Summary
The global spread of the influenza A(H1N1)pdm09 virus (pH1N1) associated with travelers from North America during the onset of the 2009 pandemic demonstrates the central role of international air travel in virus migration. To characterize risk factors for pH1N1 transmission during air travel, we investigated travelers and airline employees from four North American flights carrying ill travelers with confirmed pH1N1 infection. Of 392 passengers and crew identified, information was available for 290 (74%) passengers were interviewed. Overall attack rates for acute respiratory infection and influenza-like illness 1–7 days after travel were 5.2% and 2.4% respectively. Of 43 individuals that provided sera, 4 (9.3%) tested positive for pH1N1 antibodies, including 3 with serologic evidence of asymptomatic infection. Investigation of novel influenza aboard aircraft may be instructive. However, beyond the initial outbreak phase, it may compete with community-based mitigation activities, and interpretation of findings will be difficult in the context of established community transmission.
Keywords: H1N1, Influenza A, Air travel, Transmission risk, Aircraft, Pandemic
Background
By June 1, 2009, six weeks after the identification of the novel influenza A(H1N1)pdm09 virus (pH1N1) in North America, 62 countries and territories were reporting laboratory-confirmed cases to the World Health Organization (WHO).1 Rapidly spreading and geographically widespread cases associated with travelers from North America to Europe and Asia during the onset of the pandemic demonstrated the central role of international air travel in the global migration of the virus.2–4 By July 2009, more than 16 countries had reported primary introduction of pH1N1 by ill travelers arriving by air.3 Transmission of seasonal influenza during air travel has been documented by epidemiologic investigations and is supported by model simulations.5–9 Published studies of pH1N1 transmission among air travelers report that virus transmission during air travel is probable, but the risk of infection to susceptible passengers and crew may be limited, and the role of the aircraft cabin environment is unclear.10–12
Documented risk factors associated with seasonal influenza virus transmission include receiving or providing health care13; being in close quarters (e.g., in a household, daycare, or schools) with index patients14; and close proximity to (within 1 m) or being in crowded or enclosed environments (including aircraft cabins) with an index patient who is coughing and sneezing.9,15 To investigate the potential for person-to-person transmission of pH1N1 during air travel, the Centers for Disease Control and Prevention (CDC) conducted epidemiologic investigations among passengers who flew on flights with ill persons with laboratory-confirmed pH1N1. This report details the results of these investigations among airline employees and passengers on four North American flights in April 2009: one international flight into the United States from Mexico and three domestic flights within the United States.
Case-traveler A
On March 30, 2009, case-traveler A, a 10-year-old child from California with a medical history of asthma, developed fever (102 °F), cough, fatigue, headache, and vomiting. On April 1, 2009, case-traveler A was evaluated in an emergency department, where a nasopharyngeal swab was collected. The child was not hospitalized. An 8-year-old sibling of case-traveler A had experienced low-grade fever, sneezing, rhinorrhea, cough, and chest pain 2 weeks prior to case-traveler A’s illness onset, but was without symptoms by the date of travel. Their mother had onset of sneezing and cough without fever on April 2. On April 3, case-traveler A, reportedly unwell and feverish with no report of coughing and sneezing, traveled by commercial aircraft with the younger sibling as unaccompanied minors on a two-part flight (flights A1 and A2) from San Diego, California, to Dallas, Texas, via El Paso, Texas. Flight A1 was a 1-h 45-min flight from San Diego to El Paso and flight A2 was a 1-h 35-min flight from El Paso to Dallas, Texas. Some passengers from flight A1 disembarked in El Paso, where a new cohort embarked to join remaining passengers. The two children did not disembark. On April 15, 2009, CDC confirmed, by reverse-transcriptase polymerase chain reaction (RT-PCR) analysis of the nasopharyngeal specimen collected on April 1, that case-traveler A was the first reported positive pH1N1 case in the United States.16,17
Case-traveler B
During April 16–19, 2009, case-traveler B, a 54-year-old Kansas resident traveled round-trip from Wichita, Kansas, to Cancun, Mexico, accompanied by seven work colleagues. On April 19, case-traveler B developed diarrhea, fever, chills, cough and sore throat during the return trip. The trip consisted of flight B1, a 2-h 25-min flight from Cancun to Houston, Texas, and flight B2, a 1-h 40-min flight from Houston to Wichita. The only travelers on both flights were case-traveler B and companions. Case-traveler B’s spouse, who did not travel, developed fever, chills, cough, and myalgia on April 23. On April 24, both case-traveler B and spouse were evaluated at an outpatient clinic. On April 25, both were confirmed infected with pH1N1 by RT-PCR. None of the seven traveling companions developed acute respiratory illness nor reported infection with pH1N1; three provided serum specimens and were confirmed seronegative for pH1N1. Case-traveler B was the first confirmed case of pH1N1 reported in Kansas.
Methods
Public health authority
Section 361 of the U.S. Public Health Services Act, Part G, Quarantine and Inspection Regulations, 42 United States Code, Section 264, Parts 70 and 71, provides CDC with the regulatory authority to collect flight, passenger, and airline employee information from flight manifests to conduct public health investigations as needed.18–20 Accordingly, CDC requested that the two involved airlines provide contact information for passengers and employees and respond to questions regarding the aircraft environmental control systems.
