Abstract
Introduction
While the prognosis for most differentiated thyroid cancers (DTC) remains excellent, recurrence and in-sensitivity to radioactive iodine (RAI) lead to therapeutic challenges and poorer outcomes. In defining the pathogenesis of DTC, multiple genetic alterations have been identified in key pathways focused around receptor tyrosine kinases (RTKs) and the MAP kinase (MAPK) cascade. Sorafenib was specifically developed to target RAF kinase in the MAPK pathway. It has been shown however to have potent inhibition of several key RTKs, RAF kinase, and the V600E BRAF mutation, gaining FDA approval in November 2013 for advanced RAI-refractory DTC.
Areas covered
The authors provide a review of the targeted RAF kinase discovery strategy as well as the preclinical and clinical development of sorafenib, leading to FDA approval for DTC. The authors also provide some insight into the clinical use of sorafenib and look at important considerations for treatment.
Expert opinion
Sorafenib significantly improves progression free survival in metastatic DTC patients who are RAI-refractory. However, the overall survival benefit is still unproven and requires additional follow-up. Despite its cost and significant side effect profile, which results in dose reductions in the majority of DTC patients, sorafenib should be considered for the treatment of RAI-refractory advanced DTC patients following evaluation of their individual risk/benefit stratification.
1. Introduction
Thyroid cancer is the most common endocrine malignancy, accounting for over 90% of all endocrine cancers. It is estimated to affect over 550,000 people living in the United States with almost 63,000 new cases projected for 2014, making thyroid cancer the 9th most common cancer overall [1]. The vast majority of thyroid cancers arise from follicular epithelial cells and are further characterized into differentiated, poorly differentiated, and undifferentiated (anaplastic) subtypes. Differentiated thyroid cancer includes papillary thyroid carcinoma (PTC), follicular thyroid carcinoma (FTC) and a follicular variant Hürthle cell carcinoma, with PTC accounting for approximately 80-85% and FTC about 10% of all thyroid cancers [2, 3]. Approximately 5-9% of thyroid cancers are medullary thyroid carcinoma (MTC) and arise from the parafollicular C-cells that are responsible for calcitonin production [3].
Initial treatment for early-stage differentiated thyroid cancer (DTC) consists of surgical resection of the thyroid tumor as well as a central and/or lateral neck lymph node dissection. This central neck dissection can be done either prophylactically or therapeutically in the presence of clinically enlarged or suspicious nodes by imaging or metastatic nodes confirmed by biopsy. Prognosis in patients with DTC is excellent as the vast majority of these tumors are susceptible to the effects of radioactive iodine (RAI) following surgical resection. RAI ablation is recommended for all patients with distant metastases, gross extrathyroidal extension of tumor, tumors greater than 4cm in diameter, and tumors 1-4 cm with lymph node metastases or high risk features. After ablation, TSH suppression is administred using exogenous thyroid hormone to keep TSH levels below 0.5 mU/L for low risk patients and below 0.1 mU/L for medium and high risk patients. External beam radiation can also be used for patients with gross extrathyroidal extension or with macroscopic residual tumor after surgical resection. Following definitive treatment, patients are then followed with measurement of their serum thyroglobulin (Tg) levels to monitor for residual or recurrent disease [4]. Following this standard of care treatment, DTC patients have an excellent prognosis with a 5-year overall survival (OS) rate of 97.8%, which approaches close to 100% for patients with local disease confined to the neck [1]. However, despite optimal surgery and RAI, approximately 25% of patients will have recurrent disease, with 7% recurring with distant disease [5, 6]. The survival with metastatic disease drops to 54.7% at 5 years [1]. Importantly, 32% of metastatic tumors are RAI non-avid [7] and as a result, these patients are unable to receive subsequent RAI treatment, with an additional 5% of DTC patients having tumors that are refractory to RAI and show progression of disease within 1 year of treatment [8]. In the past, non-avid and RAI refractory DTC tumors have had relatively few options for systemic treatment aside from TSH suppression. In this setting, cytotoxic chemotherapy (most frequently doxorubicin) demonstrates low response rates which are not durable as well as high levels of off-target toxicity [9], resulting in a median survival of only 3-6 years [6].
Recent advances in the understanding of the pathogenesis of DTC has formed the groundwork for the creation of novel targeted therapy strategies focused on receptor tyrosine kinases (RTKs) and their downstream kinase pathways. Aberrant activation of the mitogen activated protein kinase (MAPK) pathway is demonstrated in a wide range of tumors, including thyroid cancers, and affects tumor progression and aggressiveness [10]. The MAPK pathway involves the RET/PTC receptor tyrosine kinase, which in turn activates a downstream kinase cascade with RAS – RAF – mitogen extracellular kinase (MEK) –and finally ERK (extracellular signal-regulated kinase) to facilitate ERK nuclear translocation where it binds to transcription factors for genes that are involved in cellular proliferation, differentiation and survival [11, 12]. PTC and FTC contain several common mutational mechanisms in which MAPK pathway is activated. In 10-20% of PTCs, a chromosomal rearrangement of RET to a partner gene of the intact tyrosine kinase domain found on the 3′ portion drives aberrant RET expression and ligand-independent dimerization of RET/PTC, which leads to constitutive activation of the MAPK pathway [13, 14]. A second mutation found in up to 44% of PTCs is a point mutation in the V600E amino acid of BRAF leading to constitutive activation of the mutated BRAF kinase. The V600E BRAF mutation has been reported to contribute to extrathyroidal extension, lymph node metastasis, tumor recurrence with loss of radioiodine avidity, and increased mortality [15, 16, 17, 18]. At a molecular level, the V600E BRAF mutation is associated with tumor angiogenesis and invasion through up-regulation of VEGF, silencing of iodide-metabolizing genes and silencing of tumor suppressor genes [17].
A second important kinase pathway in the regulation of molecular function important in tumorigenesis is the PI3K/Akt signaling pathway. With RTK activation by growth factors, RAS is activated leading to further downstream phosphorylation of PI3K which phosphorylates Akt and mechanistic target of rapamycin (mTOR) to affect cell growth, proliferation and survival. Several genetic alterations have been described in this pathway in thyroid cancer, and include PIK3CA copy gain (25% FTC, 12% PTC and 42% ATC) or less commonly mutations, RAS mutations (17% FTC, 8% PTC, and 8% ATC), and less frequently PTEN mutations, which is an inhibitor of PI3K. Overall, 55% of FTCs and 24% of PTCs contain at least one of these PI3K pathway mutations [19]. These mutations, particularly the K variant RAS mutations, are associated with more advanced thyroid cancer, a constitutively activated MAPK pathway, and increased mortality [18, 20].
Through the use of large medicinal chemistry libraries, combinatorial chemistry and high-throughput screening assays to targeting these RTKs and their downstream effectors, the small-molecule sorafenib was discovered to be highly potent in targeting multiple RTKs including the vascular endothelial growth factor receptors-1 and -2 (VEGFR1/2), platelet-derived growth factor receptor (PDGFR), FMS-related tyrosine kinase 3 (FLT3), and RAF (including V600E BRAF mutation).
2. Discovery strategy and preclinical development
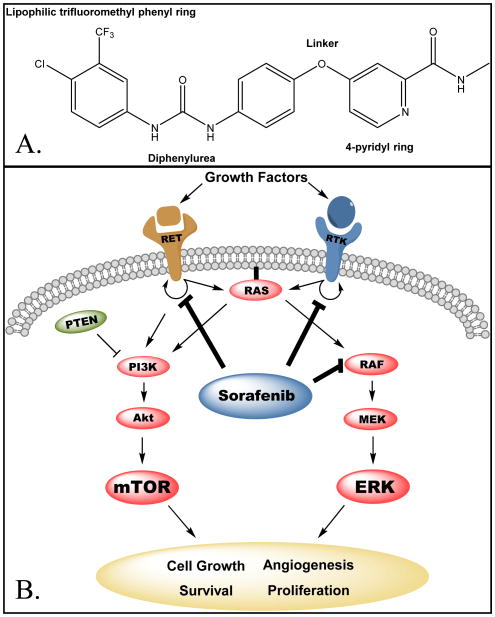
Sorafenib was initially developed by Bayer Pharmaceuticals as BAY 43-9006 in 2001, with its patent issued in 2004 from the USPTO. As growing evidence demonstrated that targeting RAF kinase was a potent oncogenic approach, Bayer, in collaboration with Onyx Pharmaceuticals, used a combination of high-throughput screening (HTS) and combinatorial chemistry to discover novel therapies that would specifically target the RAS/RAF/MEK/ERK kinases [21, 22]. Initially, 200,000 compounds were screened from medicinal chemistry directed synthesis or combinatorial libraries using a RAF kinase biochemical assay to identify molecules with activity against recombinant activated RAF kinase [21, 23]. Once a lead molecule was identified, the structure-activity relationship (SAR) of the compound to RAF kinase was evaluated and using medicinal and combinatorial chemistry (utilizing a robotic rapid parallel synthesis technique by conducting an amine-isocyanate reaction in anhydrous DMF) a library of about 1,000 analogs compounds was created [22]. These candidates were then tested in a mechanistic cellular high throughput immuneprecipitation assay in which their activity against endogenous phosphorylated MEK was determined by estradiol stimulation of a chimeric estrogen receptor and BRAF-1 protein within a mouse cell line [24, 25]. Additional rounds of combinatorial chemistry identified more potent analogs, and lead compounds demonstrating inhibition of RAF kinase activity were assessed for their anti-proliferative activity using the colon cancer cell line HCT116 [25]. The final compound BAY 43-9006 (sorafenib, Figure 1) includes a diphenylurea moiety, a 4-pyridyl ring that occupies the ATP binding pocket of the RAF-1 kinase domain, and a lipophilic trifluoromethyl phenyl ring that inserts into a hydrophobic pocket within the catalytic loop of RAF-1 [26].
