Abstract
Introduction
Children with Down syndrome (DS) experience congenital and functional medical issues that predispose them to obstructive sleep apnea (OSA). Research utilizing stringent age criteria among samples of infants with DS and OSA is limited. This study examines clinical correlates of OSA among infants with DS.
Materials and Methods
A retrospective chart review was conducted of infants ≤6 months of age referred to a DS clinic at a tertiary children’s hospital over five-years (n=177). Chi-square tests and binary logistic regression models were utilized to analyze the data.
Results
Fifty-nine infants underwent polysomnography, based on clinical concerns. Of these, 95% (56/59) had studies consistent with OSA. Among infants with OSA, 71% were identified as having severe OSA (40/56). The minimum overall prevalence of OSA among the larger group of infants was 31% (56/177). Significant relationships were found between OSA and dysphagia, congenital heart disease (CHD), prematurity, gastroesophageal reflux disease (GERD), and other functional and anatomic gastrointestinal (GI) conditions. Results indicate that odds of OSA in this group are higher among infants with GI conditions in comparison to those without. Co-occurring dysphagia and CHD predicted the occurrence of OSA in 36% of cases with an overall predictive accuracy rate of 71%.
Discussion
Obstructive sleep apnea is relatively common in young infants with DS and often severe. Medical factors including GI conditions, dysphagia and CHD may help to identify infants who are at greater risk and may warrant evaluation. Further studies are needed to assess the impact of OSA in infants with DS.
Keywords: Down syndrome, obstructive sleep apnea, sleep disordered breathing, apnea-hypopnea index, polysomnography, dysphagia
INTRODUCTION
Down syndrome (DS), caused by a trisomy of all or part of human chromosome 21, is the most common chromosomal aneuploidy, and occurs in approximately 1 in 800 live births [Mai et al., 2013]. Individuals with DS experience high rates of congenital cardiac, gastrointestinal (GI), respiratory, sensory, orthopedic and oropharyngeal complications [Breslin et al., 2014; Cleves et al., 2007]. Children with DS also experience increased sleep disturbances [Stores and Stores, 2013]. Sleep-disordered breathing (SDB), particularly obstructive sleep apnea (OSA), has gained increased attention regarding its effects on children with DS [Carter et al., 2008; Freezer et al., 1995]. Sleep-disordered breathing consists of a continuum of disorders ranging from snoring to OSA [Mitchell and Kelly, 2006]. Obstructive sleep apnea is characterized by significant obstruction of the upper airway during sleep, resulting in apneic (complete cessation of airflow) and hypopneic (significantly reduced airflow) pauses, hypoventilation, hypercarbia, and hypoxemia [Iber, 2007]. Polysomnography (PSG) is the standard method for diagnosing OSA and requires interpretation by a board certified pediatric sleep specialist [Wise et al., 2011].
Among the general pediatric population, up to 4% of children experience OSA [Beck, 2009]. Children with DS are at an increased risk for SDB when compared to children without DS, with reported prevalence rates of 31% to 75% among various clinic-based samples [Breslin et al., 2014; Stores and Stores, 2013], while increased rates among referred samples have been documented [Churchill et al., 2012]. The paucity of literature specific to children with DS and OSA within narrowed age groups has also been documented [Churchill et al., 2012]. Existing studies outlining nocturnal sleep characteristics of children with DS combine data from birth to late adolescence and results exclusive to infants are difficult to tease out [Churchill et al., 2012]. This gap in research, paired with the 2011 modifications to health screening provisions for children with DS regarding OSA [Bull and The Committee on Genetics, 2011], demonstrates the utility of additional research regarding the prevalence of OSA in younger children.
Individuals with DS possess phenotypes that may predispose them to SDB including adenotonsillar hyperplasia, midfacial and mandibular hypoplasia, hypotonia, small upper airway, macroglossia, choanal atresia, and acute cranial base angle [de Miguel-Diez et al., 2003; Ng et al., 2006]. Children with DS are more likely to experience sleep abnormalities such as increased fragmentation, numerous awakenings and arousals, leg movements, and parasomnias, regardless of the presence of OSA [Levanon et al., 1999]. Congenital heart defects are the most prevalent congenital condition among children with DS, while GI and oropharyngeal malformations are also common [Cleves et al., 2007]. The prevalence of feeding complications among children with DS has also been established [Lin et al., 2014].
