Abstract
Background
Although the Horn of Africa region has successfully eliminated endemic poliovirus circulation, it remains at risk for reintroduction. International partners assisted Kenya in identifying gaps in the polio surveillance and routine immunization programs, and provided recommendations for improved surveillance and routine immunization during the health system decentralization process.
Methods
Structured questionnaires collected information about acute flaccid paralysis (AFP) surveillance resources, training, data monitoring, and supervision at provincial, district, and health facility levels. The routine immunization program information collected included questions about vaccine and resource availability, cold chain, logistics, health-care services and access, outreach coverage data, microplanning, and management and monitoring of AFP surveillance.
Results
Although AFP surveillance met national performance standards, widespread deficiencies and limited resources were observed and reported at all levels. Deficiencies were related to provider knowledge, funding, training, and supervision, and were particularly evident at the health facility level.
Conclusions
Gap analysis assists in maximizing resources and capacity building in countries where surveillance and routine immunization lag behind other health priorities. Limited resources for surveillance and routine immunization systems in the region indicate a risk for additional outbreaks of wild poliovirus and other vaccine-preventable illnesses. Monitoring and evaluation of program strengthening activities are needed.
Keywords: decentralization, polio, program review, routine immunization, surveillance
Indigenous wild poliovirus has been eliminated in 98% of endemic countries since 1988, with a decrease from 350 000 cases in 1988 to 223 confirmed cases in 2012 [1]. The success of polio eradication efforts has been dependent on increasing population immunity through routine immunization and supplementary immunization activities and by improving surveillance for acute flaccid paralysis (AFP). AFP surveillance systems are expected to annually identify ≥2 nonpolio AFP (NPAFP) cases per 100 000 population under 15 years of age to demonstrate that their systems are sensitive enough to detect paralytic polio cases [2].
All countries within the Horn of Africa region have successfully eliminated endemic poliovirus circulation, with Kenya successfully presenting in 2005 their complete country documentation outlining polio-free status. However, the region has remained at high risk for reintroduction and has had multiple outbreaks in recent years. An outbreak of 19 cases of wild poliovirus occurred in Turkana, Kenya, in 2009, and was followed by an outbreak of 22 cases in Uganda in 2010. In 2011, another wild poliovirus case was detected in western Kenya and was found to be genetically related to the 2010 outbreak in Uganda. These outbreaks indicated missed circulation within the region over a 3-year period. As of 31 December, 203 confirmed cases of WPV had been confirmed in the Horn of Africa in 2013. All of these outbreaks underscore the regional struggle to achieve the surveillance sensitivity and population immunity necessary to prevent transmission of imported poliovirus.
In the midst of these outbreaks, the health system in Kenya has been decentralized, with both increased administrative autonomy at the district level and an increase in number of districts. Following a risk analysis performed in January 2012, which showed that 121 of 159 (77%) of districts in Kenya were at high or medium risk for a polio outbreak, the Kenya Ministry of Public Health and Sanitation (MoPHS) engaged the US Centers for Disease Control and Prevention (CDC), World Health Organization (WHO), United Nations Children’s Fund (UNICEF), and other international partners to assist in a review of the national surveillance and routine immunization systems. The purpose of the review was to identify gaps in the programs, as well as to provide recommendations toward improving detection of imported poliovirus and preventing poliovirus circulation during this period of health system decentralization [3].
METHODS
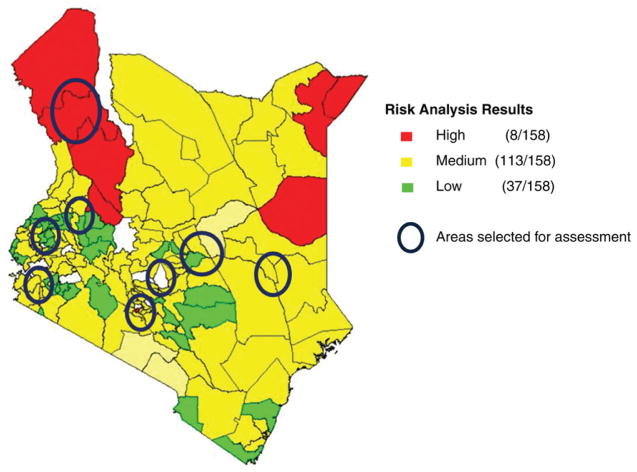
During 24–28 January 2012, 8 interview teams, consisting of personnel from MoPHS, WHO, CDC, and UNICEF, conducted a review of the national surveillance and routine immunization systems at the provincial, district, and health facility levels. At the national level, the review was conducted at the Division of Vaccines and Immunizations (DVI), Division of Disease Surveillance and Response (DDSR), Kenya Medical Research Institute (KEMRI), and the MoPHS national vaccine stock depot. Among the 159 total districts in Kenya, 22 of the 121 high-or medium-risk districts identified in the 2012 risk analysis were selected for review; these districts represented 7 of 8 total provinces (Figure 1). Districts were selected based on multiple criteria, including: (1) geography, (2) security, (3) population immunity (eg, vaccine coverage), (3) surveillance indicators (eg, nonpolio AFP rate and stool specimen adequacy), (4) presence of circulating vaccine-derived poliovirus, and (5) presence of high-risk populations. Two health facilities per district were selected for review based on the discretion of district personnel, as well as logistical considerations.
