Abstract
Apolipoprotein B Editing Complex (APOBEC3) family members are cytidine deaminases that play important roles in intrinsic responses to infection by retroviruses and have also been implicated in the control of other viruses such as parvoviruses, herpesviruses, papillomaviruses, hepatitis B virus and retrotransposons. While their direct effect on modification of viral DNA has been clearly demonstrated, whether they play additional roles in innate and adaptive immunity to viruses is less clear. Here we review the data regarding the various steps in the innate and adaptive immune response to virus infection in which APOBEC3 proteins have been implicated.
The genomes of mammals and other species contain many genes that restrict infection by viruses. Many antiviral intrinsic restriction factors were discovered through the identification of viral gene products that counteract their action. For example, Apolipoprotein B editing complex 3G (APOBEC3G), a cytidine deaminase (CDA) belonging to the activation-induced CDA (AIDCDA)/APOBEC gene family, was found because the human immunodeficiency virus (HIV)-1-encoded viral infectivity factor (Vif) blocks its activity (1, 6). Many host antiviral genes, including those in the APOBEC3 family, have undergone positive selection by infectious pathogens, resulting in polymorphisms in both regulatory and coding regions (2-5). In addition to the sequence heterogeneity found in polymorphic alleles within a given species, the number of APOBEC3 genes in different species varies due to expansion or contraction of the locus, ranging from 1 gene in mice and rats to 7 in primates (APOBEC3A, APOBEC3B, APOBEC3C, APOBECDE, APOBEC3F, APOBEC3G and APOBEC3H) (3, 5-10). Polymorphisms in the mouse Apobec3 gene have been linked to susceptibility to infection by murine retroviruses (11-13), while at least one polymorphic APOBEC3H allele (HapII) is believed to confer resistance to HIV-1 infection and disease progression; genome-wide association studies have also implicated other human APOBEC3 polymorphisms in HIV-induced disease (11, 14-19). Since HIV is a relatively recent virus in humans, it is likely that other infectious agents contributed to the positive selection of APOBEC3s.
APOBEC3 proteins inhibit replication of HIV-1 lacking vif, which encodes a 23kD protein (1, 20-23). In virus-producer cells, Vif binds APOBEC3D, APOBEC3F, APOBEC3G and APOBEC3H, targeting them for ubiquitinylation and degradation in the proteasome through interactions with a number of cellular factors, including CBF-β, Cul5 and elongins. This prevents APOBEC3 packaging and thereby overcomes the antiviral activity (24-30). In contrast, in producer cells infected with vif-deficient-HIV or with retroviruses that do not express a Vif protein, APOBEC3 proteins are packaged into virions via interaction with the nucleocapsid protein and viral RNA (31-35).
Once packaged, APOBEC3 proteins inhibit infection by deaminating deoxycytidine residues on minus strand DNA produced by reverse transcription, introducing G-to-A mutations in newly synthesized HIV-1 coding strand DNA. This leads to both degradation of reversed transcribed DNA prior to integration and to lethal G to A coding strand mutations in the integrated provirus (36). APOBEC3G most often deaminates C residues in CC motifs, while the other APOBEC3 proteins preferentially modify those found in CT motifs (20, 37, 38). Because APOBEC3G-induced mutations commonly occur in TGG motifs, deamination often converts the tryptophan codon TGG to a TAG stop codon; in contrast, the other human APOBEC3 proteins, as well as mouse APOBEC3, generate GAA or GA to AAA and AA mutations, which more frequently result in missense mutations (22, 39, 40). APOBEC3-mediated sub-lethal mutagenesis could lead to both drug-resistant and immune escape viruses and lethal mutations may result in the production of truncated viral proteins degraded by the cell’s proteolytic processing pathways, thereby increasing antigen presentation (see APOBEC3 proteins and Adaptive Immune Responses, below) (41).
APOBEC3 proteins also inhibit virus replication by CDA-independent mechanisms (42) (Fig. 1). For example, APOBEC3F lacking CDA activity is as effective as the wild type protein in inhibiting HIV-1 infection, at least in cultured cells (43). In vitro studies have suggested that APOBEC3 proteins inhibit elongation and accumulation of HIV-1, murine leukemia virus (MLV) and mouse mammary tumor virus (MMTV) reverse transcription products (44-46). APOBEC3 proteins also inhibit infection by parvoviruses, as well as retroelement mobilization, without extensively hypermutating their genomes (47-51).
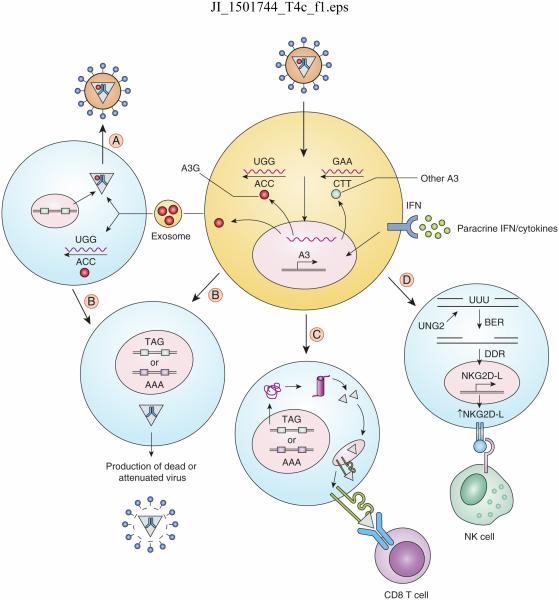
Figure 1.
Role of APOBEC3 proteins in anti-viral immunity. APOBEC3 proteins are ISGs, whose expression is induced by IFNs and other chemokines/cytokines produced by cells in response to infection. APOBEC3 molecules are either packaged into virions or produced by the target cell, leading to deamination of cytosine residues in viral reverse-transcribed DNA; this leads to the generation of stop codons, in the case of A3G (red molecule) or missense mutations with the other A3 proteins (blue molecule). Certain cells, such as B cells, may also shed APOBEC3 in exosomes, which can be transmitted to virus-infected cells and either be packaged into viruses (A) or deaminate viral reverse transcripts which then integrate into the genome. These cells, as well as cells directly infected with virions containing packaged A3 (B), produce dead or attenuated viruses. When APCs are infected (C), the introduction of mutations generates truncated or misfolded proteins, which are degraded by the proteasome and provide MHC-I epitopes leading to increased CTL responses and destruction of infected cells. In other cells (D), U residues in DNA generated by APOBEC3-mediated deamination are cleaved by UNG2, generating gaps that are acted upon by the cell’s base excision repair (BER) machinery, triggering a DNA damage response (DDR). This in turn induces increases NK ligand expression and NK-mediated killing of infected cells.