Longstanding, untreated childhood SDB can be serious, even fatal [Greene and Carroll, 1997], leading to complications in the cardiovascular, metabolic, and central nervous systems [Capdevila et al., 2008; O’Driscoll et al., 2012]. Pulmonary hypertension and cor pulmonale are among the most serious consequences of chronic hypoxia and respiratory acidosis from OSA [Breslin et al., 2014]. Cognitive development, behavior, daytime function, and quality of life are also adversely affected by SDB [Breslin et al., 2014; Mitchell and Kelly, 2006], and have been linked specifically to chronic hypoxic exposure [Bass, 2004]. Cognitive impairment associated with OSA has been linked to endothelial dysfunction due to hypoxemia, among other factors [Lal et al., 2012]. Daytime somnolence is a common result of SDB in adulthood [Katyal et al., 2013], while attention-deficit hyperactivity disorder has been associated with pediatric OSA [Huang et al., 2007]. Pediatric SDB has also been linked to sleep deprivation in parents of children with DS, resulting in reduced capacity to manage and maintain positive feelings towards children [Carter et al., 2008]. Parents of children with sleep disorders and developmental disabilities also report higher levels of maternal stress [Quine, 1991].
Until recently, screening children with DS for OSA before age 5 was uncommon [Roizen, 2003]. Current health supervision recommendations for pediatric patients with DS suggest discussing symptoms of OSA with parents by the age of 6 months, as well as overnight PSG for all children with DS by the age of four, due to the prevalence of SDB in this population [Bull and The Committee on Genetics, 2011]. Additionally, Rosen and colleagues [Rosen, 2010] propose frequent reassessment of infants with DS and OSA to monitor the necessity of ongoing interventions. Alternatives with limited efficacy such as parent questionnaires, audio and videotaping, overnight pulse oximetry, and portable monitoring have been used in lieu of overnight PSG due to its cost and limited availability. Parent reports have been found to underestimate the presence of OSA in children with DS [Lin et al., 2014; Ng et al., 2006; Shott et al., 2006], while audio and video taping cannot detect hypopneas or monitor airflow. Further, portable monitoring can detect moderate or severe apnea, but lacks utility as a screening device [Sinha and Guilleminault, 2010]. The goal of this study is to evaluate clinical correlates of OSA among young infants with DS at a specialty clinic.
MATERIALS AND METHODS
Participants
A retrospective review of medical charts was conducted with consent of the IUPUI and Clarian Institutional Review Boards. Infants referred to the Down Syndrome Program at Riley Hospital for Children were included in the study if they had a diagnosis of DS, were born between August 2005 and July 2010 and visited the program between the ages of birth to 6 months (corrected for prematurity, if indicated). Karyotypes showed DS was caused by trisomy 21 (93%), Robertsonian translocation (4%), mosaic trisomy 21 (1.5%) and unknown (1.5%, karyotype results not available, due to having been done at an outside institution). The Down Syndrome Program is a specialty consultation clinic which sees patients from throughout the state of Indiana, as well as from bordering regions of neighboring states of Kentucky, Michigan, Ohio, and Illinois. The primary clinic provides comprehensive medical, as well as developmental, consultation pertaining to the care of children birth to 21 with DS. During the study period, the clinic was staffed by three Developmental Pediatricians, Pediatric Nurse Practitioners, Dietitians, Social Workers and Family Support Staff. During a portion of the study period, a Pediatric Speech-Language Pathologist was also present for consultation. Physicians and Nurse Practitioners participated in medical assessments, including identifying clinical concerns regarding airway issues and ordering appropriate evaluations.
Chart Review
The chart review evaluated a wide array of parameters including structural cardiac defects, anatomic and functional GI conditions, feeding complications, structural and functional airway anomalies, and functional hearing issues. A total of 178 medical charts were reviewed for the initial analysis. One chart was eliminated due to insufficient information for this study. Fifty-nine infants underwent nap (n=55) and/or overnight (n=4) PSG due to symptoms of concern for sleep apnea or other airway compromise. Clinicians at this center considered ordering PSG for infants with neck hyperextension with sleep, snoring or noisy breathing with sleep and oxygen requirement for unclear indications. Indication for individual studies was not included as part of the overall data collection. Infants who did not complete PSG studies were not considered to have OSA (n=109) (see discussion). Nap PSG (≤4 hours in duration) was utilized to screen for the presence of OSA in the sample. PSG was performed at Riley Hospital for Children’s Sleep Disorders Center. The eight-room center is based in a quiet, remote location with limited windows within the institution. Studies were conducted using the Sandman Elite Version 9.1 (Embla Systems, Denver, CO, USA) and digitally recorded with the corresponding software package. Studies were then manually scored by a physician who was board-certified by the American Board of Sleep Medicine using the American Academy of Sleep Medicine (AASM) manual [Iber, 2007].
Infants were monitored by a respiratory therapist and sleep technician during the studies. Parents had the option of attending infants or waiting in nearby rooms. Parameters monitored during nap and nocturnal PSG included end-tidal carbon dioxide (CO2), digital pulse oximetry, respiratory rates, limb movement, chest and abdominal movement with thoracic and abdominal impedance belts, electrocardiogram, electrooculogram to identify rapid-eye movement sleep, electromyography to identify chin movement, electroencephalography and oronasal airflow. Sleep deprivation and sedation were not used.
