TB in people with diabetes.13 In a case control study from Tanzania, for example, diabetes was associated with fourfold increased risk of TB in HIV negative, but not positive, patients14 Because diabetes is common (affecting 8.3% of the global adult population, based on International Diabetes Federation estimates for 20148) the number of cases of TB attributable to diabetes is large. Globally, for example, diabetes is estimated to account for 15% of all adult cases of TB. Even in Africa, where diabetes prevalence in adults is estimated to be 5% (the lowest of all regions), and HIV is a major contributor to TB incidence, diabetes is still thought to account for almost 1 in 10 adult cases.8
In addition to increasing the risk of incident TB, diabetes is also a risk factor for poorer TB outcomes. A systematic review and meta-analysis found that the risk of death during TB treatment was almost twice as high in those with diabetes compared to those without, and relapse following treatment almost four times as high.15
These interactions between diabetes and TB have implications for achieving the 2035 WHO targets for TB incidence and mortality.9 Diabetes prevalence is expected to continue to increase over the coming decades, especially in LMIC.16 Conservative estimates suggest that it will increase to around 10% globally in adults in 203516 and the results of modelling suggest that this would offset the present downward trend in incidence by around 3%.8 A less conservative estimate of the increase in diabetes prevalence, suggests that it will be 13% in 2035 and this would offset the decline in TB incidence by 8%.8
The strong association between TB and diabetes, and the poorer health outcomes associated with their co-existence, naturally leads to the question of whether patients with one condition should be screened for the other. The latest WHO strategy9 recommends screening people with diabetes for active TB in settings with a high TB burden, such as where the TB incidence is 100 cases per 100 000 persons/year or more. The type of screening will depend on resources. A practical approach described in China and India is to screen all people with diabetes on each clinic visit with a symptom-based questionnaire with referral for further investigations for those who are positive.8 The WHO strategy also recommends that all people with TB are screened for diabetes, with referral for diabetes diagnosis and management for those who are positive.9
It is clear that diabetes and TB are intimately related. However, there remain many unanswered questions as to the most effective approaches to minimizing the morbidity and mortality from this interaction. These were summarized in a recent review13 and include the following: what is the effect of glycaemic control on new TB infection, active TB and TB treatment outcomes, and what are the most effective approaches to achieving glycaemic control in people with TB? what are the most feasible and valid approaches to screening for diabetes in patients with TB (noting that inflammation from TB infection may cause a transitory hyperglycaemic response)? what models of health care delivery can deliver sustainable, integrated and cost-effective care for diabetes and TB in LMIC? and is screening and prophylactic treatment of latent TB infection indicated in people with diabetes?
A significant contribution to answering some of the questions on the diagnosis of diabetes in TB, the role of glycaemic control on TB outcomes, and the best way to deliver care, is being made by the TANDEM study,17 which began in 2013. This is a study working in four endemic TB countries that are experiencing rapid rises in diabetes prevalence (Romania, Peru, South Africa and Indonesia) and supported by researchers in Germany, UK and the Netherlands.
Tuberculosis and chronic obstructive pulmonary disease
Figure 1 shows the bi-directional nature of the interaction between TB and chronic obstructive pulmonary disease (COPD). Due to the similarity between TB and COPD symptoms, there is potential for missing the diagnosis of one when they co-exist. Persons with COPD have been found in one study to have a two to threefold higher risk of developing TB,18 and a twofold increased mortality compared to non-COPD patients.19 The increased risk of TB associated with COPD is often attributed to smoking.20 However, studies have also found an association between oral corticosteroid use in COPD patients and TB risk.18 A systematic review confirmed that although this association is independent of smoking,21 the risk of COPD is further increased by tobacco smoking and low socioeconomic status, common risk factors for both COPD and TB.
The histopathological changes that occur in the lungs of TB patients can result in anatomical changes associated with both obstructive and restrictive patterns of impaired lung function of varying severity, which can persist after successful completion of TB treatment.22 The prevalence of COPD after TB treatment completion varies from 28 to 68%,23 and is further increased in persons with multiple episodes of TB.24 Childhood studies have also demonstrated this association, due to prolonged bronchial obstruction by enlarged lymph nodes during TB disease.25 In LMIC, alongside a concomitant rise in the prevalence of tobacco smoking, TB is an important contributor to poor quality of life and disability-adjusted life years lost due to COPD.26 A study in South Africa reported that the strongest predictor of chronic bronchitis was a history of TB.27 Early identification and management of chronic lung impairment is therefore crucial to minimizing the long-term negative impact of TB.
