Abstract
Increasing evidence, both functional and morphological, supports the concept of increased intestinal permeability as an intrinsic characteristic of type 1 diabetes (T1D) in both humans and animal models of the disease. Often referred to as a “leaky gut”, its mechanistic impact on the pathogenesis of T1D remains unclear. Hypotheses that this defect influences immune responses against antigens (both self and non-self) predominate, yet others argue hyperglycemia and insulitis may contribute to increased gut permeability in T1D. To address these complicated issues, we herein review the many conceptual role(s) for a leaky gut in the pathogenesis of T1D and suggest ways that if true, therapeutic interventions aimed at the gut-pancreas axis may prove promising for future therapeutic interventions.
Keywords: intestinal permeability, barrier function, diabetes, beta cell autoimmunity
Introduction
The gastrointestinal system, serving as the largest organ of the immune system and one providing an interface between the external environment and the host, has been linked with numerous autoimmune diseases (1). Included in any such listings of disease entities is type 1 diabetes (T1D) where a relationship between the gut and beta (β) cell autoimmunity has long been thought to influence the disorder’s pathogenesis (2–5). Studies of both animal models of T1D as well as humans with the disease have demonstrated the impact of environmental and dietary factors on the disorder’s incidence, gut leakiness, an altered microbiome, and a variety of concepts associated with alterations in immune regulation, supporting such an assertion.
Some seven years ago, well before the recent explosion in interest surrounding the role for the gut in T1D began, we proposed a novel concept…“The Perfect Storm hypothesis” in an attempt to address the question of how the gut influences the pathogenesis of T1D (2). With this notion, we proposed a complex interplay between intestinal microbiota, gut permeability and mucosal immunity, three facets that collectively were thought to form the unfortunate underpinnings for T1D development (2). In the time since first presenting this hypothesis, much in the way of supporting evidence has been obtained, even to the point where discussions of therapies targeting the gut for the purpose of preventing and/or delaying the development of T1D are occurring. In this mini-review, we will convey current thoughts regarding these issues and thereby consider the potential role for “gut leakiness” in the pathogenesis of T1D.
Gut leakiness in rodent models of T1D
Functional evidence
Gut leakiness, most commonly referred to as increased intestinal permeability, can be functionally evaluated by measurement of disaccharides and monosaccharides in urine following their oral administration. As early as 1999, investigations involving the spontaneously diabetic bio breeding (BB) rat model of T1D asserted an unusual degree of gastrointestinal permeability in the stomach, small intestine, and colon in this strain of animals following such testing (6). Interestingly, this increased gastric and small intestinal, but not colonic permeability appeared before the development of both insulitis and overt diabetes. Later studies demonstrated that high risk diabetes prone (BB-DP) rats had increased intestinal permeability in comparison to low risk diabetes resistant (BB-DR) rats even in the pre-diabetic phase, and also provided anatomical evidence of alterations in the tight junction protein claudin (7). In contrast, the gut infiltration by inflammatory cells (i.e., neutrophils) was much more predominant in BB-DP versus BB-DR rats, supporting the notion that increased intestinal permeability might trigger the inflammatory response in genetically-predisposed models (8).
However, the means (if any) by which this intestinal inflammation might lead to autoimmune insulitis remain unclear. It is reasonable to speculate that chronic inflammation may increase permeability to luminal antigens, thereby rendering it difficult to induce normal tolerance to orally delivered antigens and that this combined with a potential forerunner is capable of inducing the autoimmunity ultimately culminating in T1D. Deficiencies in gut natural killer (NK) cell number and function, which precedes diabetes onset in BB-DP rats (9), may also play a role in this multifaceted overall aberrancy of gut leakiness (Fig. 1).
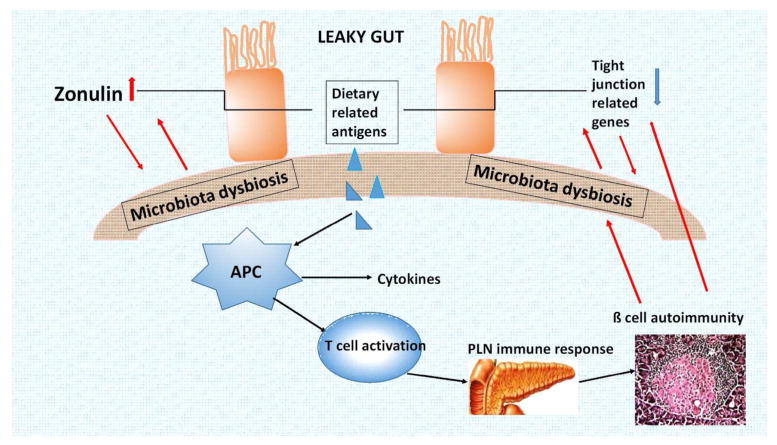
Figure 1.