Passenger and crew survey
We developed a traveler cohort survey questionnaire to evaluate the risk of disease transmission of pH1N1 to passengers and crew on 4 flights with a confirmed pH1N1 index case-traveler on board. Since seats on flights A1 and A2 were unassigned, all passengers and crew were targeted for interview; additionally, since case-traveler A was an unaccompanied minor, airport-based customer service agents who may have interacted with the case-traveler were also included. For flights B1 and B2, all coach passengers and crew were targeted.
All interviewees were asked about the development of symptoms of acute respiratory illness and influenza-like illness during 1–7 days following air travel. The survey also included questions regarding onset of other symptoms, such as acute vomiting, diarrhea, headache, and rash. To evaluate risk factors for pH1N1 infection and developing acute respiratory illness after air travel, travelers were asked about seat assignment, risk factors for community exposure to pH1N1 (e.g. presence of ill household members, travel itineraries, and activities while in Mexico, Texas, and California), and receipt of the 2008–2009 seasonal influenza vaccine [trivalent vaccine containing A/Brisbane/59/2007 (H1N1)-like, A/Brisbane/10/2007 (H3N2)-like, and B/Florida/4/2006-like antigens]. Questionnaires were developed in English and Spanish. Interviews were conducted via telephone. Medical and vaccination records were not reviewed.
Participation in the interviews was voluntary. Documented verbal informed consent for participation in the survey was obtained before each interview. Parental permission was obtained for surveys administered to minors aged less than 18 years. The protocol underwent human subjects review at CDC and was determined to be nonresearch.
State health departments were notified of these investigations via a secure web-based communications network (Epidemic Information Exchange©, CDC, Atlanta, GA) and given the option to administer the questionnaire to passengers and crew members in their jurisdiction or to allow CDC staff to contact their residents. From April 21 to May 20, 2009, public health officers from CDC, Arizona, California, Colorado, Kansas, Texas, Wisconsin, and Wyoming conducted interviews. Up to 5 attempts were made to reach each traveler.
Case and contact definitions
Case-travelers were defined as persons with virologically confirmed pH1N1 infection who traveled by air within seven days of symptom onset, the period of illness when viral shedding was most likely to occur.21,22
For flights A1 and A2, contacts were defined as persons who reported talking with, sharing hand-held electronics with, touching, or holding hands with the unaccompanied minors, or who were in close proximity to them for at least 1 h during air travel. Because flights A1 and A2 did not have assigned seating and the case-traveler sat in row 2, passengers who reported sitting “near the front” of the plane were assigned as contacts. For flights B1 and B2, close proximity was defined as being seated in the same row or two rows in front of or two rows behind the case-traveler.23 Passengers on flights B1 and B2 not seated within 2 rows of the case-traveler, and those passengers on flights A1 and A2 who sat “in the middle”, “over the wing”, or “in the back” of the aircraft were considered non-contacts.
Acute respiratory illness was defined as at least two of the following: self-reported fever or feverishness, rhinorrhea or nasal congestion, cough, or sore throat. Influenza-like illness was defined as self-reported fever or feverishness with cough or sore throat. Influenza-like illness cases were a subset of total acute respiratory illness cases. The illness was considered indeterminate if symptoms were present but the illness did not meet the acute respiratory illness or influenza-like illness definition; for analysis, these illnesses were reclassified as not acute respiratory illness or influenza-like illness.
Probable secondary air travel-associated cases were defined as individuals (contacts and non-contacts) who developed acute respiratory illness 1–7 days after the flight.
Confirmed secondary air travel-associated cases were defined as individuals (contacts and non-contacts) who developed acute respiratory illness 1–7 days after the flight with serologically confirmed pH1N1 infection.
Serology
Travelers who agreed to provide sera were referred to their local health departments for serum collection. Serum samples were tested for the presence of pH1N1-specific antibodies at CDC using microneutralization (MN) and turkey red blood cell hemagglutination-inhibition (HI) assays with a A/California/07/2009-like virus as previously described.24 Despite efforts to collect paired baseline and follow-up sera to demonstrate seroconversion, a ≥4-fold rise in antibody titer, by either assay, the earliest sample collection was 18 days after the flights. Therefore, seroconversion could not be used as a laboratory indicator of pH1N1 infection. Instead, we used a combination of threshold titers (a MN titer of ≥40 and an HI titer of ≥20) to demonstrate seropositivity. This combination of pH1N1-specific antibody titers was shown to provide 90% sensitivity and 96% specificity for detection of pH1N1 infection in U.S. individuals <60 years of age and 92% specificity in those aged 60–79 years.25
Data management and analysis
Completed questionnaires were faxed in a secure manner, or mailed by interviewers from the state health departments and CDC Quarantine Stations to CDC headquarters in Atlanta, GA, and entered into a database, excluding personal identifying information, using Microsoft Access Database Software, Version 2003, Copyright© 2003 SP2 (Microsoft Corp., USA). All identifying information on questionnaires was kept confidential and restricted only to investigators.