Tuberculosis and chronic kidney disease
The prevalence of chronic kidney disease (CKD) is increasing, and it is estimated that 70% of patients with end stage renal disease will reside in LMIC by 2030.28 This has significant implications on infrastructural and financial resources. The most common causes of CKD in LMIC are chronic glomerulonephritis and interstitial nephritis due to infections, including TB.28 Conversely, patients with CKD and patients on dialysis are at an increased risk of TB and poorer TB outcomes.29 One study in India reported a 4% incidence of TB, despite negative tuberculin skin tests (TST) in the majority.30 Similarly, a study of hemodialysis patients in Turkey also reported a 3.1% incidence of TB with almost 40% of patients having a negative TST.31 This suggests that there should be a high index of suspicion of TB in these patients regardless of TST results. Examining factors associated with treatment non-adherence or death, a study in Brazil found that socio-demographic characteristics such as younger age and alcoholism were associated with poorer outcomes.32
The co-existence of TB also complicates management of blood pressure in CKD patients, as concurrent TB treatment is associated with a decrease in the potency of antihypertensive treatment.33
Tuberculosis and the heart
Tuberculosis is the most common cause of pericarditis in Africa and other high TB burden settings, often presenting with symptoms similar to those of heart failure.34 The increase in the burden of TB pericarditis has been attributed to HIV35 and in the Western Cape, South Africa, 50% of patients with pericardial effusions have HIV.36 Given these data, TB is an important consideration in persons presenting with heart failure in high TB burden settings, especially if HIV-infection is present. However, significant challenges remain, including diagnostic difficulty due to atypical presentation and varying evidence on the optimal management of these co-morbid conditions.35
HIV
Globally, HIV is the fifth and sixth leading cause of DALYs lost and mortality, respectively.37,38 There are promising signs that the HIV pandemic is abating in high burden settings, with declining incidence and mortality rates.39 Nonetheless, the rising NCD morbidity and mortality rates alongside an established HIV epidemic make it crucial to better understand the interactions that exist with emerging NCD and disease precursors, both related to HIV directly or as a side effect of antiretroviral therapy (ART). LMIC bear a disproportionate burden of the HIV pandemic. Furthermore, HIV-infected adults on treatment have higher than expected risk of several non-AIDS disorders, including cardiovascular disease and kidney disease in addition to adverse effects associated with ART.40,41 HIV has also been identified in a case control study as an independent risk factor for stroke in urban and rural Tanzania,42 although there is a paucity of data on the nature and extent of this interaction in LMIC.
HIV and metabolic syndrome
There is conflicting evidence on an association between HIV infection and hyperglycemia (including T2DM) independent of ART.43,44 The use of ART containing protease inhibitors (PI) and nucleoside reverse transcriptase inhibitors has been associated with insulin resistance.45,46 A Cape Town survey of HIV-infected persons on ART reported a 21.9% prevalence of newly detected hyperglycemia and a significant association with efavirenz (a non-nucleoside reverse transcriptase inhibitor).47 HIV-related dyslipidemia independent of ART has been described.48 The use of ART is also associated with dyslipidemia, peripheral wasting and central fat accumulation. In particular PI and non-nucleoside reverse transcriptase inhibitors (NNRTI)-based regimens have been associated with dyslipidemia and atrophy. Protease inhibitor drugs are particularly associated with dyslipidemia, a known risk factor for cardiovascular complications49,50; patients with baseline elevated lipid levels have the greatest risk of developing dyslipidemia, especially hypertriglyceridemia.49 A study conducted in South Africa reported an association between ART and increased central fat and reduced peripheral fat; partially improved by switching from an NNRTI to a PI-based regimen.51
HIV and the heart
The most commonly reported cardiac manifestation in HIV is pericardial disease, often due to TB.52 HIV-related dilated cardiomyopathy is also common with the prevalence in the pre-ART era ranging between 18 and 43% in LMIC.53,54 The prognosis of this condition has historically been poor,55,56 although the prevalence has decreased with the roll out of ART.52 Evidence from Africa on the prevalence of echocardiographic abnormalities in asymptomatic persons with HIV is limited although documented in other regions to vary from a 34–48% prevalence of systolic and diastolic dysfunction, and >10% prevalence of dilated cardiomyopathy.57,58 Pulmonary hypertension is also associated with HIV infection, with a prevalence of between 0.5 and 5%.52 Although mortality associated with pulmonary hypertension has significantly decreased post-ART roll out, specific treatment for pulmonary hypertension is required to improve cardiac function.59 There is an increased risk of myocardial infarction in patients with HIV on ART, particularly in patients with metabolic syndrome.60 Protease inhibitor drugs have been shown to be associated with a 26% increase in the rate of myocardial infarction per year of exposure, partially due to dyslipidemia.61 This was found with some (e.g., indinavir, ritonavir-boosted lopinavir62) but not others (boosted atazanavir63). Evidence on the cardiovascular disease (CVD) risk associated with nucleoside reverse transcriptase inhibitors is conflicting; however a recent meta-analysis demonstrated no increased risk.64 There has been no proven association between T2DM and other ART drug classes. Drug-drug interactions are also important to mention due to interaction of ART with the cytochrome P450 pathway.52
HIV and chronic kidney disease
Chronic kidney disease is an important cause of morbidity and mortality in persons with HIV, including HIV-associated nephropathy and membranoproliferative glomerulonephritis, particularly in hepatitis C co-infection.65 The risk of CKD is further increased in the presence of other risk factors including older age, hypertension, diabetes, and black ethnicity.66 Although the incidence of CKD has been remarkably altered by widespread ART access, some ART regimens are associated with incident acute or chronic kidney disease.67 Studies of HIV-infected patients on ART in Taiwan and Vietnam reported a 7% prevalence of CKD, with older age, lower body weight and tenofovir use being independently associated with CKD.68,69 However, the benefits of tenofovir are considered to outweigh the nephrotoxic side effects.70
HIV and chronic obstructive pulmonary disease
Studies have shown an increased risk of COPD in HIV-infected patients. Studies conducted in the pre-ART era demonstrated an association between HIV and airway hyper-responsiveness as well as radiographic emphysema.71,72 A post-ART era study conducted in the USA showed that after adjusting for known COPD risk factors, HIV remained an independent risk factor for COPD with patients with HIV 50–60% more likely to have COPD than HIV-negative.73 These findings have been confirmed in other studies conducted in the USA24,74 and Italy.75 A French study reported a 26% prevalence of COPD among persons with HIV, 74% of which were previously undiagnosed.76 There is a paucity of data on HIV and COPD from high HIV prevalence LMIC. With increasing periods on ART, pulmonary complications are shifting from opportunistic infections to non-infectious complications such as COPD.