Abnormal breathing was categorized based on the AASM manual [Iber, 2007]. Apneas were defined as complete cessation of airflow lasting two breaths, or ten seconds. Hypopneas were defined as a marked (50%) reduction in airflow and ≥4% desaturation for a period of two breaths, or ten seconds. Apnea-Hypopnea Index (AHI) was defined as the number of apneic plus the number of hypopneic events/hour during the study. Obstructive sleep apnea was defined by an AHI of 2 or greater. While the AASM manual defines OSA as AHI greater than or equal to 1, we defined obstructive sleep apnea as an AHI of 2 or greater [Iber, 2007]. Mild OSA was defined by reports from this lab as AHI between 2 and 5, moderate OSA was defined by AHI between 6 and 10, and severe OSA was defined by AHI levels of 10 or more. One patient in this study had an AHI that would have required classification as OSA (AHI 1.1) if AASM guidelines had been used. No information on central apnea was gathered.
Analyses
To identify possible predictors of the presence of OSA among infants with DS, chi-square tests and binary logistic regression were conducted using the Statistical Package for the Social Sciences (SPSS) Version 20.0 for Windows (Chicago, IL, USA). Chi-square tests were conducted to determine clinical correlates for OSA among the sample. Regression analysis was conducted to determine possible predictors for OSA among the sample. Of the 178 charts retrospectively reviewed, one case was omitted from the regression analysis due to insufficient information, which left 177 charts fit for analysis. The dependent, dichotomous variable was whether or not infants met the criteria for OSA. Predictor variables utilized in the regression analysis included gender, the presence of congenital heart disease (CHD), dysphagia, and the presence of GI conditions. CHD included atrial septal defects, ventricular septal defects, atrioventricular canal defects, tetralogy of Fallot, aortic coarctation, and patent ductus arteriosus. Dysphagia was defined as the occurrence of aspiration or significant pharyngeal penetration during feeding as identified on videofluoroscopic swallow study. Gastrointestinal conditions included duodenal atresia, duodenal stenosis, tracheoesophageal fistula, malrotation, and Hirschsprung disease. Gastroesophageal reflux disease (GERD) and constipation were not included as GI conditions in the regression analysis to avoid multicollinearity. Factors were entered into the analysis using the forward-LR method. Statistical significance was set at P < 0.05.
RESULTS
Of the 177 infants with DS seen in the Down Syndrome Program at Riley Hospital for Children, 59 (33%) infants underwent nap (n=55) and/or overnight (n=4) PSG due to symptoms of concern for sleep apnea or other airway compromise and 56 (95%) were diagnosed with OSA. The mean gestational age of all the infants was 37.5 ± 2.1 weeks (median age 38 weeks). With the review, several prominent health diagnoses emerged (Table I). Of the 56 number of patients identified as having OSA, 26 (47%) underwent bronchoscopy. Findings on bronchoscopy included pharyngomalacia (n=6, 23%), tracheomalacia (n=9, 35%), and laryngomalacia (n=21, 81%). Additionally, one patient was identified with unilateral choanal atresia and another patient was found to have a tracheal accessory bronchus and paralaryngeal cyst. Chi-square tests outlined significant (P<0.05) relationships between OSA and other health conditions (Table I). Gender was not found to be significantly related to OSA.
Table I.
Characteristics of Infants with DS Evaluated for Obstructive Sleep Apnea at the Down Syndrome Program at Riley Hospital for Children, August 2005 to December 2011
| Full Sample (n=177) | Infants with PSG (n=59) | Infants with no identified PSG (n=118) | |
|---|---|---|---|
| Mean Estimated Gestational Age (weeks) | 37.5 ± 2.1 | 37 ± 2.4 | 37.8 ± 1.9 |
| Male | n=93 | n=32 | n=61 |
| Female | n=84 | n=26 | n=58 |
| Prematurity1* | n=37 (21%) | n=18 (30%) | n=19 (16%) |
| CHD2* | n=73 (41%) | n=30 (50%) | n=43 (36%) |
| GI Abnormalities3*,** | n=102 (57%) | n=41 (69%) | n=61 (51%) |
| GERD4* | n=66 (37%) | n=29 (49%) | n=37 (31%) |
| Constipation | n=43 (24%) | n=21 (36%) | n=22 (19%) |
| Duodenal Atresia | n=10 (5%) | n=4 (7%) | n=6 (5%) |
| Duodenal Stenosis | n=3 (1%) | n=1 (2%) | n=2 (2%) |
| Tracheoesophageal Fistula | n=0 (0%) | n=0 (0%) | n=0 (0%) |
| Malrotation | n=3(1%) | n=2 (3%) | n=1 (1%) |
| Hirschsprung Disease | n=12 (7%) | n=6 (10%) | n=6 (5%) |
| Dysphagia* | n=89 (50%) | n=42 (71%) | n=47 (40%) |
Prematurity in estimated gestational age (weeks)
Congenital heart disease including atrial septal defects, ventricular septal defects, atrioventricular canal defects, tetralogy of Fallot, aortic coarctation, and patent ductus arteriosus
Includes the presence of one or more of the following structural or functional gastrointestinal abnormalities: gastroesophageal reflux disease, constipation, duodenal atresia, duodenal stenosis, tracheoesophageal fistula, malrotation, and Hirschsprung disease.
