Abstract
Pediatric bipolar disorder is a severe mental illness whose pathophysiology is poorly understood and for which there is an urgent need for improved diagnosis and treatment. MR spectroscopy is a neuroimaging method capable of in vivo measurement of neurochemicals relevant to bipolar disorder neurobiology. MR spectroscopy studies of adult bipolar disorder provide consistent evidence for alterations in the glutamate system and mitochondrial function. In bipolar disorder, these 2 phenomena may be linked because 85% of glucose in the brain is consumed by glutamatergic neurotransmission and the conversion of glutamate to glutamine. The purpose of this article is to review the MR spectroscopic imaging literature in pediatric bipolar disorder, at-risk samples, and severe mood dysregulation, with a focus on the published findings that are relevant to glutamatergic and mitochondrial functioning. Potential directions for future MR spectroscopy studies of the glutamate system and mitochondrial dysfunction in pediatric bipolar disorder are discussed.
With an estimated lifetime prevalence of up to 5.1%,1 bipolar disorder (BD) is a disabling and often fatal brain disease characterized by recurrent episodes of depression and mania. In pediatric BD, the rate of attempted suicide is 40 times that of healthy adolescents,2 and BD is the diagnosis imparting the greatest risk for completed suicide.3 Adding to the morbidity and mortality imposed on patients and their families, the annual economic burden of BD in the United States is at least $151 billion.4 Despite decades of research, the underlying pathophysiology of BD across the life span is yet to be elucidated.5,6
The neurobiology of pediatric BD is of particular interest because up to 65% of patients with BD experience its onset before 19 years of age.7,8 Adolescence is the peak period for the first episode of mania,9 the mood state that defines the illness. In fact, the World Health Organization ranks BD as the fourth most disabling disease worldwide in persons between 10 and 24 years of age.10 Leverich et al11 found that childhood-onset BD is associated with a delay of first treatment of > 16 years. Expert consensus has identified the improved definition and diagnosis of pediatric BD, based on its underlying pathophysiology, as a critical barrier to progress in the field.12 The National Institute of Mental Health Strategic Plan13 and A Research Agenda for DSM-V14,15 both advocate attempts to discover neuroimaging biomarkers of BD. Thus, neuroimaging has an important role to play in translational research in pediatric BD.16–18
Converging lines of evidence implicate 2 related systems in the pathophysiology of BD: 1) alterations in glutamatergic neurotransmission,19–21 and 2) cerebral mitochondrial dysfunction.22–24 They are interdependent because >80% of all synapses are glutamatergic25 and approximately 85% of the energy derived from glucose in the brain is used to support glutamatergic neurotransmission and the conversion of glutamate (Glu) to glutamine (Gln).26,27
Mood-related alterations in cerebral bioenergetics would presumably have an impact on the glutamate system. Support for this is provided by the fact that inhibition of mitochondrial respiratory chain complexes I, III, and IV in an animal model of depression is reversed by administration of the N-methyl-D-aspartate glutamate receptor antagonist ketamine,28 a novel intervention for refractory BD.29,30 MR spectroscopy is a neuroimaging method capable of noninvasive interrogation of specific brain metabolites in vivo. Because it allows measurement of the chemical status of specific brain regions, MR spectroscopy is one potential method for establishing quantitative correlates of illness and treatment response in psychiatric conditions such as BD.31,32 Accordingly, MR spectroscopy has been used extensively in BD research to study both the glutamate system and brain bioenergetics. Two systematic reviews33,34 and 1 meta-analysis35 have each concluded that MR spectroscopy studies provide convincing evidence for glutamatergic abnormalities in BD. As reviewed by Stork and Renshaw,36 the MR spectroscopy literature in BD also provides consistent support for mitochondrial dysfunction. The promising nature of these findings has led to the conjecture that MR spectroscopy studies may represent “a pathway to diagnosis, novel therapeutics, and personalized medicine” in mood disorders.37
There is an urgent need for translational pediatric BD research, for the reasons enumerated above and because the data suggest that juvenile BD is continuous with adult BD.38–40 The MR spectroscopy literature in pediatric major depressive disorder was recently reviewed,41 but a review of MR spectroscopy studies of child and adolescent BD is lacking. The purpose of this article is to provide a companion review in pediatric BD, with particular attention paid to evidence for alterations in the glutamatergic system and mitochondrial dysfunction, and to discuss opportunities for further study.
MR Spectroscopy Measures Relevant to Bipolar Disorder
A technical description of MR spectroscopy methods for data acquisition and analysis is beyond the scope of this article, but excellent technical reviews are available.42,43 MR spectroscopy can be used to study a range of atomic nuclei that possess magnetic properties, including hydrogen (1H), phosphorus (31P), lithium (7Li), fluorine (19F), and carbon (13C).31 To date, the published pediatric BD literature has largely focused on 2 of these: 1H and 31P.