HIV and the brain
Neurocognitive disease and dementia
We highlight three aspects of ageing in HIV: patients with HIV are surviving for longer periods; an increasing proportion of incident HIV cases are in older persons who may perceive themselves to be at low risk of HIV; and HIV and ART are thought to be associated with acceleration of the ageing process such that illnesses associated with advanced age occur at younger ages.77 These combined processes mean that co-morbidity of neurocognitive disorders such as HIV-associated neurocognitive disorder (HAND) will become increasingly more common. A study in Nigeria reported a 21.5% prevalence of HAND in HIV-infected patients on ART for at least 1 year.78
Epilepsy and seizures
Seizures, a neurological manifestation of HIV infection, are mostly of the generalized type, and are more common in advanced stages of HIV, although they may rarely be the presenting manifestation or occur early in the course of illness.79 Reported causes include mass lesions, opportunistic infections including toxoplasmosis and cryptococcal meningitis and the direct effect of HIV on the brain (HIV encephalopathy).79 The reported incidence of new-onset seizures varies from 4–20%79,80 with a higher prevalence in LMIC, likely due to a higher prevalence of opportunistic infections. The management of co-morbid HIV and epilepsy can be challenging. Phenytoin is the most commonly prescribed anti-epileptic drug and this drug induces the CYP450 system and can result in ART failure to control HIV viral replication81 or phenytoin toxicity.82 This highlights the importance of careful monitoring of viral load and anti-epileptic drug levels, and careful selection of anti-epileptics such as levetiracetam.83 However, in many LMIC, choices of anti-epileptic drugs are limited.
Malaria
Malaria is a parasitic infection that is responsible for at least half a million deaths per year (estimated to be 660 000 in 2010, uncertainty range from 490 000 to 836 000), and around 200 million clinical cases per year (219 million in 2010, uncertainty range 154 to 289).84 Eighty percent of all deaths occur in just 14 countries, with 40% occurring in just two: Nigeria and the Democratic Republic of the Congo.84
Malaria is caused by the protozoan Plasmodium, which is transmitted between humans by the Anopheles mosquito. There are four species of Plasmodium causing disease in humans, of which vivax and falciparum are the most common; falciparum is the most deadly because of its tendency to involve the brain (cerebral malaria). Roughly 30% of the world's population lives in areas where there is a risk of falciparum malaria.85 Groups at high risk of severe and life threatening infection are those with no immunity (e.g., children, and visitors from non-endemic areas) and with impaired immunity (e.g., those with HIV/AIDS). However, immunity is relatively short lived and those returning to endemic areas after several months or years are also at increased risk because of reduced or absent immunity.84
Malaria is, of course, associated with the environmental conditions that favour the breeding of its mosquito vector. However, similar to the major NCDs it is also associated with poor socio-economic conditions, and it has been suggested that economic development per se will reduce its impact.86 Other shared risk factors with NCDs are less clear. The relationship between poor childhood nutrition, a risk factor for T2DM and cardiovascular disease, and the risk of malaria is unclear.87–90
Malaria and diabetes
There is evidence from a recent case control study conducted in urban Ghana that people with T2DM are roughly 50% more likely to show evidence, based on testing for the DNA of the parasite, of infection with falciparum malaria.91 It is important to note that participants in this study did not have clinical malaria. However, the findings do support the hypothesis that people with T2DM may be at increased risk of clinical malaria.
Clinical malaria in adults with T2DM is likely to be relatively common in endemic areas that also have a high prevalence of T2DM, such as many urban centers in Africa and Asia. However, there is a lack of evidence on whether people with T2DM who develop clinical malaria have poorer outcomes than people without T2DM.
Malaria and chronic kidney disease
Chronic kidney disease and risk of malaria
Chronic kidney disease was ranked the 18th commonest cause of death globally in 2010, estimated to have caused 736 000 deaths.92 Type 2 diabetes mellitus and hypertension are the two most important risk factors for CKD,92 and as these increase in LMIC, so will CKD. The clinical end point of CKD is end stage kidney disease, which can be defined by the requirement for life saving dialysis or renal transplantation.93 Worldwide it is estimated that 1.9 million people are undergoing some form of renal replacement therapy.94 In LMIC, it is estimated that only around a quarter of those who require renal replacement therapy receive it.
It is not known if CKD increases the risk of clinical malaria. It is known, however, that renal transplantation in malarial areas is associated with a risk of malaria in the recipients, the infection being transmitted via the donor kidney.95 Thus, it is recommended that renal transplant patients in malarial areas receive appropriate prophylaxis to eliminate the risk of this potentially life threatening complication.95
Finally, three of the drugs commonly used in malaria prophylaxis (malarone, proguanil and chloroquine) may be contraindicated in patients with CKD, depending on the level of renal impairment.96
Malaria as a cause of kidney disease
One form of malaria, caused by Plasmodium malariae, is associated with a risk of progressive renal damage (nephrotic syndrome) which even after successful eradication of the infection may progress to end stage kidney disease, and thus require renal replacement therapy.95 This condition occurs predominantly in children and young adults. While it is a well-recognized condition, good estimates of its incidence and overall contribution to end stage kidney disease are lacking. It is thought to occur in only a fraction of Plasmodium malariae infections but it is described as one of the major causes of renal disease in children living in malarial areas.97
It is estimated that falciparum malaria is associated with acute renal failure in 1–5% of cases occurring in local inhabitants in endemic areas, but that in non-immune visitors around a quarter suffer this complication.95 Successful treatment of the infection normally leads to recovery of renal function within 2–6 weeks. However, during the acute phase many patients (40–70%) require dialysis.95
Discussion of implications for health systems
This review highlights the complex interactions between established communicable and emerging NCDs in LMIC. The results emphasize the importance of re-thinking disease classifications in the context of disease prevention, promotion, treatment and care.