Gastroesophageal reflux disease
Denotes statistically significant correlate of obstructive sleep apnea, defined by AHI level of 2 or greater.
Denotes statistical significance of odds ratio P=0.002.
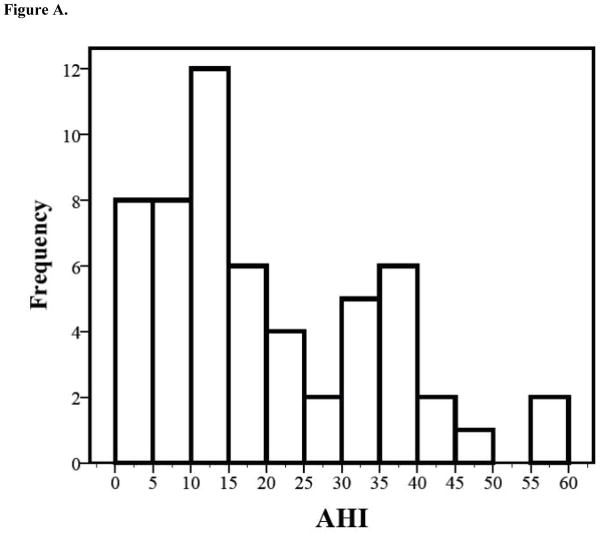
Among children who underwent PSG (n=59) the mean age was 44 ± 48 days (Table II). Fifty-six infants who completed PSG studies met the criteria for OSA (AHI ≥ 2) (95%). Additionally, 71% of infants with OSA met criteria for severe OSA as defined by AHI levels of 10 or more (n=40) (Figure A). The number of infants with OSA (n=56) compared to infants with negative PSG results (n=3) and infants with clinical features that were not indicative of OSA (n=109) yielded a prevalence rate of 31% of OSA among the sample from our clinic. Additionally, hypercapnia (average EtCO2 mean = 44.6 ± 7.9, range 33–80, n = 51; mean EtCO2 maximum = 58.5 ± 10.1; range = 44–98, n = 50.) and hypoxemia (average O2 mean = 90.6 ± 21.8, range 73–95, n = 56; mean O2 minimum = 72.9 ± 26, range = 60–97.8, n =56) were observed in the individuals in this study.
Table II.
Polysomnography Results of Infants with Down Syndrome Seen at the Down Syndrome Program at Riley Hospital for Children, August 2005 to December 2011
| All Infants (n=59)1 | Males (n=32) | Females (n=26) | |
|---|---|---|---|
| Average age at diagnosis (days)2 | 44 ± 48 | 34 ± 37 | 61 ± 57 |
| Total sleep time (minutes) | 150 ± 69 | 149 ± 63 | 151±78 |
| End-tidal CO2 mean (mm Hg) | 44 ± 8 | 46 ± 9 | 42 ± 6 |
| Mean Apnea Hypopnea Index | 19 ± 14 | 20 ±15 | 19 ±14 |
| OSA3 Criteria Not Met | 3 | 2 | 1 |
| Mild OSA (AHI 2–5) | 7 | 3 | 4 |
| Moderate OSA (AHI 6–10) | 9 | 4 | 5 |
| Severe OSA (AHI≥10) | 401 | 22 | 17 |
Missing gender for 1 infant that underwent polysomnography.
Age corrected for prematurity. Average female age at diagnosis skewed by ten infants in sample diagnosed very late (>100 days). Median female age at diagnosis was 58 days. Median male age at diagnosis was 20 days.
Obstructive sleep apnea defined by Apnea Hypopnea Index of 2 or more.
Figure A.
Infants with polysomnography results indicative of obstructive sleep apnea (OSA) (n=56) experienced varying degrees of apnea with marked variation occurring beyond the threshold for severe OSA (AHI≥10).
Binary logistic regression was conducted to identify the influence of four predictive variables (gender, CHD, GI complications, and dysphagia) on the criterion, OSA. Predictive variables were determined by analyses conducted prior to regression analysis to avoid multicollinearity, and excluded GERD and prematurity as possible predictive variables for the regression. The Hosmer-Lemeshow test was not significant at 0.135, which demonstrates the goodness-of-fit of our model to the population. The combination of dysphagia and CHD predicted the occurrence of OSA in 36% of cases with an overall positive predictive value of 71% (Wald= 17.19, df=1, P<0.001). Thus, co-occurring dysphagia and CHD were the strongest predictors of OSA in this group of infants. Further, odds ratios for correlates of OSA indicate that odds of OSA are significantly higher among infants with GI conditions when compared to infants without GI conditions (odds ratio, 2.92; 95% confidence interval, 1.75–4.09; P=0.002).
