Abstract
The importance of Bone Morphogenetic Proteins (BMPs) in the regulation of cell fate, differentiation and proliferation in the growth plate is well-known. However, in secondary cartilages (such as that in the temporomandibular joint) that grow by proliferation of prechondrocytes and differ in their pattern of growth, the role of BMPs is largely unexplored. To examine this question, we ablated Bmpr1a in the condylar cartilage of neonatal mice and assessed the consequences for mandibular condyle growth and organization at intervals over the ensuing four weeks. Bmpr1a deficiency caused significant chondrodysplasia and almost eliminated the chondrocytic phenotype in the TMJ. Expression of Sox9, collagen II, proteoglycan were all greatly reduced, and cell proliferation as detected by BrdU was almost non-existent in the knockout mice. Primary bone spongiosa formation was also disturbed and was accompanied by reduced Osterix expression. These findings strongly suggest that Bmpr1a is critical for the development and growth of the mandibular condyle via its effect on proliferation of prechondroblasts and chondrocyte differentiation.
Keywords: Bmpr1a, mandibular condyle, endochondral ossification, condylar cartilage
Introduction
Found only in mammals, the temporomandibular joint (TMJ) is a synovial joint in which translation and rotation occurs between the temporal bone and mandible during opening and closing of the mouth. Although the cartilage covering the mandibular condyle (mandibular condylar cartilage or MCC) functions both as an important site of growth and articulation, it differs considerably in its development and structure from both a growth plate and an articular cartilage. A secondary cartilage in contradistinction to primary cartilages of the limbs and cranial base, the MCC develops adjacent to the intramembranous bone of the mandibular ramus from modified periosteal cells in the region that is loaded by articulation with the temporal bone. Accordingly, its superficial layers comprise a perichondrium, deep to which lies a more typical cartilage that ossifies by endochondral ossification at its deep aspect. The perichondrium consists of thin fibrous articular layer and a deeper polymorphic layer of relatively undifferentiated prechondroblastic cells. Just deep to the polymorphic layer lies the flattened chondrocyte zone, with a zone of hypertrophic chondrocytes deep to that [1]. The polymorphic layer contains chondroprogenitor cells that in growing individuals exhibit high mitotic activity and will differentiate into chondrocytes, undergo hypertrophy, and then endochondral ossification in the layers nearest the cartilage-bone interface. Unlike chondrocytes in developing long bones, chondrocytes in the MCC rapidly mature, and exhibit neither columnar palisades of cells nor secondary ossification centers seen in the primary cartilages of long bones[2]. Although these structural features of the TMJ are well documented, much less information is available with respect to the genetic, cellular, and molecular mechanisms involved in TMJ morphogenesis and development.
Bone morphogenetic proteins (BMPs) are multi-functional growth factors that are vital not only to osteogenesis but to chondrogenesis as well. Mice with a conditional knockout of Bmp2 using Col2-Cre displayed severe defects in chondrocyte proliferation, differentiation and apoptosis in the growth plates[3]. Knockout studies of the BMP receptors-1A and -1B (both expressed throughout the growth plate) showed redundancy but double-mutant mice displayed a severe chondrodysplasia phenotype during embryonic development [4]. Conversely, overexpression of a constitutively active Bmpr1a in chondrocytes caused shortening of the columnar layer of proliferating chondrocytes in growth plate and up-regulation of maturation markers [5]. In addition to the acceleration of chondrocyte differentiation, morphological alteration of perichondrial cells was evident, possibly due to stimulation of differentiation of prechondrogenic cells.
The goal of this study was to determine whether a conditional deletion of Bmpr1a in cartilage affects postnatal growth and maturation of the MCC. After activating the transgene at 3 days postnatal, we examined morphology, gene expression (Sox9, Col2, and Osterix), and proliferation (BrdU) in mouse MCC at 1, 2, and 4 weeks of age.
Materials & Methods
Generation of aggrecan-Cre; Bmpr1a Conditional Mutant Mice
Bmpr1a flox/flox mice were mated with aggrecan -CreER mice that express CreER under control of cartilage-specific aggrecan gene regulatory sequences. Conditional Bmpr1a knockout mice were obtained by crossing homozygous Bmpr1a floxed mice (Bmpr1a flox/flox ) and Bmpr1a flox/−; aggrecan -Cre mice. To induce Cre activity, we administered a single injection of tamoxifen (7.5μl/g body wt) in postnatal day 3 and craniofacial specimens were collected from wild type and knockout mice at 1 week, 2 weeks and 4 weeks of age. To evaluate Cre activity, we crossed aggrecan -CreER mice with ROSA26R mice. All protocols were reviewed and approved by the Institutional Animal Care and Use Committee (IACUC) at our respective Institutions.