Figure 1.
A. Chemical structure of sorafenib. B. Cellular targets of sorafenib. Sorafenib blocks auto-phosphorylation of multiple RTKs, including VEGFR1/2, PDGFR, FLT3 and RET. It is also a direct inhibitor of RAF-1, wild-type BRAF and the mutant V600E BRAF. RTK, receptor tyrosine kinase; RET, ret proto-oncogene; RAF, rapidly accelerated fibrosarcoma; MEK, mitogen-activated protein kinase kinase; ERK, extracellular signal-regulated kinase; PTEN, phosphatase and tensin homolog, PI3K, phosphoinositide 3-kinase; mTOR, mechanistic target of rapamycin.
Pre-clinical data was further developed by using in vitro biochemical and cellular assays that identified sorafenib as a potent inhibitor of MAPKs, including RAF-1, BRAF, and BRAF V600E mutation, as well as an inhibitor of the autophosphorylation of upstream RTKs. VEGFR1/2 phosphorylation was inhibited in VEGF-stimulated human umbilical vascular endothelial cells (HUVEC), PDGFR was inhibited in human aortic smooth muscle cells (HAoSMC), and FLT3 in human embryonic kidney (HEK) cells. In the biochemical assays, sorafenib was not active in inhibition of epidermal growth factor receptor (EGFR). Wilhelm et al. further demonstrated sorafenib's anti-tumor activity in human cancer cell line xenografts. Daily oral treatment with 30 mg/kg or 60 mg/kg of sorafenib yielded complete stasis of tumor growth in MDA-MB-231 breast cancer tumors (BRAF and KRAS mutations), COLO-205, HT-29 (BRAF mutation) and DLD-1 (KRAS mutation) colon cancer tumors, and in the A549 non-small cell lung cancer (NSCLC) xenograft harboring a KRAS mutation [27]. The primary method of antitumor activity in these in vivo models was via inhibition of ERK in the majority of cancer types, however, there was also significant tumor reduction in cancers in which ERK was not inhibited, which was thought due to the potent anti-angiogenic effects [27]. Additionally, further study demonstrated inhibition of tumor growth in other cancer xenograft models, including ovarian, pancreatic, melanoma and thyroid [27, 28, 29, 30].
Further characterizing sorafenib treatment in thyroid cancer, in vitro and in vivo studies used thyroid cancer cell lines harboring the V600E BRAF and oncogenic RET mutations. The role of V600E BRAF was shown using BRAF siRNA to inhibit expression, leading to decreased cellular proliferation of homozygous and heterozygous V600E BRAF anaplastic thyroid cancer cell lines. Subsequent sorafenib treatment demonstrated an IC50 between 500 nmol/L and 1 μmol/L, with significant inhibition of V600E BRAF enzymatic activity and downstream phosphorylation of the MAPK cascade, as well as inhibition of cellular proliferation. Additional murine xenograft studies with the ARO cell line demonstrated significant reduction in tumor volumes compared to controls after 22 days of sorafenib treatment, as well as tumor necrosis in part due to potent anti-angiogenic effects of sorafenib [30, 31]. In further studies, the anti-tumor actions of sorafenib were confirmed using DRO mouse xenografts, however, an association to V600E BRAF mutation was not identified and rather the tumor reductions were attributed to the potent anti-angiogenic effects of sorafenib and endothelial apoptosis within tumors rather than BRAF inhibition [31, 32]. Thyroid cancer containing the oncogenic activation of RET tyrosine kinase was also evaluated in the TT medullary thyroid cancer cell line, with significant reduction of RET kinase activity at IC50 levels of only 50 nM, and arrested growth of fibroblasts transfected with oncogenic RET and thyroid cancer cells harboring RET mutation. Further in vivo studies using oncogenic RET thyroid cancer cells in murine xenografts demonstrated a significant reduction in tumor volumes compared to controls with tumor necrosis and concomitant reductions in Ki67, mitotic index and downstream RET phosphorylated proteins [28]. These studies used multiple thyroid cancer cell lines in vitro and set the groundwork for advanced clinical studies with sorafenib in thyroid cancer. However, the pre-clinical mouse data using the ARO and DRO cell lines must be interpreted with caution since these cell lines are not true thyroid cancer cell lines as reported by Schweppe et al. in 2008. This report demonstrated that the ARO and DRO cell lines described in the xenograft studies above [30, 32] have since been identified as HT-29 colon cancer and A-375 melanoma cells, respectively, based on DNA profiling analysis [33].
3. Clinical Development
Given the broad anti-tumorgenic effects of sorafenib across multiple solid tumor types, initial phase I clinical studies involved single agent sorafenib with various intermittent and continuous dosing schedules in mixed advanced solid tumor patients [34, 35, 36, 37]. The optimum regimen and maximal tolerated dose was identified as oral administration of 400 mg twice daily [38]. For patients taking 600mg twice daily, the dose limiting toxicity (DLT) was related to grade 3 skin reactions, and those taking 800 mg twice daily had DLTs of grade 3 diarrhea. Additional adverse reactions included anorexia, nausea, fatigue, alopecia, stomatitis, and pancreatitis, however, they were generally mild to moderate and easily manageable [38, 39].
Within the mixed solid tumor patients enrolled in the phase I trials, anti-tumor response was measured by Response Evaluation Criteria in Solid Tumors (RECIST) and showed a partial response (PR; decrease ≥ 30% by RECIST)) in 1.5% of patients (2/137), and stable disease (SD; between − 30% and +20% by RECIST) in 28% of patients (38/137) [34, 35, 36, 39]. Of several solid tumors to demonstrate a response, including metastatic renal cell carcinoma (RCC), hepatocellular carcinoma (HCC), non-small cell lung cancer (NSCLC), ovarian and colorectal, RCC was chosen for further phase II and III due to its association with upregulation of RAF-1, VEGF and VEGFR, hypervascularity, and poor stage 4 survival. The randomized, placebo-controlled phase III Treatment Approaches in Renal Cancer Global Evaluation Trial (TARGET) affirmed that 400mg twice daily dosing of sorafenib was safe and effective in RCC. It demonstrated a 2.1% PR rate (7/335) vs. 0% for placebo by RECIST and significantly improved progression free survival (PFS) of 167 days vs. 84 days for placebo [40, 41]. Although, at the time of initial analysis, there was no OS benefit with sorafenib treatment, the significant increase in PFS led to crossover of placebo patients into the sorafenib arm as well as provided the basis for FDA approval of sorafenib for advanced RCC in December 2005. Subsequent analysis of OS to account for the crossover effect using censorship of placebo-assigned patients did reach significance with a median survival of 17.8 months for the sorafenib group compared to 14.3 months for placebo (p = 0.0287) [41]. In RCC, sorafenib is currently used as an alternative first-line treatment for patients with clear-cell etiology with good/intermediate prognosis or those with non-clear cell RCC, and as a standard second line therapy for those previously treated with cytokines such as interferon-alpha or interleukin-2 [42].
Additional phase II trials with single agent sorafenib and multi-agent trials were conducted for other responding cancers, including HCC, NSCLC, ovarian and colorectal, which led to the multi-center randomized, placebo-controlled phase III trial for unresectable HCC. It showed a significant median OS benefit of 10.7 months for the sorafenib group and 7.9 months for placebo (p<0.001). This resulted in a 1-year survival rate of 44% for sorafenib and 33% for placebo, which equates to a 31% relative reduction of mortality at 1 year (p = 0.009) and led to cessation of the study based on the significant positive results. While once again there were no complete responders, there was a significant increase in median time to radiologic progression (5.5 months sorafenib vs. 2.8 months for placebo; p<0.001) and disease-control rate (% with a best-response rating of at least stable disease lasting more than 28 days) [43]. Following the success of sorafenib treatment in HCC, the FDA also approved the use of sorafenib in unresectable HCC in 2007. Sorefenib is currently FDA approved for systemic therapy for HCC and is now considered first-line management for treatment of advanced HCC in patients not amenable to resection or failed trans-arterial chemoembolization (TACE) [44].