DISCUSSION
This study aimed to explore OSA among a sample of young infants with DS at a specialty clinic. The average female age at diagnosis was 58 days and was skewed by ten infants in the sample that were diagnosed very late (>100 days). Median male age at diagnosis was 20 days. Infants with OSA had an average AHI of 19 ± 14, which supports the relationship between severe OSA and young age established by de Miguel-Diez and colleagues [de Miguel-Diez et al., 2003]. However, children younger than one year were not included in the aforementioned study. Gender was not predictive of OSA, which aligns with findings reported by Shires and colleagues [Shires et al., 2010], yet contradicts other findings [de Miguel-Diez et al., 2003] that found males had higher rates of OSA. Our study indicated that infants with GI issues experienced the highest likelihood of also having OSA. The regression model that most accurately predicted the presence of OSA included co-occurring dysphagia and CHD. These findings suggest that infants with these comorbidities may warrant increased suspicion and referral for OSA evaluation.
Our study yielded correlations between OSA and prematurity, CHD, dysphagia, GERD, and other GI conditions among infants in our sample. Trisomy affects early development and in a mouse model has been linked to deficits in neural crest cells [Roper et al., 2009]. The neural crest are cellular precursors and important contributors to many developmental processes including to the oropharyngeal, respiratory and gastrointestinal structures. Additionally, a single trisomic gene may affect may affect myriad structures during development [Roper and Reeves, 2006]. A relationship between the presence and severity of OSA symptoms and GERD has been previously reported [Shepherd et al., 2011]. There have been a number of proposed mechanisms linking these two conditions including physical, neuromuscular and biochemical factors [Wasilewska et al., 2012]. Further, it has been noted that in patients with comorbid GERD and OSA, treating OSA with CPAP can reduce nocturnal GERD [Kerr et al., 1992; Shepherd et al., 2011; Tawk et al., 2006] and that treating GERD with proton pump inhibitors (PPI) can reduce obstructive respiratory events [Wasilewska et al., 2012]. The causal relationship between other GI abnormalities and OSA has not been well documented, but may share similar mechanisms to those proposed for the relationship between GERD and OSA. There is, likewise, a paucity of information regarding factors that could explain an association between CHD and OSA. A previous study looking at OSA in people with DS ranging in age from 2 weeks to 51 years failed to show an association between these factors [Marcus et al. 1991].
Outcomes of predictive studies of OSA are varied. Increased levels of severity of OSA have been associated with higher body-mass index (BMI) and age levels of children with DS [Dyken et al., 2003; Ng et al., 2006; Shires et al., 2010]. Conversely, some studies have not found linear relationships between BMI and severe OSA in pediatric samples without DS [Goroza et al., 2009]. Further, young age and male gender have been found to be predictive of OSA in one sample of children with DS [de Miguel-Diez et al., 2003].
Several limitations to the study exist, and may be taken into consideration to inform future research. First, we assumed that all of the infants who did not have a sleep study (PSG) ordered did not have OSA. The prevalence of OSA in our sample was 31% which aligns with other studies [Stores and Stores, 2013], but the true prevalence could be much higher as we had a limited number of nocturnal PSGs performed among a sample with a high prevalence (95%) of OSA among infants who underwent PSG. Second, it was not possible from our dataset to determine how many children were referred for PSG, but did not complete the study as recommended.
Another possible limitation of the study is the use of nap PSG. Nap PSG has been used as a screening tool for OSA, but may significantly underestimate prevalence and severity [Aurora et al., 2011]. Additionally, this is a referral population seen at a large tertiary care children’s hospital which could increase our estimate due to higher medical complexity of patients referred. As such, the results of this study provide information regarding OSA in a specialty clinic, but cannot be generalized to the population of patients with DS at large. The information herein is valuable and can be applied to other specialty clinics that see a large number of individuals with DS. Large scale registries of individuals with DS may help to achieve population based studies [Oster-Granite et al., 2011].
Lastly, the retrospective nature of this study limits follow-up information regarding subsequent treatment following the diagnosis of OSA. However, three patients underwent tracheostomy, five patients underwent supraglottoplasty, 34 patients required supplemental oxygen at some point during the time studied, and 40 patients utilized an apnea monitor. None of the patients in the current study were documented to utilize continuous positive airway pressure (CPAP) in the home setting. The aforementioned limitations may serve as helpful information to be considered for future clinical studies. Additional areas for further study would include the natural history of OSA in infants with DS and impact of early identification and treatment on future outcomes for these infants.