The increasing prevalence of communicable/NCD multimorbidity in many LMIC settings, particularly in socio-economically disadvantaged groups suggests that this changing pattern of disease has significant implications for the health system and models of health care delivery. For example, a recent study in a peri-urban informal township near Cape Town, South Africa showed that 19% of HIV-infected patients on ART were on treatment for another chronic disease, with 77% and 17% of these patients concurrently receiving anti-hypertensive and diabetic treatment respectively.98 There is therefore a need to ensure integrated care across the continuum of care from primary to tertiary levels.
The Innovative Care for Chronic Conditions (ICCC) Framework developed by WHO addresses the increasing burden of chronic diseases in LMIC and is a tool designed to assist health systems to shift from providing predominantly acute episodic care in order to meet the increasing needs of chronic disease care.99 However, it does not explicitly incorporate the concept of co-existing and interacting multiple morbidities in these settings. A conceptual modification to this framework has been proposed that incorporates these multiple morbidities and examines the impact beyond biological interaction of these diseases.100 This modified framework could be used as a tool to help guide the development of integrated interventions at multiple levels. Models for integration of commonly occurring conditions need to be evaluated to optimize and streamline management. Integrated chronic disease models are being developed and implemented in some LMIC. An example is the integrated chronic disease management model in South Africa that includes HIV, TB, diabetes, hypertension, asthma, epilepsy, asthma, COPD and mental health illnesses. This model builds on the strengths of the HIV/TB integrated programme and focuses on a systems approach to re-structuring the primary health care system, improving efficiency using integrated clinical algorithms, clinic stationery and re-organised clinic flows as well as the integrated training of community care workers in the prevention, promotion and treatment of these diseases. In the context of multimorbidity, the model also aims to empower patients and assist with self-management of their chronic diseases. Integration of community support groups is another approach to supporting patients with multiple and interacting chronic diseases potentially improving adherence to treatment and disease outcomes.
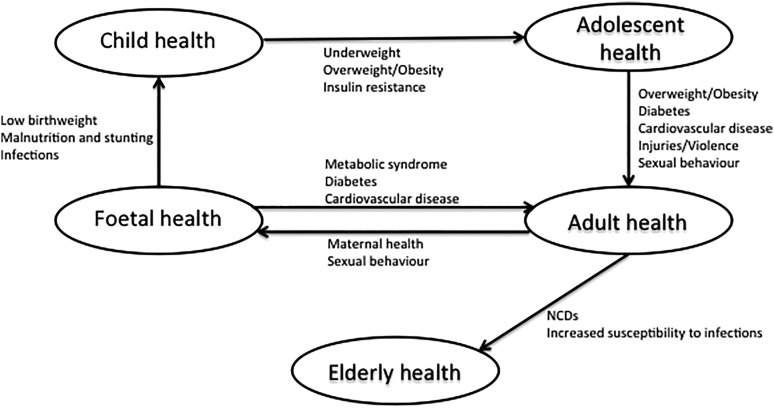
When considering interventions aimed at primary prevention, Figure 2 highlights the importance, both of considering shared risk factors (identified in Figure 1) that influence the risk and outcomes, and of using a life course approach when considering targets for intervention. Women have a disproportionally higher prevalence of certain NCD risk factors, particularly obesity and lower physical activity, and in addition show rising rates of smoking and alcohol consumption.101 Given this fact, along with the impact of maternal factors on fetal and child health, and the likelihood of strongly influencing dietary household choices, female adolescents and adults represent an important population group for intervention. These interventions should ideally occur pre-conception and cover nutrition, physical activity, contraception, high-risk behavior including high-risk sexual practices, smoking and alcohol consumption. Figure 2 also highlights the importance of focusing on the elderly as ageing is associated with increasing prevalence of NCDs and an increased susceptibility to communicable diseases. With increasing access to ART, there is increasing survival and ageing in HIV-infected persons. However, public health HIV control interventions in LMIC often focus on the younger 15–44 age group where incidence is highest, with insufficient emphasis on the older age groups. Furthermore, immunocompromise associated with ageing increases the risk of other communicable diseases including TB, potentially compounded by the increased risk of T2DM. Given the high prevalence of NCDs, older persons in LMIC are at a high risk of developing multi-morbid communicable and non-communicable conditions.102 This can result in disability, reduced quality of life, and social isolation, limiting their ability to fulfill emotional, cultural and economic roles within families and the society. Interventions that address isolation and promote social participation have been identified as potentially important in the elderly.103
Figure 2.
Life-course approach to joint communicable and non-communicable disease (NCD) prevention and control.
Limitations
As this is a critical and not a systematic review it did not aim to exhaustively identify and abstract data from all relevant literature. Rather the aim was to describe key concepts from the current literature. While we believe that we have achieved this aim, it is quite possible that other authors would have used somewhat different literature to illustrate the same concepts.
Conclusions
The aim of this review was to illustrate the overlap and interaction between communicable and NCDs, particularly in LMIC, and show how the agendas for their prevention and control are inextricably linked. There is therefore a need for those responsible for the design of health systems within individual countries to understand the distribution and interaction of communicable and NCDs within their own populations in order to appropriately plan preventive and treatment programs and services. When it comes to the provision of health care for treatment this will require breaking down barriers between departments within health ministries that have traditionally designed services and programs for communicable and NCDs separately. When it comes to prevention, it will require integrated multi-sectoral action addressing determinants across the life course.
Acknowledgments
Authors' contributions: TO and NU were responsible for conceptualizing the manuscript. TO led the writing, with both TO and NU contributing to initial drafts of the manuscript and editing. TO and NU read and approved the final manuscript. TO is guarantor of the paper.