While virion-packaged proteins appear to restrict infection of T cells, APOBEC3 proteins expressed in human and mouse myeloid and dendritic cells also can restrict incoming HIV, MMTV and MLV by both CDA-dependent and -independent means (52-57). APOBEC3 proteins have also been implicated in hyper-mutating and restricting the hepadnavirus hepatitis B virus (HBV) and human papillomavirus (HPV) in hepatocytes and keratinocytes, respectively, as well as the herpes simplex virus-1 (HSV-1) and Epstein Barr virus (EBV) in established cell lines and primary patient samples (58-60); whether this is the result of target cell APOBEC3 activity or packaged viruses in the case of these other virus families is not known.
APOBEC3 expression in cells of the immune system
The different APOBEC3 proteins are expressed to varying levels in hematopoietic cell populations, including CD4+ and CD8+ T cell subsets (e.g. naïve and memory), B cells, and myeloid cells (55, 61, 62). CD4+ T cell expression of APOBEC3 proteins clearly plays a role in virus restriction. Analysis of the deamination motifs found in HIV-1 cDNA isolated from CD4+ T cells indicates that APOBEC3G is the dominant antiviral protein in this cell type (63). Moreover, HIV-1 proviral DNA hypermutation combined with increased APOBEC3G expression levels has been linked to lower virus replication and increased CD4+ T cells counts in patients (64-67), although individuals carrying the anti-HIV-1 APOBEC3H HapII allele also display lower levels of infection and higher CD4+ T cells counts compared to individuals carrying other APOBEC3H alleles (14). More recent work suggests that both cytidine deamination and reverse transcription inhibition play a role HIV-1 restriction in primary CD4+ T cells (63).
Macrophages are also major targets of infection by HIV and other retroviruses, particularly at mucosal surfaces, serving not only as latent reservoirs but also as antigen-presenting cells (APCs) (68). However, macrophages are relatively resistant to retrovirus infection due to the expression of not only APOBEC3 proteins but additional cell host restriction factors, such as SAM domain and HD domain-containing protein 1 (SAMHD1); the latter is believed to deplete the dNTP pools available to reverse transcriptase for viral cDNA synthesis (69-72). Macrophages express APOBEC3G, APOBEC3F and APOBEC3DE and their upregulation is IFNα-dependent, although APOBEC3G restricts HIV-1 more potently than the combined effect of APOBEC3F and APOBEC3DE in this cell type (62, 73). Target-cell expression in primary macrophages may also play a role in restricting HIV as well as other retroviruses (45, 54, 55). APOBEC3A is also expressed at the high levels in monocytes, while fully differentiated macrophages express low levels (74); however, macrophage expression of APOBEC3A is strongly enhanced by IFNα treatment (55, 62, 74). This difference in APOBEC3A levels between monocytes and macrophages correlates with susceptibility to HIV-1 infection, with monocytes being more resistant to infection than macrophages; when APOBEC3A was silenced in HIV-1-infected monocytes, virus production was increased (69, 74). APOBEC3G induction by IFNα in CD14+ monocytes is also significantly higher in HIV-1-exposed but seronegative individuals than in HIV-infected individuals or healthy controls (75). Only a small percentage (≤ 6%) of HIV-1 sequences from infected macrophages contain G to A hypermutations; thus, it is possible that APOBEC3 restriction of lentiviruses in macrophages occurs primarily by CDA-independent means (55).
Immature myeloid dendritic cells (DCs) can be productively infected by HIV-1; these cells may also be used by HIV-1 as a “Trojan Horse” to allow for the transmission of virus to the lymph nodes, thus bringing it in the proximity of T cells (76, 77). Transmission of virus from DCs to T cells likely occurs by direct cell-cell transmission via immunological synapses (78, 79). Myeloid DCs however support only a limited amount of HIV replication; at least in part, this has been attributed to cell host restriction factors such as APOBEC3 proteins. Activation of myeloid DCs by IFNα results in the potent upregulation of APOBEC3A, APOBEC3F, and APOBEC3G, without the induction of signals that lead to their maturation (80-82). Unlike macrophages, HIV-1 DNA in myeloid DCs is hypermutated upon IFNα treatment; transmission from myeloid DCs to T cells is significantly reduced compared to IFN-naïve cells (80).
B cell expression of APOBEC3G is higher than that seen in monocytes (62); nevertheless, because B cells lack the entry receptors required for HIV-1 infection, APOBEC3G is unlikely to play a role in cell-intrinsic restriction. B cells, however, are a major source of exosome production upon CD40L and IL4 stimulation (83). APOBEC3G has been shown to be a major exosomal component and exosomes bearing APOBEC3G can confer anti-HIV-1 activity to Jurkat T cells (84). APOBEC3G levels are upregulated upon treatment of B cells with a combination of agonists such as CD40L, IL-4 and anti-HLA- class II antibodies and autologous CD4+ T cells co-cultured with B cells activated with these agonists are significantly less infected by HIV-1 (85). This suggests that APOBEC3G derived from exosomes produced by activated B cells confers antiviral activity to CD4+ T cells (85). B cell-expressed APOBEC3 proteins may also act on other viruses, which infect this cell type, such as EBV (58).
APOBEC3 and the innate immune response
As discussed, APOBEC3 genes are interferon-stimulated genes (ISGs) whose expression is increased in response to various stimuli, including the ligands of Toll-like receptors TLR3, TLR4 and TLR7 (52, 80, 81, 86-90). Thus, the activation of innate immune responses that occurs upon infection with many different viruses could lead to increased APOBEC3 activity. This might especially be important for combating viruses acquired at mucosal sites of infection, where sentinel cell targets such as macrophages and dendritic cells are poised to mount such responses.
Expression of IFNs and other cytokines/chemokines is induced by virus infection, the result in part of cytosolic nucleic acid sensors that detect single- and double-stranded RNA and DNA (reviewed in (91)). Studies have shown that reverse-transcribed retroviral DNA is recognized by several sensors, including cyclic GMP-AMP synthase and members of the absent in melanoma 2 (AIM2)-like receptor (ALR) family that subsequently signal through the stimulator of interferon genes (STING) pathway (92-94). As described above, APOBEC3 proteins inhibit reverse transcription in addition to introducing CDA-induced mutations. APOBEC3-mediated inhibition of reverse transcriptions would thus seemingly limit cytosolic sensing and the IFN-induced antiviral response. Indeed, we recently showed that in mice, APOBEC3 in macrophages limits levels of retroviral reverse transcripts that trigger cytosolic sensing (94). However, reverse transcripts that escaped this blockade activate the STING pathway via cytosolic sensing and induce type 1 IFN expression. This in turn increased expression of ISGs like APOBEC3, reducing virus loads in vivo. Because human APOBEC3 proteins also block reverse transcription in sentinel cells and lentivirus reverse transcripts trigger STING via cytosolic sensing, it is likely that similar interactions between the two restriction pathways occur during HIV infection. Interestingly, DNA viruses like herpesviruses also induce IFN production via the cytosolic sensor-STING pathway and would be predicted to induce antiviral APOBEC3 expression that could then act on these viruses. This interplay between innate immune sensors and APOBEC3 is likely to afford multiple layers of protection to host against infection by multiple different virus families.