Our findings highlight the increased prevalence and comorbidities of OSA among young infants with DS in a specialty clinic. Obstructive sleep apnea may further impact health and development of infants with DS. These findings justify further study as well as the evaluation procedures and recommended screening for even very young infants with DS. Our findings support the AAP 2011 Health Supervision for Children with Down Syndrome recommendations to discuss with parents “at least once during the first 6 months of life, the symptoms of obstructive sleep apnea, including heavy breathing, snoring, uncommon sleep positions, frequent night awakening, daytime sleepiness, apneic pauses, and behavior problems that could be associated with poor sleep (and to) refer to a physician with expertise in pediatric sleep disorders for examination and further evaluation of a possible sleep disorder if any of the above mentioned symptoms occur” [Bull and Committee on Genetics, 2011]. Medical factors including prematurity, CHD, GERD, GI conditions, and dysphagia may also help to identify infants who are at greater risk for OSA and who may warrant evaluation.
Table III.
Effects of Dysphagia and Congenital Heart Disease on Obstructive Sleep Apnea1 among Infants with Down Syndrome Seen at the Down Syndrome Program at Riley Hospital for Children, August 2005 to December 2011
| B2 | S.E.2 | Wald2 | df2 | Sig.2 | Exp(B)2 | ||
|---|---|---|---|---|---|---|---|
| Step 13 | Dysphagia | −1.392 | 0.349 | 15.942 | 1 | 0.000* | 0.249 |
| Constant | −0.112 | 0.212 | 0.281 | 1 | 0.596 | 0.894 | |
| Step 24 | Congenital Heart Disease5 | −0.816 | 0.350 | 5.446 | 1 | 0.020 | 0.442 |
| Dysphagia | −1.492 | 0.360 | 17.190 | 1 | 0.000* | 0.225 | |
| Constant | 0.390 | 0.306 | 1.630 | 1 | 0.202 | 1.477 | |
Obstructive sleep apnea defined by Apnea Hypopnea Index of 2 or more.
B- regression coefficient for the constant; S.E.- standard error around coefficient for the constant; Wald chi-square- tests null hypothesis that the constant equals 0; df- degrees of freedom for Wald chi-square test; Sig.- null hypothesis rejected when p-value is <0.001; Exp(B)- odds ratio
Variable entered in regression analysis on step 1, dysphagia.
Variables entered in regression analysis on step 2, dysphagia and congenital heart disease. The combination of dysphagia and congenital heart disease demonstrates increased statistical significance in regard to the criterion, obstructive sleep apnea.
Including atrial septal defects, ventricular septal defects, atrioventricular canal defects, tetralogy of Fallot, aortic coarctation, and patent ductus arteriosus.
Denotes statistical significance.
Acknowledgments
Funding Source: NIH Grant, DE021034, NSF Grant, DGE0742475 and IUPUI Undergraduate Research Opportunity Grants. The funding sources had no involvement in the study design; the collection, analysis, and interpretation of data; the writing of the report; and the decision to submit the manuscript for publication.
Footnotes
Financial Disclosure: Ms. Goffinski and Dr. Roper have no conflicts of interest to disclose. Dr. Stanley discloses relationship with F. Hoffmann-La Roche Ltd. (Principal Investigator)
References
- Aurora R, Zak R, Karippot A, Lamm C, Morgenthaler T, Auerbach S, Bista S, Casey K, Chowdhuri S, Kristo D, Ramar K. Practice parameters for the respiratory indications for polysomnography in children. Sleep. 2011;34:379–388. doi: 10.1093/sleep/34.3.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bass JL. The effect of chronic or intermittent hypoxia on cognition in childhood: A review of the evidence. Pediatrics. 2004;114:805–816. doi: 10.1542/peds.2004-0227. [DOI] [PubMed] [Google Scholar]
- Beck S, Marcus CL. Pediatric polysomnography. Sleep Med Clin. 2009;4:393–406. doi: 10.1016/j.jsmc.2009.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breslin J, Spano G, Bootzin R, Anand P, Nadel L, Edgin J. Obstructive sleep apnea syndrome and cognition in Down syndrome. Dev Med Child Neurol. 2014;56:657–64. doi: 10.1111/dmcn.12376. [DOI] [PubMed] [Google Scholar]