Funding: This work was supported by a Carnegie Corporation Postdoctoral Fellowship; a Harry Crossley Senior Clinical Fellowship; a Wellcome Trust funded Clinical Infectious Disease Research Initiative clinical fellow postdoctoral award; and the University of the West Indies.
Competing interests: None declared.
Ethical approval: Not required.
References
- 1.World Bank Group. Data. Country and Lending Groups. http://data.worldbank.org/about/country-and-lending-groups [accessed 29 March 2015.
- 2.Setel PW, Saker L, Unwin N et al. . Is it time to reassess the categorization of disease burdens in low-income countries? Am J Public Health 2004;94:384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Omran AR. The epidemiologic transition: a theory of the epidemiology of population change. Milbank Q 2005;83:731–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nelson MC, Rogers J. The epidemiologic transition revisited, or what happenes if we look beneath the surface? Health Transit Rev 1997;7:235–55. [Google Scholar]
- 5.Santosa A, Wall S, Fottrell E et al. . The development and experience of epidemiological transition theory over four decades: a systematic review. Glob Health Action 2014;7:23574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grant MJ, Booth A. A typology of reviews: an analysis of 14 review types and associated methodologies. Health Info Libr J 2009;26:91–108. [DOI] [PubMed] [Google Scholar]
- 7.WHO. Global Tuberculosis Report 2014. Geneva: World Health Organization; 2014. [Google Scholar]
- 8.Lönnroth K, Roglic G, Harries AD. Improving tuberculosis prevention and care through addressing the global diabetes epidemic: from evidence to policy and practice. Lancet Diabetes Endocrinol 2014;2:730–9. [DOI] [PubMed] [Google Scholar]
- 9.WHO. Global Strategy and Targets for Tuberculosis Prevention, Care, and Control After 2015. WHO 67th World Health Assembly 2014 Geneva: World Health Organization; 2014. [Google Scholar]
- 10.Patra J, Jha P, Rehm J, Suraweera W. Tobacco smoking, alcohol drinking, diabetes, low body mass index and the risk of self-reported symptoms of active tuberculosis: individual participant data (IPD) meta-analyses of 72,684 individuals in 14 high tuberculosis burden countries. PLoS ONE 2014;9:e96433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jeon CY, Murray MB. Diabetes mellitus increases the risk of active tuberculosis: a systematic review of 13 observational studies. PLoS Med 2008;5:e152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stevenson CR, Critchley JA, Forouhi NG et al. . Diabetes and the risk of tuberculosis: a neglected threat to public health? Chronic Illn 2007;3:228–45. [DOI] [PubMed] [Google Scholar]
- 13.Riza AL, Pearson F, Ugarte-Gil C et al. . Clinical management of concurrent diabetes and tuberculosis and the implications for patient services. Lancet Diabetes Endocrinol 2014;2:740–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Faurholt-Jepsen D, Range N, Praygod G et al. . Diabetes is a risk factor for pulmonary tuberculosis: a case-control study from Mwanza, Tanzania. PLoS ONE 2011;6:e24215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baker MA, Harries AD, Jeon CY et al. . The impact of diabetes on tuberculosis treatment outcomes: a systematic review. BMC Med 2011;9:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.International Diabetes Federation. Diabetes Atlas. Give edition. http://www.idf.org/diabetesatlas [accessed 17 November 2013].
- 17.Van Crevel R, Dockrell HM. TANDEM: understanding diabetes and tuberculosis. Lancet Diabetes Endocrinol 2014;2:270–2. [DOI] [PubMed] [Google Scholar]
- 18.Lee C-H, Lee M-C, Shu C-C et al. . Risk factors for pulmonary tuberculosis in patients with chronic obstructive airway disease in Taiwan: a nationwide cohort study. BMC Infect Dis 2013;13:194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Inghammar M, Ekbom A, Engström G et al. . COPD and the risk of tuberculosis--a population-based cohort study. PLoS ONE 2010;5:e10138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lin H-H, Ezzati M, Murray M. Tobacco smoke, indoor air pollution and tuberculosis: a systematic review and meta-analysis. PLoS Med 2007;4:e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Allwood BW, Myer L, Bateman ED. A systematic review of the association between pulmonary tuberculosis and the development of chronic airflow obstruction in adults. Respiration 2013;86:76–85. [DOI] [PubMed] [Google Scholar]
- 22.Pasipanodya JG, Miller TL, Vecino M et al. . Pulmonary impairment after tuberculosis. Chest 2007;131:1817–24. [DOI] [PubMed] [Google Scholar]
- 23.Willcox PA, Ferguson AD. Chronic obstructive airways disease following treated pulmonary tuberculosis. Respir Med 1989;83:195–8. [DOI] [PubMed] [Google Scholar]
- 24.Hnizdo E, Singh T, Churchyard G. Chronic pulmonary function impairment caused by initial and recurrent pulmonary tuberculosis following treatment. Thorax 2000;55:32–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jordan TS, Spencer EM, Davies P. Tuberculosis, bronchiectasis and chronic airflow obstruction. Respirology 2010;15:623–8. [DOI] [PubMed] [Google Scholar]
- 26.Maguire GP, Anstey NM, Ardian M et al. . Pulmonary tuberculosis, impaired lung function, disability and quality of life in a high-burden setting. Int J Tuberc Lung Dis 2009;13:1500–6. [PubMed] [Google Scholar]