Radiography and Micro-computed Tomography (Micro-CT)
Both the wild-type (WT) and Bmpr1a CKO mandibles were radiographed with a Faxitron model MX-20 System (Faxitron X-Ray LLC, Lincolnshire, IL, USA). Skulls fixed in buffered 4% paraformaldehyde overnight at 4°C were subjected to micro-computed tomography (μCT) with a CT40 SCANCO Medical System (Southeastern, PA, USA) with a 36-mm holder at 45 kV of energy, 12-μm scanning thickness, and medium resolution. Two-dimensional slice images were selected and used to generate three-dimensional reconstructions with the following parameters: filter width sigma = 0.8, support level = 1.0, and threshold = 173. The same values were used to analyze wild-type and mutant samples at each specified time point. Three-dimensional images were rotated at specific angles to generate a lateral view of the mandibles and condyles.
Skeletal Preparation and Morphometric Analysis
Mouse heads were dissected and fixed with 95% EtOH for 24 hrs at room temperature. After removal of soft tissues, Mandibles were photographed and mandibular length (see Suppl Data) measured (4 samples in each group) using Image J software. Statistical significance was determined by an independent-sample t-test using SPSS 12.0. A P value of < 0.05 was considered statistically significant.
Histology and Immunohistochemistry
Mandibular condyles fixed in 4% paraformaldehyde were decalcified, embedded in paraffin, sectioned at 3–4 μm, and stained with H&E or safranin O (proteoglycans). Other sections were evaluated by immunohistochemistry using the following antibodies: rabbit anti-Sox9 polyclonal antibody (Santa Cruz Biotechnology, USA, 1:100); rabbit anti-Osterix (Santa Cruz Biotechnology, USA, 1:400); mouse anti-Col2 monoclonal antibody (Santa Cruz Biotechnology USA, 1:50); mouse anti-Col X ( Quartett, USA, 1:25); rabbit anti-BMPr1A (Allele Biotechnology, USA,1:100). Detection of immunoreactivity for all preparations was done using a 3,3-diaminobenzidine kit (Vector Laboratories, Burlingame, CA) according to the instructions of the manufacturer. BrdU (Sigma, USA, 10μl/g body wt) was injected into the mice two times; the second injection was 24 hours later than the first one and the mice were sacrificed 2 hours after the second injection.
Results
At each stage examined, the wild type MCC displayed a polymorphic (P) cell layer, a flattened chondrocyte (F) zone, and a hypertrophic chondrocyte (H) zone (Fig. 1A left). To determine the activity of the aggrecan-Cre, we crossed this Cre line with Rosa26R mice. X-gal staining showed that the aggrecan-cre was strongly active in the flattened chondrocyte and hypertrophic zones, with a few cells in the polymorphic zone (Fig. 1A left); no aggrecan-cre activity was detectable in the control (Fig. 1A right). Immunoreactivity for Bmpr1a was strong in the flattened chondrocyte and hypertrophic zones of the MCC, but less pronounced in the polymorphic zone (Fig. 1B left). No immunoreactivity was present in the knockout mice (Fig. 1B right).
Fig 1.
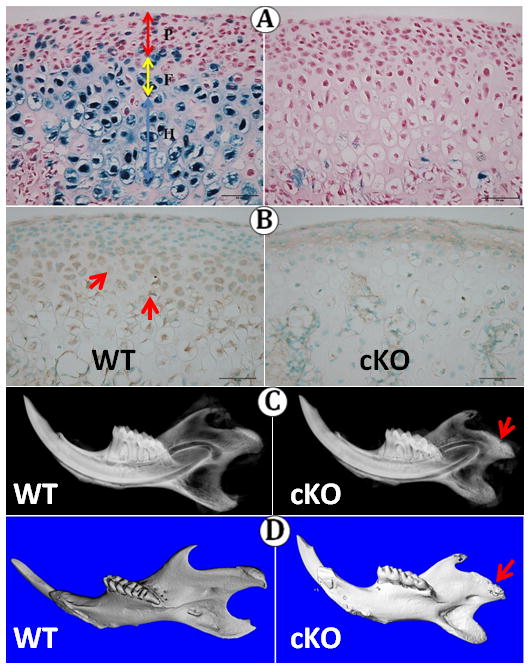
Condyles and mandibles at four weeks of age. A: X-gal staining in Aggrecan-cre with (left) and without (right) Rosa26R. B: Bmpr1a immunoreactivity (red arrows) in WT and knockout mice. C–D: X-ray and micro-CT Images demonstrating the shorter (red arrow) condyle in BMPr1A-deficient mice. P, polymorphic layer; F, Flattened layer; H, Hypertrophic layer.