APOBEC3G has also been linked to increased destruction of HIV-1 infected cells by natural killer (NK) cells (95). Cells infected with HIV lacking or encoding defective Vifs unable to bind APOBEC3G have higher levels of the NKG2D ligands such as ULBPs and PLAP, resulting in more efficient NK cell-mediated lysis. The increase in lysis was linked to higher uracil content in HIV reverse transcripts caused by APOBEC3G-mediated cytidine deamination. Uracils are removed from DNA via cleavage by uracil-DNA glycosylase which then activates the base-excision-repair pathway. This in turn generates gaps or breaks in DNA and subsequently induces the DNA-damage response, a known inducer of NKG2D ligand expression (95). Thus, another antiviral function of APOBEC3G may be to recruit lymphocyte effectors such as NK cells, which in turn eliminate infected cells. Whether other human APOBEC3 proteins also affect NK recognition of HIV or other virus-infected cells remains to be determined.
APOBEC3 and the adaptive immune response
Clearly, APOBEC3 proteins play a role in the intrinsic/innate response to and the subsequent control of early virus infection. There is increasing evidence that APOBEC3 proteins also contribute to the adaptive immune response, namely by affecting the generation of cytotoxic T lymphocytes (CTLs) that recognize viral peptides and perhaps B cell production of antiviral antibodies. Because APOBEC3 expression is induced by virus infection of APCs, as discussed above, this may afford additional layers of host protection.
It is well-known that viruses causing persistent infection, like HIV-1, generate escape variants. HIV may take advantage of non-lethal mutations induced by APOBEC3 proteins to generate such CTL escape variants (96); APOBEC3G/F hotspots in the HIV genome are enriched in immunogenic CTL epitopes and deamination at these hotspots diminished CD8+ T cell responses to infected cells (39, 40). Thus, it may be beneficial for the virus to preserve APOBEC3G mutation hotspots at locations that influence the presentation and recognition of T cell epitopes and thereby aid in immune escape. In contrast, the host may strengthen its intrinsic ability to control HIV-1 infection by selecting for immune escape variants; there are known CD8 epitopes in Vif itself and Vif immune escape variants would be predicted to more weakly counteract APOBEC3s (97, 98).
CTL responses to viruses may also be increased through APOBEC3G-generated stop codons leading to premature truncation of proteins and degradation via antigen-processing pathways in HIV-1 infected cells (99, 100). Indeed, the virus-specific CTL response to APOBEC3G-expressing, HIV-infected APCs is increased compared with APCs that don’t express the CDA (100). Moreover, the activation of HIV-specific CTLs was stronger with vif-deficient HIV-infected APCs. However, a recent report that longitudinally followed pol and env sequences in a large cohort of patients chronically infected with HIV-1 found that the vast majority of the G-to-A mutations fell into the recognition motif for APOBEC3F and the other APOBEC3 proteins and not that of APOBEC3G (39). In this case, missense mutations in viral proteins could also lead to misfolding and protein degradation. Indeed, for many viruses including HIV-1, there is an inverse correlation between CTL response and viremia and disease progression (101, 102). Thus, the effects of APOBEC3 proteins on the CTL response can have a significant impact on retrovirus infection and evolution.
B cell production of virus-neutralizing antibodies may also be altered by APOBEC3 proteins. APOBEC3 knockout mice generate neutralizing antibodies against Friend murine leukemia virus (FV) with significantly slower kinetics than wild-type mice, although the responses in APOBEC3 knockout mice eventually become as high as those in wild type animals (13, 103) . This effect on the antiviral humoral immune response is likely due to APOBEC3-mediated suppression of virus infection at early times, thereby limiting the number of virus-producing cells in the early stages of infection. This may reduce antigen load and the induction of immune tolerance or prevent FV-mediated damage of critical hematopoietic lineage cells required for generating an immune response (103, 104).
APOBEC3 may play also a role in somatic hypermutation of antibodies. AID is the primary CDA involved in somatic hypermutation of immunoglobulin genes that results in the diverse repertoire of antibodies needed to clear infections (105). One study found that the Ig heavy-chain (IgH) sequences of FV-specific monoclonal antibodies derived from APOBEC3 knockout mice had significantly lower levels of C-to-T and G-to-A somatic hypermutation and lower virus-binding ability than those from wild type mice (106). However, it is not clear that Ig somatic mutations are critical for the control of FV, since AID knockout mice produce virus-neutralizing IgM antibodies and control infection (107). Nevertheless, since the human APOBEC3 protein APOBEC3A and APOBEC3B have been implicated in mutating genomic DNA, it is possible that they contribute to the development of neutralizing antibodies during virus infection (108, 109).
Conclusions
Organisms adapt to infectious agents by developing protective responses and conversely, infectious agents develop adaptive countermeasures to these responses. Host defenses against infectious agents include various mechanisms of innate immunity (e.g. NK cells, the Toll-like receptors and interferons) and adaptive immunity (humoral and cell-mediated). APOBEC3 proteins have clear roles in conferring intrinsic host resistance to infection to different viruses due to their ability to directly act on viral genomes, thereby generating mutations or blocking viral nucleic acid synthesis. There is also a body of evidence, particularly in the case of retroviruses, that APOBEC3 deaminase activity plays additional anti-viral roles in innate and adaptive immunity. Whether these additional mechanisms operate in the anti-viral response to other virus families remains to be determined. Moreover, it will be important to demonstrate that these additional roles for APOBEC3 proteins function in the context of virus infection in vivo.
Acknowledgments
This work was supported by PHS grant R01-AI-085015, NIH T32-CA115299 and F32-AI100512 and a Mathilde Krim Fellowship in Basic Biomedical Research (amfAR 108993-57-RKHF).