With this figure, we propose a working hypothesis for the role of gut leakiness in T1D pathogenesis. The intercellular spaces between intestinal epithelials become wide (existence of leaky gut) in type 1 diabetes, due to zonulin up-regulation and tight junction related gene down-regulation. The increased permeability to lumenal antigens, on the one hand, makes it difficult to induce normal tolerance to orally delivered antigens; on the other hand, leads to gut microbiota dysbiosis. The microbiota imbalance, in turn, exacerbates gut leakiness. Dietary related antigens give rise to an autoimmune cascade, leading to β cell autoimmunity and insulitis, and the resulting upregulation of pro-inflammatory cytokines further aggravates intestinal barrier dysfunction, forming a vicious cycle and accelerating the development of T1D.
Structural evidence
Additional evidence supporting the concept of gut leakiness, albeit more indirect than the aforementioned functional studies, largely derives from histological and structural information including mucosal architecture, crypt length, expression levels of tight junction proteins, inflammatory infiltration status, intestinal peroxidase activity, glucagon-like peptide 1 (GLP-1) levels and mucin content (10). As a collective, these facets provide strong support to the notion that gut damage and dysfunction is an inherent characteristic of BB-DP rats.
Recently, a thorough study described both structural and functional aberrations affording increased intestinal permeability in young normoglycemic BB-DP rats, accompanied subsequently by a typical inflammatory reaction in the mucosa as well as loss of nitrergic neurons and motor function in gut (11). Altered small intestinal motor control has also been observed in diabetic BB-DP rats (12). With such a large number of gut-associated abnormalities, it is clear why we and others have offered the proposal that the origin of T1D might reside in the gut (5, 13).
Evidence from methods alleviating and aggravating gut permeability
The observation that gut leakiness precedes diabetes onset in BB-DP rats supports the potential for a cause-effect relationship between gut physiology/inflammation and the onset of T1D. The questions then become those of how and why. How might, in a sequential order, various gut aberrancies, inflammation result in disease, and why does this occur?
Dietary factors were amongst the first candidates demonstrating the ability to modulate gut permeability. Hydrolyzed casein diets, gluten-free diets, and oral administration of butyrate and glucagon-like peptide 2 (GLP-2), the latter two having the physiological ability to restore impaired intestinal barrier function, have been evaluated in both BB-DP rats and non-obese diabetic (NOD) mice. The impact of these interventions on disease development did, however, vary in terms of their effectiveness. Autoimmune diabetes was reduced by 50% with both hydrolyzed casein-diet (14, 15) and gluten-free diet (16). Yet, in vivo studies in these animal models using either butyrate (17) or GLP-2 (18) did not provided such pronounced effects. The reasons for inconsistency between such studies are unclear, but it would appear that the strongest protection from disease was observed with casein hydrolyte and thought to be through its influence on the intestinal barrier (19). Strikingly, even maternal gluten-free diets in NOD mice reduced inflammation and diabetes incidence in the offspring (20), with increased expression of tight junction-related genes in the gut and increased frequency of FoxP3 positive regulatory T cells in pancreas, suggesting that dietary gluten may influence the gut-level priming of immune cells capable of influencing autoimmunity against pancreatic β cells.
It is conceivable that regulation of gut barrier could protect or accelerate diabetes development. In this regard, cereal diets have been demonstrated to promote diabetes development in NOD mice in comparison to a hydrolyzed casein diet (15); although, no direct evidence on decreased gut permeability was observed. Experiments involving the administration of enteric bacterial pathogens to NOD mice, with or without gut barrier-disrupting effects, have further elaborated on the relationship between barrier function and immune activation. Specifically, infection with wild-type C. rodentium accelerated the development of insulitis in concert with infection-induced barrier disruption; whereas, insulitis development was not altered in NOD mice infected with the non-barrier-disrupting DeltaespF strain (21). Additional mechanistic studies also noted that activation and proliferation of pancreatic-draining lymph node T cells, especially diabetogenic CD8+ T cells, occurred after loss of intestinal barrier function and contributed to insulitis development. These findings indicate that interaction of the intestinal microbes with gut immune system is a critical pathogenic factor modifying progression to T1D in those genetically predisposed (22).
In sum, a collective of studies utilizing animal models of T1D indicate that the manifestation of autoimmune disease can be modified by factors that influence the gut immune system, thereby forming the basis for addressing similar questions in humans.
Gut leakiness in human patients with T1D
Functional evidence
Based, in part, on the aforementioned studies in animal models, both functional and histological aspects of the small bowel have been subject to investigation in human T1D, albeit such attempts have been limited in number. With respect to intestinal permeability, clearance of lactulose and mannitol has been investigated in patients with T1D with diverse results in terms of their outcome. An early study of 31 T1D patients showed an increase of intestinal permeability to monosaccharide mannitol, indicative of an injury of the integral surface mucosa (23). A subsequent study in pediatric T1D patients did not display any difference of gut permeability to lactulose and mannitol, except for in those patients with the HLA-DQB 1*02 allele (i.e., high-risk for celiac disease (CD)) who absorbed significantly more mannitol (24). The largest sample size study to date tested 81 subjects with islet autoimmunity (including preclinical, new-onset and long-term T1D) and found all such groups showed a similar increase in intestinal permeability to the disaccharide lactulose, indicative of a damaged barrier, relative to controls (25). However, a similar permeability to mannitol, indicative of an integral surface mucosa, was observed for all groups (25). These results have instilled some to suggest that this leakiness is a primary feature of T1D and perhaps the initial steps of evolution to celiac disease (23).