- Bull MJ The Committee on Genetics. Health supervision for children with Down syndrome. Pediatrics. 2011;128:393–406. doi: 10.1542/peds.2011-1605. [DOI] [PubMed] [Google Scholar]
- Capdevila OS, Kheirandish-Gozal L, Dayyat E, Gozal D. Pediatric obstructive sleep apnea: Complications, management, and long-term outcomes. Proc Am Thorac Soc. 2008;5:274–282. doi: 10.1513/pats.200708-138MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter M, McCaughey E, Annaz D, Hill CM. Sleep problems in a Down syndrome population. Arch Dis Child. 2008;94:308–310. doi: 10.1136/adc.2008.146845. [DOI] [PubMed] [Google Scholar]
- Churchill SS, Kieckhefer GM, Landis CA, Ward TM. Sleep measurement and monitoring in children with Down syndrome: A review of the literature, 1960–2010. Sleep Med Rev. 2012;16:477–488. doi: 10.1016/j.smrv.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleves MA, Hobbs CA, Cleves PA, Tilford JM, Bird TM, Robbins JM. Congenital defects among liveborn infants with Down syndrome. Birth Defects Res A Clin Mol Teratol. 2007;79:657–663. doi: 10.1002/bdra.20393. [DOI] [PubMed] [Google Scholar]
- de Miguel-Diez J, Villa-Asensi JR, lvarez-Sala AJL. Prevalence of sleepdisordered breathing in children with Down syndrome: Polygraphic findings in 108 children. Sleep. 2003;26:1006–1009. doi: 10.1093/sleep/26.8.1006. [DOI] [PubMed] [Google Scholar]
- Dyken M, Lin-Dyken D, Poulton S, Zimmerman M, Sedars E. Prospective polysomnographic analysis of obstructive sleep apnea in down syndrome. Arch Pediatr Adolesc Med. 2003;157:655–660. doi: 10.1001/archpedi.157.7.655. [DOI] [PubMed] [Google Scholar]
- Freezer NJ, Bucens IK, Robertson CF. Obstructive sleep apnoea presenting as failure to thrive in infancy. J Paediatr Child Health. 1995;31:172–175. doi: 10.1111/j.1440-1754.1995.tb00779.x. [DOI] [PubMed] [Google Scholar]
- Goroza E, Sagy M, Sagy N, Bock K. Severity assessment of obstructive sleep apnea syndrome (OSAS) in pediatric patients. Clin Pediatr (Phila) 2009;48:528–533. doi: 10.1177/0009922809332584. [DOI] [PubMed] [Google Scholar]
- Greene M, Carroll J. Consequences of sleep-disordered breathing in childhood. Curr Opin Pulm Med. 1997;3:456–463. doi: 10.1097/00063198-199711000-00013. [DOI] [PubMed] [Google Scholar]
- Huang YS, Guilleminault C, Li HY, Yang CM, Wu YY, Chen NH. Attention-deficit/hyperactivity disorder with obstructive sleep apnea: a treatment outcome study. Sleep Med. 2007;8:18–30. doi: 10.1016/j.sleep.2006.05.016. [DOI] [PubMed] [Google Scholar]
- Iber C, editor. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specification. Westchester, IL: American Academy of Sleep Medicine; 2007. [Google Scholar]
- Katyal V, Pamula Y, Martin AJ, Daynes CN, Kennedy JD, Sampson WJ. Craniofacial and upper airway morphology in pediatric sleep-disordered breathing: Systematic review and meta-analysis. Am J Orthod Dentofacial Orthop. 2013;143:20–30. e23. doi: 10.1016/j.ajodo.2012.08.021. [DOI] [PubMed] [Google Scholar]
- Kerr P, Shoenut J, Millar T, Buckle P, Kryger M. Nasal CPAP reduces gastroesophageal reflux in obstructive sleep apnea syndrome. Chest. 1992;101:1539–1544. doi: 10.1378/chest.101.6.1539. [DOI] [PubMed] [Google Scholar]
- Lal C, Strange C, Bachman D. Neurocognitive impairment in obstructive sleep apnea. Chest. 2012;141:1601–1610. doi: 10.1378/chest.11-2214. [DOI] [PubMed] [Google Scholar]
- Levanon A, Tarasiuk A, Tal A. Sleep characteristics in children with Down syndrome. J Pediatr. 1999;134:755–760. doi: 10.1016/s0022-3476(99)70293-3. [DOI] [PubMed] [Google Scholar]
- Lin SC, Davey MJ, Horne RS, Nixon GM. Screening for obstructive sleep apnea in children with Down syndrome. J Pediatr. 2014;165:117–22. doi: 10.1016/j.jpeds.2014.02.032. [DOI] [PubMed] [Google Scholar]
- Mai CT, Kucik JE, Isenburg J, Feldkamp ML, Marengo LK, Bugenske EM, Thorpe PG, Jackson JM, Correa A, Rickard R, Alverson CJ, Kirby RS National Birth Defects Prevention N. Selected birth defects data from population-based birth defects surveillance programs in the United States, 2006 to 2010: featuring trisomy conditions. Birth Defects Res A Clin Mol Teratol. 2013;97:709–725. doi: 10.1002/bdra.23198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell RB, Kelly J. Behavior, neurocognition and quality-of-life in children with sleep-disordered breathing. Int J Pediatr Otorhinolaryngol. 2006;70:395–406. doi: 10.1016/j.ijporl.2005.10.020. [DOI] [PubMed] [Google Scholar]