References
- 1.Sheehy AM, Gaddis NC, Choi JD, Malim MH. Isolation of a human gene that inhibits HIV-1 infection and is suppressed by the viral Vif protein. Nature. 2002;418:646–650. doi: 10.1038/nature00939. [DOI] [PubMed] [Google Scholar]
- 2.Sobieszczyk ME, Lingappa JR, McElrath MJ. Host genetic polymorphisms associated with innate immune factors and HIV-1. Curr Opin HIV AIDS. 2011;6:427–434. doi: 10.1097/COH.0b013e3283497155. [DOI] [PubMed] [Google Scholar]
- 3.Ross SR. Are viruses inhibited by APOBEC3 molecules from their host species? PLoS Pathog. 2009;5:e1000347. doi: 10.1371/journal.ppat.1000347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Emerman M, Malik HS. Paleovirology--modern consequences of ancient viruses. PLoS Biol. 2010;8:e1000301. doi: 10.1371/journal.pbio.1000301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Daugherty MD, Malik HS. Rules of engagement: molecular insights from host-virus arms races. Annual review of genetics. 2012;46:677–700. doi: 10.1146/annurev-genet-110711-155522. [DOI] [PubMed] [Google Scholar]
- 6.Sawyer SL, Emerman M, Malik HS. Ancient adaptive evolution of the primate antiviral DNA-editing enzyme APOBEC3G. PLoS biology. 2004;2:E275. doi: 10.1371/journal.pbio.0020275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.OhAinle M, Kerns JA, Malik HS, Emerman M. Adaptive evolution and antiviral activity of the conserved mammalian cytidine deaminase APOBEC3H. J. Virol. 2006;80:3853–3862. doi: 10.1128/JVI.80.8.3853-3862.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Compton AA, Hirsch VM, Emerman M. The host restriction factor APOBEC3G and retroviral Vif protein coevolve due to ongoing genetic conflict. Cell host & microbe. 2012;11:91–98. doi: 10.1016/j.chom.2011.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sanville B, Dolan MA, Wollenberg K, Yan Y, Martin C, Yeung ML, Strebel K, Buckler-White A, Kozak CA. Adaptive evolution of Mus Apobec3 includes retroviral insertion and positive selection at two clusters of residues flanking the substrate groove. PLoS Pathog. 2010;6:e1000974. doi: 10.1371/journal.ppat.1000974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.LaRue RS, Andresdottir V, Blanchard Y, Conticello SG, Derse D, Emerman M, Greene WC, Jonsson SR, Landau NR, Lochelt M, Malik HS, Malim MH, Munk C, O'Brien SJ, Pathak VK, Strebel K, Wain-Hobson S, Yu XF, Yuhki N, Harris RS. Guidelines for naming nonprimate APOBEC3 genes and proteins. J Virol. 2009;83:494–497. doi: 10.1128/JVI.01976-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miyazawa M, Tsuji-Kawahara S, Kanari Y. Host genetic factors that control immune responses to retrovirus infections. Vaccine. 2008;26:2981–2996. doi: 10.1016/j.vaccine.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 12.Okeoma CM, Petersen J, Ross SR. Expression of murine APOBEC3 alleles in different mouse strains and their effect on mouse mammary tumor virus infection. J Virol. 2009;83:3029–3038. doi: 10.1128/JVI.02536-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Santiago ML, Montano M, Benitez R, Messer RJ, Yonemoto W, Chesebro B, Hasenkrug KJ, Greene WC. Apobec3 encodes Rfv3, a gene influencing neutralizing antibody control of retrovirus infection. Science. 2008;321:1343–1346. doi: 10.1126/science.1161121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ooms M, Brayton B, Letko M, Maio SM, Pilcher CD, Hecht FM, Barbour JD, Simon V. HIV-1 Vif adaptation to human APOBEC3H haplotypes. Cell Host Microbe. 2013;14:411–421. doi: 10.1016/j.chom.2013.09.006. [DOI] [PubMed] [Google Scholar]
- 15.An P, Bleiber G, Duggal P, Nelson G, May M, Mangeat B, Alobwede I, Trono D, Vlahov D, Donfield S, Goedert JJ, Phair J, Buchbinder S, O'Brien SJ, Telenti A, Winkler CA. APOBEC3G genetic variants and their influence on the progression to AIDS. J. Virol. 2004;78:11070–11076. doi: 10.1128/JVI.78.20.11070-11076.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.An P, Johnson R, Phair J, Kirk GD, Yu XF, Donfield S, Buchbinder S, Goedert JJ, Winkler CA. APOBEC3B deletion and risk of HIV-1 acquisition. J Infect Dis. 2009;200:1054–1058. doi: 10.1086/605644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reddy K, Winkler CA, Werner L, Mlisana K, Abdool Karim SS, Ndung'u T. APOBEC3G expression is dysregulated in primary HIV-1 infection and polymorphic variants influence CD4+ T-cell counts and plasma viral load. AIDS. 2010;24:195–204. doi: 10.1097/QAD.0b013e3283353bba. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kanari Y, Clerici M, Abe H, Kawabata H, Trabattoni D, Caputo SL, Mazzotta F, Fujisawa H, Niwa A, Ishihara C, Takei YA, Miyazawa M. Genotypes at chromosome 22q12-13 are associated with HIV-1-exposed but uninfected status in Italians. AIDS. 2005;19:1015–1024. doi: 10.1097/01.aids.0000174447.48003.dd. [DOI] [PubMed] [Google Scholar]
- 19.Duggal NK, Fu W, Akey JM, Emerman M. Identification and antiviral activity of common polymorphisms in the APOBEC3 locus in human populations. Virology. 2013;443:329–337. doi: 10.1016/j.virol.2013.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bishop KN, Holmes RK, Sheehy AM, Davidson NO, Cho SJ, Malim MH. Cytidine deamination of retroviral DNA by diverse APOBEC proteins. Curr Biol. 2004;14:1392–1396. doi: 10.1016/j.cub.2004.06.057. [DOI] [PubMed] [Google Scholar]
- 21.Wiegand HL, Doehle BP, Bogerd HP, Cullen BR. A second human antiretroviral factor, APOBEC3F, is suppressed by the HIV-1 and HIV-2 Vif proteins. EMBO J. 2004;23:2451–2458. doi: 10.1038/sj.emboj.7600246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liddament MT, Brown WL, Schumacher AJ, Harris RS. APOBEC3F properties and hypermutation preferences indicate activity against HIV-1 in vivo. Curr. Biol. 2004;14:1385–1391. doi: 10.1016/j.cub.2004.06.050. [DOI] [PubMed] [Google Scholar]
- 23.Zheng YH, Irwin D, Kurosu T, Tokunaga K, Sata T, Peterlin BM. Human APOBEC3F is another host factor that blocks human immunodeficiency virus type 1 replication. J. Virol. 2004;78:6073–6076. doi: 10.1128/JVI.78.11.6073-6076.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mehle A, Strack B, Ancuta P, Zhang C, McPike M, Gabuzda D. Vif overcomes the innate antiviral activity of APOBEC3G by promoting its degradation in the ubiquitin-proteasome pathway. J. Biol. Chem. 2004;279:7792–7798. doi: 10.1074/jbc.M313093200. [DOI] [PubMed] [Google Scholar]