1H-MR Spectroscopy
The most common spectroscopic imaging method used in BD research is 1H-MR spectroscopy because the scans can be obtained on standard low-field clinical systems. Glutamatergic 1H-MR spectroscopy metabolites include Glu, Gln, γ-aminobutyric acid (GABA) and N-acetyl aspartylglutamate.44 At the magnetic field strengths used in clinical research, separation of the Glu and Gln resonance is unreliable42; however their combined peak (Glx) can be accurately quantified and therefore is most commonly reported. Although by convention Glx is defined as Glu + Gln, GABA may also contribute to the total Glx signal.45 However, when conventional MR spectroscopy methods are used, the contribution of GABA to Glx is considered very small.46
Significant findings in 1H-MR spectroscopy measures considered indicators of mitochondrial dysfunction in BD include the following: decreased NAA, decreased total creatine, increased total choline (tChol), increased Glx, and increased mIns.36 NAA is synthesized inside neuronal mitochondria from L-aspartate + acetyl coenzyme A by the enzyme L-aspartate-N-acetyl trans-ferase in an energy-dependent process, suggesting that decreased NAA concentrations are consistent with impaired mitochondrial bioenergetics.36,47 The concentration of NAA by in vivo 1H-MR spectroscopy methods is consistently higher than that found by careful 1H-nuclear MR analysis of freeze-clamped animal brain tissue, suggesting additional contributions to the “NAA” in vivo peak.48 The total creatine peak is composed of phosphocreatine (PCr), a temporal and spatial buffer of adenosine triphosphate (ATP), and creatine (PCr+Cre). tChol is a trimethylamine peak that is composed of phosphocholine, a membrane phospholipid precursor; glycerophosphocholine, a membrane phospholipid breakdown product; and choline, acetylcholine, carnitine, and acetyl-L-carnitine. The replicated finding of increased tChol in adult BD is hypothesized to be due to increased phospholipid turnover resulting from mitochondrial dysfunction.36 The Glx peak contains contributions from glutamate, glutamine, and γ-aminobutyric acid. The largest contributors to the Glu resonance are Glu in metabolic pathways and, to a much lesser degree, the neurotransmitter Glu. Increased Glx in BD is hypothesized to reflect Glu-induced neuronal hyperactivation,36 which places abnormally large demands on neuronal and glial energy metabolism.46
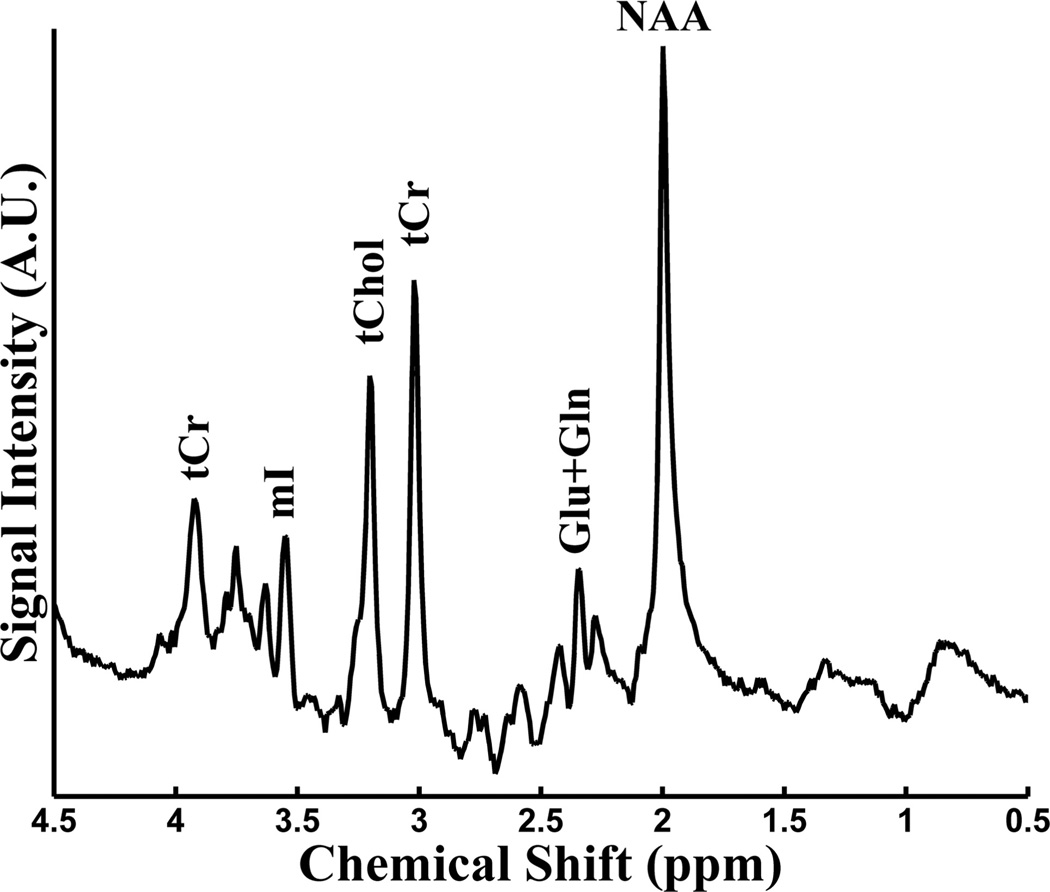
Notably, the classic medication lithium has a significant normalizing effect on Glx in BD.49 The mIns resonance consists primarily (>95%) of the cyclic sugar alcohol mIns, with minor contributions from inositol sugar phosphate compounds and glycine.50 The relevance of mIns to BD stems from its status as a potential indicator of altered membrane metabolism resulting from mitochondrial dysfunction36 and the fact that a decrease in mIns is associated with administration of the BD medication lithium.36 A representative 1H-MR spectroscopy spectrum is shown in Fig 1.
FIG 1.
Representative proton-1 MR spectroscopy (1H-MRS) spectrum acquired with parameters TR/TE = 2000/31 ms on a 3T MR imaging scanner.
31P MR Spectroscopy
Another MR spectroscopy technique used in BD research is 31P-MR spectroscopy, which requires specialized hardware and software, such as a dual-tuned 1H-31P radio-frequency coil, a broadband radio-frequency power amplifier, and customized pulse sequences. Investigator experience with 31P-MR spectroscopy pulse-sequence design and data processing/ analysis is also essential because MR imaging system manufacturers do not typically supply these tools. Yet 31P-MR spectroscopy may provide unique insights into BD neurobiology because it is a validated method for in vivo measurement of the ultimate mitochondrial process: ATP synthesis.51 Few 31P-MR spectroscopy studies of adult BD have been reported,52 and to date, there are just 3 published 