Intestinal biopsy evidence
Morphological and histological studies, though limited in number, have more readily associated gut leakiness with immunologic activity or immunoregulatory mechanisms. Patients with T1D, similar to newly detected CD, exhibited parallel expression of tight junction protein 1 (26). This finding might provide a good explanation for a second study which found that even structurally normal intestine in pediatric T1D patients without CD had enhanced immunologic activity, with increased expressions of HLA-DR, HLA-DP, and intercellular adhesion molecule-1, as well as increased frequency of IL-1α and IL-4-positive cells in the small intestinal mucosa (27). However, for T1D patients without intestinal symptoms, biopsy of gut has, in certain countries, been difficult to perform for ethical reasons; also, for patients with gut symptoms who had negative biopsies, new indicators might help to discern subclinical abnormalities. As such, some have questioned whether other biomarkers of inflammation and leakiness commonly used in intestinal diseases could be applied in T1D, as indirect measures.
Additional markers for intestinal permeability
Zonulin, the only human protein discovered to date that is known to reversibly regulate intestinal permeability by modulating intercellular tight junctions (28–30). In particular, zonulin upregulation seems to precede the onset of the T1D, providing a potential link between increased intestinal permeability, environmental exposure to non-self-antigens, and the development of autoimmunity in genetically susceptible individuals (31).
Other possible candidates, that mainly serve as biomarkers for intestinal inflammatory reactions, might also serve as new parameters for analysis. For example, calprotectin and S100A12, two members of the Ca2+-binding S100 family of proteins, have been investigated as a noninvasive marker of gut inflammation and were highly correlated with the activity of inflammatory bowel disease (32, 33). Serum and urine levels of claudin-3 and intestinal fatty acid binding protein (I-FABP) were also demonstrated to correlate with injury to the intestinal mucosa common to inflammatory bowel diseases (34). Therefore, these and other biomarkers (summarized in Table 1) should be further evaluated for their associations with T1D and as a potential means for identifying a leaky gut in those with the disease.
Table 1.
Factors that may be reflective of gut permeability and/or inflammation.
| Biomarker | Source | Indication | Comment |
|---|---|---|---|
| Calprotectin | Stool | Necrotizing enterocolitis, intestinal inflammation | High concentration in leukocytes |
| Endotoxin | Plasm | Endotoxemia due to translocation from intestine | Prone to high false positive results |
| Citrulline | Plasm | Intestinal surface area and overall correlation to function | |
| Claudin 3 | Urine; blood | Necrotizing enterocolitis | Tight junction protein, water soluble High levels found in impaired intestinal barrier |
| D-lactate | Plasm | Product of bacterial fermentation | function |
| Endotoxin core antibodies | Plasm | Detects anti-endotoxin immunoglobulins following translocation of gut derive endotoxins | Indirect information on gut barrier function |
| Interleukin-8 | Stool; blood | Important neutrophil chemoattractant found in several cell types including intestinal epithelium | |
| Intestinal fatty acid binding protein (IFAB) | Urine; blood | Necrotizing enterocolitis, intestinal epithelial breakdown and marker of intestinal ischemia | Marker of epithelial integrity. |
| Lactulose/Mannitol ratio | Urine | Permeability marker, primarily paracellular | Need to collect urine samples over time |
| 3-O-Methyglucose uptake | Urine | Absorptive function | Done in conjunction with lactulose/mannitol |
| Poly-R-478 | Blood; urine | Permeability marker | Correlated to C-14 PEG (large molecule) |
| S100A12 | Stool | Released from neutrophils | Damage associated molecule pattern (DAMP) molecule |
Potential factors that may contribute to a leaky gut
The role of dietary factors and microbiota
As discussed above for mice, specific diets, such as hydrolyzed casein and gluten-free diets, have the physiological ability to restore impaired intestinal barrier function in humans while cow's milk, which may contain adjuvant autoantigens, might have the opposite effect (35). These different effects of diet may, in part, be due to dietary effects on gut microbiota patterns, or instead, they may control intestinal inflammation through a mechanism involving GLP-2-driven improvement of gut permeability (36, 37) .
Emerging studies assessing the role of gut microbiome in T1D have, in fact, made reference to the potential influence on T1D susceptibility (38). Most of these studies, albeit limited, focus on the role of butyrate and/or lactate producing bacteria, as well as the role for short chain fatty acid ingestion influencing said bacteria, in turn influencing permeability (32). The interaction of gut microbiota and permeability had been emphasized in our and others previous reviews (39, 40).