Analyses were conducted using SAS, Version 9.2 Copyright© 2008 (SAS Institute Inc. Cary, NC, USA). For descriptive results, categorical variables were presented as proportions, and continuous variables were described by the mean or the median and range. Because of the small sample sizes and since many pH1N1 cases were afebrile, acute respiratory illness, rather than influenza-like illness, was considered the primary outcome measure. We created five age categories, two seat-proximity categories (contacts and non-contacts), and two duration-of-exposure categories (traveling on one flight with a case-traveler vs. both flights).
Because our investigation focused on the possibility of transmission during air travel, the eight passengers known to have had interaction with the case-travelers outside of air travel (the sibling of case-traveler A, and the seven work colleagues of case-traveler B), were excluded from the analyses. Passengers reporting onset of acute respiratory illness prior to 24 h after the flight were also excluded from the risk analyses.
Univariate analysis was used to describe the association between developing acute respiratory illness in the 1–7 days following the flight and traveler demographics, 2008–09 vaccination status, traveler status (passenger vs. employee), seating proximity to the case-traveler, interaction with the case-traveler, duration of Mexico trip, and traveling on one or both flights. A Fisher’s exact test or chi-squared (X2) test was used for categorical variables. Continuous variables were compared by using the t-test. An unadjusted multivariate model was used.
Results
Epidemiologic investigation
Flights A1 and A2 carried 127 and 138 passengers, respectively. Excluding case-traveler A and the sibling, we identified a total of 225 individuals for interview: 211 passengers and 14 airline employees. Thirteen (92.9%) of 14 employees and 155 (73.5%) passengers were contacted. All employees and 146 (94.2%) passengers agreed to be interviewed. Interviews were conducted during April 21–May 20, 2009; the average duration between flight date and interview date was 28.9 days (range: 18–47 days).
Of 146 passengers interviewed who traveled on flights A1 or A2, 39 (26.7%) flew on both flights. Of 140 reporting seat location, 46 (32.9%) passengers were classified as contacts (Table 1). Among all interviewees, 144 (98.6%) passengers provided complete illness information; two were excluded for inadequate information about onset dates or symptoms. No employees reported acute respiratory illness or influenza-like illness with onset from April 4 to April 10, 2009. Four passengers reported onset of acute respiratory illness prior to the flight date, two of whom indicated symptom onset within 4 days prior to traveling, but did not report if they were symptomatic during the flights. Eight (5.6%) passengers reported having acute respiratory illness 1–7 days after the flights; 4 (2.8%) had symptoms consistent with influenza-like illness. Three (37.5%) of these 8 passengers who met the case definition of a probable secondary air travel-associated cases also met the definition of a contact; the attack rate among contacts was 7%.
Table 1.
Demographic and in-flight characteristics among passengers and crew on 4 flights into and within the United States that had travelers with confirmed influenza A(H1N1)pdm09 infection onboard, April 2009.
| Travel contact characteristics | Flights A1 and A2
|
Flights B1 and B2
|
||
|---|---|---|---|---|
| Passengers no. (%) | Crew no. (%) | Passengers no. (%) | Crew no. (%) | |
| Sex: male | 77/145 (53.1) | 5/13 (38.5) | 60/124 (48.4) | 6/9 (66.7) |
| Median age, years (range) | 48 (3–97) | 43 (24–52) | 39 (2–68) | 40 (24–58) |
| Resident of California or Texas | 119/145 (82.1) | 8/13 (61.5) | 35/123 (28.5) | 4/9 (44.4) |
| Seasonal influenza vaccinea | 55/142 (38.7) | 5/13 (38.5) | 25/121 (20.7) | 3/9 (33.3) |
| Traveler-contact by seating proximityb | 46/140 (32.9) | – | 24/124 (19.4) | – |
| Reported interaction with index patientc | 9/133 (6.8) | 3/13 (23.1) | – | – |
| Talked with index patient | 6/8 (75) | 3/3 (100) | – | – |
| Touched belongings of index patient | 2/8 (25) | 1/3 (33.3) | – | – |
Received influenza vaccine between October, 2008 and March, 2009.
For flights A1 and A2, passengers who reported sitting “near the front” of the aircraft. For flights B1 and B2, passengers seated within 2 rows of the index case-traveler.
One passenger reported interaction, but did not specify details of that interaction.
Flights B1 and B2 carried 129 and 46 coach passengers, respectively. Excluding case-traveler B and the seven travel companions, we identified a total of 167 travelers for interview: 9 airline employees and 158 passengers. All employees were interviewed; 128 (81.0%) passengers were contacted of whom 124 (96.9%) agreed to be interviewed. Interviews were conducted from April 28 to May 18, 2009, on average 14 days after the flights (range: 9–29 days).