- Ng D, Hui H, Chan C, Kwok K, Chow P, Cheung J. Obstructive sleep apnoea in children with Down syndrome. Singapore Med J. 2006;47:774–779. [PubMed] [Google Scholar]
- O’Driscoll D, Horne R, Davey M, Hope S, Anderson V, Trinder J, Walker A, Nixon G. Cardiac and sympathetic activation are reduced in children with down syndrome and sleep disordered breathing. Sleep. 2012;35:1269–1275. doi: 10.5665/sleep.2084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oster-Granite ML, Parisi MA, Abbeduto L, Berlin DS, Bodine C, Bynum D, Capone G, Collier E, Hall D, Kaeser L, Kaufmann P, Krischer J, Livingston M, McCabe LL, Pace J, Pfenninger K, Rasmussen SA, Reeves RH, Rubinstein Y, Sherman S, Terry SF, Whitten MS, Williams S, McCabe ER, Maddox YT. Down syndrome: national conference on patient registries, research databases, and biobanks. Mol Genet Metab. 2011;104:13–22. doi: 10.1016/j.ymgme.2011.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quine L. Sleep problems in children with mental handicap. J Ment Defic Res. 1991;35:269–290. doi: 10.1111/j.1365-2788.1991.tb00402.x. [DOI] [PubMed] [Google Scholar]
- Roizen NJ. The early interventionist and the medical problems of the child with Down syndrome. Infants Young Child. 2003;16:88–95. [Google Scholar]
- Roper RJ, Reeves RH. Understanding the basis for Down syndrome phenotypes. PLoS Genet. 2006;2:e50. doi: 10.1371/journal.pgen.0020050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roper RJ, VanHorn JF, Cain CC, Reeves RH. A neural crest deficit in Down syndrome mice is associated with deficient mitotic response to Sonic hedgehog. Mech Dev. 2009;126:212–219. doi: 10.1016/j.mod.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen D. Some infants with Down syndrome spontaneously outgrow their obstructive sleep apnea. Clin Pediatr. 2010;49:1068–1071. doi: 10.1177/0009922810378037. [DOI] [PubMed] [Google Scholar]
- Shepherd K, James A, Musk A, Hunter M, Hillman D, Eastwood P. Gastro-oesophageal reflux symptoms are related to the presence and severity of obstructive sleep apnoea. J Sleep Res. 2011;20:241–249. doi: 10.1111/j.1365-2869.2010.00843.x. [DOI] [PubMed] [Google Scholar]
- Shires CB, Anold SL, Schoumacher RA, Dehoff GW, Donepudi SK, Stocks RM. Body mass index as an indicator of obstructive sleep apnea in pediatric Down syndrome. Int J Pediatr Otorhinolaryngol. 2010;74:768–772. doi: 10.1016/j.ijporl.2010.03.050. [DOI] [PubMed] [Google Scholar]
- Shott S, Amin R, Chini B, Heubi C, Hotze S, Akers R. Obstructive sleep apnea: Should all children with Down syndrome be tested? Arch Otolaryngol Head Neck Surg. 2006;132:432–436. doi: 10.1001/archotol.132.4.432. [DOI] [PubMed] [Google Scholar]
- Sinha D, Guilleminault C. Sleep disordered breathing in children. Indian J Med Res. 2010;131:311–320. [PubMed] [Google Scholar]
- Stores G, Stores R. Sleep disorders and their clinical significance in children with Down syndrome. Dev Med Child Neurol. 2013;55:126–130. doi: 10.1111/j.1469-8749.2012.04422.x. [DOI] [PubMed] [Google Scholar]
- Tawk M, Goodrich S, Kinasewitz G, Orr W. The effect of 1 week of continuous positive airway pressure treatment in obstructive sleep apnea patients with concomitant gastroesophageal reflux. Chest. 2006;130:1003–1008. doi: 10.1378/chest.130.4.1003. [DOI] [PubMed] [Google Scholar]
- Wasilewska J, Semeniuk J, Cudowska B, Klukowski M, Debrowska K, Kamarski M. Respiratory response to proton pump inhibitor treatment in children with obstructive sleep apnea syndrome and gastroesophageal reflux disease. Sleep Med. 2012;13:824–830. doi: 10.1016/j.sleep.2012.04.016. [DOI] [PubMed] [Google Scholar]
- Wise M, Nichols C, Grigg-Damberger M, Marcus C, Witmans M, Kirk V, D’Andrea L, Hoban T. Respiratory indications for polysomnography in children: an evidence-based review. Sleep. 2011;34:398A–398AW. doi: 10.1093/sleep/34.3.389. [DOI] [PMC free article] [PubMed] [Google Scholar]