Figure 1.
Geographic areas of Kenya selected for the acute flaccid paralysis surveillance and Expanded Program on Immunization review, 2012.
For data collection, standardized questionnaires were developed. The structured questionnaires collected information about AFP surveillance resources, training, data monitoring and supervision at all levels. Information on surveillance officer knowledge and documentation of AFP identification, reporting of AFP cases, and specimen collection were collected at the provider level. The routine immunization program information collected included questions about vaccine and resource availability, cold chain, logistics, healthcare services and access, outreach coverage data, microplanning, and overall management and monitoring of the system. Respondents were also asked to produce documentation to confirm the presence of guidelines, frequency of meetings, and other objective measures. Teams administered the questionnaires to a total of 70 key informants and documented observed cold chain and coverage charting.
RESULTS
National and Provincial Level
The national NPAFP rate of 3.1 per 100 000 population <15 years of age met WHO standards in 2011. However, national-level respondents reported that insufficient surveillance funding, related to insufficient allocation of funds for outbreak response, was contributing to deficiencies in training, supervision, active surveillance, specimen transport, communications, and meeting coordination. Further, an increase in the number of districts led to a dilution of trained healthcare and surveillance officers throughout the system; this challenge was compounded by high staff turnover and decreased accountability.
Training and Feedback
Kenya has transitioned from disease-specific surveillance to the regional Integrated Disease Surveillance and Response (IDSR) system, with AFP surveillance now being supported by IDSR. The transition has necessitated new data collection forms and feedback processes. National-level staff reported concerns about the challenges in training all IDSR employees on AFP surveillance; missing and incomplete data collection forms were noted. The most recent formal AFP surveillance training was held in 2006, and no refresher training had been held in the previous 12 months. Additionally, there was no standardized protocol for the dissemination of laboratory results from AFP specimens back to the health centers that sent the specimens.
Coverage
In 2011, national coverage with the third dose of oral poliovirus vaccine (OPV3) was 88%, which did not meet the WHO target of 90%. Coverage and performance reports were received at the district level from 95% of health facilities in the previous 12 months, and 98% of districts provided feedback on these reports on schedule [4]. Outreach activities were not conducted as planned nationwide from 2009 to 2011, due to budget shortfalls at the national level; the national goal of 80% of facilities conducting outreach was not met. The national immunization program reported a major need for assistance in microplanning and estimation of target populations to ensure optimal coverage of the target population.
District and Health Facility Level
We visited 22 district offices and 41 health facilities in 7 provinces. Due to poor access and security concerns, we were unable to visit 3 health facilities from 2 districts in Eastern province.
Resources for AFP Surveillance
Insufficient financial resources for key elements of surveillance infrastructure, including active case searches and follow up, specimen transportation, and monitoring and supervision, were reported in 14 (64%) districts (Table 1). Staff frequently cited using their own money for fuel, stool specimen delivery, and airtime for mobile telephones to complete surveillance tasks due to funding gaps or delays in reimbursements. Stool specimen collection kits for AFP surveillance were available in only 13 (59%) districts, causing gaps in specimen collection or improvisation (eg, use of glass jars) at the facility level (Table 1). Improvisation reportedly caused inadequate specimens, poor temperature regulation in transit, and biosafety hazards at receipt.
Table 1.