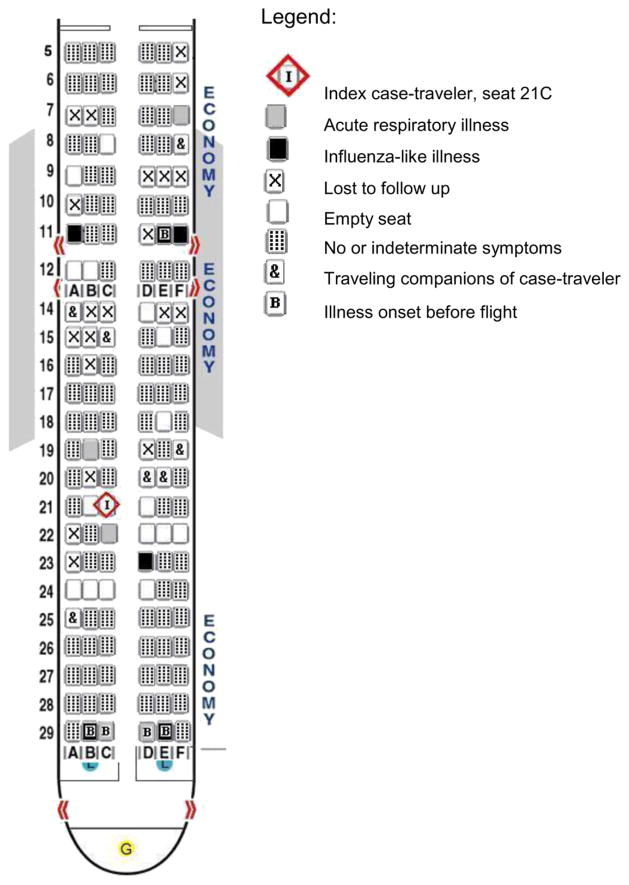
None of the employees reported developing acute respiratory illness within 7 days after working flights B1 and B2. Because passenger seat assignments were available for both flights, we were able to create seating charts for flights B1 and B2. Only the seating chart for flight B1 is included (Fig. 1). Combined, 24 (19.4%) of 124 passengers were seated within 2 rows of the case-traveler and classified as contacts; the remaining 97 (80.0%) were classified as non-contacts. Of the 124 passengers interviewed, 6 reported acute respiratory illness prior to the flight date. Five of these were on flight B1 from Mexico; 4 traveled together in Mexico, reported symptom onset 4 days before the flight, and sat in row 29 (Fig. 1); the fifth sat in 11E and had symptom onset 2 days before the flight.
Figure 1.
Flight B1 on April 19, 2009, from Cancun, Mexico, to Houston, Texas: Boeing 737 seating chart of coach passengers reporting symptoms from April 12 to April 26, 2009.
From both flights B1 and B2, seven passengers (5.6%) reported having acute respiratory illness within 7 days after travel, 3 (2.4%) reported developing influenza-like illness. Three (42.9%) of the 7 probable secondary air travel related cases were contacts on flight B1, and 3 were non-contacts on B1; 2 of the non-contact probable cases (seats 11A and 11F in Fig. 1) were identified as household members of the passenger in 11E who reported acute respiratory illness onset prior to the flight. We identified 4 passengers (seats 7F, 19B, 22C, and 23D) on flight B1 who developed acute respiratory illness within one to seven days following the flight and were not epidemiologically linked to a case of acute respiratory illness with onset before the flight. Three of these 4 probable secondary cases sat within 2 rows of case-traveler B. The remaining probable case was a non-contact on flight B2, who reported no prior exposure to someone with acute respiratory illness and sat 5 rows in front of case-traveler B. The attack rate among contacts on the two flights was 13%.
Combined, among 290 travelers from the 4 flights for whom information was available, 15 (5.2%) had symptoms that met the case definition of acute respiratory illness within 1–7 days following the flight, and 7 (2.4%) reported influenza-like illness. Developing acute respiratory illness 1–7 days after flights was not significantly associated with seasonal influenza vaccination status, seating proximity to an index case, or direct interaction with the index case-travelers (Table 2). Among passengers on flights B1 and B2, there were 4 children under 5 years of age; 2 developed acute respiratory illness prior to the flight and 2, non-contacts, developed acute respiratory illness within 1–7 days after the flight (Table 2).
Table 2.
Univariate analysis of risk factors for developing acute respiratory illnessa among passengers and crew on 4 flights carrying 2 influenza A(H1N1)pdm09 index case-travelers.
| Risk factor | Flights A1 and A2
|
Flights B1 and B2
|
||
|---|---|---|---|---|
| No. acute respiratory illness cases/no. total | Risk ratio (95% CI) | No. acute respiratory illness cases/no. total | Risk ratio (95% CI) | |
| Age | ||||
| <5 yrs | 0/1 | 4.4b (0.4–53.1) | 2/2 | 25.0 (10.6–59.0) |
| ≥ 5 yrs [referent (R)] | 8/149 | 5/125 | ||
| Sex | ||||
| Female | 4/74 | 1.4 (0.3–6.1) | 3/62 | 0.8 (0.2–3.4) |
| Male (R) | 3/78 | 4/65 | ||
| Vaccinated | ||||
| No | 6/93 | 1.8 (0.4–8.8) | 7/90 | 4.2 (0.3–72.7) |
| Yes (R) | 2/57 | 0/27 | ||
| Traveler status | ||||
| Crew | 0/13 | 0.6b (0.0–9.7) | 0/9 | 0.8b (0.0–12.9) |
| Passenger (R) | 8/140 | 7/118 | ||
| Seating proximityc | ||||
| Contact | 3/45 | 1.2 (0.3–4.7) | 3/24 | 3.0 (0.7–12.7) |
| Non-contact (R) | 5/89 | 4/97 | ||
| Interaction with index-traveler | ||||
| Yes | 1/10 | 1.8 (0.2–12.9) | – | – |
| No (R) | 7/123 | – | ||
| Flights taken | ||||
| Both A1, A2 | 2/40s | 0.9 (0.2–4.2) | – | – |
| Only one (R) | 6/107 | – | ||
| Duration of Mexico trip | ||||
| ≥6 days | – | – | 4/74 | 0.8 (0.2–4.0) |
| <6 days (R) | – | 2/29 | ||
Acute respiratory illness defined as ≥ of 2 rhinorrhea, sore throat, cough, fever or feverishness with symptoms onset 1–7 days after air travel.