Clinical trials to evaluate sorafenib as a therapeutic strategy to target thyroid cancer stemmed from evidence identifying VEGFR, BRAF and RAS as key proteins in the MAPK tumorgenic pathways in these tumor cells, and the preclinical studies identifying significant anti-tumorgenic responses in thyroid cancer cell lines. Sorafenib significantly inhibits the VEGFR RTK with potent anti-angiogenic effects as well as inhibits BRAF, which plays a central role in the MAPK pathway with downstream phosphorylation and activation of ERK. The first phase II trial in patients with metastatic or unresectable thyroid carcinoma who no longer had effective curative measures was conducted in 2008 by Gupta-Abramson et al. and included thirty patients with measurable disease by RECIST, and included differentiated (90% of patients), poorly differentiated, MTC, and ATC patients (with each category including at least 1 patient). Patients were treated with 400 mg twice daily sorafenib, with dose decreases as necessary due to adverse reactions. The overall PR rate was 23% (CI 10% - 42%, p=0.0005). SD rate was 53% (CI 34%-72%). The median overall PFS for all thyroid cancer types was 79 weeks, and 84 weeks for differentiated thyroid cancers alone. Thyroglobulin levels were followed for 19 patients, and 17 of them (95%) had a marked mean reduction of 70% within 4 months of initiation of treatment. The single anaplastic thyroid cancer patient had rapid progression of her disease and withdrew after only 4 days of treatment due to medical complications related to her rapid progression [45].
The second phase II trial by Kloos et al. published in 2009 focused on iodine refractory PTC patients either chemotherapy naïve or having prior chemotherapy, as well as non-PTC thyroid cancers. A total of 58 patients were enrolled with 73% having PTC. Within the PTC chemotherapy naïve group (n=33), 15% of patients had a PR by RECIST (n=5) of a median duration of 9 months, 57% had SD lasting at least 6 months (n=19), and 12% had progressive disease (PD, n=4). The median PFS was 16 months (ranging 8-27.5 months). Within the group of PTC patients with prior chemotherapy (n=8), a single patient (13%) had a PR lasting 6 months, whereas 75% (n=6) had SD (4 of which had SD at least 6 months), and a single patient had PD. The median PFS was 10 months (ranging 4 to 48 months). The non-PTC patients included Hürthle cell (HTC), FTC and ATC, none of which had a PR, though 9 (82%) HTC/FTC patients demonstrated SD, of which 6 of these lasted greater than 6 months, and a single ATC patient had SD lasting at least six months, whereas the 3 other ATC patients had PD. Within this patient population, neither Tg levels at baseline nor during treatment consistently correlated with objective tumor response. Looking at BRAF as well as VEGF/VEGFR for the PTC patients, 64% had V600E and 14% had K601E BRAF mutations, and of 10 paired biopsy samples, 40% had major reduction in levels of immunoactive pVEGFR, pERK, and VEGF based on IHC. However, given the small sample size and large percentage of BRAF mutations, conclusions could not be drawn regarding the efficacy of sorafenib in PTC with BRAF mutations. [46]
The initial phase II trials had promising results with DTC and PTC, and additional phase II trials were also conducted evaluating poorly differentiated subtypes such as medullary thyroid cancer (MTC) and ATC. The first of two studies that describe response to sorafenib in MTC had a PR of 6% (lasting almost 21 months), and 88% of patients had SD with more than half of these lasting over 15 months. The PFS was 17.9 months [47]. The second study had a PR of 13% at 6 months, and 25% at 12 months for MTC patients. Comparatively, within that second study, DTC PR rates were 16% and 18% at 6 and 12 months, respectively. The PFS at 2 years was 84% for MTC, compared to 62% for DTC [48]. The only study to focus on ATC enrolled 20 patients, all of which had received previous chemotherapy. Two (10%) patients had a PR and 5 (25%) had SD for a clinical benefit rate of 35%. The median PFS was 1.9 months, with 15% having PFS at 6 months and 10% PFS at 12 months. Median OS in the ATC cohort was 3.9 months, with 30% and 20% survival at 6 and 12 months, respectively. It is also important to note that the 2 partial responders had papillary thyroid features, one was originally papillary prior to anaplastic transformation, and the other had focal areas of papillary features on biopsy [49]. This study demonstrated responses and survival similar to the 3-5 month median survival for ATC with current chemotherapy strategies therefore failing to demonstrate a significant improvement in OS related to standard of care in this more aggressive disease.
Following the multiple phase II trials that demonstrated a significant extension of PFS, and PR rates ranging 14.3-27.5% for DTC [50], a multi-center, randomized, double-blind and placebo-controlled phase III trial (DECISION) was conducted [51]. Treatment with 400 mg twice daily sorafenib was investigated in locally advanced and metastatic DTC patients (papillary, follicular, and poorly differentiated) who had been iodine refractory and had disease progression in the last 14 months by RECIST. Patients who had previously received targeted or chemotherapy were excluded. With 417 patients enrolled, 207 received sorafenib and 209 received placebo. Using RECIST, sorafenib treatment had a significant improvement in PFS compared to placebo (10.8 months vs. 5.8 months), which equated to a 41% reduction in risk of progression during the study. The PR rate was 12.2% for sorafenib compared to 0.5% for placebo, with a median PR duration of 10.2 months (95% CI 7.4 – 16.6). Of those without a PR, an additional 41.8% of the sorafenib group had durable SD lasting greater than 6 months, compared to 33.2% for placebo. Although the PR rate was lower than the initial phase II trials in DTC, the overall clinical impact on disease control was 54.1% with sorafenib compared to 33.8% with placebo. There was no difference in OS between the two groups, thought this may have been confounded by the majority of patients in the placebo group crossing over to receive open-label sorafenib upon disease progression [52]. The response rates and overall PFS for the primary clinical phase II studies and the DECISION phase III trial are summarized in Table 1 with an overall 20.7% PR rate (95% CI 14.1-27.2%), a 54% SD rate (95% CI 33.6%-74.3%) and a median PFS of 16.1 months (95% CI 13.3-18.8) [45, 46, 48, 51, 53, 54, 55]. When the groups were analyzed based on BRAF and RAS mutations, the BRAF mutation patients had the longest median PFS (20.5 months vs. 9.4 months for placebo), however, mutational status was not predictive of sorafenib benefit as both wildtype BRAF and RAS patients also had significant increases in PFS compared to placebo. When thyroglobulin levels were analyzed, significant decreases compared to placebo were observed in the sorafenib treated group, with the lowest levels in the partial responders [51].
Table 1. Comparison of Overall Response Rates and Adverse Events of Sorefenib in Differentiated Thyroid Cancer.
| Gupta-Abramson et al. 2008 [45] | Kloos et al. 2009 [46] | Ahmed et al. 2011 [48] | Capdevila et al. 2012 [53] | Hoftijzer et al. and Schneider et al. 2012 [54, 55] | Brose et al. 2014 [51] | Overall (95% CI) | ||
|---|---|---|---|---|---|---|---|---|
| Number of DTC Patients Evaluated | RESPONSE RATE | |||||||
| n=23 | n=46 | n=16 | n=16 | n=26 | n=196 | n=323 | ||
| Partial Response Rate | 30% | 13% | 19% | 19% | 31% | 12.2% | 20.7% (14.1% - 27.2%) | |
| Stable Disease | 65% | 74% | 81% | 50% | 12% | 41.8% | 54.0 (33.6% - 74.3%) | |
| Progressive Disease | 4% | 13% | 0% | 25% | 58% | 46% | 24.3% (5.8% - 42.9%) | |
| Median PFS - Months (95% CI) | 19.3* | 16 (chemotherapy naïve) (8 - 27.5) | 19* | 13.3 (4.5 - 16.5) | 18 (7 - 29) | 10.8* | 16.1 (13.3 - 18.8) | |
| OVERALL INCIDENCE OF ADVERSE EVENTS (GRADE 3/4)** | ||||||||
| Number of Patients | n=30 | n=56 | n=34 | n=34 | n=31 | n=207 | Any Reaction (95% CI) | Grade 3/4 (95% CI) |
| n=392 | ||||||||
| Hand Foot Skin Reaction | 93% (10%) | 63% (7%) | 79% (44%) | 62% (24%) | 71% (23%) | 76% (20%) | 74.2% (64.8% - 83.7%) | 20.2% (9.7% - 30.6%) |
| Diarrhea | 80% (7%) | 75% (4%) | 77% (3%) | 62% (15%) | 52% (6%) | 69%(6%) | 69.1% (60.6% - 77.7%) | 6.1% (2.7% - 9.5%) |
| Alopecia | 43% (0%) | 79% (0%) | 74% (0%) | 26% (12%) | 52% (0%) | 67% (0%) | 62.8% (46.8% - 78.7%) | 1% (-2.8% - 4.9%) |
| Rash/Derm Other | 80% (10%) | 79% (4%) | 88% (6%) | 35% (6%) | 55% (16%) | 50% (5%) | 58.9% (42.3% - 75.5%) | 6.1% (2.4% - 9.8%) |