Characteristics Regarding AFP Surveillance and Routine Immunizations Among Surveyed Districts, Kenya, 2012
| Indicator | Districts (n) | Districts Responding, % (n/22) |
|---|---|---|
| Insufficient financial resources for key surveillance elements | 14 | 64 |
| Calculated nonpolio AFP rate | 5 | 23 |
| Tracked number of nonpolio AFP cases | 9 | 41 |
| Surveillance feedback reports observed | 17 | 77 |
| Timely reporting | 8 | 36 |
| Conducted supervisory visits | 10 | 45 |
| Written documentation of supervisor feedback | 7 | 32 |
| Surveillance discussed at supervisory visits | 13 | 59 |
| Surveillance guidelines observed | 21 | 95 |
| Operational plan observed | 14 | 64 |
| Adequate number of specimen collection kits observed | 13 | 59 |
| Conducted immunization services | 17 | 77 |
| Population maps/tables for immunization planning observed | 13 | 59 |
| District contained populations not receiving immunization services | 17 | 77 |
| Vaccine interruptions at the national level | 18 | 81 |
| Monitoring chart observed | 16 | 73 |
| Monitoring chart was current | 9 | 41 |
| Calculated drop-out rate | 8 | 36 |
| Vaccine stock-outs at the district level | 18 | 81 |
| Unreliable transport | 13 | 59 |
Total districts = 22.
Abbreviation: AFP, acute flaccid paralysis.
Training for AFP Surveillance
Inadequately trained workforces deployed to the expanding number of districts and high staff turnover were repeatedly reported as problems by the districts and health facilities. Deficits in training were more pronounced at the health-facility level where only 19 (46%) facilities had refresher trainings on surveillance within the previous 12 months (Table 2). Surveillance staff in only 5 (23%) districts knew their respective NPAFP rate (Table 1). Staff responsible for AFP surveillance in 8 (20%) facilities could not state the accurate case definition for acute flaccid paralysis (Table 2). Only 17 (41%) facilities engaged the traditional healers and private practitioners in case notification activities (Table 2). Twenty-one (95%) districts had a copy of the technical surveillance guidelines, and 14 (64%) maintained an operational plan for surveillance (Table 1).
Table 2.
Characteristics Regarding AFP Surveillance and Routine Immunizations Among Surveyed Facilities, Kenya, 2012
| Indicator | Facilities (n) | Number of Facilities Responding (N) | n/N (%) |
|---|---|---|---|
| Received quarterly supervisory visits in previous 12 mo | 18 | 41 | 44 |
| Conducted surveillance refresher trainings in last 12 mo | 19 | 41 | 41 |
| Conducted daily immunizations | 33 | 36 | 92 |
| Provided vaccine any time an eligible child presented at the facility | 28 | 33 | 85 |
| Routine immunization microplans observed | 9 | 41 | 22 |
| Routine immunization microplans reviewed on a schedule | 1 | 36 | 3 |
| Catchment area map observed | 14 | 37 | 38 |
| Conducted outreach sessions | 26 | 39 | 67 |
| Conducted all planned outreach sessions | 10 | 39 | 26 |
| Surveillance staff stated accurate AFP case definition | 33 | 41 | 80 |
| Surveillance discussed at supervisory visits | 22 | 41 | 54 |
| Registered new births | 8 | 41 | 22 |
| Tracked immunization defaulters | 16 | 41 | 39 |
| Conducted mobilization sessions for immunization | 22 | 41 | 53 |
| Inventory log was consistent with vaccine supply | 12 | 41 | 29 |
| Calculated wastage rate | 17 | 41 | 41 |
| BCG stock-outs occurred | 15 | 41 | 37 |
| Insufficient supply of syringes | 8 | 32 | 25 |
| Insufficient supply of diluent | 7 | 41 | 17 |
Total health facilities = 41.
Abbreviations: AFP, acute flaccid paralysis; BCG, bacillus Calmette-Guérin.
Supervision for AFP Surveillance
Twelve (55%) districts had completed planned supervisory visits to health facilities (Table 1). Health facilities in 7 (32%) districts had documentation of written supervisor feedback to ensure mitigation of identified deficiencies (Table 1). Among health facilities, 18 (44%) received the planned quarterly supportive supervision visits from the district in the past 12 months (Table 2). The most common reasons reported for not implementing planned supervisory visits were lack of funds for fuel, lack of transport, and staff shortages.
Data Monitoring for AFP Surveillance
Eight (36%) districts received timely and complete reporting from health facilities (Table 1). Staff in only 9 (41%) districts reported the number of AFP cases that matched the records in the national database. Thirteen (59%) districts reported discussing surveillance in review meetings with health facilities. For health facilities, 22 (54%) reported discussing surveillance during supervisory visits from the districts (Table 2). Seventeen (77%) districts were able to present a recent feedback report for surveillance in their district (Table 1).