The influence of age
As expected, results in BB-DP animal models demonstrated that intestinal permeability measured both in vivo and ex vivo decreased as age increased. Importantly, this trend existed in all rat strains, whether T1D prone BB-DP, T1D resistant BB-DR or wild-type Wistar rats. However, for humans, only healthy aging has been studied to address this question, and these efforts found that the small intestinal mucosal barrier did not deteriorate with age per se (41).
The role of maternal status
Maternal obesity and the influence of maternal antibiotic usage have both been evaluated for their influence on gut barrier in their offspring. NOD offspring from obese mothers had higher intestinal permeability and reduced villi/crypt ratios in the ileum (42). While theoretical, this enhanced gut permeability in offspring of obese mothers might predispose them to the development of T1D and other gut permeability-associated diseases. The use of antibiotic in mothers around parturition may also transiently modify maternal fecal microbiota and then selectively modulate colonic permeability in their offspring (43).
Other possible factors
The status of hyperglycemia might also be one of the factors influencing intestinal permeability. One clue emanates from studies suggesting increased intestinal permeability in T2D (44) where circulatory zonulin levels were significantly increased and positive correlations were seen with inflammatory markers as well as poor glycemic/lipid control (45). Moreover, the diabetic state could affect the innervation of the gut (46), a concept suggesting that abnormalities in glucose metabolism might contribute to altered permeability in T1D. Finally, a role for certain enteroviruses has been established in modulating intestinal permeability; although, inconsistent results have been displayed with or without enteroviruses detected in the intestine of patients with T1D (47–49).
Future directions and possible intervention trials
As noted in the introduction, evidence in support of a “leaky gut” in T1D has expanded, and while the issue is still debated, ongoing discussions are occurring regarding the potential efficacy of gut directed therapies for T1D prevention/reversal (50). Amongst the most discussed examples of such therapies are presented here.
Dietary intervention
Diets which are supposed to improve gut barrier, including hydrolyzed formula (51–54), delayed exposure to gluten (55), gluten-free foods (56), and omega-3 polyunsaturated fatty acids (57) have been implemented in both animal models and T1D patients for prevention and intervention. Sadly, to date, no definite benefit toward T1D prevention has been uncovered, yet their potential to disrupt the formation of disease associated autoantibodies remains in question.
Oral probiotic or antibiotic therapy
Both oral probiotics, such as Bifidobacterium and Lactobacillus species, as well as non-absorbable antibiotics inducing “gut decontamination” could potentially improve intestinal barrier function at multiple levels, as shown through animal studies (58, 59). Beyond this, probiotic or antibiotic administration has been shown to prevent diabetes development in NOD mice (60), with decreasing gut permeability and modulation of local immunity proposed as the primary mechanism of therapeutic action. These studies also provided evidence demonstrating that their effect on prevention was associated with an increased production of IL-10 from Peyer's patches and spleen, alongside of increased IL-10 expression in the pancreas (60). Taken collectively, this implies a gut–pancreas axis strategy might prove beneficial in T1D prevention or treatment.
GLP-1 based therapy
Given the optimistic impact of GLP-1 based therapy in T2D, this class of drugs has shown a promising effect in the preservation of beta cell function for T1D patients (61, 62). Although the exact mechanisms are unclear and should be multi-targeted, their action on gut microbiota and intestinal permeability may prove therapeutically beneficial.
Just like other intervention therapies, agents aiming at the leaky gut in NOD mice showed much more promising results than in humans. However, some specific diet and probiotics have shown beneficial results, although some with only with minimal effect (55, 56, 63). Considering certain aspects of the T1D related physiology in NOD mice might not be shared with humans, we could see a situation where responses to therapies may be somewhat inconsistent in terms of their translation. Hence, future longitudinal studies with regular follow-up of intestinal permeability and morphology, aiming primarily at beta cell function or T1D incidence would provide valuable evidence towards this issue.
Conclusions
Multiple studies suggest that aberrant functional integrity of the gut, as well as its impact on the immune system, in settings of T1D may indeed contribute to the development of this disease. These studies have encouraged the search for treatments interfering with this permeability for the purpose of T1D prevention. Our understanding of the function of the gut immune system in humans is, however, limited and the use of drugs (e.g., oral antigens or immune adjuvants) that modify the function of the gut immune system may not be benign. Therefore, while certainly justifiable as an area for active investigation, we would suggest that much more in the way of functional and mechanistic information must be derived before therapeutic interventions could proceed with confidence.
Acknowledgments
The authors wish to that Dr. Amanda Posgai for her exceptional editorial assistance in terms of developing this mini-review. The concepts underlying the opinions highlighted within this article were supported with funding provided by the National Insititues of Health (AI42288), JDRF, The American Diabetes Association, and the Jeffrey Keene Family Professorship.