- 25.Yu X, Yu Y, Liu B, Luo K, Kong W, Mao P, Yu XF. Induction of APOBEC3G ubiquitination and degradation by an HIV-1 Vif-Cul5-SCF complex. Science. 2003;302:1056–1060. doi: 10.1126/science.1089591. [DOI] [PubMed] [Google Scholar]
- 26.Xiao Z, Ehrlich E, Yu Y, Luo K, Wang T, Tian C, Yu XF. Assembly of HIV-1 Vif-Cul5 E3 ubiquitin ligase through a novel zinc-binding domain-stabilized hydrophobic interface in Vif. Virology. 2006;349:290–299. doi: 10.1016/j.virol.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 27.Marin M, Rose KM, Kozak SL, Kabat D. HIV-1 Vif protein binds the editing enzyme APOBEC3G and induces its degradation. Nat. Med. 2003;9:1398–1403. doi: 10.1038/nm946. [DOI] [PubMed] [Google Scholar]
- 28.Munk C, Jensen BE, Zielonka J, Haussinger D, Kamp C. Running Loose or Getting Lost: How HIV-1 Counters and Capitalizes on APOBEC3-Induced Mutagenesis through Its Vif Protein. Viruses. 2012;4:3132–3161. doi: 10.3390/v4113132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jager S, Kim DY, Hultquist JF, Shindo K, LaRue RS, Kwon E, Li M, Anderson BD, Yen L, Stanley D, Mahon C, Kane J, Franks-Skiba K, Cimermancic P, Burlingame A, Sali A, Craik CS, Harris RS, Gross JD, Krogan NJ. Vif hijacks CBF-beta to degrade APOBEC3G and promote HIV-1 infection. Nature. 2012;481:371–375. doi: 10.1038/nature10693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang W, Du J, Evans SL, Yu Y, Yu XF. T-cell differentiation factor CBF-beta regulates HIV-1 Vif-mediated evasion of host restriction. Nature. 2012;481:376–379. doi: 10.1038/nature10718. [DOI] [PubMed] [Google Scholar]
- 31.Huthoff H, Autore F, Gallois-Montbrun S, Fraternali F, Malim MH. RNA-dependent oligomerization of APOBEC3G is required for restriction of HIV-1. PLoS Pathog. 2009;5:e1000330. doi: 10.1371/journal.ppat.1000330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Apolonia L, Schulz R, Curk T, Rocha P, Swanson CM, Schaller T, Ule J, Malim MH. Promiscuous RNA binding ensures effective encapsidation of APOBEC3 proteins by HIV-1. PLoS Pathog. 2015;11:e1004609. doi: 10.1371/journal.ppat.1004609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Burnett A, Spearman P. APOBEC3G multimers are recruited to the plasma membrane for packaging into human immunodeficiency virus type 1 virus-like particles in an RNA-dependent process requiring the NC basic linker. J Virol. 2007;81:5000–5013. doi: 10.1128/JVI.02237-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Svarovskaia ES, Xu H, Mbisa JL, Barr R, Gorelick RJ, Ono A, Freed EO, Hu WS, Pathak VK. Human apolipoprotein B mRNA-editing enzyme-catalytic polypeptide-like 3G (APOBEC3G) is incorporated into HIV-1 virions through interactions with viral and nonviral RNAs. J. Biol. Chem. 2004;279:35822–35828. doi: 10.1074/jbc.M405761200. [DOI] [PubMed] [Google Scholar]
- 35.Okeoma CM, Lovsin N, Peterlin BM, Ross SR. APOBEC3 inhibits mouse mammary tumour virus replication in vivo. Nature. 2007;445:927–930. doi: 10.1038/nature05540. [DOI] [PubMed] [Google Scholar]
- 36.Cullen BR. Role and mechanism of action of the APOBEC3 family of antiretroviral resistance factors. J Virol. 2006;80:1067–1076. doi: 10.1128/JVI.80.3.1067-1076.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Beale RC, Petersen-Mahrt SK, Watt IN, Harris RS, Rada C, Neuberger MS. Comparison of the differential context-dependence of DNA deamination by APOBEC enzymes: correlation with mutation spectra in vivo. J Mol Biol. 2004;337:585–596. doi: 10.1016/j.jmb.2004.01.046. [DOI] [PubMed] [Google Scholar]
- 38.Langlois MA, Beale RC, Conticello SG, Neuberger MS. Mutational comparison of the single-domained APOBEC3C and double-domained APOBEC3F/G anti-retroviral cytidine deaminases provides insight into their DNA target site specificities. Nucleic acids research. 2005;33:1913–1923. doi: 10.1093/nar/gki343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Armitage AE, Deforche K, Welch JJ, Van Laethem K, Camacho R, Rambaut A, Iversen AK. Possible footprints of APOBEC3F and/or other APOBEC3 deaminases, but not APOBEC3G, on HIV-1 from patients with acute/early and chronic infections. J Virol. 2014;88:12882–12894. doi: 10.1128/JVI.01460-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim EY, Lorenzo-Redondo R, Little SJ, Chung YS, Phalora PK, Maljkovic Berry I, Archer J, Penugonda S, Fischer W, Richman DD, Bhattacharya T, Malim MH, Wolinsky SM. Human APOBEC3 induced mutation of human immunodeficiency virus type-1 contributes to adaptation and evolution in natural infection. PLoS Pathog. 2014;10:e1004281. doi: 10.1371/journal.ppat.1004281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jern P, Russell RA, Pathak VK, Coffin JM. Likely role of APOBEC3G-mediated G-to-A mutations in HIV-1 evolution and drug resistance. PLoS Pathog. 2009;5:e1000367. doi: 10.1371/journal.ppat.1000367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Newman EN, Holmes RK, Craig HM, Klein KC, Lingappa JR, Malim MH, Sheehy AM. Antiviral function of APOBEC3G can be dissociated from cytidine deaminase activity. Curr. Biol. 2005;15:166–170. doi: 10.1016/j.cub.2004.12.068. [DOI] [PubMed] [Google Scholar]
- 43.Holmes RK, Koning FA, Bishop KN, Malim MH. APOBEC3F can inhibit the accumulation of HIV-1 reverse transcription products in the absence of hypermutation: Comparisons with APOBEC3G. J. Biol. Chem. 2007;282:2587–2595. doi: 10.1074/jbc.M607298200. [DOI] [PubMed] [Google Scholar]