- 27.Ehrlich RI, White N, Norman R et al. . Predictors of chronic bronchitis in South African adults. Int J Tuberc Lung Dis 2004;8:369–76. [PubMed] [Google Scholar]
- 28.Barsoum RS. Chronic kidney disease in the developing world. N Engl J Med 2006;354:10. [DOI] [PubMed] [Google Scholar]
- 29.Hussein M, Mooij J. Tuberculosis and chronic renal disease. Saudi J Kidney Dis Transpl 2002;320–30. [PubMed] [Google Scholar]
- 30.Venkata RK, Kumar S, Krishna RP et al. . Tuberculosis in chronic kidney disease. Clin Nephrol 2007;67:217–20. [DOI] [PubMed] [Google Scholar]
- 31.Ates G, Yildiz T, Danis R et al. . Incidence of tuberculosis disease and latent tuberculosis infection in patients with end stage renal disease in an endemic region. Ren Fail 2010;32:91–5. [DOI] [PubMed] [Google Scholar]
- 32.Reis-Santos B, Gomes T, Horta BL, Maciel EL. The outcome of tuberculosis treatment in subjects with chronic kidney disease in Brazil: a multinomial analysis. J Bras Pneumol 39:585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sharma AP, Sural S, Gupta A et al. . Effect of antitubercular medications on blood pressure control in chronic kidney disease patients with tuberculosis: a prospective cohort study. J Nephrol 2006;19:771–7. [PubMed] [Google Scholar]
- 34.Desai HN. Tuberculous pericarditis. A review of 100 cases. S Afr Med J 1979;55:877–80. [PubMed] [Google Scholar]
- 35.Mayosi BM, Burgess LJ, Doubell AF. Tuberculous pericarditis. Circulation 2005;112:3608–16. [DOI] [PubMed] [Google Scholar]
- 36.Reuter H, Burgess LJ, Doubell AF. Epidemiology of pericardial effusions at a large academic hospital in South Africa. Epidemiol Infect 2005;133:393–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Murray CJL, Vos T, Lozano R et al. . Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012;380:2197–223. [DOI] [PubMed] [Google Scholar]
- 38.Lozano R, Naghavi M, Foreman K et al. . Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012;380:2095–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.WHO, UNAID, UNICEF. Global HIV/AIDS Response: Epidemic update and health sector progress towards Universal Access – Progress Report 2011. Geneva: World Health Organization; 2011. http://www.unaids.org/sites/default/files/media_asset/20111130_UA_Report_en_1.pdf [accessed 1 December 2014]. [Google Scholar]
- 40.Subbaraman R, Chaguturu SK, Mayer KH et al. . Adverse effects of highly active antiretroviral therapy in developing countries. Clin Infect Dis 2007;45:1093–1101. [DOI] [PubMed] [Google Scholar]
- 41.Dillon DG, Gurdasani D, Riha J et al. . Association of HIV and ART with cardiometabolic traits in sub-Saharan Africa: a systematic review and meta-analysis. Int J Epidemiol 2013;42:1754–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Walker RW, Jusabani A, Aris E et al. . Stroke risk factors in an incident population in urban and rural Tanzania: a prospective, community-based, case-control study. Lancet Glob Health 1:e282–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Butt AA, McGinnis K, Rodriguez-Barradas MC et al. . HIV infection and the risk of diabetes mellitus. AIDS 2009;23:1227–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brown TT, Cole SR, Li X et al. . Antiretroviral therapy and the prevalence and incidence of diabetes mellitus in the multicenter AIDS cohort study. Arch Intern Med 2005;165:1179–84. [DOI] [PubMed] [Google Scholar]
- 45.De Wit S, Sabin CA, Weber R et al. . Incidence and risk factors for new-onset diabetes in HIV-infected patients: the Data Collection on Adverse Events of Anti-HIV Drugs (D:A:D) study. Diabetes Care 2008;31:1224–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Samaras K. Prevalence and pathogenesis of diabetes mellitus in HIV-1 infection treated with combined antiretroviral therapy. J Acquir Immune Defic Syndr 2009;50:499–505. [DOI] [PubMed] [Google Scholar]
- 47.Dave JA, Lambert EV, Badri M et al. . Effect of nonnucleoside reverse transcriptase inhibitor-based antiretroviral therapy on dysglycemia and insulin sensitivity in South African HIV-infected patients. J Acquir Immune Defic Syndr 2011;57:284–9. [DOI] [PubMed] [Google Scholar]
- 48.Grunfeld C, Kotler DP, Hamadeh R et al. . Hypertriglyceridemia in the acquired immunodeficiency syndrome. Am J Med. 1989;86:27–31. [DOI] [PubMed] [Google Scholar]
- 49.Montes ML, Pulido F, Barros C et al. . Lipid disorders in antiretroviral-naive patients treated with lopinavir/ritonavir-based HAART: frequency, characterization and risk factors. J Antimicrob Chemother 2005;55:800–4. [DOI] [PubMed] [Google Scholar]
- 50.Anastos K, Lu D, Shi Q et al. . Association of serum lipid levels with HIV serostatus, specific antiretroviral agents, and treatment regimens. J Acquir Immune Defic Syndr 2007;45:34–42. [DOI] [PubMed] [Google Scholar]
- 51.Goedecke JH, Micklesfield LK, Levitt NS et al. . Effect of different antiretroviral drug regimens on body fat distribution of HIV-infected South African women. AIDS Res Hum Retroviruses 2013;29:557–63. [DOI] [PubMed] [Google Scholar]
- 52.Thienemann F, Sliwa K, Rockstroh JK. HIV and the heart: the impact of antiretroviral therapy: a global perspective. Eur Heart J 2013; 34:3538–46. [DOI] [PubMed] [Google Scholar]