The Conditional Bmpr1a Knockout Reduces Overall Mandibular Size
As seen in the X-ray and micro-CT images (Fig. 1 C, D), the mandibles of the Bmpr1a knockout mice appeared noticeably smaller than the wild-type mandibles. The condylar head was flattened and reduced in height in the knockout mice. Statistical analysis (Supplementary Fig) showed that mandibular length (mm) was significantly (p< 0.05) reduced in knockout mice (WT: 11.95 ±0.01, cKO: 9.21 ±0.01, n=4 in each group).
Zonal Organization and Chondrogenic Gene Expression Is Altered in Bmpr1a - deficient Condyles
By 1 week of age, the morphology of the knockout condylar cartilage was drastically altered compared to the wild type MCC (Fig. 2A). The extent of the cartilage was much reduced, and proteoglycans (Fig. 2B) and Collagen II (Fig. 2C) were greatly attenuated. There were almost no mature chondrocytes apparent in the knockout MCC and Col IIexpression was limited to a small island near the cartilage-bone interface (Fig. 2C right).
Fig 2.
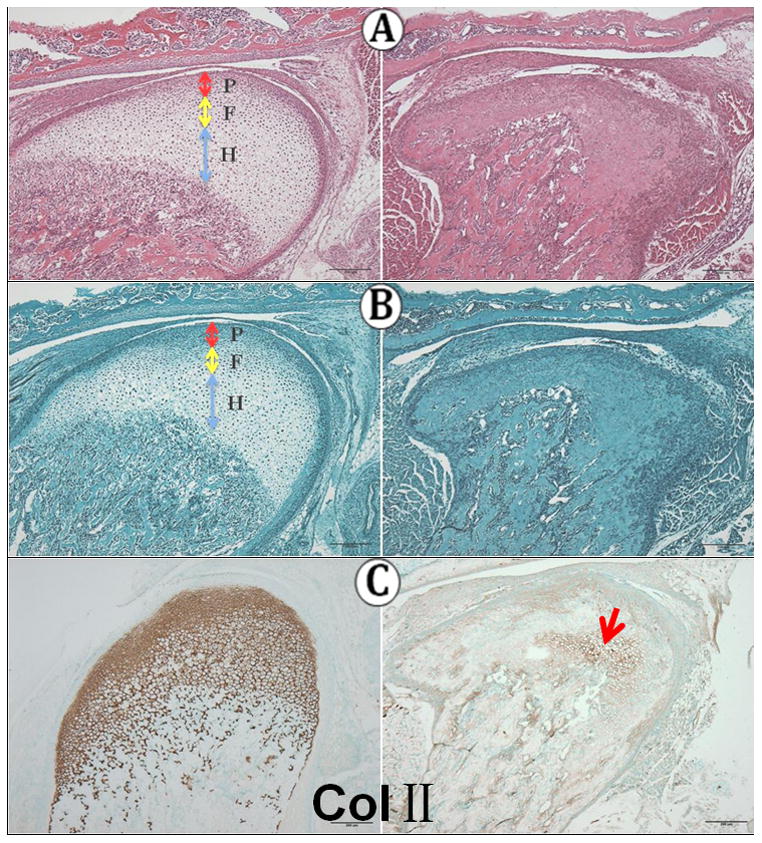
One week of age. A: H&E staining; B: Safranin O staining; C ColII immunoreactivity.