Planning for Routine Immunization
Only 13 (59%) districts had a map or table indicating which facilities in the district offered immunization (Table 1). Ten (25%) facilities had plans and maps for immunization outreach services. Only 9 (22%) health facilities had microplans for routine immunization, and only 1 (3%) reported reviewing microplans as an activity conducted by the district during supervisory visits (Table 2). Only 14 (37%) of the 37 health facilities with responses reported having a current map of their catchment area, with only 6 (16%) identifying locations of outreach or mobile immunization sessions; lack of updated maps was reported in at least 1 facility in every province (Table 2).
Logistics and Cold Chain for Routine Immunization
Only 29% (12/41) of facilities had inventory logs that were consistent with their vaccine supply, and 17 (41%) facilities knew how to calculate a vaccine wastage rate (Table 2). Vaccine supply at the district level was affected by stock-outs in 18 (82%) districts and unreliable transportation in 13 (59%) districts (Table 1). The length of stock-outs varied from 2 weeks to 3 months at the district level. Among the health facilities, 15 (37%) reported stock-outs of bacillus Calmette-Guérin (BCG); stock-outs of tetanus toxoid, pneumococcal conjugate vaccine, oral poliovirus vaccine, and pentavalent vaccine were also mentioned (Table 2). In addition, 8 (25%) facilities reported not having enough syringes, while 7 (17%) did not have sufficient diluents (Table 2). Stock-outs of sharps containers and vaccination cards were also reported.
Service Delivery of Routine Immunization
Among the 22 districts surveyed, 17 (77%) reported that the majority of health facilities within the district offered immunization services (Table 1). Although up to 40% of the population may be seeking healthcare and routine vaccinations outside the public sector [5], only 9 (41%) districts maintained comprehensive lists of traditional healers or private practitioners in their catchment areas. Seventeen (77%) districts reported that some populations within their catchment area did not receive immunization services (Table 1). Eighteen (81%) districts had immunization service interruptions within the past 12 months (Table 1). Among 36 health facilities with responses, 33 (92%) reported providing immunizations on a daily basis (Table 2). Among the 33 facilities who provided immunizations daily, 28 (85%) reported providing all recommended vaccines (ie, measles and BCG) to infants presenting at their facility on any day and at any time (Table 2). While 26 of 39 (67%) facilities reportedly conducted outreach sessions, only 10 held all planned outreach sessions (Table 2). Among the 41 health facilities, 8 (20%) reported registering newborns for immunization, 16 (39%) reported tracking defaulters, and 22 (53%) reported performing social mobilization activities prior to sessions (Table 2). Vaccine stock-outs, hard-to-reach populations, and refusals based on religious beliefs were the main challenges cited for immunization service delivery.
Data Monitoring for Routine Immunization
Among the 22 districts, 16 (73%) had vaccine coverage monitoring charts, but only 9 (41%) were up to date and accurate. Eight (36%) districts were able to calculate a dropout rate from their data (Table 1).
DISCUSSION
This evaluation of the AFP surveillance and routine immunization programs in Kenya in 2012 highlighted gaps at the district-and health-facility levels within the context of a health system that rapidly decentralized after the elimination of endemic poliovirus. Deficiencies were reported and observed at multiple levels of the health system and were most commonly related to the challenges of funding, training, and supervision.
Shortcomings with surveillance and routine immunization activities were particularly evident at the local level. Although AFP surveillance met national performance standards, widespread deficiencies and the limited resources were observed and reported at subnational levels. This evaluation demonstrated inadequate financial and human resources for supervision, training, active surveillance, and specimen transport. The routine immunization program deficits included service interruptions, leading to gaps in vaccination coverage, vaccine shortages, lack of microplanning, inadequate use of vaccination data and monitoring tools for quality assurance, and lack of adequate staff training. Decentralization and a change from multiple surveillance systems to IDSR have the potential to improve the overall system. However, adequate funding and training are needed for optimal implementation [6, 7].
The data collected during this evaluation led to recommendations to the national program to strengthen surveillance through trainings and prioritizing of hard-to-reach and migratory populations, use of short message service (SMS) reporting technologies, active surveillance at the community level, environmental surveillance, and strengthening partnerships with other health programs. Recommendations for routine immunization strengthening included establishing a process for routine immunization microplanning at district level, coordinating with other programs to integrate services and share data, engaging outside partners, prioritizing areas based on community risk and monitoring data, implementing vaccine forecasting at the facility level, monitoring vaccine distribution to prevent stock-outs, and creating a program to recognize high-achieving individuals or facilities. Recommendations were also made in the areas of financing, advocacy, and supplemental immunization campaign planning.