| Fatigue | 63% (3%) | 82% (16%) | 59% (9%) | 56% (15%) | NR | 50% (6%) | 57.3% (46.6% - 68.1%) | 8.3% (3.5% - 13.2%) |
| Weight Loss | 60% (10%) | 82% (5%) | 29% (0%) | NR | 58% (10%) | 47% (6%) | 52.9% (9.7% - 30.6%) | 5.9% (2.3% - 9.4%) |
| Hypertension | 43% (13%) | 43% (4%) | 21% (6%) | 18% (0%) | 42% (16%) | 41% (10%) | 37.5% (27.9% - 47.1%) | 8.4% (3.5% - 13.3%) |
| Anemia | NR | 50% (0%) | 6% (3%) | NR | 35% (0%) | NR | 33.9% (8.4% - 59.3%) | 0.8% (-1.1% - 2.7%) |
| Anorexia | 20% (3%) | 57% (0%) | 29% (0%) | 21% (9%) | NR | 32% (2%) | 33.5% (20.3% - 46.8%) | 2.5% (-0.7% - 5.7%) |
| Arthralgia/Myalgias | 77% (0%) | 82% (11%) | 35% (9%) | 3% (0%) | NR | 14% (0.5%) | 30.5% (-1.1% - 62.1%) | 2.8% (-1.9% - 7.4%) |
| Hypocalcemia | NR | 57% (4%) | NR | NR | 48 % (0%) | 19% (6%) | 29.3% (6.5% - 52%) | 4.8% (1.5% - 8.1%) |
| Mucositis | 47% (0%) | 16% (2%) | 27% (9%) | 47% (3%) | 48% (10%) | 23% (1%) | 28.3% (16.8% - 39.8%) | 2.6% (-0.8% - 5.9%) |
| Deaths | 1 (3%) | 1 (2%) | 0 (0%) | 1 (3%) | 1 (3%) | 12 (1 treatment related) (6%) | 4.1% (2.5% - 5.6%) | |
Confidence intervals were not reported by Gupta-Abramson et al., Ahmed et al. did not reach median PFS endpoint at 19 months, Brose et al provided hazard ratio 0.59 (CI 0.45-0.76)
Adverse events include all enrolled patients irrespective of thyroid cancer classification
PFS, progression free survival; NR, not reported; CI, confidence interval; DTC, differentiated thyroid cancer
As seen in the phase I and II trials, the most common adverse reactions were dermatologic (hand-foot skin reaction (HFSR), rash, desquamation) and diarrhea. Fatigue, anorexia, weight loss, hypertension and arthralgias were also common. Adverse reactions and their frequency are summarized in Table 1. In the DECISION phase III trial [51], the most common adverse reaction was HFSR, which occurred in 76.3% of patients (vs 9.6% for placebo), with 20.3% having grade 3 reactions and none with grade 4. Overall, HFSR generally occurs within the first 6 weeks of initiating treatment, [56] with cumulative dose exposure increasing overall prevalence [57], but incidence decreases as treatment continues [58]. Diarrhea was the second most common reaction experienced by 68.6% of patients, with only 5.3% grade 3 and 0.5% grade 4. Diarrhea has been shown to correlate with young age and treatment duration, and becomes more frequent as cumulative sorafenib exposure increases [58]. Alopecia and rash/desquamation followed with 67.1% and 50.2% of patients having any reaction, respectively. Additional grade 4 reactions included fatigue (0.5%), oral mucositis (0.5%) and fever (0.5%). Although most reactions were either grade 1 or grade 2, a majority of patients (64.3%) did require dose adjustments down from the starting 400mg BID dose, with 18.8 % of patients withdrawing from the study due to these adverse reactions. These results are similar to the phase II trials described as 56% of patients had dose reductions and 16% withdrew from treatment [50]. The most important laboratory abnormalities included hypocalcemia in 18.8% of patients (5.8 % grade 3 and 3.4% grade 4), increased liver enzymes, and an increase in serum TSH levels [51]. It is important to note that sorafenib can impair TSH suppression, and that 41% of patients in the DECISION trial had elevation of their TSH above 0.5 mU/L with a median maximal TSH of 1.6 mU/L, and 25% with TSH levels greater than 4.4 mU/L. As a result, it is now recommended that TSH levels should be monitored monthly to adjust thyroid replacement dosage as needed to continue TSH suppression while on sorafenib treatment [59].
Pharmacokinetic profiles were developed in phase I and II trials and showed significant inter-patient variation of pharmacokinetics, including significant accumulation (2.5 to 7 fold) of sorafenib in plasma over the course of continuous treatment [34] with steady state levels achieved after 7 days [36]. The average time to maximal concentration (tmax) was 3 hours (ranging 1.0 – 12.3 hours), with elimination half life (t1/2) ranging from 25 to 48 hours [59]. Initial maximal concentrations (Cmax) were between 2.3-3.0 mg/L on day 1, and increased over treatment duration to 5.4-10.0 mg/L on the last day of dosing. The area under the curve for the first 12 hours (AUC0-12) was 18.0-24.0 mg·h/L on day 1 and 47.8-76.6 mg·h/L on the last day of dosing [35, 36, 37, 38]. The mean accumulation ratio for Cmax was 3.8 and for AUC was 5.7. Taking sorafenib with a fatty meal inhibits the bioavailability by 29% and it is not recommended to be taken with food [59]. Initial studies saw no significance between dose amount or extent of sorafenib exposure and drug-related adverse events [37, 39], but additional pharmacokinetic studies demonstrated increased likelihood for adverse reactions with higher AUC0-12. While patients were experiencing grade 3 or 4 adverse reactions, they had significantly higher median AUC0-12 than other patients without severe reactions, or when they were compared to themselves at other times during treatment [57].
Sorafenib is 99.5% bound to plasma proteins (primarily albumin) and metabolized in the liver by CYP3A4 mediated oxidation and UGT1A9 mediated glucuronidation [59, 60]. This creates two major metabolites following Michaelis-Menten kinetics, the N-oxide via oxidation of the pyridine nitrogen and N-hydroxymethyl via oxidation of the terminal amido methyl group. The N-oxide metabolite is the most common (about 65%) and has potency similar to sorafenib. A potent inhibitor of CYP3A4, ketoconazole, did not alter the AUC following sorafenib administration [60], however, the potent inducer of CYP3A4 rifampin did result in a mean reduction of AUC after a single dose by 37% [59]. It is recommended to avoid concomitant use of CYP3A4 inducers when taking sorafinib. Although in vitro data demonstrated sorafenib as an inhibitor of some CYP450 isoforms, these were not clinically meaningful at 400 mg BID dosing and did not significantly affect plasma concentrations for midazolam, dextromethorphan or omeprazole [59]. Although initial studies did not demonstrate an increase in INR with concomitant sorafenib and warfarin use, infrequently patients taking warfarin have reported elevated INR or bleeding after sorafenib initiation, and INR should be regularly monitored [59]. Age and gender did not have a clinically meaningful effect on pharmacokinetics, however, it has been demonstrated that Asians had a mean AUC 30% lower than for Caucasians [59].
Sorafenib is primarily excreted in feces (77%) and urine (19%), and undergoes enterohepatic circulation. No adjustments in dose are needed based on patients' age or gender. Patients with mild, moderate and severe hepatic and renal impairment have sorafenib AUC concentrations within range for those without hepatic or renal impairment after a single dose, however, those with severe hepatic or renal impairment could not tolerate the recommended 400 mg BID dosing due to significant DLTs. For these patients, starting dose reductions have been recommended based on the level of impairment, with an initial dose of 200 mg BID for moderate impairment and 200 mg daily for severe impairment and titration based on adverse reactions [61]. Dose adjustments are not necessary from the recommended 400 mg BID for patients with mild to moderate hepatic or renal impairment [59].
4. Post-launch
Following the positive results of the DECISION trial, the FDA approved sorafenib for the treatment of locally recurrent or metastatic, progressive and iodine-refractory DTC treatment in November 2013, with European approval following in May 2014. A continued point of concern is the significant side effect profile of sorafenib in thyroid patients. Patients in the DECISION trial taking sorafenib had an overall lower quality of life (QOL) as measured by the Functional Assessment of Cancer Therapy-General (FACT-G) survey as well as health status by EQ-5D and visual analog scale (VAS) when compared to patients taking placebo. This decrease was first seen at the start of their second treatment cycle (day 28), and although the magnitude of treatment effect differences was small, this QOL decrease remained significantly lower than patients taking placebo for the remaining trial period [62]. Despite this decrease in quality of life, in depth analysis of the DECISION trial adverse event data demonstrates that the majority of adverse reactions were grade 1 to grade 2, occurred early in the treatment cycle, and were manageable over time [63].