Logit estimators using a correction of 0.5 in every cell that contains zero.
For flights A1 and A2, passengers who reported sitting “near the front” of the aircraft. For flights B1 and B2, passengers seated within 2 rows of the index case-traveler.
Serology
During telephone interviews for all flights, 207 (71.4%) of 290 individuals consented to provide a serum specimen for confirmatory testing of pH1N1 infection. The CDC influenza laboratory received specimens for 48 (23.2%) of the 207 individuals and provided final serologic results for 43 individuals, 4 of whom were seropositive (9.3%). Samples from 5 individuals could not be tested due to inadequate quality or insufficient quantity. Serum collection occurred on average 38 days (range 18–73 days) after the flights.
Laboratory results were available for 4 of 25 travelers who reported onset of an acute respiratory illness from one week before travel to one week after; one of whom was seropositive. This passenger, on flight B2, sat 5 rows in front of the index case-traveler, developed symptoms 1 day after the flight, and tested negative for influenza by rapid test. The remaining 3 tested negative for pH1N1 antibodies.
The remaining 3 seropositive individuals were asymptomatic. An airport-based customer service agent from flight A1 who interacted with case-traveler A had sera drawn 3 and 6 weeks after the flight; both specimens tested positive for pH1N1 antibodies. This contact also reported having 2 children in the household who developed fever and cough on April 7, 2009. Those household members were not tested for pH1N1 infection. The other 2 seropositive individuals were passengers on flight B2 whose specimens were obtained May 26, 2009. The first traveled alone and was not a contact of case-traveler B. The second sat in the same row as case-traveler B, separated by the aisle; the traveler’s spouse who sat in the adjacent seat also remained asymptomatic and tested negative for pH1N1 antibodies (serum collected May 26).
Aircraft environmental assessment
The same single-cabin Boeing-737 was used for flights A1 and A2. The airline reported that the aircraft environmental control system was operating under normal conditions: cabin air exchange rate of 20–30 air changes per hour; greater than 50% fresh air from outside; the remainder re-circulated air, filtered using high-efficiency particulate air filters (99.93% efficiency rate at 0.3 microns). The aircraft for flight B1 was a 2-cabin Boeing-737. The aircraft for flight B2 was an Embraer RJ-145, single cabin, 50-seat regional jet aircraft. The airline operating flights B1 and B2 reported both were operating under normal conditions, with environmental control system similar to those found on the majority of commercial aircraft operating in the United States.26
Discussion
This report details the findings and challenges of the first investigations of pH1N1 transmission among airline travelers arriving into and traveling within the United States. Among travelers evaluated and considered at-risk for pH1N1 infection and who were not ill on the day of the flight nor travel companions of the index case-travelers, the overall attack rates of acute respiratory illness and influenza-like illness, occurring 1–7 days after the flights were 5.2% and 2.4% respectively. The acute respiratory illness attack rate was 7% among passenger-contacts on flights A1 and A2 and was 13% among passenger-contacts for flights B1 and B2, comparable to attack rates reported among household contacts of confirmed pH1N1 cases.27
Among passengers arriving from Mexico, we identified clusters of acute respiratory illness and influenza-like illness among household and traveler-contacts not epidemiologically linked to the index case-traveler. Though none were tested to confirm pH1N1 infection, these observations replicate published findings of travelers in groups, who are more likely to contribute to pH1N1 transmission than non-epidemiologically linked air travelers.10,11
Limitations to this investigation included potential misclassification and recall bias regarding symptoms, dates of onset of illness, and aircraft seating location. In-cabin assessments of the association between illness and proximity to the index case were complicated by the lack of assigned seating on flights A1 and A2. Further complicating the incabin risk assessment was the identification of passengers reporting having acute respiratory illness and influenza-like illness with onset before and during the flights. It is possible that one or more of these passengers who reported symptoms during travel may also have been infectious with pH1N1 during travel. Additionally, travel companions of the case-travelers may have had asymptomatic pH1N1 infection and been another source of transmission. Collection of nasopharyngeal swab specimens and acute- and convalescent-phase sera to confirm pH1N1 infection among traveler-contacts was limited due to the lag time between flight dates, laboratory confirmation of index case-travelers, and conduct of the epidemiologic investigation, as well as the limited availability of local public health staff during the burgeoning pH1N1 outbreaks in U.S. cities. Without laboratory confirmation, self-reported symptoms are nonspecific with respect to seasonal or pH1N1 influenza, and their interpretation is limited.28,29
We used acute respiratory illness as a measure of illness for travelers exposed to an index case-traveler infected with pH1N1. Previous studies showed that the incidence of confirmed pH1N1 among persons with acute respiratory illness is low. In a study of 79 symptomatic travelers (90% with acute respiratory illness, 66% with fever) who flew on commercial aircraft from North America to Sweden (during April 24 through June 10, 2009), 5% were confirmed infected with pH1N1 and 34% were confirmed infected with rhinovirus.28 Assessing surveillance tools during the early pH1N1 epidemic period in Singapore, investigators concluded that the high levels of “background noise” within acute respiratory illness surveillance data provide poorer resolution of influenza epidemic activity than does influenza-like illness.30 Just as the number of secondary cases of acute respiratory illness considerably over-represents the true incidence of pH1N1 infection among passengers, it is also likely that some asymptomatic or mildly symptomatic passengers with pH1N1 infection, or those with atypical symptoms such as diarrhea, were not identified. From our investigation of travel contacts, serologic testing identified 3 individuals with evidence of asymptomatic pH1N1 infection.