31P-MR spectroscopy investigations in the pediatric BD literature.53–55 31P-MR spectroscopy measures relevant to mitochondrial function include phosphomonoesters, phosphodiesters, β-nucleoside triphosphate, PCr, inorganic phosphate (Pi), and intracellular pH.52 The phosphomonoesters signal contains contributions from the membrane precursors phos-phoethanolamine and phosphocholine, in addition to sugar and inositol phosphates.36 Phosphomonoesters are the building block precursors of neuronal membrane phospholipids. Both increased and decreased phosphomonoesters has been observed in studies of BD; thus, changes in the phosphomonoesters resonance may be state-dependent—with increased phosphomonoesters in depression and mania reflecting increased membrane phospholipid turnover, with decreased phosphomonoesters in subjects with euthymic BD possibly reflecting an opposite quiescence.36
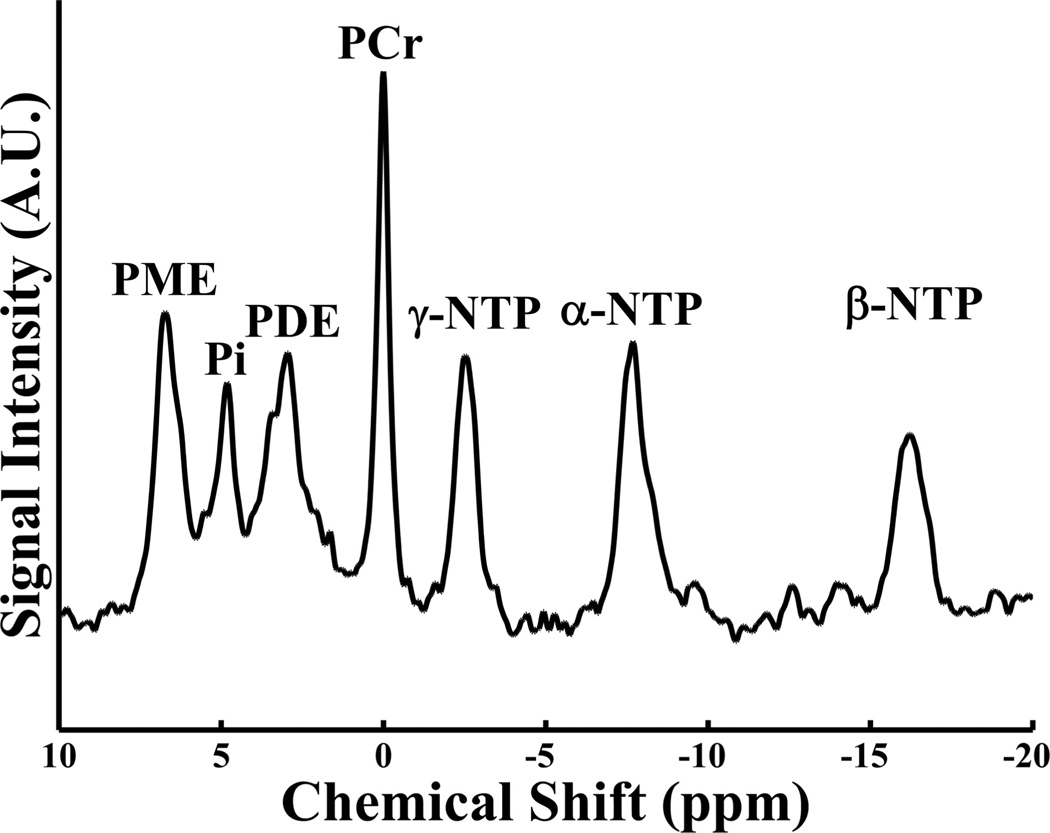
The phosphodiester signal, made up of contributions from glycerophosphocholine, glycerophosphoethanolamine, and mobile phospholipids, represents the breakdown products of phospholipid membranes.52 PCr is the buffer storage form of ATP and serves as the substrate reservoir for the creatine kinase reaction.56 In the mitochondria, this reaction reversibly converts PCr into ATP + creatine in a 1:1 molar ratio.57,58 Neuronal energy demands are met through a shift in reaction equilibrium, which is designed to maintain constant ATP concentrations.59–61 β-nucleoside triphosphate predominately measures β-ATP levels and is, therefore, used as a proxy measure of relative ATP concentrations. Pi and adenosine diphosphate are the products of the ATP hydrolysis reaction and are released when ATP is consumed. Intracellular pH can be calculated by using a modified Henderson-Has-selbalch equation and the resonance Pi relative to PCr.62 Figure 2 presents a representative 31P-MR spectroscopy spectrum.
FIG 2.
Representative phosphorus 31 MR spectroscopy (31P-MRS) spectrum acquired without proton decoupling on a 3T MR imaging scanner.
MATERIALS AND METHODS
Search Strategy
A computer-assisted literature search by using PubMed and MEDLINE data bases of the National Library of Medicine was conducted to identify reports focusing on pediatric BD samples studied with MR spectroscopy. The following terms were included in the search: “MR spectroscopy,” “bipolar disorder,” “pediatric or child or adolescent or juvenile or early-onset.” A backward search of bibliographic references from the identified references was performed to ensure inclusion of relevant articles; a forward citation search for identified studies was also performed. We also included reports from MR spectroscopy studies of at-risk youth who were the offspring of parents with BD and subjects with severe mood dysregulation,63 also known as disruptive mood dysregulation disorder,64 a new mental illness of childhood published in the Diagnostic and Statistical Manual of Mental Disorders, 5th ed,65 the diagnostic manual for US psychiatrists, in May 2013. All relevant articles published in English were included, and due to the small number of studies, no MR spectroscopy methodologic exclusion criteria were applied.
RESULTS
Overview of the Literature
The literature searches yielded 55 citations, of which 26 contained original neuroimaging acquired from subjects younger than 18 years of age. The sample characteristics, scanning acquisition methods, voxel location, and MR spectroscopy metabolite results are presented in Tables 1–4, and the key findings related to glutamatergic and mitochondrial function are summarized below. In addition to the modest number of reports and diversity of study methods and samples, this literature is in its infancy: The first MR spectroscopy study of pediatric BD was published in 2000. Despite this, the investigations conducted to date point to numerous important directions for further study.
Table 1.