The Horn of Africa’s history of sustained transmission of wild poliovirus postimportation is indicative of gaps in the surveillance and routine immunization programs. The gaps identified in Kenya’s surveillance and routine immunization systems in 2012 forewarned partners of the risk of an impending regional wild poliovirus outbreak in these high-risk districts, which subsequently occurred in North Eastern province. Detection of surveillance gaps within the national program led to planning for systemic modifications and trainings to improve the suboptimal areas identified by the evaluation. Although these modifications had begun by the Horn of Africa Technical Advisory Group meeting in December 2012, they were unable to prevent the 2013 importation-related outbreak, likely due to the long-term accumulation of susceptible populations during the post-elimination era.
The methods used to evaluate the Kenyan program may be applicable to other countries in the region looking to maximize resources and capacity building, especially in countries where prioritization of AFP surveillance and routine immunization have lagged behind other health priorities. Limited resources devoted to the surveillance and routine immunization systems in the region indicate a risk for additional outbreaks of wild poliovirus and other vaccine-preventable illnesses, as well as the need for monitoring and evaluation of program-strengthening activities. Kenya traditionally serves as a leader in their region as the country’s health programs aim to identify and address modifiable risk factors for poliovirus importation and continued transmission.
This evaluation had several limitations. Because of the purposive sampling at all levels, findings are not meant to be generalizable beyond the sites visited. Recall bias may be present, as structured questionnaires required the recall of both routine and on-the-job duties as well as intermittent activities. The time-frame available for data collection activities limited the possible number of districts included and led to targeting of the nonrepresentative at-risk sample. Though these limitations make results of this activity specific to the areas where it was conducted, replication of these efforts can create a better understanding of the risks posed by wild poliovirus importation and the steps needed locally to avert transmission of imported poliovirus. Continued yearly risk analyses of surveillance and population factors at the district level should provide actionable data for advocacy, financial forecasting, and planning for needed program inputs.
Similar if not larger gaps in surveillance and routine immunizations exist in other countries in the Horn of Africa, which likely contributed to the ongoing polio outbreak. This evaluation highlights the importance of systematically assessing programs on a regular basis and the importance of continued vigilance toward AFP surveillance and routine immunization to prevent reintroduction of poliovirus to nonendemic regions such as the Horn of Africa. The expected timeframe for sustainable system strengthening in response to weaknesses at the local level is lengthy and requires human and financial resources, but it is a necessary step to achieving global polio eradication.
Acknowledgments
We would like to acknowledge Dr Mohamed Dahir Duale, former WHO Kenya Expanded Programme on Immunization focal person and current representative for Garissa County in the Kenyan National Assembly. This effort would not have been possible without his hard work, guidance, and commitment to the children of Kenya. Thank you to the local teams that made this work possible, including Wycliffe Matini, Omar Abdi, Mawati Samuel, Yusuf Ajack, Peter Kamau, Peter Okoth, Florence Yonga, Linus Ndegwa, Ondari Mogeni and Eric Gogstad.
Footnotes
Supplement sponsorship. This article is part of a supplement entitled “The Final Phase of Polio Eradication and Endgame Strategies for the Post-Eradication Era,” which was sponsored by the Centers for Disease Control and Prevention.
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. [Accessed 14 November 2013];Global Polio Eradication Initiative. 2013 http://apps.who.int/immunization_monitoring/en/diseases/poliomyelitis/afpextract.cfm.
- 2.World Health Organization. Field guide for supplementary activities aimed at achieving polio eradication. Geneva: World Health Organization; 1997. Expanded Programme on Immunization. [Google Scholar]
- 3. [Accessed 4 December 2013];Kenya Open-Source Government Data. 2013 https://www.opendata.go.ke/Health-Sector/Vol-II-Table-14-Population-by-main-type-of-disabil/sgq4-86hq.
- 4.WHO and UNICEF working group for monitoring national immunization coverage. Data Update: A Summary of Global Immunization Coverage through 2012. UNICEF and WHO; Oct, 2013. [Google Scholar]
- 5.Kenya National Bureau of Statistics (KNBS) and ICF Macro. Kenya demographic and health survey 2008–09. Calverton, Maryland: KNBS and ICF Macro; 2010. [Google Scholar]
- 6.Nsubuga P, White ME, Thacker SB, et al. Public health surveillance: a tool for targeting and monitoring interventions. In: Jamison DT, Breman JG, Measham AR, et al., editors. Disease control priorities in developing countries. 2. Washington, DC: The World Bank; 2006. [PubMed] [Google Scholar]
- 7.White ME, McDonnell SM. Public health surveillance in low- and middle-income countries. In: Teutsch SM, Churchill RE, editors. Principles and practices of public health surveillance. New York: Oxford University Press; 2000. [Google Scholar]