Abbreviations
- T1D
type 1 diabetes
- β
beta
- BB-DP
bio breeding diabetes prone
- BB-DR
bio breeding diabetes resistant
- NK
natural killer
- GLP-1
glucagon-like peptide 1
- GLP-2
glucagon-like peptide 2
- NOD
non-obese diabetic
- Treg
regulatory T cells
- CD
celiac disease
- I-FABP
intestinal fatty acid binding protein
References
- 1.Fasano A. Leaky gut and autoimmune diseases. Clin Rev Allergy Immunol. 2012;42:71–8. doi: 10.1007/s12016-011-8291-x. [DOI] [PubMed] [Google Scholar]
- 2.Vaarala O, Atkinson MA, Neu J. The "perfect storm" for type 1 diabetes: the complex interplay between intestinal microbiota, gut permeability, and mucosal immunity. Diabetes. 2008;57:2555–62. doi: 10.2337/db08-0331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vaarala O. Leaking gut in type 1 diabetes. Curr Opin Gastroenterol. 2008;24:701–6. doi: 10.1097/MOG.0b013e32830e6d98. [DOI] [PubMed] [Google Scholar]
- 4.de Kort S, Keszthelyi D, Masclee AA. Leaky gut and diabetes mellitus: what is the link? Obes Rev. 2011;12:449–58. doi: 10.1111/j.1467-789X.2010.00845.x. [DOI] [PubMed] [Google Scholar]
- 5.Vaarala O. Is the origin of type 1 diabetes in the gut? Immunol Cell Biol. 2012;90:271–6. doi: 10.1038/icb.2011.115. [DOI] [PubMed] [Google Scholar]
- 6.Meddings JB, Jarand J, Urbanski SJ, Hardin J, Gall DG. Increased gastrointestinal permeability is an early lesion in the spontaneously diabetic BB rat. Am J Physiol. 1999;276:G951–7. doi: 10.1152/ajpgi.1999.276.4.G951. [DOI] [PubMed] [Google Scholar]
- 7.Neu J, Reverte CM, Mackey AD, Liboni K, Tuhacek-Tenace LM, Hatch M, et al. Changes in intestinal morphology and permeability in the biobreeding rat before the onset of type 1 diabetes. J Pediatr Gastroenterol Nutr. 2005;40:589–95. doi: 10.1097/01.mpg.0000159636.19346.c1. [DOI] [PubMed] [Google Scholar]
- 8.Hardin JA, Donegan L, Woodman RC, Trevenen C, Gall DG. Mucosal inflammation in a genetic model of spontaneous type I diabetes mellitus. Can J Physiol Pharmacol. 2002;80:1064–70. doi: 10.1139/y02-138. [DOI] [PubMed] [Google Scholar]
- 9.Todd DJ, Forsberg EM, Greiner DL, Mordes JP, Rossini AA, Bortell R. Deficiencies in gut NK cell number and function precede diabetes onset in BB rats. J Immunol. 2004;172:5356–62. doi: 10.4049/jimmunol.172.9.5356. [DOI] [PubMed] [Google Scholar]
- 10.Malaisse WJ, Courtois P, Scott FW. Insulin-dependent diabetes and gut dysfunction: the BB rat model. Horm Metab Res. 2004;36:585–94. doi: 10.1055/s-2004-825920. [DOI] [PubMed] [Google Scholar]
- 11.Vanuytsel T, Vanormelingen C, Vanheel H, Masaoka T, Salim Rasoel S, Tóth J, et al. From intestinal permeability to dysmotility: the biobreeding rat as a model for functional gastrointestinal disorders. PLoS One. 2014;9:e111132. doi: 10.1371/journal.pone.0111132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Demedts I, Masaoka T, Kindt S, De Hertogh G, Geboes K, Farré R, et al. Gastrointestinal motility changes and myenteric plexus alterations in spontaneously diabetic biobreeding rats. J Neurogastroenterol Motil. 2013;19:161–70. doi: 10.5056/jnm.2013.19.2.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vaarala O. The role of the gut in beta-cell autoimmunity and type 1 diabetes: a hypothesis. Pediatr Diabetes. 2000;1:217–25. doi: 10.1046/j.1399543x.2000.010408.x. [DOI] [PubMed] [Google Scholar]
- 14.Visser JT, Lammers K, Hoogendijk A, Boer MW, Brugman S, Beijer-Liefers S, et al. Restoration of impaired intestinal barrier function by the hydrolysed casein diet contributes to the prevention of type 1 diabetes in the diabetes-prone BioBreeding rat. Diabetologia. 2010;53:2621–8. doi: 10.1007/s00125-010-1903-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Patrick C, Wang GS, Lefebvre DE, Crookshank JA, Sonier B, Eberhard C, et al. Promotion of autoimmune diabetes by cereal diet in the presence or absence of microbes associated with gut immune activation, regulatory imbalance, and altered cathelicidin antimicrobial Peptide. Diabetes. 2013;62:2036–47. doi: 10.2337/db12-1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Funda DP, Kaas A, Bock T, Tlaskalová-Hogenová H, Buschard K. Gluten-free diet prevents diabetes in NOD mice. Diabetes Metab Res Rev. 1999;15:323–7. doi: 10.1002/(sici)1520-7560(199909/10)15:5<323::aid-dmrr53>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 17.Li N, Hatch M, Wasserfall CH, Douglas-Escobar M, Atkinson MA, Schatz DA, et al. Butyrate and type 1 diabetes mellitus: can we fix the intestinal leak? J Pediatr Gastroenterol Nutr. 2010;51:414–7. doi: 10.1097/MPG.0b013e3181dd913a. [DOI] [PubMed] [Google Scholar]