Collection of serum specimens from participating travelers from all flights was attempted; however, the proportion of participants that provided specimens was low. Furthermore, the late timing of specimen collection precluded our ability to use seroconversion, the gold standard for serologic diagnosis, for confirmation of pH1N1 infection. Nevertheless sensitive and specific serologic criteria were applied to identify pH1N1 infected persons. Seropositive results among asymptomatic contacts and seronegative results among those reporting acute respiratory illness reinforce the limitations of self-reported symptoms alone as a marker for influenza infection.
Published studies of pH1N1 transmission among contacts, following exposure, report secondary attack rates of acute respiratory illness and influenza-like illness ranging from 13 to 53% in household settings31–33; 15% influenza-like illness among campers in two camp programs34; 11% confirmed or suspected pH1N1 among 1346 cadets exposed in a military facility22; 11% influenza-like illness attack rate among crew of a docked naval ship35; and 35% self-reported illness among 2686 high school students in New York City.36 These studies of pH1N1 transmission in other settings reflect attack rates of acute respiratory illness and ILI that are 2–10 times higher than were found among our passenger cohort.
Although international air travel played a role in the global spread of pH1N1, our investigation did not demonstrate in-flight transmission of pH1N1 and it appears that the risk of spread of pH1N1 to passengers and crew during air travel is low during flights of relatively short duration. Flights of longer duration, such as intercontinental flights, may pose a greater risk of transmission, particularly given greater potential for patients on longer flights to develop initial symptoms while on-board as has been found in other investigations.9,11 However, other investigators who attempted contact investigations during the initial phase of the 2009 pandemic concluded that tracing close contacts among flight passengers was not effective, as timely provision of post-exposure prophylaxis could not be achieved in most cases.37
Investigation of transmission of novel influenza strains aboard aircraft during the initial phases of an epidemic or pandemic may be instructive. Definitive identification of index case-travelers and timely and complete clinical and confirmatory laboratory diagnostic data from traveler-contacts are required to fully inform attack rate calculations and provide true estimates of disease transmission risk within the aircraft cabin. However, the ongoing conduct of passenger contact investigations beyond the initial phase of an outbreak may require too many resources, compete with community-based infection control activities, and render interpretation of findings difficult in the context of established community transmission. Any benefit of aircraft contact investigations for novel influenza viruses needs to be judged against the substantial resource investment required, the impact of shifting those resources from local public health activities, and the characteristics of the disease, including transmissibility and case fatality rates.
Acknowledgments
The authors would like to thank the state public health agencies in Arizona, California, Colorado, Kansas, Texas, Wisconsin, and Wyoming who mobilized local resources in response to these investigations. Special gratitude to our CDC colleagues in the Influenza Division for their participation and guidance, especially the work of Carolyn Bridges, Saumil Doshi, Lyn Finelli, Seema Jain, and Tim Uyeki. We would like to thank members of the Division of Global Migration and Quarantine pH1N1 Investigation Team including Clive Brown, Greg Burgess, Marty Cetron, Sheila Sheffler De La Cruz, Naomi Guzman, Peter Houck, Chris Hurst, Petra Illig, Laura Leidel, Daniel Rodriguez, William Schluter, Madeleine Short, and Danitza Tomianovic. We would also like to thank members of the Influenza Serology Working Group including Yaohui Bai, Peter Browning, Li Cronin, Hanan Dababneh, Heather Noland, Conrad Quinn, Vic Veguilla, Jarad Schiffer, Stephen Soroka, and Leilani Thomas.
Footnotes
Disclaimer
The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention.
Sponsorship
None.
Conflicts of interest
None declared.
References
- 1.World Health Organization. [accessed 15.09.09];Influenza A (H1N1) update 42. 2009 Jun 1; Available at: http://www.who.int/csr/don/2009_06_01a/en/index.html.