In addition, a recent phase II sorafenib study in DTC demonstrated a high incidence of fatal events while on sorafenib treatment. The general adverse reactions were similar to other studies, with HFSR occurring most frequently and the main DLT, however, three study group patients died of upper respiratory tract hemorrhage with trachea-esophageal neoplastic infiltration, and two died from cardiac arrest [64]. The bleeding events are likely due to a combination of VEGF signaling inhibition leading to endothelial dysfunction in renewal and healing, tumor necrosis and erosion, and subjecting the tumor and vasculature to previous radiation [65]. It was shown that there is a 1.86 relative risk increase of bleeding with sorafenib in RCC and HCC patients [66], but bleeding events had not been described as a major adverse reaction in the clinical trials with thyroid cancer patients. Regarding the cardiovascular events, there was a 1.9% incidence of cardiac ischemia and infarction in DTC patients, and even higher in the RCC and HCC populations (2.9% and 2.7% respectively), all three of which were significantly higher than in the placebo group [59]. It is thought the mechanism of cardiac toxicity is related to RAF-1 inhibition leading to enhanced cardiac myocyte apoptosis and fibrosis, which when combined with VEGFR signaling disruption in the setting of HTN (another common adverse reaction) may exacerbate myocyte contractile dysfunction, fibrosis and heart failure [67]. Another important adverse event related to sorafenib treatment and other RAF inhibitors is the increased risk of skin cancer, specifically squamous cell carcinoma (SCC). Sorafenib induces keratinocyte proliferation and paradoxically, increased activation of the MAPK pathway in normal skin [68]. This increase in SCC was seen in the DECISION trial, with 7 patients developing SCC, one of which also had melanoma, with no cases of SCC in the placebo group. Careful monitoring and skin examinations are important for patients who take sorafenib, with a low threshold for biopsy of concerning skin lesions.
Another important consideration in the use of sorafenib is its current economic burden. Although many more individuals in the United States are enrolling in insurance programs, the current wholesale cost of $113 per 200 mg tablet is not insignificant [69]. Extrapolated for 400mg twice daily dosing, a 30-day supply of sorafenib treatment is over $13,000 and over $162,000 yearly. Advanced DTC patients treated with sorafenib had a median PFS of 10.8 months in the DECISION trial, and even longer in several phase II trials, so it is not unreasonable that patients would remain on treatment for a year or longer. This amount of cost burden, whether directly to patients, or indirectly through their insurance carriers should be taken into consideration when making individualized therapeutic decisions.
Current post-market evaluation of the drug also includes ongoing trials using sorafenib in combination with other targeted therapies. Initial results of a phase II combination therapy of sorafenib and the mTOR inhibitor, everolimus (a derivative or sirolimus), were presented at the 2013 American Society of Clinical Oncology (ASCO) annual meeting. Results were promising with PR rates >50% for DTC, and in particular a 67% PR rate for Hürthle cell carcinoma [70]. There are also at least two other clinical trials actively recruiting to test this combination on advanced FTC or DTC. Another clinical trial combining sorafenib and the mTOR inhibitor temsirolimus is currently active with closed recruitment and expected primary outcome data collection in December 2014 [71].
Currently, the only other medication FDA approved for metastatic and advanced DTC is doxorubicin, but poor response rates and high levels of adverse reactions related to toxicity have limited its use [9]. In addition to targeted therapy by sorafenib, another small molecule RTK inhibitor lenvatinib (E7080, Eisai Inc, New Jersey) has recently been evaluated in clinical phase III trials (SELECT) for RAI-refractory advanced DCT and presented as the ASCO 2014 meeting. Lenvatinib primarily inhibits VEGFR1-3, FGFR1-4, PDGFRβ, RET and KIT signaling pathways. A total of 392 patients were enrolled and randomized 2:1 for lenvatinib or placebo. Patients taking lenvatinib had a significant median PFS of 18.3 months compared to 3.6 months for placebo, with 1.5% (n=4) of patients demonstrating a complete response, and 63.2% (n=165) of patients with PR versus 0% and 1.5% for placebo, respectively. Overall survival endpoints have not yet been reached and were not significant between lenvatinib and placebo. Adverse reactions, while manageable, were frequent and consisted most commonly of HTN (68%), diarrhea (59%), anorexia (50%), weight loss (46%) and nausea (41%), with grade III HTN occurring in 43% of patients. Dose reductions occurred in 78.5% of patients, 14.2% of patients discontinued the study due to adverse events and several patient deaths were attributed to treatment-related toxicities [72]. Despite the lack of OS improvement thus far, and questions raised from the frequent adverse reactions and several toxicity related deaths that occurred during trials, the fact that some patients achieved complete response and the overall high rate of partial responders make lenvatinib a promising additional targeted therapy for RAI-refractory advanced DTC. It is currently under New Drug Application Priority Review status by the FDA with a proposed review deadline in April, 2015.
5. Conclusion
Locally advanced and metastatic DTC that is refractory to RAI ablation has been a challenge to treat with poor OS compared to iodine-avid disease. Sorafenib was developed to specifically target RAF-1, a key protein kinase within the receptor tyrosine kinase and MAPK cascade as a result of improved understanding of the pathogenesis of tumors. Additionally, sorafenib targets multiple receptor tyrosine kinases and has potent anti-angiogenic effects across multiple cancers, including DTC. Sorafenib is the first targeted therapy approved for advanced DTC, and the only other therapy besides doxorubicin. It has shown significant improvements in PFS compared to placebo, although an OS benefit has yet to be demonstrated. While the overall side effect profile is generally well tolerated, a majority of patients have required dose reductions or interruptions, and many had to withdraw from studies due to toxicities. Development of new skin cancers on therapy remain a surveillance concern. Despite these side effects, options outside of clinical trials for advanced RAI-refractory DTC are very limited, and as a FDA-approved drug, sorafenib presents a viable therapeutic strategy to be offered to patients with advanced disease and tumor-morbidity in an attempt to decrease tumor burden and progression. As with any therapeutic option, an individualized risk/benefit analysis including drug side-effects, cost, and tumor-benefit should be performed when deciding on optimal treatment for patients with advanced thyroid cancer.
6. Expert Opinion
For most patients with differentiated thyroid cancer, treatment and prognostic options are excellent with surgery and radioactive iodine ablation. Unfortunately, given the heterogeneity of the disease and the existing of poorly differentiated subtypes, some tumors will progress and become iodine-insensitive. When this occurs, treatment options become limited and patients eventually succumb to their disease. Through improvements in our understanding of the molecular and genetic events that drive cancer and its progression, and through advances in high throughput chemical compound screening and molecular modeling, development of targeted therapeutics has revitalized the war on cancer. This effort has lead to compounds that are orally tolerated and significantly impact progression-free survival as well as lead to partial tumor responses in a meaningful percentage of patients suffering from tumor-associated symptoms and morbidity. Regarding DTC, aside from TSH suppression, the approval of sorafenib in the treatment of RAI-refractory advanced disease is the first major advance in systemic therapeutics since the approval of doxorubicin in the 1970s. By advancements in our understanding of the molecular pathogenesis of DTC, sorafenib was developed to specifically target and inhibit RTKs regulating thyroid cancer cell proliferation and survival pathways. As such, patients in clinical trials have demonstrated PR rates and improvements in PFS not experienced with doxorubicin and external beam radiation. Although an OS benefit has yet to be demonstrated, the Phase III DECISION trial showed a significant improvement in PFS and the potential benefit to decrease disease-related morbidity from advanced tumor progression. As with other targeted therapeutics, the potential benefits of sorafenib must be weighed against its cost and toxicity profile as part of clinical decision-making. In this case, to achieve a PFS of 10.9 months or more may result in a potentially substantial cost of treatment at over $162,000 per year. Additionally, off-target toxicities can be dose-limiting and include HFSR and other cutaneous reactions including the development of new squamous cell carcinomas, as well as HTN, diarrhea, anorexia, nausea and weight loss. While most of these reactions are low grade toxicities and largely manageable with patient education, preventative measures, and symptomatic treatment, dose-reduction may be necessary if side effects become more severe, and many patients report decreases in their quality of life compared to no treatment [63, 73]. In fact, during the large multicenter DECISION trial, over 75% of patients taking sorafenib required a dose reduction, and almost 15% of patients discontinued treatment due to significant adverse events. This may be in part a result of the increase in median sorafenib serum concentrations in DTC patients compared to RCC and HCC patients when taking the same dose [74]. The reason behind this increase is unclear, although the concomitant TSH suppression therapy that many DTC patients receive may reduce lean body mass and in HCC patients, sarcopenia was associated with more severe toxicities from sorafenib [75]. Even with the dose reductions in the DECISION trial, the benefit in PFS persisted and suggests that 400 mg twice daily dosing in the DTC populations may not be necessary to maintain therapeutic benefits. This finding has already been described in HCC, where 400 mg total daily dosing had comparable survival benefit with fewer adverse events [76]. It is also important to recognize the less common, but more severe risks of bleeding and cardiotoxicity. While less prevalent in the generally healthier advanced DTC population compared to HCC and RCC patients, it is important to identify patients on anti-coagulants like warfarin, as well as those with a history of previous radiation or cardiac disease. While not a complete contraindication, these factors may increase the risk of a severe adverse event with sorafenib in these patients and may change the risk/benefit balance for determining therapy choices.