- 44.MacMillan AL, Kohli RM, Ross SR. APOBEC3 inhibition of mouse mammary tumor virus infection: the role of cytidine deamination versus inhibition of reverse transcription. J Virol. 2013;87:4808–4817. doi: 10.1128/JVI.00112-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stavrou S, Nitta T, Kotla S, Ha D, Nagashima K, Rein AR, Fan H, Ross SR. Murine leukemia virus glycosylated Gag blocks apolipoprotein B editing complex 3 and cytosolic sensor access to the reverse transcription complex. Proc Natl Acad Sci U S A. 2013;110:9078–9083. doi: 10.1073/pnas.1217399110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sanchez-Martinez S, Aloia AL, Harvin D, Mirro J, Gorelick RJ, Jern P, Coffin JM, Rein A. Studies on the restriction of murine leukemia viruses by mouse APOBEC3. PLoS ONE. 2012;7:e38190. doi: 10.1371/journal.pone.0038190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jonsson SR, LaRue RS, Stenglein MD, Fahrenkrug SC, Andresdottir V, Harris RS. The restriction of zoonotic PERV transmission by human APOBEC3G. PLoS One. 2007;2:e893. doi: 10.1371/journal.pone.0000893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Narvaiza I, Linfesty DC, Greener BN, Hakata Y, Pintel DJ, Logue E, Landau NR, Weitzman MD. Deaminase-independent inhibition of parvoviruses by the APOBEC3A cytidine deaminase. PLoS Pathog. 2009;5:e1000439. doi: 10.1371/journal.ppat.1000439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Muckenfuss H, Hamdorf M, Held U, Perkovic M, Lower J, Cichutek K, Flory E, Schumann GG, Munk C. APOBEC3 proteins inhibit human LINE-1 retrotransposition. J Biol Chem. 2006;281:22161–22172. doi: 10.1074/jbc.M601716200. [DOI] [PubMed] [Google Scholar]
- 50.Stenglein MD, Harris RS. APOBEC3B and APOBEC3F inhibit L1 retrotransposition by a DNA deamination-independent mechanism. J. Biol. Chem. 2006;281:16837–16841. doi: 10.1074/jbc.M602367200. [DOI] [PubMed] [Google Scholar]
- 51.Chen H, Lilley CE, Yu Q, Lee DV, Chou J, Narvaiza I, Landau NR, Weitzman MD. APOBEC3A is a potent inhibitor of adeno-associated virus and retrotransposons. Curr Biol. 2006;16:480–485. doi: 10.1016/j.cub.2006.01.031. [DOI] [PubMed] [Google Scholar]
- 52.Okeoma CM, Low A, Bailis W, Fan HY, Peterlin BM, Ross SR. Induction of APOBEC3 in vivo causes increased restriction of retrovirus infection. J Virol. 2009;83:3486–3495. doi: 10.1128/JVI.02347-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Low A, Okeoma CM, Lovsin N, de las Heras M, Taylor TH, Peterlin BM, Ross SR, Fan H. Enhanced replication and pathogenesis of Moloney murine leukemia virus in mice defective in the murine APOBEC3 gene. Virology. 2009;385:455–463. doi: 10.1016/j.virol.2008.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stavrou S, Crawford D, Blouch K, Browne EP, Kohli RM, Ross SR. Different modes of retrovirus restriction by human APOBEC3A and APOBEC3G in vivo. PLoS Pathog. 2014;10:e1004145. doi: 10.1371/journal.ppat.1004145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Koning FA, Goujon C, Bauby H, Malim MH. Target cell-mediated editing of HIV-1 cDNA by APOBEC3 proteins in human macrophages. J Virol. 2011;85:13448–13452. doi: 10.1128/JVI.00775-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vetter ML, D'Aquila RT. Cytoplasmic APOBEC3G restricts incoming Vif-positive human immunodeficiency virus type 1 and increases two-long terminal repeat circle formation in activated T-helper-subtype cells. J Virol. 2009;83:8646–8654. doi: 10.1128/JVI.00020-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Langlois MA, Kemmerich K, Rada C, Neuberger MS. The AKV murine leukemia virus is restricted and hypermutated by mouse APOBEC3. J Virol. 2009;83:11550–11559. doi: 10.1128/JVI.01430-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Suspene R, Aynaud MM, Koch S, Pasdeloup D, Labetoulle M, Gaertner B, Vartanian JP, Meyerhans A, Wain-Hobson S. Genetic editing of herpes simplex virus 1 and Epstein-Barr herpesvirus genomes by human APOBEC3 cytidine deaminases in culture and in vivo. J Virol. 2011;85:7594–7602. doi: 10.1128/JVI.00290-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vartanian JP, Guetard D, Henry M, Wain-Hobson S. Evidence for editing of human papillomavirus DNA by APOBEC3 in benign and precancerous lesions. Science. 2008;320:230–233. doi: 10.1126/science.1153201. [DOI] [PubMed] [Google Scholar]
- 60.Vartanian JP, Henry M, Marchio A, Suspene R, Aynaud MM, Guetard D, Cervantes-Gonzalez M, Battiston C, Mazzaferro V, Pineau P, Dejean A, Wain-Hobson S. Massive APOBEC3 editing of hepatitis B viral DNA in cirrhosis. PLoS Pathog. 2010;6:e1000928. doi: 10.1371/journal.ppat.1000928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Refsland EW, Stenglein MD, Shindo K, Albin JS, Brown WL, Harris RS. Quantitative profiling of the full APOBEC3 mRNA repertoire in lymphocytes and tissues: implications for HIV-1 restriction. Nucleic Acids Res. 2011;38:4274–4284. doi: 10.1093/nar/gkq174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Koning FA, Newman EN, Kim EY, Kunstman KJ, Wolinsky SM, Malim MH. Defining APOBEC3 expression patterns in human tissues and hematopoietic cell subsets. J Virol. 2009;83:9474–9485. doi: 10.1128/JVI.01089-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gillick K, Pollpeter D, Phalora P, Kim EY, Wolinsky SM, Malim MH. Suppression of HIV-1 infection by APOBEC3 proteins in primary human CD4(+) T cells is associated with inhibition of processive reverse transcription as well as excessive cytidine deamination. J Virol. 2013;87:1508–1517. doi: 10.1128/JVI.02587-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Land AM, Ball TB, Luo M, Pilon R, Sandstrom P, Embree JE, Wachihi C, Kimani J, Plummer FA. Human immunodeficiency virus (HIV) type 1 proviral hypermutation correlates with CD4 count in HIV-infected women from Kenya. J Virol. 2008;82:8172–8182. doi: 10.1128/JVI.01115-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jin X, Brooks A, Chen H, Bennett R, Reichman R, Smith H. APOBEC3G/CEM15 (hA3G) mRNA levels associate inversely with human immunodeficiency virus viremia. J Virol. 2005;79:11513–11516. doi: 10.1128/JVI.79.17.11513-11516.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pace C, Keller J, Nolan D, James I, Gaudieri S, Moore C, Mallal S. Population level analysis of human immunodeficiency virus type 1 hypermutation and its relationship with APOBEC3G and vif genetic variation. J Virol. 2006;80:9259–9269. doi: 10.1128/JVI.00888-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kieffer TL, Kwon P, Nettles RE, Han Y, Ray SC, Siliciano RF. G-->A hypermutation in protease and reverse transcriptase regions of human immunodeficiency virus type 1 residing in resting CD4+ T cells in vivo. J Virol. 