Cross-sectional MRS studies of pediatric bipolar disorder
| Study | Sample | Methods (Device/FS/TR/TE/Sequence) | Region-of-lnterest Voxel Size and Location |
Findings |
|---|---|---|---|---|
| Castillo et al, 200066 | 10 Patients with BD, mood state not reported, unmedicated, (mean age, 8 yr), 10 non-aged-matched healthy volunteers |
Not stated/1.5T/3000/30/PRESS | 8 mL or 27 mL in the left and right frontal lobe, 8 mL or 27 mL in the left and right temporal lobe |
↑ Glx/tCre in BD in the frontal lobe and basal ganglia; No significant difference in NAA/tCre or tChol/tCre |
| Davanzo et al, 200178 | 11 Patients with BD, mixed state (n = 9), manic state (n = 2), medicated (n = 9), unmedicated (n = 2), (mean age, 11.4 yr), 11 age- and gender-matched healthy volunteers |
Signaa/1.5T/1500/135/PRESS | 8 mL in the anterior cingulate cortex |
Trend (P = .054) toward ↑ mIns/tCre in BD |
| Moore and Galloway, 200250 | 9 Youth with BD with mean serum lithium level = −0.87 mEq/L, mood state not reported, (mean age, 13.4 yr), 18 adults with BD with mean serum lithium level = 0.78 mEq/L, mood state not reported, (mean age, 37.3 yr) |
Signaa/1.5T/25,000/not stated/1D-ISIS Lithium-7 MRS was used to measure brain lithium concentrations in vivo |
60-mm axial section centered on the superior edge of the ventricles |
Mean brain lithium concentration = 0.52 mEq/L in pediatric patients with BD; mean brain lithium concentration = 0.92 mEq/L in adult patients with BD; lower brain-to-serum concentration ratiosb in youth with BD compared with adults: 0.58 vs 0.92 (P < .02); brain-to-serum concentration ratio correlated positively with age (r = 0.44; P < .02); formula for brain-to-serum lithium concentration ratiob; (brain lithium in mEq/L)/ (serum lithium in mEq/L) |
| Chang et al, 200370 | 15 Patients with BD with at least 1 parent with BD, mood state euthymic (n = 15), medicated (n = 14), unmedicated (n = 1), (mean age, 12.6 yr), 11 healthy volunteers (mean age, 12.6 yr) |
Signaa/3T/2000/35/PRESS | 8 mL in left and right dorsolateral prefrontal cortices |
↓ NAA/tCre in BD in the right dorsolateral prefrontal cortex (P < .02) |
| Davanzo et al, 200375 | 10 Patients with BD, mood state manic (n = 2), mood state mixed (n = 8), medicated (n = 7), unmedicated (n = 3), (mean age, 9.8 yr), 10 patients with intermittent explosive disorder, (mean age, 9.6 yr), 13 healthy volunteers, (mean age, 11.7 yr) |
Signaa/1.5T/3000/30/PRESS | 8 mL in the anterior cingulated cortex, 8 mL in the occipital cortex |
↑ mIns and mIns/tCre in anterior cingulate in BD compared with both intermittent explosive disorder and healthy volunteers (P <0.02); ↑ tChol/tCre in anterior cingulate in BD compared with healthy volunteers (P = .05); no significant group differences in occipital cortex mIns, mIns/tCre, or tChol/tCre |
| Sassi et al, 200571 | 14 Patients with BD, mood state euthymic (n = 13), mood state depressed (n = 1), medicated (n = 13), unmedicated (n = 1), (mean age, 15.5 yr), 18 healthy volunteers, (mean age, 17.3 yr) |
Signaa/1.5T/1500/20/STEAM | 8 mL in the left dorsolateral prefrontal cortex |
↓ NAA in BD (P = .03); tChol was negatively correlated with the number of previous BD episodes (r = −0.86; P = .0001) |
| Moore et al, 200767 | 10 Unmedicated patients with BD mania, (mean age, 11.1 yr), 8 patients with BD on risperidone; mean dose = 2.09 mg; mean duration = 101 weeks (mean age, 10.9 yr) |
Signaa/1.5T/2000/30/Probe-P(PRESS)and MRSI; TR/TE not stated |
4.8 mL in the anterior cingulate cortex |
↓ Glx/tCre in BD mania compared with BD risperidone-treatment responders (P < .05) |
| Moore et al, 200768 | 22 Patients with BD, euthymic state (n = 2), depressed state (n = 1), manic state (n = 10), mixed state (n = 9), medicated (n = 15), unmedicated (n = 7), (mean age, 12.6 yr), 10 healthy volunteers, (mean age, 12.3 yr) |
Varianc 4T/2000/30/PRESS | 8 mL in the anterior cingulate cortex |
↓ Gin in unmedicated BD (n = 7; mean age, 12.9 yr) compared with both medicated BD (P = .003) and healthy volunteers (P = .027); no significant difference in Glu among the 3 groups |
| Olvera et al, 200772 | 35 Patients with BD, (mean age, 13.2 yr), mood state not reported, medicated (n = 24), unmedicated (n = 11), 36 healthy volunteers, (mean age, 13.7 yr) |
lnterab/l-5T/6000/30/PRESS | 8 mL in the left dorsolateral prefrontal cortex |
↓ NAA in BD (P = .04); no significant difference in Glu, tCre, tChol, or mIns |
| Patel et al, 200874 | 28 Unmedicated depressed patients with BD, (mean age, 15.5 yr), 10 healthy volunteers, (mean age, 14.6 yr) |
Signaa/1.5T/2000/35/Probe-P(PRESS) | 8 mL in the anterior cingulate cortex, and the left and right ventral lateral prefrontal cortices |
↑ NAA in BD in the anterior cingulate cortex, (P = .0002) and the left (P = .0008) and right (P = .002) ventral lateral prefrontal cortices; ↑ tChol in BD in the left (P = .001) and right (P = .01) ventral lateral prefrontal cortices; ↑ mIns in BD in the right (P = .002) ventral lateral prefrontal cortex; in general, patients with BD had higher neurometabolite concentrations than healthy volunteers |
| Caetano et al, 201173 | 43 Patients with BD, euthymic state (n = 8), depressed state (n = 11), manic state (n = 12), mixed state (n = 12), medicated (n = 31), unmedicated (n = 12), (mean age, 13.2 yr), 38 healthy volunteers, (mean age, 13.9 yr) |
lnterb/1-5T/1500/272/MRSI | 8 mL in bilateral medial prefrontal cortex, dorsolateral prefrontal cortex, anterior and posterior cingulate cortices, and occipital lobe |
↓ NAA in BD in the left (P = .002) and right (P = .004) medial prefrontal cortices; ↓ GPC + PC in BD in left (P = .006) and right (P = .002) right) medial prefrontal cortices; ↓ PCr+Cr in BD in the left medial prefrontal cortex (P = .005); ↓ NAA in BD in the left dorsolateral prefrontal cortex (P = .013); ↓ PCr+Cr in BD (P = .011) in the left dorsolateral prefrontal cortex |
| Shi et al, 201253 | 14 Depressed patients with BD, medicated (n = 6), unmedicated (n = 8), (mean age, 15.6 yr), 24 healthy volunteers, (mean age, 15.7 yr) |
Triod/3T/3000/2.3/31P-MRSI; No 1H decoupling; dual-tuned 1H and 31P head coil |
15.625 mL in the frontal lobe | ↓ Pi/TP in unmedicated BD vs healthy controls; ↑ PCr/Pi ratio in unmedicated BD compared with healthy volunteers (24%; P = .013) and with medicated BD (39%; P = .002) |
| Sikoglu et al, 201354 | 8 Patients with BD, mood state euthymic (n = 3), mood state mixed, manic, or depressed (n = 5), medicated (n = 8), unmedicated (n = 0), (mean age, 16.9 yr), 8 healthy volunteers, (mean age, 15.5 yr) |
Varianc/4T/3000/1.2/31P-MRSI; no 1H decoupling; dual-tuned 1H and 31P head coil |
540 mL global sagittal whole- brain slab, 108 mL in the frontal lobe |
↓ Global Pi/TP in BD (P= .021); ↑ PCr/Pi global ratio in BD (P= .05); Frontal pHi increased with age in BD (Spearman ρ = 0.64) |
| Weber et al, 201355 | 19 Unmedicated patients with manic BD, (mean age, 15.5 yr), 14 unmedicated patients with euthymic BD, (mean age, 16.1 yr), 20 healthy volunteers, (mean age, 15.4 yr) |
Varianc/4T/500/notstated/31P-MRSI and Varianc/4T/2000/23/PRESS; no 1H decoupling; dual-tuned 1H and 31P head coil |
11-mL effective voxel size in the anterior cingulate cortex |
↓ pHi in manic BD vs HC (P = .03); ↓ ADP in manic BD vs HC (P = .01) |
Note:—TR and TE are expressed in milliseconds. FS indicates field strength; 1D–ISIS, adiabatic 1D image-selected in vivo spectroscopy; PRESS, point-resolved spectroscopic sequence; Probe-P(PRESS), proton brain examination single-voxel PRESS; MRSI, multivoxel MRS imaging; 31P-MRSI, multivoxel phosphorus MRS imaging; ↓, decreased; ↑, increased; 31P-MRS, phosphorus 31 MRS; ADP, adenosine diphosphate; GPC, glycerophosphocholine; PC, phosphocholine; pHi, intracellular pH; tCre, total creatine; TP, total 31P signal
GE Healthcare, Milwaukee, Wisconsin
Philips Healthcare, Best, the Netherlands
Varian, Palo Alto, California
Siemens, Erlangen, Germany
Table 4.
