Abstract
As part of a retrospective analysis of 616 individuals followed from incident HIV-infection for up to 18 years as part of the San Diego Primary Infection Cohort we found 16 subjects who started antiretroviral therapy (ART) within the first 4 months of infection and subsequently interrupted ART after being virologically suppressed for a median of 1.75 years. No subject maintained sustained virologic control after interruption of ART, even when treatment was started during the earliest stages of HIV-infection. Median time to HIV RNA rebound after ART interruption was 0.9 months (range: 0.2 – 6 months).
Introduction
Antiretroviral therapy (ART) suppresses viral replication in most HIV-infected individuals with a substantial impact on morbidity and mortality [1]. However, ART does not eradicate latently infected cells [2], and plasma viremia rebounds after treatment is interrupted [3]. Starting ART during the earliest stages of HIV infection is associated with a smaller HIV reservoir [4], reduced cellular HIV transcription [5], and preserved immune response [6]. A delayed viral rebound has been observed for variable periods of follow-up (generally 6 up to 48 months) in a small proportion of individuals after cessation of ART initiated during the earliest phases of HIV infection [7-9]. However, studies with longer follow-up (18 months up to several years) showed that virus control was not maintained in most individuals after early ART interruption [10-14]. In 2010, the French “Virological and Immunological Studies in Controllers after Treatment Interruption” (VISCONTI) group reported a selected subset of individuals in whom HIV RNA remained below 50 copies/ml for several years after the interruption of prolonged ART that was initiated during primary HIV infection (median duration 75 months) [15]. The same group calculated that the probability of maintaining viral control at 24 months post early treatment interruption could be as high as 15%, which is much higher than observed in persons who exhibit spontaneous control, without ART. This study has revived considerable interest in using early and prolonged ART to achieve long-term infection control and may have important implications in the search for a functional HIV cure. Similar follow-up studies with larger number of individuals [16-19] confirmed the existence of post treatment controllers at a lower frequency <10%.
Here, we performed a retrospective analysis including clinical and virologic data collected from participants of the San Diego Primary Infection Cohort (SD PIC) over 18 years of follow-up. Our objective was to find post-treatment controllers among those participants who interrupted ART after being virologically suppressed.
Methods and Results
Since 1996, the SD PIC has enrolled 616 individuals diagnosed during acute and very early HIV infection and followed for several years into chronic infection. The estimated date of HIV infection (EDI) is determined for each individual using established algorithms [20]. Importantly, the duration of HIV infection at the time ART was started was calculated differently for our cohort compared to the VISCONTI group. We calculated the interval between EDI (as described in [20]) and start of ART, while the French group used time from documented primary HIV infection (defined as the date of a negative/incomplete HIV-1 western blot and a positive p24 Ag test, or a positive HIV antibody test with a negative one within the previous 3 months). This corresponds to a difference of 3-5 weeks between our two estimates of ART start. For the purpose of this analysis, we added 4 weeks to our EDI (referred to “adjusted EDI” in the text).
We used the following inclusion criteria for this retrospective analysis of SD PIC participants: (i) ART start within 16 weeks (i.e. 4 months) from the “adjusted EDI” (n=235, 38%), (ii) reached suppressed HIV RNA levels (<50 or <400 copies/ml depending on sensitivity of the assay used) within 36 weeks of ART initiation (n=116, 50%), (iii) maintained suppressed HIV RNA for at least 24 weeks, with one blip <200 copies/ml allowed (n=109, 94%), (iv) documented treatment interruption (for any reason other than virologic failure) (n=16, 15%). If exact information about timing of ART interruption was missing, a manual review of clinical charts was performed and subjects were excluded from this analysis if the ART interruption date could not be documented.
In our cohort, we were able to identify 109 individuals who started ART within 16 weeks from our “adjusted EDI” and subsequently achieved suppressed HIV RNA in blood plasma (median time from ART initiation to HIV RNA suppression was 16 weeks), and remained suppressed for at least 24 weeks. Sixteen had a documented episode of voluntary ART interruption (see figure S1 for selection criteria). Table 1 summarizes the main characteristics of the 16 individuals who did interrupt ART (supplemental table 1 shows individual characteristics). Half of study participants started ART within 8.6 weeks from adjusted EDI and were treated for 1.75 years before ART interruption.