2005;79:1975–1980. doi: 10.1128/JVI.79.3.1975-1980.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Koppensteiner H, Brack-Werner R, Schindler M. Macrophages and their relevance in Human Immunodeficiency Virus Type I infection. Retrovirology. 2012;9:82. doi: 10.1186/1742-4690-9-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Berger G, Durand S, Fargier G, Nguyen XN, Cordeil S, Bouaziz S, Muriaux D, Darlix JL, Cimarelli A. APOBEC3A is a specific inhibitor of the early phases of HIV-1 infection in myeloid cells. PLoS Pathog. 2011;7:e1002221. doi: 10.1371/journal.ppat.1002221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Laguette N, Rahm N, Sobhian B, Chable-Bessia C, Munch J, Snoeck J, Sauter D, Switzer WM, Heneine W, Kirchhoff F, Delsuc F, Telenti A, Benkirane M. Evolutionary and functional analyses of the interaction between the myeloid restriction factor SAMHD1 and the lentiviral Vpx protein. Cell Host Microbe. 2012;11:205–217. doi: 10.1016/j.chom.2012.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cassetta L, Kajaste-Rudnitski A, Coradin T, Saba E, Della Chiara G, Barbagallo M, Graziano F, Alfano M, Cassol E, Vicenzi E, Poli G. M1 polarization of human monocyte-derived macrophages restricts pre and postintegration steps of HIV-1 replication. AIDS. 2013;27:1847–1856. doi: 10.1097/QAD.0b013e328361d059. [DOI] [PubMed] [Google Scholar]
- 72.Lahouassa H, Daddacha W, Hofmann H, Ayinde D, Logue EC, Dragin L, Bloch N, Maudet C, Bertrand M, Gramberg T, Pancino G, Priet S, Canard B, Laguette N, Benkirane M, Transy C, Landau NR, Kim B, Margottin-Goguet F. SAMHD1 restricts the replication of human immunodeficiency virus type 1 by depleting the intracellular pool of deoxynucleoside triphosphates. Nat Immunol. 2012;13:223–228. doi: 10.1038/ni.2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chaipan C, Smith JL, Hu WS, Pathak VK. APOBEC3G restricts HIV-1 to a greater extent than APOBEC3F and APOBEC3DE in human primary CD4+ T cells and macrophages. J Virol. 2013;87:444–453. doi: 10.1128/JVI.00676-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Peng G, Greenwell-Wild T, Nares S, Jin W, Lei KJ, Rangel ZG, Munson PJ, Wahl SM. Myeloid differentiation and susceptibility to HIV-1 are linked to APOBEC3 expression. Blood. 2007;110:393–400. doi: 10.1182/blood-2006-10-051763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Biasin M, Piacentini L, Lo Caputo S, Kanari Y, Magri G, Trabattoni D, Naddeo V, Lopalco L, Clivio A, Cesana E, Fasano F, Bergamaschi C, Mazzotta F, Miyazawa M, Clerici M. Apolipoprotein B mRNA-editing enzyme, catalytic polypeptide-like 3G: a possible role in the resistance to HIV of HIV-exposed seronegative individuals. J Infect Dis. 2007;195:960–964. doi: 10.1086/511988. [DOI] [PubMed] [Google Scholar]
- 76.Cavrois M, Neidleman J, Kreisberg JF, Fenard D, Callebaut C, Greene WC. Human immunodeficiency virus fusion to dendritic cells declines as cells mature. J Virol. 2006;80:1992–1999. doi: 10.1128/JVI.80.4.1992-1999.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Coleman CM, Wu L. HIV interactions with monocytes and dendritic cells: viral latency and reservoirs. Retrovirology. 2009;6:51. doi: 10.1186/1742-4690-6-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chen BK. T cell virological synapses and HIV-1 pathogenesis. Immunol Res. 2012;54:133–139. doi: 10.1007/s12026-012-8320-8. [DOI] [PubMed] [Google Scholar]
- 79.Yamamoto T, Tsunetsugu-Yokota Y, Mitsuki YY, Mizukoshi F, Tsuchiya T, Terahara K, Inagaki Y, Yamamoto N, Kobayashi K, Inoue J. Selective transmission of R5 HIV-1 over X4 HIV-1 at the dendritic cell-T cell infectious synapse is determined by the T cell activation state. PLoS Pathog. 2009;5:e1000279. doi: 10.1371/journal.ppat.1000279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mohanram V, Skold AE, Bachle SM, Pathak SK, Spetz AL. IFN-alpha induces APOBEC3G, F, and A in immature dendritic cells and limits HIV-1 spread to CD4+ T cells. J Immunol. 2013;190:3346–3353. doi: 10.4049/jimmunol.1201184. [DOI] [PubMed] [Google Scholar]
- 81.Peng G, Lei KJ, Jin W, Greenwell-Wild T, Wahl SM. Induction of APOBEC3 family proteins, a defensive maneuver underlying interferon-induced anti-HIV-1 activity. J. Exp. Med. 2006;203:41–46. doi: 10.1084/jem.20051512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Peng G, Greenwell-Wild T, Nares S, Jin W, Lei KJ, Rangel ZG, Munson PJ, Wahl SM. Myeloid differentiation and susceptibility to HIV-1 are linked to APOBEC3 expression. Blood. 2007;110:393–400. doi: 10.1182/blood-2006-10-051763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.McLellan AD. Exosome release by primary B cells. Crit Rev Immunol. 2009;29:203–217. doi: 10.1615/critrevimmunol.v29.i3.20. [DOI] [PubMed] [Google Scholar]
- 84.Khatua AK, Taylor HE, Hildreth JE, Popik W. Exosomes packaging APOBEC3G confer human immunodeficiency virus resistance to recipient cells. J Virol. 2009;83:512–521. doi: 10.1128/JVI.01658-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Seidl T, Whittall T, Babaahmady K, Lehner T. B-cell agonists up-regulate AID and APOBEC3G deaminases, which induce IgA and IgG class antibodies and anti-viral function. Immunology. 2012;135:207–215. doi: 10.1111/j.1365-2567.2011.03524.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wang X, Chao W, Saini M, Potash MJ. A common path to innate immunity to HIV-1 induced by Toll-like receptor ligands in primary human macrophages. PLoS One. 2011;6:e24193. doi: 10.1371/journal.pone.0024193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mehta HV, Jones PH, Weiss JP, Okeoma CM. IFN-alpha and lipopolysaccharide upregulate APOBEC3 mRNA through different signaling pathways. J Immunol. 2012;189:4088–4103. doi: 10.4049/jimmunol.1200777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chen K, Huang J, Zhang C, Huang S, Nunnari G, Wang FX, Tong X, Gao L, Nikisher K, Zhang H. Alpha interferon potently enhances the anti-human immunodeficiency virus type 1 activity of APOBEC3G in resting primary CD4 T cells. J. Virol. 2006;80:7645–7657. doi: 10.1128/JVI.00206-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sarkis PT, Ying S, Xu R, Yu XF. STAT1-independent cell type-specific regulation of antiviral APOBEC3G by IFN-alpha. J. Immunol. 2006;177:4530–4540. doi: 10.4049/jimmunol.177.7.4530. [DOI] [PubMed] [Google Scholar]