Cross-sectional and repeated-measures MRS studies of severe mood dysregulation
| Study | Sample | Methods (Device/FS/TR/TE/Sequence) |
Region-of-lnterest Voxel Size and Location |
Findings |
|---|---|---|---|---|
| Dickstein et al, 200893 | 36 Euthymic unmedicated patients with severe mood dysregulation, (mean age, 12.2 yr), 43 healthy volunteers, (mean age, 12.9 yr) |
Signaa/1.5T/2000/30/PRESS | 8 mL in the right frontal cortex, 8 mL in the left temporal cortex, 8 mL in the central parieto-occipital lobe, 8 mL in the left parietal lobe |
↓ mIns/tCre in severe mood dysregulation in the left temporal lobe (P = .03); no significant group differences in mIns/tCre, NAA/tCre, or Glx/tCre in the other regions of interest; females with severe mood dysregulation showed trends toward ↓ mIns/tCre in the left temporal cortex (P = .07), ↑ tCre in the left temporal cortex (P = .08), and ↑ Glx/tCre in the left parietal lobe (P = .08) |
| Dickstein et al, 200994 | 25 Euthymic unmedicated patients with severe mood dysregulation, randomized to 6 weeks of treatment with lithium (n = 14; mean age, 10.8 yr; mean serum lithium level = 0.82 mmol/L) or placebo (n = 11; mean age, 12.1 yr) |
Signa/1.5T/2000/30/PRESS | 8 mL in the right frontal cortex, 8 mL in the left temporal cortex, 8 mL in the central parieto-occipital lobe, 8 mL in the left parietal lobe, scans obtained at baseline and 6 weeks |
There was a significant Group × Time interaction for Glx/ tCre in the parieto-occipital lobe (P = .01), with ↑ Glx/tCre in the lithium group and ↓ Glx/tCre in the placebo group; Group × Time interactions for mIns/tCre, NAA/tCre, and Glx/ tCre in the other regions of interest were nonsignificant |
Note:—TR and TE are expressed in milliseconds. FS indicates field strength; PRESS, point-resolved spectroscopic sequence; ↓, decreased; ↑ , increased; tCre, total creatine
GE Healthcare, Milwaukee, Wisconsin,
Cross-Sectional MR Spectroscopy Studies of Pediatric BD
Cross-Sectional Studies of the Glutamate System
A summary of the cross-sectional MR spectroscopy studies comparing patients with pediatric BD versus healthy controls (HC) is shown in Table 1. Castillo et al66 were the first to study juvenile patients with BD and controls in 2000, reporting that patients with BD showed elevated Glx in the bilateral frontal lobes and basal ganglia. In a cross-sectional study of stably medicated pediatric patients with BD versus patients with mania, Glx was decreased in mania; this finding, the authors noted, could represent anterior cingulate cortex (ACC) hypometabolism.67 In another study, decreased ACC Gln was found in pediatric patients with manic BD, compared with both controls and stably medicated patients,68 while there were no differences in Glu.
Cross-Sectional Studies of Mitochondrial Function
The frontal lobes have been the subject of numerous case-control studies in pediatric BD, with several investigators reporting decreased NAA concentrations in subjects with pediatric BD compared with controls.69–73 In one report whose findings were in the opposing direction, Patel et al74 reported increased NAA in the ACC in patients with pediatric BD depression. Two studies reported increased mIns in pediatric BD, compared with both HC74 and intermittent explosive disorder.75 One study of pediatric mania found reduced tChol levels compared with HC,75 while a study of pediatric BD depression found an increase in tChol.74
As noted above, 3 recent studies of pediatric BD used 31P-MR spectroscopy to study cerebral energy metabolism: Shi et al53 focused on the frontal lobe, Sikoglu et al54 reported global or “whole brain” findings, and Weber et al55 interrogated the ACC. Most interesting, none of the studies reported a significant difference between BD and HC in β-nucleoside triphosphate (a proxy measure for ATP), but 2 investigators reported a significant decrease in Pi and an increased PCr/Pi ratio,53,54 neither of which has been reported in studies of adult BD. In the most recent 31P-MR spectroscopy study of pediatric BD, manic subjects had reduced intracellular pH and lower adenosine diphosphate concentrations in the ACC.55
Longitudinal Treatment MR Spectroscopy Studies of Pediatric BD and High-Risk Samples
Longitudinal Studies of the Glutamate System
Strawn et al76 treated pediatric patients with BD in a manic or mixed episode with valproic acid and performed 1H-MR spectroscopy at baseline, day 7, and day 28 (Table 2). In subjects who achieved clinical remission with valproic acid, the investigators found a decreased baseline Glx and a correlation between a change in the Young Mania Rating Scale77 scores and decreased Glu in the left ventro-lateral prefrontal cortex.76
Table 2.