Table 1.
| Overall characteristics | Data | NC (n = 16) |
|---|---|---|
| Age (years), median (range) | 35 (29.5 – 44.5) | |
| Sex Male, n (%) | 16 (100) | |
| White ethnicity, n (%) | 13 (81.25) | |
| MSM, n (%) | 15 (93.75) | |
| Year (of enrollment), median (range) | 2001 (1997 – 2010) | |
| Symptomatic PHI, n (%) | 11 (64.71) | |
| Protective alleles (HLA-B*27 or HLA-B*57), n (%) | 1 (6.7) | |
| Risk alleles (HLA-B*07 or HLA-B*35), n (%) | 4 (26.7) | |
| CD4/μl at presentation, median (IQR) | 425 (333.5 – 620) | |
| HIV-RNA at presentation, log10/ml, median (IQR) | 5.43 (4.77 – 5.84) | |
| Nadir CD4/μl, median (IQR) | 406 (289 – 620) | |
| Peak HIV-RNA, log10/ml, median (IQR) | 5.46 (4.84 – 6.10) | |
| Characteristics at the last visit before ART initiation | ||
| CD4/μl, median (IQR) | 524.5 (375 – 694.5) | |
| CD4/CD8 ratio, median (IQR) | 0.60 (0.40 – 0.97) | |
| HIV-RNA, log10/ml, median (IQR) | 4.75 (4.22 – 5.38) | |
| Adjusted EDI to ART initiation (months), median (IQR) | 1.97 (1.68 – 2.12) | |
| Characteristics at the last visit before ART interruption | ||
| ART duration (years), median (IQR) | 2.04 (1.00 – 4.65) | |
| Undetectability duration during ART (years)*, median (IQR) | 1.75 (0.82 – 4.31) | |
| Ratio ART duration (years) / time before ART initiation (weeks), median (IQR) |
0.31 (0.11 – 0.55) | |
| CD4/μl, median (IQR) | 743.5 (572 – 859.5) | |
| CD4/CD8 ratio, median (IQR) | 1.10 (0.77 – 1.55) | |
| PI-based regimen (at the time of ART interruption) | 12 (75%) | |
| Characteristics at the first visit after ART interruption | ||
| Time to rebound (months), median (IQR) | 0.9 (0.5 – 1.6) | |
| CD4/μl change per month, median (IQR) | − 14.30 (−44.28 to 5.48) | |
| HIV RNA, log10/ml, median (IQR) | 3.52 (2.70 – 4.50) | |
| ART resumption (%) | 7 (43.7) | |
NC: Non-controllers (rebound within 6 months), EDI: Estimated date of infection, MSM: men who have sex with men, ART: antiretroviral therapy, PI: protease Inhibitor
Single blips (VL < 200 copies/ml) allowed
None of the SD PIC participants who met the described criteria for inclusion (0%, 95% CI: 0% – 14.3%) demonstrated sustained control of HIV after interruption of their ART. This estimate was not statistically different from the VISCONTI estimate (15,6%, CI: 5.3 - 32.8%, Fisher Exact p=0.15).
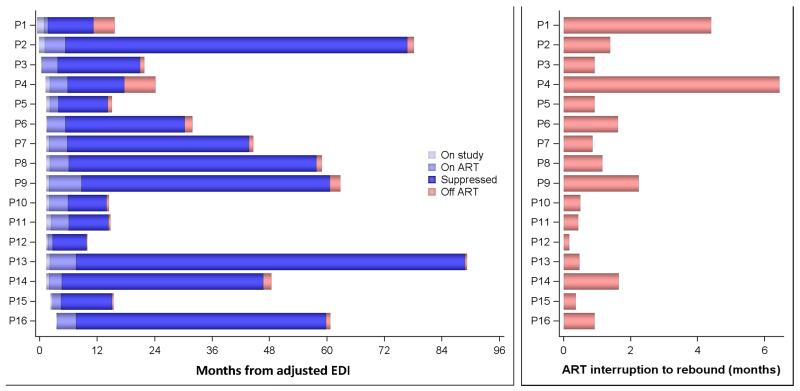
In fact, 100% of participants who stopped ART had detectable HIV RNA within a median of 50 days from ART interruption (range 6 to 197 days), see figure 1. None of the tested variables (inclusive baseline and peak HIV RNA viral load, baseline and nadir CD4 count, timing of ART initiation and time on ART) was associated with longer time to viral rebound. However, our analysis was limited because of the retrospective study design and the imprecise time to rebound estimates due to sparse sampling.