- 2.Hahné S, Donker T, Meijer A, Timen A, van Steenbergen J, Osterhaus A, et al. Epidemiology and control of influenza A(H1N1)V in the Netherlands: the first 115 cases. Euro Surveill. 2009;14(27) doi: 10.2807/ese.14.27.19267-en. [DOI] [PubMed] [Google Scholar]
- 3.Khan K, Arino J, Hu W, Raposo P, Sears J, Calderon F, et al. Spread of a novel influenza A (H1N1) virus via global airline transportation. N Eng J Med. 2009;361(2):212–4. doi: 10.1056/NEJMc0904559. [DOI] [PubMed] [Google Scholar]
- 4.Shimada T, Gu Y, Kamiya H, Komiya N, Odaira F, Sunagawa T, et al. Epidemiology of influenza A(H1N1)V virus infection in Japan, May–June 2009. Euro Surveill. 2009;14(24) doi: 10.2807/ese.14.24.19244-en. [DOI] [PubMed] [Google Scholar]
- 5.Brownstein JS, Wolfe CJ, Mandl KD. Empirical evidence for the effect of airline travel on inter-regional influenza spread in the United States. PLoS Med. 2006;3(10):e401. doi: 10.1371/journal.pmed.0030401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Klontz KC, Hynes NA, Gunn RA, Wilder MH, Harmon MW, Kendal AP. An outbreak of influenza A/Taiwan/1/86 (H1N1) infections at a naval base and its association with airplane travel. Am J Epidemiol. 1989;129(2):341–8. doi: 10.1093/oxfordjournals.aje.a115137. [DOI] [PubMed] [Google Scholar]
- 7.Marsden AG. Outbreak of influenza-like illness related to air travel. Med J Aust. 2003;179(3):172–3. doi: 10.5694/j.1326-5377.2007.tb01296.x. [DOI] [PubMed] [Google Scholar]
- 8.Mazumdar S, Chen Q. A one-dimensional analytical model for airborne contaminant transport in airliner cabins. Indoor Air. 2009;19(1):3–13. doi: 10.1111/j.1600-0668.2008.00553.x. [DOI] [PubMed] [Google Scholar]
- 9.Moser MR, Bender TR, Margolis HS, Noble GR, Kendal AP, Ritter DG. An outbreak of influenza aboard a commercial airliner. Am J Epidemiol. 1979;110(1):1–6. doi: 10.1093/oxfordjournals.aje.a112781. [DOI] [PubMed] [Google Scholar]
- 10.Baker MG, Thornley CN, Mills C, Roberts S, Perera S, Peters J, et al. Transmission of pandemic A/H1N1 2009 influenza on passenger aircraft: retrospective cohort study. BMJ. 2010;340:c2424. doi: 10.1136/bmj.c2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Han K, Zhu X, He F, Liu L, Zhang L, Ma H, et al. Lack of airborne transmission during outbreak of pandemic (H1N1) 2009 among tour group members, China, June 2009. Emerg Infect Dis. 2009;15(10):1578–81. doi: 10.3201/eid1510.091013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ooi PL, Lai FYL, Low CL, Lin R, Wong C, Hibberd M, et al. Clinical and molecular evidence for transmission of novel influenza A(H1N1/2009) on a Commercial Airplane. Arch Intern Med. 2010;170(10):913–5. doi: 10.1001/archinternmed.2010.127. [DOI] [PubMed] [Google Scholar]
- 13.Pachucki CT, Pappas SAW, Fuller GF, Krause SL, Lentino JR, Schaaff DM. Influenza A among hospital personnel and patients. Implications for recognition, prevention, and control. Arch Intern Med. 1989;149(1):77–80. [PubMed] [Google Scholar]
- 14.Viboud C, Boëlle PY, Cauchemez S, Lavenu A, Valleron AJ, Flahault A, et al. Risk factors of influenza transmission in households. Br J Gen Pract. 2004;54(506):684–9. [PMC free article] [PubMed] [Google Scholar]
- 15.Brankston G, Gitterman L, Hirji Z, Lemieux C, Gardam M. Transmission of influenza A in human beings. Lancet Infect Dis. 2007;7(4):257–65. doi: 10.1016/S1473-3099(07)70029-4. [DOI] [PubMed] [Google Scholar]
- 16.Dawood FS, Jain S, Finelli L, Shaw MW, Lindstrom S, Garten RJ, et al. Emergence of a novel swine-origin influenza A (H1N1) virus in humans. N Engl J Med. 2009;360(25):2605–15. doi: 10.1056/NEJMoa0903810. [DOI] [PubMed] [Google Scholar]
- 17.Fry AM, Hancock K, Patel M, Gladden M, Doshi S, Blau DM, et al. The first cases of 2009 pandemic influenza A (H1N1) virus infection in the United States: a serologic investigation demonstrating early transmission. Influenza Other Respi Viruses. 2012 May;6(3):e48–53. doi: 10.1111/j.1750-2659.2012.00339.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Presidential Documents. Executive Order 13375 of April 1, 2005. Amendment to Executive Order 13295 relating to certain influenza viruses and quarantinable communicable diseases. [accessed 18.08.09];Federal Register. 2005 70(64):17299. Available at: http://edocket.access.gpo.gov/2005/pdf/05-6907.pdf. [Google Scholar]
- 19.US Department of Health and Human Services. Title 42, Part 71. Foreign quarantine. Washington, DC: Government Printing Office; 2003. [accessed 18.08.09]. Available at: http://www.access.gpo.gov/nara/cfr/waisidx_03/42cfr71_03.html. [Google Scholar]
- 20.US Department of Health and Human Services. Title 42, Part 70. Interstate quarantine. Washington, DC: Government Printing Office; 2003. [accessed 18.08.09]. Available at: http://www.access.gpo.gov/nara/cfr/waisidx_03/42cfr70_03.html. [Google Scholar]
- 21.Lee N, Chan PK, Hui DS, Rainer TH, Wong E, Choi KW, et al. Viral loads and duration of viral shedding in adult patients hospitalized with influenza. J Infect Dis. 2009;200(4):492–500. doi: 10.1086/600383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Witkop CT, Duffy MR, Macias EA, Gibbons TF, Escobar JD, Burwell KN, et al. Novel influenza A (H1N1) outbreak at the U.S. Air Force Academy: epidemiology and viral shedding duration. Am J Prev Med. 2010;38(2):121–6. doi: 10.1016/j.amepre.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 23.World Health Organization. [accessed 10.11.09];WHO technical advice for case management of influenza A(H1N1) in air transport. 2009 May 13; Available at: http://www.who.int/ihr/travel/A(H1N1)_air_transport_guidance.pdf.