- 18.Hadjiyanni I, Li KK, Drucker DJ. Glucagon-like peptide-2 reduces intestinal permeability but does not modify the onset of type 1 diabetes in the nonobese diabetic mouse. Endocrinology. 2009;150:592–9. doi: 10.1210/en.2008-1228. [DOI] [PubMed] [Google Scholar]
- 19.Visser JT, Bos NA, Harthoorn LF, Stellaard F, Beijer-Liefers S, Rozing J, et al. Potential mechanisms explaining why hydrolyzed casein-based diets outclass single amino acid-based diets in the prevention of autoimmune diabetes in diabetes-prone BB rats. Diabetes Metab Res Rev. 2012;28:505–13. doi: 10.1002/dmrr.2311. [DOI] [PubMed] [Google Scholar]
- 20.Hansen CH, Krych L, Buschard K, Metzdorff SB, Nellemann C, Hansen LH, et al. A maternal gluten-free diet reduces inflammation and diabetes incidence in the offspring of NOD mice. Diabetes. 2014;63:2821–32. doi: 10.2337/db13-1612. [DOI] [PubMed] [Google Scholar]
- 21.Lee AS, Gibson DL, Zhang Y, Sham HP, Vallance BA, Dutz JP. Gut barrier disruption by an enteric bacterial pathogen accelerates insulitis in NOD mice. Diabetologia. 2010;53:741–8. doi: 10.1007/s00125-009-1626-y. [DOI] [PubMed] [Google Scholar]
- 22.Wen L, Ley RE, Volchkov PY, Stranges PB, Avanesyan L, Stonebraker AC, et al. Innate immunity and intestinal microbiota in the development of Type 1 diabetes. Nature. 2008;455:1109–13. doi: 10.1038/nature07336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carratù R, Secondulfo M, de Magistris L, Iafusco D, Urio A, Carbone MG, et al. Altered intestinal permeability to mannitol in diabetes mellitus type I. J Pediatr Gastroenterol Nutr. 1999;28:264–9. doi: 10.1097/00005176-199903000-00010. [DOI] [PubMed] [Google Scholar]
- 24.Kuitunen M, Saukkonen T, Ilonen J, Akerblom HK, Savilahti E. Intestinal permeability to mannitol and lactulose in children with type 1 diabetes with the HLA-DQB1*02 allele. Autoimmunity. 2002;35:365–8. doi: 10.1080/0891693021000008526. [DOI] [PubMed] [Google Scholar]
- 25.Bosi E, Molteni L, Radaelli MG, Folini L, Fermo I, Bazzigaluppi E, et al. Increased intestinal permeability precedes clinical onset of type 1 diabetes. Diabetologia. 2006;49:2824–7. doi: 10.1007/s00125-006-0465-3. [DOI] [PubMed] [Google Scholar]
- 26.Uibo R, Panarina M, Teesalu K, Talja I, Sepp E, Utt M, et al. Celiac disease in patients with type 1 diabetes: a condition with distinct changes in intestinal immunity? Cell Mol Immunol. 2011;8:150–6. doi: 10.1038/cmi.2010.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Westerholm-Ormio M, Vaarala O, Pihkala P, Ilonen J, Savilahti E. Immunologic activity in the small intestinal mucosa of pediatric patients with type 1 diabetes. Diabetes. 2003;52:2287–95. doi: 10.2337/diabetes.52.9.2287. [DOI] [PubMed] [Google Scholar]
- 28.Fasano A. Intestinal permeability and its regulation by zonulin: diagnostic and therapeutic implications. Clin Gastroenterol Hepatol. 2012;10:1096–100. doi: 10.1016/j.cgh.2012.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fasano A. Zonulin and its regulation of intestinal barrier function: the biological door to inflammation, autoimmunity, and cancer. Physiol Rev. 2011;91:151–75. doi: 10.1152/physrev.00003.2008. [DOI] [PubMed] [Google Scholar]
- 30.Watts T, Berti I, Sapone A, Gerarduzzi T, Not T, Zielke R, et al. Role of the intestinal tight junction modulator zonulin in the pathogenesis of type I diabetes in BB diabetic-prone rats. Proc Natl Acad Sci U S A. 2005;102:2916–21. doi: 10.1073/pnas.0500178102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sapone A, de Magistris L, Pietzak M, Clemente MG, Tripathi A, Cucca F, et al. Zonulin upregulation is associated with increased gut permeability in subjects with type 1 diabetes and their relatives. Diabetes. 2006;55:1443–9. doi: 10.2337/db05-1593. [DOI] [PubMed] [Google Scholar]