Longitudinal MRS studies of pediatric bipolar disorder and youth at risk for bipolar disorder
| Study | Sample | Methods (Device/FS/TR/TE/Sequence) |
Region-of-lnterest Voxel Size and Location |
Findings |
|---|---|---|---|---|
| Davanzo et al, 200178 | 11 Patients with BD mania, medicated (n = 9), unmedicated (n = 2), (mean age, 11.4 yr), Treatment with lithium for 1 week (mean lithium level = 0.64 mEq/L), Scans obtained at baseline and day 7 |
Signaa/1.5T/3000/30/PRESS | 8 mL in the anterior cingulate cortex | ↓ mIns/tCre was associated with acute lithium treatment (P = .047); ↓ mIns/tCre in lithium responders (P < .012); mIns/tCre was not significantly different in lithium nonresponders (P < .655); NAA/tCre was the metabolite least affected by lithium |
| DelBello et al, 200681 | 19 Unmedicated manic or mixed patients with BD treated with olanzapine, 10–20 mg/day for 4 weeks, (mean age, 14.7 yr), 10 healthy volunteers, (mean age, 15 yr), |
Signa/1.5T/2000/35/PRESS | 8 mL in the medial ventral prefrontal cortex, 8 mL in the left and right ventral lateral prefrontal cortices, Scans obtained at baseline, day 7, and day 28 |
↑ tChol in medial ventral prefrontal cortex (P = .0001) associated with olanzapine treatment; ↑ baseline tChol in the medial ventral prefrontal cortex associated with remission at 4 weeks (P = .001); ↑ NAA for 4 weeks in the medial ventral prefrontal cortex in olanzapine remitters (n = 11) vs ↓ NAA in nonremitters (n = 8) (P = .006); ↑ NAA for 4 weeks was associated with reduction in YMRS scores (r = 0.68; P = .004) |
| Patel et al, 200679 | 28 Unmedicated depressed patients with BD, (mean age, 15.5 yr), Treatment with lithium for 42 days, titrated to serum levels of 1.0–1.2 mEq/L |
Signa/1.5T/2000/35/PRESS | 8 mL in the medial prefrontal cortex, 8 mL in the left and right lateral prefrontal cortices, Scans obtained at baseline, day 7, and day 42 |
mIns in medial prefrontal cortex did not significantly differ among baseline, day 7, or day 42; ↓ baseline mIns in medial prefrontal cortex in lithium remitters (n = 8) vs non-remitters (n = 20) (P = .003) |
| Chang et al, 200983 | 10 Offspring of BD parents with mood symptoms but not BD, medicated (n = 3), unmedicated (n = 7), (mean age, 11.3 yr), Treatment with divalproex for 12 weeks; mean serum VPA level = 82 µg/mL |
Signa/3T/2000/35/ PRESS | 8 mL in the left and right dorsolateral prefrontal cortices, Scans obtained at baseline and 12 weeks |
No significant difference in NAA in the left (P = .88) or right (P = .13) dorsolateral prefrontal cortex associated with 12 weeks of divalproex treatment; Effect size for decreased NAA = 0.94 for the right dorsolateral prefrontal cortex; Exploratory analyses showed no significant differences in tChol or mIns |
| Strawn et al, 201276 | 25 Unmedicated manic or mixed patients with BD (mean age, 14.5), Treatment with divalproex for 28 days, titrated to a serum VPA level of 85-125 µg/mL |
Varianb/4T/3000/23/PRESS | 8 mL in the anterior cingulate cortex (gray matter), 8 mL in left and right ventrolateral prefrontal cortices (white matter), Scans obtained at baseline, day 7, and day 28 |
↓ Glx at baseline in the left ventrolateral prefrontal cortex in treatment remitters (P = .01); In divalproex treatment remitters, change in Glu in the left ventrolateral prefrontal cortex correlated with change in YMRS score (r = 0.82; P = .03) |
| Chang et al, 201282 | 26 Unmedicated depressed patients with BD, Treatment with either quetiapine (n = 16) or placebo (n = 10) for 8 weeks, (mean age, 15.6 yr) |
Signa/3T/2000/26/ PRESS and Varian/4T/2000/26/PRESS |
8 mL in the anterior cingulate cortex, 8 mL in the left dorsolateral prefrontal cortex, 8 mL in the right dorsolateral prefrontal cortex |
↓, Posttreatment mIns in the ACC in quetiapine responders (5 of 16 patients); no significant change in NAA between the quetiapine and placebo groups |
Note:—TR and TE are expressed in milliseconds. FS indicates field strength; PRESS, point-resolved spectroscopic sequence; ↓, decreased; ↑ , increased; tCre, total creatine; VPA, valproic acid; YMRS, Young Mania Rating Scale
GE Healthcare, Milwaukee, Wisconsin
Varian, Palo Alto, California
Longitudinal Studies of Mitochondrial Function
Repeated-measures MR spectroscopy studies of treatment response have been conducted in pediatric BD and in high-risk samples of children with a parent with BD. Davanzo et al78 treated pediatric inpatients with manic BD with lithium and found that decreased mIns was associated with treatment, an effect that was stronger in treatment responders. Patel et al79 treated adolescents with BD depression with lithium and found that mIns concentrations did not significantly change from baseline, though pretreatment cortical mIns was significantly lower in patients who achieved remission. The same investigators later reported a significant decrease in NAA levels in response to treatment with lithium.80 DelBello et al81 treated pediatric patients with mania with olanzapine and found that increased tChol was associated with treatment; in addition, treatment remitters demonstrated increased NAA while nonremitters showed a decrease in NAA. Chang et al82 randomized subjects with pediatric BD depression to quetiapine or placebo and found that decreased mIns levels were associated with a positive treatment response. One repeated-measures MR spectroscopy study has been performed with a high-risk sample of youth with subsyndromal mood symptoms and at least 1 parent with BD: Chang et al83 found no significant alterations in NAA, tChol, or mIns associated with valproic acid treatment. However, a large effect size was noted for decreased posttreatment NAA in the right dorsolateral prefrontal cortex.83
Cross-Sectional MR Spectroscopy Studies of Youth at Risk for BD
Cecil et al69 conducted a study of mood-disordered children with a familial risk for BD and found increased mIns and decreased NAA in the high-risk subjects compared with HC (Table 3). Gallelli et al84 studied children of parents with BD in 3 groups: those with BD, those with subsyndromal BD symptoms, and HC. Measuring NAA, mIns, and tChol, the investigators found no significant between-group differences. Singh et al85 studied an at-risk sample of offspring of parents with BD, focusing first on the ACC and then on the cerebellar vermis.86 In the ACC, the authors found decreased absolute Glu concentrations in BD compared with both HC and offspring with subsyndromal BD symptoms.85 Then, studying at-risk offspring without BD and HC, Singh et al86 reported decreased cerebellar mIns and tChol. Finally, Wozniak et al87 measured ACC Glu in children with at least 1 parent with BD, dividing their sample into children with high-versus-low scores on a Child Behavior Checklist88 profile proposed as a correlate of pediatric BD.89,90 No group differences in ACC Glu were found, but in the high-score group, there was a positive correlation between Glu levels and Child Behavior Checklist profile scores.87
Table 3.