The discovery of the BRAF mutation in thyroid cancer in 2003 has lead to significant research over the past decade on the role of the MAPK and PI3K/Akt pathways in DTC pathogenesis. This has advanced our understanding and characterization of the genetic mutations and proteins involved in this disease. Ongoing research in this area has lead to new target identification including the recent characterization of the EIF1AX mutation (eukaryotic translation initiation factor 1A, X-linked) that mediates formation of the 40S pre-initiation complex for protein translation [77]. As novel targeted therapeutics are developed against these key regulatory pathways in thyroid cancer, such as the mTOR inhibitors, and the RTK inhibitor lenvatinib, combination strategies with sorafenib and newer inhibitors may create an additive or synergystic effect to improve efficacy and lower toxicity using lower doses in combination. Ongoing clinical trials with mTOR inhibitors may in-fact demonstrate an OS benefit where one has not yet been observed with sorafenib alone. In addition, a beneficial result with the ongoing phase III SELECT trial comparing lenvatinib with sorafenib could lead to lenvatinib's approval by FDA for the treatment of advanced DTC which could help guide decisions regarding first-line treatment. Having additional therapeutic options for patients would be of significant benefit in this disease as some patients may have a greater indication to use one drug over the other. Also, if or when their disease shows progression on one drug, there is still a reasonable potential for a response with the other drug.
Although the current management guidelines set forth by the American Thyroid Association and the National Comprehensive Cancer Network for advanced, RAI-refractory DTC have yet to be updated following the FDA's approval of sorafenib, there is clear evidence that a clinical benefit exists for sorafenib treatment over standard cytotoxic chemotherapy. Ongoing and future trials will continue to define the role of sorafenib in multi-treatment regimens targeting the RTK and MAPK pathways, and whether synergistic or additive effects can be obtained to further improve patient outcomes. In the end, the real decision for therapy choice in patients with progressive, iodine-resistant DTC must include a careful consideration of the each patient's individual disease burden, symptoms, comorbidities and prognosis weighed against the cost, toxicities and lack of OS benefit observed with sorafenib. Of the adjuvant treatment options available to patients with advanced DTC non-responsive to RAI ablation, sorafenib had been shown to be superior to other treatment choices previously available. As such, sorafenib should be considered in these DTC patients if the benefits and goals of care outweigh its cost concerns and adverse risks. Defining optimal combination strategies and dosing in combination may improve outcomes and lower toxicities for patients with metastatic or progressing DTC. With ongoing research in this area, the era of molecular and personalized medicine has catalyzed the field of oncology providing exciting new therapies with improved outcomes for patients with advanced thyroid cancers. Challenges, however, remain in minimizing toxicities and cost-burden for more manageable long-term use of these exciting compounds.
Article Highlights.
Differentiated thyroid cancer, accounting for more than 90% of thyroid cancers, has excellent prognosis when confined locally, however, metastatic disease that is refractory to radioactive iodine remains a challenge even against newer targeted chemotherapeutics.
Sorafenib is the first targeted therapy approved for the treatment of advanced differentiated thyroid cancer refractory to radioactive iodine.
Sorafenib in clinical trials significantly improves progression free survival, but not overall survival for differentiated thyroid cancer patients.
Sorafenib has an increase in adverse reaction severity in differentiated thyroid cancer patients compared to other cancers, which is not well understood, and often requires dose reductions.
Sorafenib's associated adverse reactions include hand-foot skin reaction, rash, diarrhea, fatigue, anorexia, weight loss, hypertension and arthralgias, as well as abnormalities of calcium and loss of adequate thyroid stimulating hormone suppression.
Consideration of patients with advanced thyroid cancer for sorafenib therapy should be individualized to weigh its benefits against its side effect profile, cost, and lack of overall survival benefit while basing decisions on the goals of care and clinical judgment of the provider.
This box summarizes key points contained in the article.
Acknowledgments
The authors are supported by funds from the University of Michigan Comprehensive Cancer center and the University Of Michigan Department Of Surgery.
Footnotes
Financial and Competing Interests Disclosure: The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed
Contributor Information
Peter T White, Email: ptwhite@umich.edu, University of Michigan Health System, Department of Surgery, 1500 E Medical Center Dr SPC 5332, Taubman Center Floor 2 Reception F, Ann Arbor, MI, USA 48109, 734-936-5738.
Mark S Cohen, University of Michigan Health System, Department of Surgery, 1500 E Medical Center Dr SPC 5332, Taubman Center Floor 2 Reception F, Ann Arbor, MI, USA 48109, 734-936-5738.
Bibliography
Papers of special note have been highlighted as either of interest (*) or of considerable interest (**) to readers.
- 1.Howlader N, Noone AM, Krapcho M, Garshell J, Miller D, Altekruse SF, Kosary CL, Yu M, Ruhl J, Tatalovich Z, Mariotto A, Lewis DR, Chen HS, Feuer EJ, Cronin KA, editors. SEER Cancer Statistics Review. National Cancer Institute; Bethesda, MD: 1975-2011. http://seer.cancer.gov/csr/1975_2011/, based on November 2013 SEER data submission, posted to the SEER web site, April 2014. [Google Scholar]
- 2.Hundahl SA, Fleming ID, Fremgen AM, Menck HR. A National Cancer Data Base report on 53,856 cases of thyroid carcinoma treated in the U.S., 1985-1995 [see comments] Cancer. 1998;83:2638–48. doi: 10.1002/(sici)1097-0142(19981215)83:12<2638::aid-cncr31>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 3.Davies L, Welch HG. Increasing incidence of thyroid cancer in the United States, 1973-2002. JAMA. 2006;295:2164–7. doi: 10.1001/jama.295.18.2164. [DOI] [PubMed] [Google Scholar]
- 4.Cooper DS, Doherty GM, Haugen BR, et al. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2009;19:1167–214. doi: 10.1089/thy.2009.0110. [DOI] [PubMed] [Google Scholar]
- 5.Mazzaferri EL, Massoll N. Management of papillary and follicular (differentiated) thyroid cancer: new paradigms using recombinant human thyrotropin. Endocr Relat Cancer. 2002;9:227–47. doi: 10.1677/erc.0.0090227. [DOI] [PubMed] [Google Scholar]
- 6.Mazzaferri EL, Jhiang SM. Long-term impact of initial surgical and medical therapy on papillary and follicular thyroid cancer. Am J Med. 1994;97:418–28. doi: 10.1016/0002-9343(94)90321-2. [DOI] [PubMed] [Google Scholar]
- 7.Durante C, Haddy N, Baudin E, et al. Long-term outcome of 444 patients with distant metastases from papillary and follicular thyroid carcinoma: benefits and limits of radioiodine therapy. J Clin Endocrinol Metab. 2006;91:2892–9. doi: 10.1210/jc.2005-2838. [DOI] [PubMed] [Google Scholar]
- 8.Xing M, Haugen BR, Schlumberger M. Progress in molecular-based management of differentiated thyroid cancer. Lancet. 2013;381:1058–69. doi: 10.1016/S0140-6736(13)60109-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sherman SI. Cytotoxic chemotherapy for differentiated thyroid carcinoma. Clin Onc. 2010;22:464–8. doi: 10.1016/j.clon.2010.03.014. [DOI] [PubMed] [Google Scholar]
- 10.Hoshino R, Chatani Y, Yamori T, et al. Constitutive activation of the 41-/43-kDa mitogen-activated protein kinase signaling pathway in human tumors. Oncogene. 1999;18:813–22. doi: 10.1038/sj.onc.1202367. [DOI] [PubMed] [Google Scholar]
- 11.MacCorkle RA, Tan TH. Mitogen-activated protein kinases in cell-cycle control. Cell Biochem Biophys. 2005;43:451–61. doi: 10.1385/CBB:43:3:451. [DOI] [PubMed] [Google Scholar]
- 12.Robinson MJ, Cobb MH. Mitogen-activated protein kinase pathways. Curr Op Cell Bio. 1997;9:180–6. doi: 10.1016/s0955-0674(97)80061-0. [DOI] [PubMed] [Google Scholar]