Figure 1.
Panel A summarizes ART history (initiation to interruption) for each individual participant; Panel B shows individual times to HIV RNA rebound.
Discussion
The recent report of the VISCONTI post-treatment controllers sparked considerable interest among the HIV community. The 15% prevalence of post-treatment controllers in the VISCONTI study is much higher than both previous reports in similar populations and observations of spontaneous viral control in natural history studies. In our cohort of 109 recently HIV-infected individuals who initiated ART within 16 weeks of their EDI and achieved sustained virologic control for a median of 1.75 years, we were able to find 16 individuals who interrupted therapy while their HIV RNA viral load was undetectable in blood plasma (<50-400 copies/ml). All of them rebounded to detectable HIV RNA levels by the following visit, achieving an average of 3.5 log10 HIV RNA copies/ml within 6-197 days from ART interruption. Of note, because of the small sample size we obtained large confidence intervals around our negative result (0% – 14.3%), which are overlapping with the confidence intervals reported by the VISCONTI group (5.3-32.8%) and therefore we were not able to detect a significant difference from the reported VISCONTI group. A similar analysis performed among participants of the Seattle primary infection cohort found 22 subjects who interrupter ART (out of 389) and only one was able to control viral load to levels <500 copies/ml for at least 24 months [21]. Based on our data, we calculated that a sample size of 34 (or 54) individuals interrupting ART would be necessary to detect a statistically significant difference from the VISCONTI cohort with a 76% power (or 90% power respectively), using a Fisher’s exact test with α = 0.05.
The not significantly different frequency of post-treatment control between the VISCONTI and SD PIC cohort (15% versus 0%) could have several explanations. Most importantly, all of the SD PIC participants started ART later compared to the VISCONTI cohort (median of 8.6 weeks compared to 2.3 and 5.8 weeks for VISCONTI controller and non-controller, respectively) and also had a shorter duration on ART (2.0 years compared to 5 years for the VISCONTI controllers). This resulted in a low ratio of ART duration/time before ART initiation of 0.3, which was much lower that any of the VISCONTI groups (controller, transient controller and non controller). This ratio was the main predictor of post-treatment control in the VISCONTI group. Regarding HLA data, one out of 16 participants interrupting ART (6.7%) carried two protective HLA alleles (HLA-B*27 and HLA-B*57) while four out of 16 (26.7%) carried at least one HLA risk allele (HLA-B*07 and/or HLA-B*35). This relatively high prevalence of risk alleles in our population of early-infected individuals (compared to previous reports [22, 23]) is similar or less compared to the prevalence reported in the VISCONTI cohort (29%) and in the Seattle primary infection cohort (33%) [21]. This might be a consequence of more severe seroconversion symptoms reported in people carrying HLA risk alleles, making them more likely to be identified during primary infection.
Additionally, VISCONTI post-treatment controllers also had a very small HIV DNA reservoir [19], confirming that the initiation of early ART decreases the pool of latently infected cells [4]. However, a small reservoir alone does not explain why these participants did not experience viral rebound after ART interruption since re-emergence of plasma HIV RNA in HIV-infected individuals treated early and who have small reservoirs has been repeatedly reported [10-14]. One interesting characteristic of the VISCONTI cohort is the relatively small contribution of long-lived central memory T cells to the HIV reservoir compared to previous reports [24]. This suggests that perhaps the distribution of the viral reservoir may play an important role in controlling infection in the absence of ART. Future studies are needed to confirm the VISCONTI findings and further explore the mechanisms of post-treatment control.