Cross-sectional MRS studies of youth at risk for bipolar disorder
| Study | Sample | Methods (Device/FS/TR/TE/Sequence) |
Region-of-lnterest Voxel Size and Location |
Findings |
|---|---|---|---|---|
| Cecil et al, 200369 | 9 Patients with euthymic mood disorder with at least 1 parent with BD, medicated (n = 1), unmedicated (n = 8), (mean age, 9.9 yr), 10 healthy volunteers, (mean age, 10.6 yr) |
Signaa/1.5T/2000/35/PRESS | 8 mL in the cerebellar vermis, 8 mL in the medial frontal cortex, 8 mL in frontal lobe white matter |
↑ mIns/tCre levels in patients with mood disorder in the medial frontal cortex (16%); ↓ NAA/tCre in patients with mood disorder in the cerebellar vermis (8%); no significant differences in NAA/tCre, tChol/tCre, or mIns/tCre in frontal lobe white matter |
| Gallelli et al, 200584 | 60 Offspring of parents with BD, 32 patients with BD, medicated (n = 28), unmedicated (n = 4), (mean age, 14.1 yr), 28 patients with subsyndromal BD symptoms, medicated (n = 20), unmedicated (n = 8), (mean age, 12.2 yr), mood state not reported, though “most subjects were not experiencing a manic or depressive episode at the time of scan,” 26 unaffected controls, (mean age, 14.2 yr) |
Signa/3T/2000/35/ PRESS | 8 mL in the left and right dorsolateral prefrontal cortices |
No significant group difference in NAA/tCre in the left (P = .99) or right (P = .75) dorsolateral prefrontal cortex; no significant group difference in mIns/tCre in the left (P = .15) or right (P = .19) dorsolateral prefrontal cortex; no significant group difference in tChol/tCre in the left (P = .31) or right (P = .46) dorsolateral prefrontal cortex |
| Singh et al, 201085 | 20 Patients with euthymic BD, medicated (n = 17), unmedicated (n = 3), (mean age, 15.9 yr), 20 euthymic patients with subsyndromal BD, medicated (n = 17), unmedicated (n = 3), (mean age, 12.9), 20 healthy volunteers, (mean age, 15.1 yr) |
Signa/3T/2000/35/ PRESS | 8 mL in the anterior cingulate cortex |
↓ Glu in BD compared with both healthy volunteers (P < .04) and subsyndromal BD (P < .04); no significant group differences in NAA or mIns |
| Singh et al, 201186 | 22 Euthymic youth at risk for BD with a parent with BD, medicated (n = 17), unmedicated (n = 5), psychostimulants discontinued 24 hours prior to scan, (mean age, 13.3 yr), 25 healthy volunteers, (mean age, 13.5 yr) |
Signa/3T/2000/35/PRESS | 8 mL in the cerebellar vermis |
↓ mIns in children at risk for BD (P < .01); ↓ tChol in children at risk for BD (P < .01) |
| Wozniak et al, 201287 |
24 Euthymic youth at risk for BD, with a parent with BD, medicated (n = 11), unmedicated (n = 13), (mean age, 11.8 yr), 13 healthy volunteers (mean age, 11.5 yr) |
Varianb/4T/2000/30/PRESS | 8 mL in the anterior cingulate cortex |
Positive correlation between emotional dysregulation score and Glu (Pearson correlation = 0.659; P < .02) |
Note:—TR and TE are expressed in milliseconds. FS indicates field strength; PRESS, point-resolved spectroscopic sequence; ↓, decreased; ↑ , increased; tCre, total creatine
GE Healthcare, Milwaukee, Wisconsin
Varian, Palo Alto, California
Cross-Sectional and Repeated-Measures MR Spectroscopy Studies of Severe Mood Dysregulation
The publication of the Diagnostic and Statistical Manual of Mental Disorders, 5th ed65 in May 2013 introduced disruptive mood dysregulation disorder as a new mood disorder of childhood (Table 4). Designed to differentiate children who present with severe, nonepisodic irritability from those with BD,91 disruptive mood dysregulation disorder is closely related to the severe mood dysregulation syndrome defined by Leibenluft63 and Leibenluft et al.92 Dickstein et al93 conducted a series of prescient MR spectroscopy investigations of severe mood dysregulation. In a case-control study, the investigators used 1H-MR spectroscopy to interrogate the frontal, temporal, and parietal cortices in severe mood dysregulation versus HC; their main finding was reduced mIns in the temporal cortex, though female subjects with severe mood dysregulation showed trends toward increased temporal tCr and Glx. The authors then conducted a randomized controlled trial of lithium in severe mood dysregulation, selecting this intervention on the basis of its potential effects on irritability, aggression, and neurometabolism.94 MR spectroscopy scans were acquired at baseline and repeated following 6 weeks of treatment with lithium or a placebo. A significant treatment Group × Time interaction was found for parieto-occipital Glx, which increased in the lithium group and decreased in placebo group.94
DISCUSSION
Pediatric BD is a prevalent and disabling illness, for which progress in timely diagnosis and effective treatment is urgently needed. Research in psychiatry is increasingly focused on biomarker discovery,95,96 and a consensus is emerging that MR neuroimaging investigations,16 including multimodal approaches that include MR spectroscopy,17 offer significant promise in elucidating the pathophysiology of BD.97
Findings Related to the Glutamate System in Pediatric BD
It has been proposed that MR spectroscopy studies of the glutamate system may hold the key to elucidating the pathophysiology of BD and to identifying novel treatment interventions.37 In adults, a consistent pattern has emerged in meta-analyses of the MR spectroscopy mood disorder literature: increased cerebral Glx levels in BD33,35 and decreased Glx in major depressive disorder.33,98 In comparison, there have been relatively few pediatric BD studies of the glutamate system. In line with the adult BD literature, Castillo et al66 reported elevated Glx in pediatric BD compared with HC. Moore et al67 reported decreased Glx in BD mania compared with subjects with BD stably treated with risperidone. Using high-field 4T scans, these investigators were also able to parse the Gln and Glu resonances in the ACC. Most intriguing, they found decreased Gln in untreated youths with BD compared with both stably medicated patients with BD and HC.67 Taken together with the fact that there was no difference in Glu among the 3 groups,67 this finding suggests that the Gln/Glu ratio measured in adult BD investigations99,100 merits further study in pediatric BD. Finally, in a longitudinal valproic acid treatment study, Strawn et al found decreased baseline Glx in treatment remitters and decreases in Young Mania Rating Scale scores correlated with decreased Glu concentrations.76 The authors concluded that the predictive value of MR spectroscopy neuroimaging “may relate to a disturbance in either glutamine or GABA, or in the homeostatic equilibrium of Glu and glutamine,”76 providing further support for the exploration of the Gln/Glu ratio as a potential biomarker in pediatric BD.