- 13.Santoro M, Melillo RM, Fusco A. RET/PTC activation in papillary thyroid carcinoma: European Journal of Endocrinology Prize Lecture. Eur J Endocrinol. 2006;155:645–53. doi: 10.1530/eje.1.02289. [DOI] [PubMed] [Google Scholar]
- 14.Zhu Z, Ciampi R, Nikiforova MN, Gandhi M, Nikiforov YE. Prevalence of RET/PTC rearrangements in thyroid papillary carcinomas: effects of the detection methods and genetic heterogeneity. J Clin Endocrinol Metab. 2006;91:3603–10. doi: 10.1210/jc.2006-1006. [DOI] [PubMed] [Google Scholar]
- 15.Xing M, Westra WH, Tufano RP, et al. BRAF mutation predicts a poorer clinical prognosis for papillary thyroid cancer. J Clin Endocrinol Metab. 2005;90:6373–9. doi: 10.1210/jc.2005-0987. [DOI] [PubMed] [Google Scholar]
- 16.Xing M, Alzahrani AS, Carson KA, et al. Association between BRAF V600E mutation and mortality in patients with papillary thyroid cancer. JAMA. 2013;309:1493–501. doi: 10.1001/jama.2013.3190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xing M. BRAF mutation in papillary thyroid cancer: pathogenic role, molecular bases, and clinical implications. Endocr Rev. 2007;28:742–62. doi: 10.1210/er.2007-0007. [DOI] [PubMed] [Google Scholar]
- 18.Pak K, Suh S, Kim SJ, Kim IJ. Prognostic Value of Genetic Mutations in Thyroid Cancer: A Meta-Analysis. Thyroid. 2014 Sep 22; doi: 10.1089/thy.2014.0241. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 19.Hou P, Liu D, Shan Y, et al. Genetic alterations and their relationship in the phosphatidylinositol 3-kinase/Akt pathway in thyroid cancer. Clin Cancer Res. 2007;13:1161–70. doi: 10.1158/1078-0432.CCR-06-1125. [DOI] [PubMed] [Google Scholar]
- 20.Zou M, Baitei EY, Alzahrani AS, et al. Concomitant RAS, RET/PTC, or BRAF mutations in advanced stage of papillary thyroid carcinoma. Thyroid. 2014;24:1256–66. doi: 10.1089/thy.2013.0610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wilhelm S, Carter C, Lynch M, et al. Discovery and development of sorafenib: a multikinase inhibitor for treating cancer. Nat Rev Drug Discov. 2006;5:835–44. doi: 10.1038/nrd2130. [DOI] [PubMed] [Google Scholar]
- 22.Smith RA, Barbosa J, Blum CL, et al. Discovery of heterocyclic ureas as a new class of raf kinase inhibitors: identification of a second generation lead by a combinatorial chemistry approach. Bioorg & Med Chem Lett. 2001;11:2775–8. doi: 10.1016/s0960-894x(01)00571-6. [DOI] [PubMed] [Google Scholar]
- 23.Lyons JF, Wilhelm S, Hibner B, Bollag G. Discovery of a novel Raf kinase inhibitor. Endocr-Relat cancer. 2001;8:219–25. doi: 10.1677/erc.0.0080219. [DOI] [PubMed] [Google Scholar]
- 24.Wilhelm S, Chien DS. BAY 43-9006: preclinical data. Curr Pharm Des. 2002;8:2255–7. doi: 10.2174/1381612023393026. [DOI] [PubMed] [Google Scholar]
- 25*.Lowinger TB, Riedl B, Dumas J, Smith RA. Design and discovery of small molecules targeting raf-1 kinase. Curr Pharm Des. 2002;8:2269–78. doi: 10.2174/1381612023393125. Review of the discovery of sorafenib. [DOI] [PubMed] [Google Scholar]
- 26.Wan PT, Garnett MJ, Roe SM, et al. Mechanism of activation of the RAF-ERK signaling pathway by oncogenic mutations of B-RAF. Cell. 2004;116:855–67. doi: 10.1016/s0092-8674(04)00215-6. [DOI] [PubMed] [Google Scholar]
- 27**.Wilhelm SM, Carter C, Tang L, et al. BAY 43-9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer res. 2004;64:7099–109. doi: 10.1158/0008-5472.CAN-04-1443. Preclinical data of sorafenib and its targeting effects on MAPK signaling, and tumor responses in murine human tumor xenograft models. [DOI] [PubMed] [Google Scholar]
- 28.Carlomagno F, Anaganti S, Guida T, et al. BAY 43-9006 inhibition of oncogenic RET mutants. J. Natl Cancer Inst. 2006;98:326–34. doi: 10.1093/jnci/djj069. [DOI] [PubMed] [Google Scholar]
- 29.Sharma A, Trivedi NR, Zimmerman MA, et al. Mutant V599EB-Raf regulates growth and vascular development of malignant melanoma tumors. Cancer Res. 2005;65:2412–21. doi: 10.1158/0008-5472.CAN-04-2423. [DOI] [PubMed] [Google Scholar]
- 30.Salvatore G, De Falco V, Salerno P, et al. BRAF is a therapeutic target in aggressive thyroid carcinoma. Clin Cancer Res. 2006;12:1623–9. doi: 10.1158/1078-0432.CCR-05-2378. [DOI] [PubMed] [Google Scholar]
- 31.Fallahi P, Ferrari SM, Santini F, et al. Sorafenib and thyroid cancer. BioDrugs. 2013;27:615–28. doi: 10.1007/s40259-013-0049-y. [DOI] [PubMed] [Google Scholar]
- 32.Kim S, Yazici YD, Calzada G, et al. Sorafenib inhibits the angiogenesis and growth of orthotopic anaplastic thyroid carcinoma xenografts in nude mice. Mol Cancer Ther. 2007;6:1785–92. doi: 10.1158/1535-7163.MCT-06-0595. [DOI] [PubMed] [Google Scholar]
- 33.Schweppe RE, Klopper JP, Korch C, et al. Deoxyribonucleic acid profiling analysis of 40 human thyroid cancer cell lines reveals cross-contamination resulting in cell line redundancy and misidentification. J Clin Endocrinol Metab. 2008;93:4331–41. doi: 10.1210/jc.2008-1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Strumberg D, Richly H, Hilger RA, et al. Phase I clinical and pharmacokinetic study of the Novel Raf kinase and vascular endothelial growth factor receptor inhibitor BAY 43-9006 in patients with advanced refractory solid tumors. J Clin Oncol. 2005;23:965–72. doi: 10.1200/JCO.2005.06.124. [DOI] [PubMed] [Google Scholar]
- 35.Clark JW, Eder JP, Ryan D, et al. Safety and pharmacokinetics of the dual action Raf kinase and vascular endothelial growth factor receptor inhibitor, BAY 43-9006, in patients with advanced, refractory solid tumors. Clin Cancer Res. 2005;11:5472–80. doi: 10.1158/1078-0432.CCR-04-2658. [DOI] [PubMed] [Google Scholar]
- 36.Awada A, Hendlisz A, Gil T, et al. Phase I safety and pharmacokinetics of BAY 43-9006 administered for 21 days on/7 days off in patients with advanced, refractory solid tumours. Br J Cancer. 2005;92:1855–61. doi: 10.1038/sj.bjc.6602584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moore M, Hirte HW, Siu L, et al. Phase I study to determine the safety and pharmacokinetics of the novel Raf kinase and VEGFR inhibitor BAY 43-9006, administered for 28 days on/7 days off in patients with advanced, refractory solid tumors. Ann Oncol. 2005;16:1688–94. doi: 10.1093/annonc/mdi310. [DOI] [PubMed] [Google Scholar]
- 38.Strumberg D, Awada A, Hirte H, et al. Pooled safety analysis of BAY 43-9006 (sorafenib) monotherapy in patients with advanced solid tumours: Is rash associated with treatment outcome? Eur J Cancer. 2006;42:548–56. doi: 10.1016/j.ejca.2005.11.014. [DOI] [PubMed] [Google Scholar]
- 39.Strumberg D, Clark JW, Awada A, et al. Safety, pharmacokinetics, and preliminary antitumor activity of sorafenib: a review of four phase I trials in patients with advanced refractory solid tumors. Oncologist. 2007;12:426–37. doi: 10.1634/theoncologist.12-4-426. [DOI] [PubMed] [Google Scholar]
- 40.Kane RC, Farrell AT, Saber H, et al. Sorafenib for the treatment of advanced renal cell carcinoma. Clin Cancer Res. 2006;12:7271–8. doi: 10.1158/1078-0432.CCR-06-1249. [DOI] [PubMed] [Google Scholar]
- 41.Escudier B, Eisen T, Stadler WM, et al. Sorafenib for treatment of renal cell carcinoma: Final efficacy and safety results of the phase III treatment approaches in renal cancer global evaluation trial. J Clin Oncol. 2009;27:3312–8. doi: 10.1200/JCO.2008.19.5511. [DOI] [PubMed] [Google Scholar]
- 42.Escudier B, Eisen T, Porta C, et al. Renal cell carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2012;23(3):vii65–vii71. doi: 10.1093/annonc/mds227. [DOI] [PubMed] [Google Scholar]
- 43.Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–90. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 44.Bruix J, Sherman M. Management of hepatocellular carcinoma: An update. Hepatology. 2011;53(3):1020–1022. doi: 10.1002/hep.24199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gupta-Abramson V, Troxel AB, Nellore A, et al. Phase II trial of sorafenib in advanced thyroid cancer. J Clin Oncol. 2008;26:4714–9. doi: 10.1200/JCO.2008.16.3279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kloos RT, Ringel MD, Knopp MV, et al. Phase II trial of sorafenib in metastatic thyroid cancer. J Clin Oncol. 2009;27:1675–84. doi: 10.1200/JCO.2008.18.2717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lam ET, Ringel MD, Kloos RT, et al. Phase II clinical trial of sorafenib in metastatic medullary thyroid cancer. J Clin Oncol. 2010;28:2323–30. doi: 10.1200/JCO.2009.25.0068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ahmed M, Barbachano Y, Riddell A, et al. Analysis of the efficacy and toxicity of sorafenib in thyroid cancer: a phase II study in a UK based population. Eur J Endocrinol. 2011;165:315–22. doi: 10.1530/EJE-11-0129. [DOI] [PubMed] [Google Scholar]