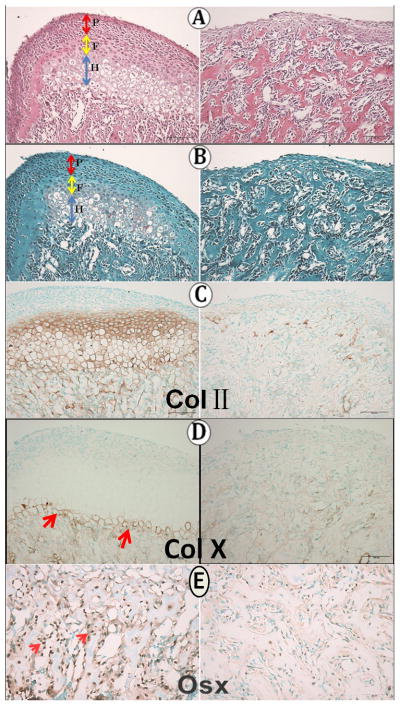
By two weeks of age, the appearance of the Bmpr1a–deficient MCC was largely unchanged. Essentially no cartilage was apparent in Bmpr1a -deficient condyles (Fig. 3 A, B). While Collagen II immunoreactivity was present throughout the flattened chondrocyte and hypertrophic zones of wild type MCC, only discrete islands of Collagen II immunoreactivity were seen in the knockout MCC, and these were in the subchondral bone (Fig. 3C). Collagen X immunoreactivity, present in the last 2–3 cell layers in wild type mice, was minimal in knockout mice (Fig. 3D). Compared to the wild type mice, the expression of Osterix was greatly reduced in the subchondral bone in knockout mice (Fig. 3E), an indication of delayed osteoblast maturation.
Fig 3.
Two weeks of age. A–B no indication of proteoglycans in the knockout MCC; C: ColII immunoreactivity; D: col X immunoreactivity;E: Osterix immunoreactivity (small red arrows).
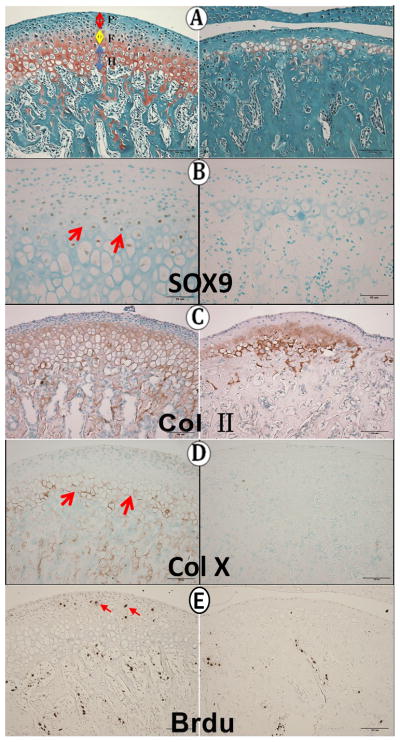
In four-week old mice, chondrocytes were again apparent in the knockout MCC, but the layer was very much thinner than in wild type condyles (Fig. 4A) and Safranin O reactivity was minimal in the knockout MCC. Immunoreactivity for Sox9, a gene necessary for the chondrogenic pathway, was present in the flattened chondrocyte zone in wild type MCC but was absent in knockout MCC (Fig. 4B). Collagen II immunoreactivity was present in the knockout MCC, but reduced and extended into the subchondral bone trabeculae (Fig. 4C). Collagen X immunoreactivity was essentially absent in knockout condyles (Fig. 4D). In wild type condyles, BrdU-labelled cells were present in the polymorphic layer and in the subchondral bone, but no labeled cells were present in the knockout cartilage, appearing in reduced numbers only in the subchondral bone (Fig. 4E).
Fig 4.
Four weeks of age. A: Safranin O staining; B: Sox9 immunoreactivity; C: Col II immunoreactivity; D: col X immunoreactivity; E: BrdU immunoreactivity (red arrows).
Discussion
Our data provide strong evidence that Bmpr1a is required for normal development and growth of the postnatal mandibular condylar cartilage. The MCC was drastically reduced in size and extent as early as 4 days after activation of the transgene and this phenotype persisted in similar form through one month of age. Immunostaining for chondrogenic marker genes Sox9, Collagen II, and Collagen X confirmed the essential cessation of the chondrogenic pathway in Bmpr1a -deficient mice. Sox9, essential for chondrocyte differentiation, directly regulates chondrogenic genes such as Col2a1, Col10a2, and aggrecan, and its near absence indicates that new cartilage formation at the MCC is no longer occurring in the knockout mice. Proliferation in the polymorphic zone was similarly arrested in the knockout MCC, strongly suggesting a role for Bmpr1a in its regulation.
The striking attenuation and near loss of cartilage at the condylar head in Bmpr1a -deficient mice mirrors in many aspects the phenotype seen in the growth plate of these animals [6]. This is interesting in light of the very different developmental origins of the growth plate and MCC: e.g., in the growth plate, proliferation takes place in differentiated chondrocytes whereas in the MCC it is limited to prechondroblasts of the polymorphic zone, a part of the perichondrium. However, the results of our study are very different from those of Yoon and co-workers, who found no apparent phenotype in the growth plate of mice in which Bmpr1a was deleted from cartilage using Col2Cre[4]. Their study was in prenatal mice, so it is possible that Bmpr1a exerts a stronger effect in postnatal cartilage.