- 53.Twagirumukiza M, Nkeramihigo E, Seminega B et al. . Prevalence of dilated cardiomyopathy in HIV-infected African patients not receiving HAART: a multicenter, observational, prospective, cohort study in Rwanda. Curr HIV Res 2007;5:129–37. [DOI] [PubMed] [Google Scholar]
- 54.Luo L, Ye Y, Liu Z et al. . Assessment of cardiac diastolic dysfunction in HIV-infected people without cardiovascular symptoms in China. Int J STD AIDS 2010;21:814–8. [DOI] [PubMed] [Google Scholar]
- 55.Sliwa K, Carrington MJ, Becker A et al. . Contribution of the human immunodeficiency virus/acquired immunodeficiency syndrome epidemic to de novo presentations of heart disease in the Heart of Soweto Study cohort. Eur Heart J 2012;33:866–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chillo P, Bakari M, Lwakatare J. Echocardiographic diagnoses in HIV-infected patients presenting with cardiac symptoms at Muhimbili National Hospital in Dar es Salaam, Tanzania. Cardiovasc J Africa 2012;23:90–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Thöni GJ, Schuster I, Walther G et al. . Silent cardiac dysfunction and exercise intolerance in HIV+ men receiving combined antiretroviral therapies. AIDS 2008;22:2537–40. [DOI] [PubMed] [Google Scholar]
- 58.Reinsch N, Kahlert P, Esser S et al. . Echocardiographic findings and abnormalities in HIV-infected patients: results from a large, prospective, multicenter HIV-heart study. Am J Cardiovasc Dis 2011;1:176–84. [PMC free article] [PubMed] [Google Scholar]
- 59.Degano B, Guillaume M, Savale L et al. . HIV-associated pulmonary arterial hypertension: survival and prognostic factors in the modern therapeutic era. AIDS 2010;24:67–75. [DOI] [PubMed] [Google Scholar]
- 60.Young F, Critchley JA, Johnstone LK, Unwin NC. A review of co-morbidity between infectious and chronic disease in Sub Saharan Africa: TB and diabetes mellitus, HIV and metabolic syndrome, and the impact of globalization. Global Health 2009;5:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.D:A:D Study Group Sabin CA, Worm SW et al. . Use of nucleoside reverse transcriptase inhibitors and risk of myocardial infarction in HIV-infected patients enrolled in the D:A:D study: a multi-cohort collaboration. Lancet 2008;371:1417–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lang S, Mary-Krause M, Cotte L et al. . Impact of individual antiretroviral drugs on the risk of myocardial infarction in human immunodeficiency virus-infected patients: a case-control study nested within the French Hospital Database on HIV ANRS cohort CO4. Arch Intern Med 2010;170:1228–38. [DOI] [PubMed] [Google Scholar]
- 63.Monforte AD, Reiss P, Ryom L et al. . Atazanavir is not associated with an increased risk of cardio- or cerebrovascular disease events. AIDS 2013;27:407–15. [DOI] [PubMed] [Google Scholar]
- 64.Ding X, Andraca-Carrera E, Cooper C et al. . No association of abacavir use with myocardial infarction: findings of an FDA meta-analysis. J Acquir Immune Defic Syndr 2012;61:441–7. [DOI] [PubMed] [Google Scholar]
- 65.Szczech LA, Gupta SK, Habash R et al. . The clinical epidemiology and course of the spectrum of renal diseases associated with HIV infection. Kidney Int 2004;66:1145–52. [DOI] [PubMed] [Google Scholar]
- 66.Winston J, Deray G, Hawkins T et al. . Kidney disease in patients with HIV infection and AIDS. Clin Infect Dis 2008;47:1449–57. [DOI] [PubMed] [Google Scholar]
- 67.Berns JS, Kasbekar N. Highly active antiretroviral therapy and the kidney: an update on antiretroviral medications for nephrologists. Clin J Am Soc Nephrol 2006;1:117–29. [DOI] [PubMed] [Google Scholar]
- 68.Hsieh M-H, Lu P-L, Kuo M-C et al. . Prevalence of and associated factors with chronic kidney disease in human immunodeficiency virus-infected patients in Taiwan. J Microbiol Immunol Infect 2013; pii:S1684–1182(13)00158–8.check page numbers [DOI] [PubMed] [Google Scholar]
- 69.Mizushima D, Tanuma J, Kanaya F et al. . WHO antiretroviral therapy guidelines 2010 and impact of tenofovir on chronic kidney disease in Vietnamese HIV-infected patients. PLoS One 2013;8:e79885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kalyesubula R, Perazella MA. Nephrotoxicity of HAART. AIDS Res Treat 2011;2011:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Diaz PT, King MA, Pacht ER et al. . Increased susceptibility to pulmonary emphysema among HIV-seropositive smokers. Ann Intern Med 2000;132:369–72. [DOI] [PubMed] [Google Scholar]
- 72.O'Donnell CR, Bader MB, Zibrak JD et al. . Abnormal airway function in individuals with the acquired immunodeficiency syndrome. Chest 1988;94:945–8. [DOI] [PubMed] [Google Scholar]
- 73.Crothers K, Butt AA, Gibert CL et al. . Increased COPD among HIV-positive compared to HIV-negative veterans. Chest 2006;130:1326–33. [DOI] [PubMed] [Google Scholar]
- 74.Gingo MR, Balasubramani GK, Rice TB et al. . Pulmonary symptoms and diagnoses are associated with HIV in the MACS and WIHS cohorts. BMC Pulm Med 2014;14:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Madeddu G, Fois AG, Calia GM et al. . Chronic obstructive pulmonary disease: an emerging comorbidity in HIV-infected patients in the HAART era? Infection 2012;41:347–53. [DOI] [PubMed] [Google Scholar]
- 76.Makinson A, Hayot M, Eymard-Duvernay S et al. . High prevalence of undiagnosed COPD in a cohort of HIV-infected smokers. Eur Resp J 2014;pii: erj01549–2014. [DOI] [PubMed] [Google Scholar]