Supplementary Material
Supplemental Figure 1: Flow chart summarizing selection criteria for this study.
Acknowledgements
We are grateful to all the participants in the San Diego Primary Infection Cohort.
Financial Disclosure
This work was supported by the Department of Veterans Affairs, the James B. Pendleton Charitable Trust and the grants from the National Institutes of Health: AI100665, MH097520, DA034978, AI036214, AI007384, AI027763, AI106039, AI100665, DA034978, AI43638, AI074621, AI106039, 7-UM1 AI068636-07, P30-AI027763, UL1TR000100, P30 AI036214, MH101012, and UL1TR000100.
SJL has received grant support from Gilead Sciences. DMS has received grant support from ViiV Pharmaceuticals and consultant fees from Gen-Probe and Testing Talent Services. DDR has served as a consultant for Chimerix, Gilead, Gen-Probe, Heras, Merck, HIV Immunotherapeutics Institute, and Monogram.
Footnotes
Competing Interests
SG and CA do not have any commercial or other associations that might pose a conflict of interest.
References
- 1.Palella FJ, Jr., Baker RK, Moorman AC, et al. Mortality in the highly active antiretroviral therapy era: changing causes of death and disease in the HIV outpatient study. J Acquir Immune Defic Syndr. 2006;43(1):27–34. doi: 10.1097/01.qai.0000233310.90484.16. [DOI] [PubMed] [Google Scholar]
- 2.Wong JK, Hezareh M, Gunthard HF, et al. Recovery of replication-competent HIV despite prolonged suppression of plasma viremia. Science. 1997;278(5341):1291–5. doi: 10.1126/science.278.5341.1291. [DOI] [PubMed] [Google Scholar]
- 3.Hoen B, Fournier I, Lacabaratz C, et al. Structured treatment interruptions in primary HIV-1 infection: the ANRS 100 PRIMSTOP trial. J Acquir Immune Defic Syndr. 2005;40(3):307–16. doi: 10.1097/01.qai.0000182628.66713.31. [DOI] [PubMed] [Google Scholar]
- 4.Strain MC, Little SJ, Daar ES, et al. Effect of treatment, during primary infection, on establishment and clearance of cellular reservoirs of HIV-1. J Infect Dis. 2005;191(9):1410–8. doi: 10.1086/428777. [DOI] [PubMed] [Google Scholar]
- 5.Schmid A, Gianella S, von Wyl V, et al. Profound Depletion of HIV-1 Transcriptionally Active PBMC by Early cART during Primary HIV-1 Infection but Not by Treatment during Chronic Infection: Results of the Zurich Primary HIV Infection Study. PLoS One. 2010;5(10):e13310. doi: 10.1371/journal.pone.0013310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oxenius A, Price DA, Easterbrook PJ, et al. Early highly active antiretroviral therapy for acute HIV-1 infection preserves immune function of CD8+ and CD4+ T lymphocytes. Proc Natl Acad Sci U S A. 2000;97(7):3382–7. doi: 10.1073/pnas.97.7.3382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ortiz GM, Nixon DF, Trkola A, et al. HIV-1-specific immune responses in subjects who temporarily contain virus replication after discontinuation of highly active antiretroviral therapy. J Clin Invest. 1999;104(6):R13–8. doi: 10.1172/JCI7371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rosenberg ES, Altfeld M, Poon SH, et al. Immune control of HIV-1 after early treatment of acute infection. Nature. 2000;407(6803):523–6. doi: 10.1038/35035103. [DOI] [PubMed] [Google Scholar]
- 9.Lisziewicz J, Rosenberg E, Lieberman J, et al. Control of HIV despite the discontinuation of antiretroviral therapy. N Engl J Med. 1999;340(21):1683–4. doi: 10.1056/NEJM199905273402114. [DOI] [PubMed] [Google Scholar]