- 24.Hancock K, Veguilla V, Lu X, Zhong W, Butler EN, Sun H, et al. Cross-reactive antibody responses to the 2009 pandemic H1N1 influenza virus. N Engl J Med. 2009;361(20):1945–52. doi: 10.1056/NEJMoa0906453. [DOI] [PubMed] [Google Scholar]
- 25.Veguilla V, Hancock K, Schiffer J, Gargiullo P, Lu X, Aranio D, et al. Sensitivity and specificity of serologic assays for detection of human infection with 2009 pandemic H1N1 virus in U.S. populations. J Clin Microbiol. 2011;49(6):2210–5. doi: 10.1128/JCM.00229-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bull K. Cabin air filtration: helping to protect occupants from infectious diseases. Travel Med Infect Dis. 2008;6(3):142–4. doi: 10.1016/j.tmaid.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 27.Cauchemez S, Donnelly CA, Reed C, Ghani AC, Fraser C, Kent CK, et al. Household transmission of 2009 pandemic influenza A (H1N1) virus in the United States. N Engl J Med. 2009;361(27):2619–27. doi: 10.1056/NEJMoa0905498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Follin P, Lindqvist A, Nystrom K, Lindh M. A variety of respiratory viruses found in symptomatic travellers returning from countries with ongoing spread of the new influenza A(H1N1) virus strain. Euro Surveill. 2009;14(24) doi: 10.2807/ese.14.24.19242-en. [DOI] [PubMed] [Google Scholar]
- 29.Monto AS, Gravenstein S, Elliott M, Colopy M, Schweinle J. Clinical signs and symptoms predicting influenza infection. Arch Intern Med. 2000;160(21):3243–7. doi: 10.1001/archinte.160.21.3243. [DOI] [PubMed] [Google Scholar]
- 30.Ong JB, Chen MI, Cook AR, Lee HC, Lee VJ, Lin RT, et al. Real-time epidemic monitoring and forecasting of H1N1-2009 using influenza-like illness from general practice and family doctor clinics in Singapore. PLoS ONE. 2010;5(4):e10036. doi: 10.1371/journal.pone.0010036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.France AM, Jackson M, Schrag S, Lynch M, Zimmerman C, Biggerstaff M, et al. Household transmission of 2009 influenza A (H1N1) virus after a school-based outbreak in New York City, April–May 2009. J Infect Dis. 2010;201(7):984–92. doi: 10.1086/651145. [DOI] [PubMed] [Google Scholar]
- 32.Morgan OW, Parks S, Shim T, Blevins PA, Lucas PM, Sanchez R, et al. Household transmission of pandemic (H1N1) 2009, San Antonio, Texas, USA, April-May 2009. Emerg Infect Dis. 2010;16(4):631–7. doi: 10.3201/eid1604.091658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Suess T, Buchholz U, Dupke S, Grunow R, an der Heiden M, Heider A, et al. Shedding and transmission of novel influenza virus A/H1N1 infection in households–Germany, 2009. Am J Epidemiol. 2010;171(11):1157–64. doi: 10.1093/aje/kwq071. [DOI] [PubMed] [Google Scholar]
- 34.Tsalik EL, Hendershot EF, Sangvai DG, Cunningham HM, Cunningham CK, Lopez-Marti MG, et al. Clinical presentation and response to treatment of novel influenza A H1N1 in a university-based summer camp population. J Clin Virol. 2010;47(3):286–8. doi: 10.1016/j.jcv.2009.12.012. [DOI] [PubMed] [Google Scholar]
- 35.Crum-Cianflone NF, Blair PJ, Faix D, Arnold J, Echols S, Sherman SS, et al. Clinical and epidemiologic characteristics of an outbreak of novel H1N1 (swine origin) influenza A virus among United States military beneficiaries. Clin Infect Dis. 2009;49(12):1801–10. doi: 10.1086/648508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lessler J, Reich NG, Cummings DA. Outbreak of 2009 pandemic influenza a (H1N1) at a New York City school. N Engl J Med. 2009;361(27):2628–36. doi: 10.1056/NEJMoa0906089. [DOI] [PubMed] [Google Scholar]
- 37.Swaan CM, Appels R, Kretzschmar ME, van Steenbergen JE. Timeliness of contact tracing among flight passengers for influenza A/H1N1 2009. BMC Infect Dis. 2011;11:355. doi: 10.1186/1471-2334-11-355. [DOI] [PMC free article] [PubMed] [Google Scholar]