- 90.Stopak KS, Chiu YL, Kropp J, Grant RM, Greene WC. Distinct patterns of cytokine regulation of APOBEC3G expression and activity in primary lymphocytes, macrophages, and dendritic cells. J. Biol. Chem. 2007;282:3539–3546. doi: 10.1074/jbc.M610138200. [DOI] [PubMed] [Google Scholar]
- 91.Goubau D, Deddouche S, Reis e Sousa C. Cytosolic sensing of viruses. Immunity. 2013;38:855–869. doi: 10.1016/j.immuni.2013.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lahaye X, Satoh T, Gentili M, Cerboni S, Conrad C, Hurbain I, El Marjou A, Lacabaratz C, Lelievre JD, Manel N. The capsids of HIV-1 and HIV-2 determine immune detection of the viral cDNA by the innate sensor cGAS in dendritic cells. Immunity. 2013;39:1132–1142. doi: 10.1016/j.immuni.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 93.Gao D, Wu J, Wu YT, Du F, Aroh C, Yan N, Sun L, Chen ZJ. Cyclic GMP-AMP synthase is an innate immune sensor of HIV and other retroviruses. Science. 2013;341:903–906. doi: 10.1126/science.1240933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Stavrou S, Blouch K, Kotla S, Bass A, Ross SR. Nucleic acid recognition orchestrates the anti-viral response to retroviruses. Cell Host Microbe. 2015;17:478–488. doi: 10.1016/j.chom.2015.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Norman JM, Mashiba M, McNamara LA, Onafuwa-Nuga A, Chiari-Fort E, Shen W, Collins KL. The antiviral factor APOBEC3G enhances the recognition of HIV-infected primary T cells by natural killer cells. Nat Immunol. 2011;12:975–983. doi: 10.1038/ni.2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Pillai SK, Wong JK, Barbour JD. Turning up the volume on mutational pressure: is more of a good thing always better? (A case study of HIV-1 Vif and APOBEC3) Retrovirology. 2008;5:26. doi: 10.1186/1742-4690-5-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Addo MM, Yu XG, Rosenberg ES, Walker BD, Altfeld M. Cytotoxic T-lymphocyte (CTL) responses directed against regulatory and accessory proteins in HIV-1 infection. DNA Cell Biol. 2002;21:671–678. doi: 10.1089/104454902760330219. [DOI] [PubMed] [Google Scholar]
- 98.Kiepiela P, Ngumbela K, Thobakgale C, Ramduth D, Honeyborne I, Moodley E, Reddy S, de Pierres C, Mncube Z, Mkhwanazi N, Bishop K, van der Stok M, Nair K, Khan N, Crawford H, Payne R, Leslie A, Prado J, Prendergast A, Frater J, McCarthy N, Brander C, Learn GH, Nickle D, Rousseau C, Coovadia H, Mullins JI, Heckerman D, Walker BD, Goulder P. CD8+ T-cell responses to different HIV proteins have discordant associations with viral load. Nat Med. 2007;13:46–53. doi: 10.1038/nm1520. [DOI] [PubMed] [Google Scholar]
- 99.Monajemi M, Woodworth CF, Zipperlen K, Gallant M, Grant MD, Larijani M. Positioning of APOBEC3G/F mutational hotspots in the human immunodeficiency virus genome favors reduced recognition by CD8+ T cells. PLoS One. 2014;9:e93428. doi: 10.1371/journal.pone.0093428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Casartelli N, Guivel-Benhassine F, Bouziat R, Brandler S, Schwartz O, Moris A. The antiviral factor APOBEC3G improves CTL recognition of cultured HIV-infected T cells. J Exp Med. 2010;207:39–49. doi: 10.1084/jem.20091933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Shankar P, Russo M, Harnisch B, Patterson M, Skolnik P, Lieberman J. Impaired function of circulating HIV-specific CD8(+) T cells in chronic human immunodeficiency virus infection. Blood. 2000;96:3094–3101. [PubMed] [Google Scholar]
- 102.Ogg GS, Jin X, Bonhoeffer S, Dunbar PR, Nowak MA, Monard S, Segal JP, Cao Y, Rowland-Jones SL, Cerundolo V, Hurley A, Markowitz M, Ho DD, Nixon DF, McMichael AJ. Quantitation of HIV-1-specific cytotoxic T lymphocytes and plasma load of viral RNA. Science. 1998;279:2103–2106. doi: 10.1126/science.279.5359.2103. [DOI] [PubMed] [Google Scholar]
- 103.Tsuji-Kawahara S, Chikaishi T, Takeda E, Kato M, Kinoshita S, Kajiwara E, Takamura S, Miyazawa M. Persistence of viremia and production of neutralizing antibodies differentially regulated by polymorphic APOBEC3 and BAFF-R loci in friend virus-infected mice. J Virol. 2010;84:6082–6095. doi: 10.1128/JVI.02516-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Takeda E, Tsuji-Kawahara S, Sakamoto M, Langlois MA, Neuberger MS, Rada C, Miyazawa M. Mouse APOBEC3 restricts friend leukemia virus infection and pathogenesis in vivo. J Virol. 2008;82:10998–11008. doi: 10.1128/JVI.01311-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Peled JU, Kuang FL, Iglesias-Ussel MD, Roa S, Kalis SL, Goodman MF, Scharff MD. The biochemistry of somatic hypermutation. Annu Rev Immunol. 2008;26:481–511. doi: 10.1146/annurev.immunol.26.021607.090236. [DOI] [PubMed] [Google Scholar]
- 106.Halemano K, Guo K, Heilman KJ, Barrett BS, Smith DS, Hasenkrug KJ, Santiago ML. Immunoglobulin somatic hypermutation by APOBEC3/Rfv3 during retroviral infection. Proc Natl Acad Sci U S A. 2014;111:7759–7764. doi: 10.1073/pnas.1403361111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kato M, Tsuji-Kawahara S, Kawasaki Y, Kinoshita S, Chikaishi T, Takamura S, Fujisawa M, Kawada A, Miyazawa M. Class switch recombination and somatic hypermutation of virus-neutralizing antibodies are not essential for control of friend retrovirus infection. J Virol. 2015;89:1468–1473. doi: 10.1128/JVI.02293-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Landry S, Narvaiza I, Linfesty DC, Weitzman MD. APOBEC3A can activate the DNA damage response and cause cell-cycle arrest. EMBO Rep. 2011;12:444–450. doi: 10.1038/embor.2011.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Burns MB, Temiz NA, Harris RS. Evidence for APOBEC3B mutagenesis in multiple human cancers. Nat Genet. 2013;45:977–983. doi: 10.1038/ng.2701. [DOI] [PMC free article] [PubMed] [Google Scholar]