- 32.de Goffau MC, Luopajärvi K, Knip M, Ilonen J, Ruohtula T, Härkönen T, et al. Fecal microbiota composition differs between children with beta -cell autoimmunity and those without. Diabetes. 2013;62:1238–44. doi: 10.2337/db12-0526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pang T, Leach ST, Katz T, Day AS, Ooi CY. Fecal biomarkers of intestinal health and disease in children. Front Pediatr. 2014;2:6. doi: 10.3389/fped.2014.00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thuijls G, Derikx JP, van Wijck K, Zimmermann LJ, Degraeuwe PL, Mulder TL, et al. Non-invasive markers for early diagnosis and determination of the severity of necrotizing enterocolitis. Ann Surg. 2010;251:1174–80. doi: 10.1097/SLA.0b013e3181d778c4. [DOI] [PubMed] [Google Scholar]
- 35.Vaarala O. Is type 1 diabetes a disease of the gut immune system triggered by cow's milk insulin? Adv Exp Med Biol. 2005;569:151–6. doi: 10.1007/1-4020-3535-7_22. [DOI] [PubMed] [Google Scholar]
- 36.Cani PD, Possemiers S, Van de Wiele T, Guiot Y, Everard A, Rottier O, et al. Changes in gut microbiota control inflammation in obese mice through a mechanism involving GLP-2-driven improvement of gut permeability. Gut. 2009;58:1091–103. doi: 10.1136/gut.2008.165886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brugman S, Klatter FA, Visser JT, Wildeboer-Veloo AC, Harmsen HJ, Rozing J, et al. Antibiotic treatment partially protects against type 1 diabetes in the Bio-Breeding diabetes-prone rat. Is the gut flora involved in the development of type 1 diabetes? Diabetologia. 2006;49:2105–8. doi: 10.1007/s00125-006-0334-0. [DOI] [PubMed] [Google Scholar]
- 38.Alkanani AK, Hara N, Gottlieb PA, Ir D, Robertson CE, Wagner BD, et al. Alterations in Intestinal Microbiota Correlate with Susceptibility to Type 1 Diabetes. Diabetes. 2015 doi: 10.2337/db14-1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Atkinson MA, Chervonsky A. Does the gut microbiota have a role in type 1 diabetes? Early evidence from humans and animal models of the disease. Diabetologia. 2012;55:2868–77. doi: 10.1007/s00125-012-2672-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Clarke G, Stilling RM, Kennedy PJ, Stanton C, Cryan JF, Dinan TG. Minireview: Gut microbiota: the neglected endocrine organ. Mol Endocrinol. 2014;28:1221–38. doi: 10.1210/me.2014-1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Valentini L, Ramminger S, Haas V, Postrach E, Werich M, Fischer A, et al. Small intestinal permeability in older adults. Physiol Rep. 2014;2:e00281. doi: 10.14814/phy2.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xue Y, Wang H, Du M, Zhu MJ. Maternal obesity induces gut inflammation and impairs gut epithelial barrier function in nonobese diabetic mice. J Nutr Biochem. 2014;25:758–64. doi: 10.1016/j.jnutbio.2014.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Arnal ME, Zhang J, Erridge C, Smidt H, Lallès JP. Maternal antibiotic-induced early changes in microbial colonization selectively modulate colonic permeability and inducible heat shock proteins, and digesta concentrations of alkaline phosphatase and TLR-stimulants in swine offspring. PLoS One. 2015;10:e0118092. doi: 10.1371/journal.pone.0118092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Horton F, Wright J, Smith L, Hinton PJ, Robertson MD. Increased intestinal permeability to oral chromium (51 Cr) -EDTA in human Type 2 diabetes. Diabet Med. 2014;31:559–63. doi: 10.1111/dme.12360. [DOI] [PubMed] [Google Scholar]
- 45.Jayashree B, Bibin YS, Prabhu D, Shanthirani CS, Gokulakrishnan K, Lakshmi BS, et al. Increased circulatory levels of lipopolysaccharide (LPS) and zonulin signify novel biomarkers of proinflammation in patients with type 2 diabetes. Mol Cell Biochem. 2014;388:203–10. doi: 10.1007/s11010-013-1911-4. [DOI] [PubMed] [Google Scholar]
- 46.Spångéus A, Suhr O, El-Salhy M. Diabetic state affects the innervation of gut in an animal model of human type 1 diabetes. Histol Histopathol. 2000;15:739–44. doi: 10.14670/HH-15.739. [DOI] [PubMed] [Google Scholar]
- 47.Oikarinen M, Tauriainen S, Oikarinen S, Honkanen T, Collin P, Rantala I, et al. Type 1 diabetes is associated with enterovirus infection in gut mucosa. Diabetes. 2012;61:687–91. doi: 10.2337/db11-1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Oikarinen M, Tauriainen S, Honkanen T, Oikarinen S, Vuori K, Kaukinen K, et al. Detection of enteroviruses in the intestine of type 1 diabetic patients. Clin Exp Immunol. 2008;151:71–5. doi: 10.1111/j.1365-2249.2007.03529.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mercalli A, Lampasona V, Klingel K, Albarello L, Lombardoni C, Ekström J, et al. No evidence of enteroviruses in the intestine of patients with type 1 diabetes. Diabetologia. 2012;55:2479–88. doi: 10.1007/s00125-012-2591-4. [DOI] [PubMed] [Google Scholar]