- 77.High KP, Brennan-Ing M, Clifford DB et al. . HIV and aging. J Acquir Immune Defic Syndr 2012;60:S1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yusuf AJ, Hassan A, Mamman AI et al. . Prevalence of HIV-associated neurocognitive disorder (HAND) among patients attending a tertiary health facility in Northern Nigeria. J Int Assoc Provid AIDS Care 2014; pii: 23259557414553839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Garg RK. HIV infection and seizures. Postgrad Med J 1999;75:387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Satishchandra P, Sinha S. Seizures in HIV-seropositive individuals: NIMHANS experience and review. Epilepsia 2008;49:33–41. [DOI] [PubMed] [Google Scholar]
- 81.Epilepsy and HIV-a dangerous combination. Lancet Neurol 2007;6:747. [DOI] [PubMed] [Google Scholar]
- 82.Birbeck GL, French JA, Perucca E et al. . Evidence-based guideline: Antiepileptic drug selection for people with HIV/AIDS: report of the Quality Standards Subcommittee of the American Academy of Neurology and the Ad Hoc Task Force of the Commission on Therapeutic Strategies of the International League Against Epilepsy. Neurology 2012;78:139–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Siddiqi O, Birbeck GL. Safe Treatment of seizures in the setting of HIV/AIDS. Curr Treat Options Neurol 2013;15:529–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.WHO. Malaria: fact sheet no. 94. Geneva: World Health Organization; 2013. [Google Scholar]
- 85.Gething PW, Patil AP, Smith DL et al. . A new world malaria map: Plasmodium falciparum endemicity in 2010. Malar J 2011;10:378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tusting LS, Willey B, Lucas H et al. . Socioeconomic development as an intervention against malaria: a systematic review and meta-analysis. Lancet 2013;382:963–72. [DOI] [PubMed] [Google Scholar]
- 87.Perez-Escamilla R, Dessalines M, Finnigan M et al. . Household food insecurity is associated with childhood malaria in rural Haiti. J Nutr 2009;139:2132–8. [DOI] [PubMed] [Google Scholar]
- 88.Mitangala PN, D'Alessandro U, Donnen P et al. . Malaria infection and nutritional status: results from a cohort survey of children from 6–59 months old in the Kivu province, Democratic Republic of the Congo. Rev Epidemiol Sante Publique 2013;61:111–20. [DOI] [PubMed] [Google Scholar]
- 89.Deribew A, Alemseged F, Tessema F et al. . Malaria and under-nutrition: a community based study among under-five children at risk of malaria, south-west Ethiopia. PLoS One 2010;5:e10775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Crookston BT, Alder SC, Boakye I et al. . Exploring the relationship between chronic undernutrition and asymptomatic malaria in Ghanaian children. Malar J 2010;9:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Danquah I, Bedu-Addo G, Mockenhaupt FP. Type 2 diabetes mellitus and increased risk for malaria infection. Emerg Infect Dis 2010;16:1601–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Jha V, Garcia-Garcia G, Iseki K et al. . Chronic kidney disease: global dimension and perspectives. Lancet 2013;382:260–72. [DOI] [PubMed] [Google Scholar]
- 93.White SL, Chadban SJ, Jan S et al. . How can we achieve global equity in provision of renal replacement therapy? Bull World Health Organ 2008;86:229–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Anand S, Bitton A, Gaziano T. The gap between estimated incidence of end-stage renal disease and use of therapy. PLoS One 2013;8:e72860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Elsheikha HM, Sheashaa HA. Epidemiology, pathophysiology, management and outcome of renal dysfunction associated with plasmodia infection. Parasitol Res 2007;101:1183–90. [DOI] [PubMed] [Google Scholar]
- 96.NICE. Malaria prophylaxis. London: National Institute for Health and Care Excellence; 2012. http://cks.nice.org.uk/malaria-prophylaxis [accessed 15 December 2014]. [Google Scholar]
- 97.Collins WE, Jeffery GM. Plasmodium malariae: parasite and disease. Clin Microbiol Rev 2007;20:579–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Oni T, Youngblood E, Boulle A et al. . Patterns of HIV, TB, and non-communicable disease multi-morbidity in peri-urban South Africa- a cross sectional study. BMC Infect Dis 2015;15:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.WHO. Diabetes Programme. Innovative care for chronic conditions: Building blocks for action: WHO global report 2002. Geneva: World Health Organization; 2002. http://www.who.int/diabetes/publications/icccreport/en/ [accessed 1 December 2014]. [Google Scholar]
- 100.Oni T, McGrath N, BeLue R et al. . Chronic diseases and multi-morbidity - a conceptual modification to the WHO ICCC model for countries in health transition. BMC Public Health 2014;14:575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.WHO. Global Status Report on Non-Communicable Diseases 2010. Geneva: World Health Organization; 2011. http://www.who.int/nmh/publications/ncd_report_full_en.pdf [accessed 20 January 2015]. [Google Scholar]
- 102.WHO. Global Health and Aging. Geneva: World Health Organization; 2011. http://www.who.int/ageing/publications/global_health.pdf [accessed 20 January 2015]. [Google Scholar]
- 103.Holmes WR, Joseph J. Social participation and healthy ageing: a neglected, significant protective factor for chronic non communicable conditions. Global Health 2011;7:43. [DOI] [PMC free article] [PubMed] [Google Scholar]