- 10.Kaufmann DE, Lichterfeld M, Altfeld M, et al. Limited durability of viral control following treated acute HIV infection. PLoS Med. 2004;1(2):e36. doi: 10.1371/journal.pmed.0010036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gianella* S, von Wyl* V, Fischer* M, Niederoest B, Joos B, Günthard HF. Impact of Early Antiretroviral Therapy during Primary HIV-1 Infection on Cell-associated HIV-1 DNA and Plasma HIV-1 RNA. Antiviral Therapy. 2011;16(4):535–45. doi: 10.3851/IMP1776. [DOI] [PubMed] [Google Scholar]
- 12.von Wyl* V, Gianella* S, Fischer* M, et al. Early antiretroviral therapy during primary HIV-1 infection results in a transient reduction of the viral setpoint upon treatment interruption. PLOS One. 2011;6(11) doi: 10.1371/journal.pone.0027463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hogan CM, Degruttola V, Sun X, et al. The setpoint study (ACTG A5217): effect of immediate versus deferred antiretroviral therapy on virologic set point in recently HIV-1-infected individuals. J Infect Dis. 2012;205(1):87–96. doi: 10.1093/infdis/jir699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fidler S, Porter K, Ewings F, et al. Short-course antiretroviral therapy in primary HIV infection. N Engl J Med. 2013;368(3):207–17. doi: 10.1056/NEJMoa1110039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hocqueloux L, Prazuck T, Avettand-Fenoel V, et al. Long-term immunovirologic control following antiretroviral therapy interruption in patients treated at the time of primary HIV-1 infection. AIDS. 2010;24(10):1598–601. doi: 10.1097/qad.0b013e32833b61ba. [DOI] [PubMed] [Google Scholar]
- 16.Goujard C, Girault I, Rouzioux C, et al. HIV-1 control after transient antiretroviral treatment initiated in primary infection: role of patient characteristics and effect of therapy. Antivir Ther. 2012;17(6):1001–9. doi: 10.3851/IMP2273. [DOI] [PubMed] [Google Scholar]
- 17.Lodi S, Meyer L, Kelleher AD, et al. Immunovirologic control 24 months after interruption of antiretroviral therapy initiated close to HIV seroconversion. Arch Intern Med. 2012;172(16):1252–5. doi: 10.1001/archinternmed.2012.2719. [DOI] [PubMed] [Google Scholar]
- 18.Williams JP, Hurst J, Stohr W, et al. HIV-1 DNA predicts disease progression and post-treatment virological control. eLife. 2014;3:e03821. doi: 10.7554/eLife.03821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saez-Cirion A, Bacchus C, Hocqueloux L, et al. Post-treatment HIV-1 controllers with a long-term virological remission after the interruption of early initiated antiretroviral therapy ANRS VISCONTI Study. PLoS Pathog. 2013;9(3):e1003211. doi: 10.1371/journal.ppat.1003211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Le T, Wright EJ, Smith DM, et al. Enhanced CD4+ T-cell recovery with earlier HIV-1 antiretroviral therapy. N Engl J Med. 2013;368(3):218–30. doi: 10.1056/NEJMoa1110187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maenza J, Tapia K, Holte S, et al. How often does treatment of primary HIV lead to post-treatment control? Antivir Ther. 2015 doi: 10.3851/IMP2963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carrington M, O’Brien SJ. The influence of HLA genotype on AIDS. Annu Rev Med. 2003;54:535–51. doi: 10.1146/annurev.med.54.101601.152346. [DOI] [PubMed] [Google Scholar]
- 23.Itescu S, Mathur-Wagh U, Skovron ML, et al. HLA-B35 is associated with accelerated progression to AIDS. J Acquir Immune Defic Syndr. 1992;5(1):37–45. [PubMed] [Google Scholar]
- 24.Chomont N, El-Far M, Ancuta P, et al. HIV reservoir size and persistence are driven by T cell survival and homeostatic proliferation. Nature Medicine. 2009;15(8):893–900. doi: 10.1038/nm.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1: Flow chart summarizing selection criteria for this study.