Although the effect of BMPs on MCC development and growth has been little investigated, mice deficient in Dlx5, a downstream mediator of BMPs, demonstrate a marked craniofacial phenotype [7] including smaller and misformed mandibles and reduced extent of the condylar cartilage, similar to our study. TGF-β also exerts important effects on mandible development with the condylar cartilage being delayed in its formation and greatly attenuated in mice with conditional inactivation of Tgfbr2 in the neural crest (Tgfbr2fl/fl;Wnt1-Cre) [8]. The authors suggest that TGF-β and Dlx5 have opposing functions that interact to determine cell fate in the polymorphic cells of the MCC. Since BMP signaling up-regulates Dlx expression [9], the effects of BMPs may be modulated by TGF-β.
Our genetic model showed not only a severe cartilage defect in knockout condyles but also some changes in subchondral bone. Immunoreactivity for Osterix, a transcription factor that is essential for osteoblast differentiation, was greatly reduced in Bmpr1a -deficient mice, as was proliferation as assessed by BrdU immunohistochemistry. Although disruption of Bmpr1a in osteoblasts has been shown to increase bone mass [10], it is unclear why Bmpr1a deletion in chondrocytes should affect the bone undrerlying the MCC. Future studies should address the reason for this surprising finding.
Supplementary Material
Acknowledgments
This study was supported by NIH grants DE018486 and R56DE022789 to JQF, and State Key Laboratory of Oral Diseases Open Funding (SKLODOF2010-03) to JQF.
References
- 1.Luder HU, Leblond CP, von der Mark K. Cellular stages in cartilage formation as revealed by morphometry, radioautography and type II collagen immunostaining of the mandibular condyle from weanling rats. Am J Anat. 1988;182:197–214. doi: 10.1002/aja.1001820302. [DOI] [PubMed] [Google Scholar]
- 2.Shibata S, Suda N, Suzuki S, et al. An in situ hybridization study of Runx2, Osterix, and Sox9 at the onset of condylar cartilage formation in fetal mouse mandible. J Anat. 2006;208:169–177. doi: 10.1111/j.1469-7580.2006.00525.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shu B, Zhang M, Xie R, et al. BMP2, but not BMP4, is crucial for chondrocyte proliferation and maturation during endochondral bone development. J Cell Sci. 2011;124:3428–3440. doi: 10.1242/jcs.083659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yoon BS, Ovchinnikov DA, Yoshii I, et al. Bmpr1a and Bmpr1b have overlapping functions and are essential for chondrogenesis in vivo. Proc Natl Acad Sci U S A. 2005;102:5062–5067. doi: 10.1073/pnas.0500031102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kobayashi T, Lyons KM, McMahon AP, et al. BMP signaling stimulates cellular differentiation at multiple steps during cartilage development. Proc Natl Acad Sci U S A. 2005;102:18023–18027. doi: 10.1073/pnas.0503617102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jing J, Ren Y, Zong Z, et al. BMP Receptor 1A Determines the Cell Fate of the Postnatal Growth Plate. Int J Biol Sci. 2013;9:895–906. doi: 10.7150/ijbs.7508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jeong J, Li X, McEvilly RJ, et al. Dlx genes pattern mammalian jaw primordium by regulating both lower jaw-specific and upper jaw-specific genetic programs. Development. 2008;135:2905–2916. doi: 10.1242/dev.019778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oka K, Oka S, Hosokawa R, et al. TGF-β-mediated Dlx5 signaling plays a crucial role in osteo-chondroprogenitor cell lineage determination during mandible development. Dev Biol. 2008;321:303–309. doi: 10.1016/j.ydbio.2008.03.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nishimura R, Hata K, Ikeda F, et al. Signal transduction and transcriptional regulation during mesenchymal cell differentiation. J Bone Min Metab. 2008;26:203–212. doi: 10.1007/s00774-007-0824-2. [DOI] [PubMed] [Google Scholar]
- 10.Kamiya N, Ye L, Kobayashi T, et al. Disruption of BMP signaling in osteoblasts through type IA receptor (BMPRIA) increases bone. J Bone Min Res. 2008;23:2007–2017. doi: 10.1359/JBMR.080809. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.