- 50.Daft JG, Lorenz RG. Role of the gastrointestinal ecosystem in the development of type 1 diabetes. Pediatr Diabetes. 2015 doi: 10.1111/pedi.12282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Knip M, Akerblom HK, Becker D, Dosch HM, Dupre J, Fraser W, et al. Hydrolyzed infant formula and early beta-cell autoimmunity: a randomized clinical trial. Jama. 2014;311:2279–87. doi: 10.1001/jama.2014.5610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Akerblom HK, Virtanen SM, Ilonen J, Savilahti E, Vaarala O, Reunanen A, et al. Dietary manipulation of beta cell autoimmunity in infants at increased risk of type 1 diabetes: a pilot study. Diabetologia. 2005;48:829–37. doi: 10.1007/s00125-005-1733-3. [DOI] [PubMed] [Google Scholar]
- 53.Knip M, Virtanen SM, Seppa K, Ilonen J, Savilahti E, Vaarala O, et al. Dietary intervention in infancy and later signs of beta-cell autoimmunity. The New England journal of medicine. 2010;363:1900–8. doi: 10.1056/NEJMoa1004809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Paronen J, Knip M, Savilahti E, Virtanen SM, Ilonen J, Akerblom HK, et al. Effect of cow's milk exposure and maternal type 1 diabetes on cellular and humoral immunization to dietary insulin in infants at genetic risk for type 1 diabetes. Finnish Trial to Reduce IDDM in the Genetically at Risk Study Group. Diabetes. 2000;49:1657–65. doi: 10.2337/diabetes.49.10.1657. [DOI] [PubMed] [Google Scholar]
- 55.Hummel S, Pfluger M, Hummel M, Bonifacio E, Ziegler AG. Primary dietary intervention study to reduce the risk of islet autoimmunity in children at increased risk for type 1 diabetes: the BABYDIET study. Diabetes Care. 2011;34:1301–5. doi: 10.2337/dc10-2456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hummel M, Bonifacio E, Naserke HE, Ziegler AG. Elimination of dietary gluten does not reduce titers of type 1 diabetes-associated autoantibodies in high-risk subjects. Diabetes Care. 2002;25:1111–6. doi: 10.2337/diacare.25.7.1111. [DOI] [PubMed] [Google Scholar]
- 57.Norris JM, Yin X, Lamb MM, Barriga K, Seifert J, Hoffman M, et al. Omega-3 polyunsaturated fatty acid intake and islet autoimmunity in children at increased risk for type 1 diabetes. Jama. 2007;298:1420–8. doi: 10.1001/jama.298.12.1420. [DOI] [PubMed] [Google Scholar]
- 58.Hsieh CY, Osaka T, Moriyama E, Date Y, Kikuchi J, Tsuneda S. Strengthening of the intestinal epithelial tight junction by Bifidobacterium bifidum. Physiol Rep. 2015;3 doi: 10.14814/phy2.12327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ghosh SS, Bie J, Wang J, Ghosh S. Oral supplementation with non-absorbable antibiotics or curcumin attenuates western diet-induced atherosclerosis and glucose intolerance in LDLR−/− mice--role of intestinal permeability and macrophage activation. PLoS One. 2014;9:e108577. doi: 10.1371/journal.pone.0108577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Calcinaro F, Dionisi S, Marinaro M, Candeloro P, Bonato V, Marzotti S, et al. Oral probiotic administration induces interleukin-10 production and prevents spontaneous autoimmune diabetes in the non-obese diabetic mouse. Diabetologia. 2005;48:1565–75. doi: 10.1007/s00125-005-1831-2. [DOI] [PubMed] [Google Scholar]
- 61.Kielgast U, Holst JJ, Madsbad S. Antidiabetic actions of endogenous and exogenous GLP-1 in type 1 diabetic patients with and without residual beta -cell function. Diabetes. 2011;60:1599–607. doi: 10.2337/db10-1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kielgast U, Krarup T, Holst JJ, Madsbad S. Four weeks of treatment with liraglutide reduces insulin dose without loss of glycemic control in type 1 diabetic patients with and without residual beta-cell function. Diabetes Care. 2011;34:1463–8. doi: 10.2337/dc11-0096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ljungberg M, Korpela R, Ilonen J, Ludvigsson J, Vaarala O. Probiotics for the prevention of beta cell autoimmunity in children at genetic risk of type 1 diabetes--the PRODIA study. Ann N Y Acad Sci. 2006;1079:360–4. doi: 10.1196/annals.1375.055. [DOI] [PubMed] [Google Scholar]