- 49.Savvides P, Nagaiah G, Lavertu P, et al. Phase II trial of sorafenib in patients with advanced anaplastic carcinoma of the thyroid. Thyroid. 2013;23:600–4. doi: 10.1089/thy.2012.0103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Thomas L, Lai SY, Dong W, et al. Sorafenib in metastatic thyroid cancer: a systematic review. Oncologist. 2014;19:251–8. doi: 10.1634/theoncologist.2013-0362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51**.Brose MS, Nutting CM, Jarzab B, et al. Sorafenib in radioactive iodine-refractory, locally advanced or metastatic differentiated thyroid cancer: a randomised, double-blind, phase 3 trial. Lancet. 2014;384:319–28. doi: 10.1016/S0140-6736(14)60421-9. Pivotal phase III trial demonstrating benefit against placebo and leading to FDA approval. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Brose MS, Jarzab B, Elisei R, et al. Updated overall survival analysis of patients with locally advanced or metastatic radioactive iodine-refractory differentiated thyroid cancer (RAI-rDTC) treated with sorafenib on the phase 3 DECISION trial. Abstract from ASCO Annual Meeting (2014) J Clin Oncol. 2014;32:5s. suppl; abstr 6060ˆ. [Google Scholar]
- 53.Capdevila J, Iglesias L, Halperin I, et al. Sorafenib in metastatic thyroid cancer. Endocr-Related Cancer. 2012;19:209–16. doi: 10.1530/ERC-11-0351. [DOI] [PubMed] [Google Scholar]
- 54.Hoftijzer H, Heemstra KA, Morreau H, et al. Beneficial effects of sorafenib on tumor progression, but not on radioiodine uptake, in patients with differentiated thyroid carcinoma. Eur J Endocrinol. 2009;161:923–31. doi: 10.1530/EJE-09-0702. [DOI] [PubMed] [Google Scholar]
- 55.Schneider TC, Abdulrahman RM, Corssmit EP, Morreau H, Smit JW, Kapiteijn E. Long-term analysis of the efficacy and tolerability of sorafenib in advanced radio-iodine refractory differentiated thyroid carcinoma: final results of a phase II trial. Eur J Endocrinol. 2012;167:643–50. doi: 10.1530/EJE-12-0405. [DOI] [PubMed] [Google Scholar]
- 56.Zhang L, Zhou Q, Ma L, Wu Z, Wang Y. Meta-analysis of dermatological toxicities associated with sorafenib. Clin Exp Dermatol. 2011;36:344–50. doi: 10.1111/j.1365-2230.2011.04060.x. [DOI] [PubMed] [Google Scholar]
- 57.Azad NS, Aragon-Ching JB, Dahut WL, et al. Hand-foot skin reaction increases with cumulative sorafenib dose and with combination anti-vascular endothelial growth factor therapy. Clin Cancer Res. 2009;15:1411–6. doi: 10.1158/1078-0432.CCR-08-1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Boudou-Rouquette P, Ropert S, Mir O, et al. Variability of sorafenib toxicity and exposure over time: a pharmacokinetic/pharmacodynamic analysis. Oncologist. 2012;17:1204–12. doi: 10.1634/theoncologist.2011-0439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bayer HealthCare Pharmaceuticals. NEXAVAR Prescribing Information [online] Whippany, NJ: Bayer HealthCare Pharmaceuticals, Inc.; 2013. Available at http://labeling.bayerhealthcare.com/html/products/pi/Nexavar_PI.pdf. [Google Scholar]
- 60.Ghassabian S, Rawling T, Zhou F, et al. Role of human CYP3A4 in the biotransformation of sorafenib to its major oxidized metabolites. Biochem pharmacol. 2012;84:215–23. doi: 10.1016/j.bcp.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 61.Miller AA, Murry DJ, Owzar K, et al. Phase I and pharmacokinetic study of sorafenib in patients with hepatic or renal dysfunction: CALGB 60301. J Clin Oncol. 2009;27:1800–5. doi: 10.1200/JCO.2008.20.0931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Schlumberger M, Jarzab B, Elisei R, et al. Phase III randomized, double-blinded, placebo controlled trial of sorafenib in locally advanced or metastatic patients with radioactive iodine (RAI)-refractory differentiated thyroid cancer (DTC) - exploratory analysis of patient-reported outcomes. Abstract from 83rd ATA meeting 2013. Thyroid. 2013 Oct;23(S1):A-1–A-114. doi: 10.1089/thy.2013.2310.abs. [DOI] [Google Scholar]
- 63.Worden FP, Fassnacht M, Yuankai S, et al. Safety and tolerability of sorafenib for treatment of locally advanced or metastatic radioactive iodine-refractory differentiated thyroid cancer (RAI-rDTC): Detailed analyses from the phase III DECISION trial. Abstract from ASCO annual meeting (2014) J Clin Oncol. 2014;32:5s. suppl; abstr 6062ˆ. [Google Scholar]
- 64.Marotta V, Ramundo V, Camera L, et al. Sorafenib in advanced iodine-refractory differentiated thyroid cancer: efficacy, safety and exploratory analysis of role of serum thyroglobulin and FDG-PET. Clin Endocrinol. 2013;78:760–7. doi: 10.1111/cen.12057. [DOI] [PubMed] [Google Scholar]
- 65.Kamba T, McDonald DM. Mechanisms of adverse effects of anti-VEGF therapy for cancer. Br J Cancer. 2007;96:1788–95. doi: 10.1038/sj.bjc.6603813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Je Y, Schutz FA, Choueiri TK. Risk of bleeding with vascular endothelial growth factor receptor tyrosine-kinase inhibitors sunitinib and sorafenib: a systematic review and meta-analysis of clinical trials. Lancet Oncol. 2009;10:967–74. doi: 10.1016/S1470-2045(09)70222-0. [DOI] [PubMed] [Google Scholar]
- 67.Force T, Krause DS, Van Etten RA. Molecular mechanisms of cardiotoxicity of tyrosine kinase inhibition. Nat Rev Cancer. 2007;7:332–44. doi: 10.1038/nrc2106. [DOI] [PubMed] [Google Scholar]
- 68.Arnault JP, Mateus C, Escudier B, et al. Skin tumors induced by sorafenib; paradoxic RASRAF pathway activation and oncogenic mutations of HRAS, TP53, and TGFBR1. Clin Cancer Res. 2012;18:263–72. doi: 10.1158/1078-0432.CCR-11-1344. [DOI] [PubMed] [Google Scholar]
- 69.RED BOOK online® product details for Nexavar. [Last accessed 28 October 2014];Average wholesale price per unit. Online through Micromedex® 2.0. [Google Scholar]
- 70.Sherman EJ, Ho AL, Fury MG, et al. Phase II study of everolimus and sorafenib for the treatment of metastatic thyroid cancer. Abstract from ASCO annual meeting (2013) J Clin Oncol. 2013;31 suppl; abstr 6024. [Google Scholar]
- 71. [Last accessed 28 October 2014];Phase II Study Evaluating the Combination of Temsirolimus and Sorafenib in the Treatment of Radioactive Iodine Refractory Thyroid Cancer. Available at ClinicalTrials.gov study identifier NC01025453. [Google Scholar]
- 72.Schlumberger M, Tahara M, Wirth LJ, et al. A phase 3, multicenter, double-blind, placebo-controlled trial of lenvatinib (E7080) in patients with 131I-refractory differentiated thyroid cancer (SELECT). Abstract from ASCO Annual Meeting (2014) J Clin Oncol. 2014;32:5s. suppl; abstr LBA6008. [Google Scholar]
- 73*.Brose MS, Frenette CT, Keefe SM, Stein SM. Management of sorafenib-related adverse events: a clinician's perspective. Semin Oncol. 2014;41(Suppl 2):S1–s16. doi: 10.1053/j.seminoncol.2014.01.001. Summary of clinical management of sorafenib adverse events. [DOI] [PubMed] [Google Scholar]
- 74.Bastholt L, Bose MS, Jarzab B, et al. Population PK modeling and exposure-response analyses of sorafenib in patients with radioactive iodine-refractory differentiated thyroid cancer (RAI-rDTC) in the phase III DECISION trial. Abstract from ASCO Annual Meeting (2014) J Clin Oncol. 2014;32:5s. suppl; abstr 6061ˆ. [Google Scholar]
- 75.Mir O, Coriat R, Blanchet B, et al. Sarcopenia predicts early dose-limiting toxicities and pharmacokinetics of sorafenib in patients with hepatocellular carcinoma. PloS one. 2012;7:e37563. doi: 10.1371/journal.pone.0037563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Morimoto M, Numata K, Kondo M, et al. Field practice study of half-dose sorafenib treatment on safety and efficacy for hepatocellular carcinoma: A propensity score analysis. Hepatol Res. 2014 doi: 10.1111/hepr.12354. [DOI] [PubMed] [Google Scholar]
- 77.Agrawal N, Akbani R, Aksoy BA, et al. Integrated Genomic Characterization of Papillary Thyroid Carcinoma. Cell. 159:676–90. doi: 10.1016/j.cell.2014.09.050. DOI: http://dx.doi.org/10.101. [DOI] [PMC free article] [PubMed] [Google Scholar]