Findings Related to Mitochondrial Dysfunction in Pediatric BD
There are 5 reports of decreased cortical NAA concentrations in pediatric BD,69–73 and 1 study of adolescent BD depression that found increased NAA in the left ventral lateral prefrontal cortex, right ventral lateral prefrontal cortex, and ACC.74 In addition, Castillo et al66 reported no difference in cortical NAA between BD and HC, though the study may have been limited by its sample size. Most interesting, Chang et al70 found normal NAA levels in the dorsolateral prefrontal cortex of youth at risk for BD who had not yet experienced mania, suggesting that alterations in NAA may be a marker for fully syndromal cases of in BD.101 Longitudinal studies of NAA have shown mixed results: increased prefrontal NAA in BD manic olanzapine remitters81; decreased prefrontal NAA in depressed adolescents with BD treated with lithium80; and no significant change in NAA following treatment with lithium,78 quetiapine in BD depression,82 or youth at risk for BD treated with valproic acid.83 While additional work will be required to confirm the role of NAA in pediatric BD, the 5 casecontrol studies69–73 showing decreased NAA are in agreement with the findings in the adult literature.
Studies of mIns have reported an increase in pediatric BD in both the manic75,78 and depressed mood state.74 In addition, Davanzo et al78 showed that decreased ACC mIns is associated with a positive response to acute lithium treatment, a finding that is consistent with the molecular mechanism common to mood-stabilizing medications.102
To date, only three 31P-MR spectroscopy studies of pediatric BD have been published. Shi et al53 studied 14 depressed subjects with BD and 24 HC and found that unmedicated BD had decreased ACC Pi compared with both HC (17%; P = .038) and medicated BD (24%; P = .022). In a study of 8 subjects with BD and 8 HC, Sikoglu et al54 reported that compared with HC, subjects with BD had reduced global Pi. The relevance of Pi to mitochondrial dysfunction in BD may be the fact that Pi is a regulator of oxidative phosphorylation, the metabolic pathway for ATP production.78,103 Furthermore, it is thought that the only Pi that is detectable by nuclear MR imaging is involved in oxidative phos-phorylation.104,105 Mammalian cells in which oxidative phos-phorylation is impaired can reduce the concentration of free Pi via compartmentation to the inner mitochondrial membrane, which immobilizes the phosphorus ions and renders them invisible to MR spectroscopy.106–108 In addition, Pi has a direct effect in vitro on glucose use in cortical neurons.109
It has been posited that decreased ATP consumption leads to a fall in cytosolic Pi, to a level that balances ATP synthesis and use,110,111 thus stabilizing the phosphorylation potential of the cell.107,108 Additional support for the potential relevance of Pi in the pathophysiology of BD is provided by the observation that the activity of sodium–potassium adenosine triphosphatase (Na+ / K ± ATPase), an enzyme partially regulated by Pi,97–101,112–114 is altered in patients with BD.115 In the third and most recent 31P-MR spectroscopy study of pediatric BD, Weber et al55 found reduced intracellular pH and decreased adenosine diphosphate concentrations in the ACC. These results are in line with the consistent finding of reduced intracellular pH in studies of adult BD36 and with the discovery of Chance et al116 that adenosine diphosphate is one of the principal controllers of oxidative metabolism.
Limitations of Pediatric MR Spectroscopy Studies
Despite the consistent difference in the glutamatergic entity Glx between patients with BD and controls found by 3 independent analyses of the adult MR spectroscopy literature33–35 and the reports of glutamatergic alterations in pediatric BD discussed here, there are uncertainties associated with MR spectroscopy measures of Glu. In the brain, Glu plays at least 3 key roles: It is the major excitatory neurotransmitter, it serves as the precursor for the major inhibitory neurotransmitter GABA, and it is involved in the synthesis of smaller metabolites, including glutathione, as well as larger peptides and proteins.117 Glu is involved in a variety of metabolic pathways, including the neuronal tricarboxylic acid cycle, the astrocytic tricarboxylic acid cycle, pyruvate carboxylation, and the glutamate-glutamine cycle that links neuronal and astrocytic metabolism.118 This metabolic compartmentation leads to spatial uncertainty because Glu is present in glutamatergic neurons, GABAergic neurons, and astrocytes, in addition to extracellular spaces. The published studies of pediatric BD do not parse these multiple Glu pools in terms of either function or location.
Another important limitation of the extant pediatric BD MR spectroscopy literature is the static nature of the measurements reported. Static measures are likely to be insufficient in generating a comprehensive picture of BD pathophysiology because brain metabolism is predominantly made up of dynamic processes (ie, enzyme-catalyzed reactions and the transfer of chemical groups through metabolic pathways). Therefore, elucidation of BD etiopathogenesis may require the use of techniques capable of dynamic in vivo measures. Two examples of these that may find application in the study of BD are magnetization transfer, which can be used to measure the Kf of the creatine kinase reaction,56 and dynamic 13C MR spectroscopy, which has been validated as a method for studying neuronal bioenergetics and Glu neurotrans-mission and cycling.119
Future Translational Directions in MR Spectroscopy Studies of Pediatric BD
Improved diagnosis and treatment for pediatric BD are urgently needed: While the evidence suggests that pediatric bipolar illness is continuous with adult BD,38–40 the delay to first appropriate treatment experienced by individuals with childhood-onset BD averages more than 16 years.11 To date, MR spectroscopy studies of pediatric BD comprise a small, albeit rapidly expanding, literature. On the basis of its ability to measure neurochemical entities relevant to glutamatergic and mitochondrial function in vivo, MR spectroscopy will play a vital role in future translational studies of pediatric BD diagnosis, treatment development, and personalized medicine.37,120 Cross-sectional studies are needed to determine whether MR spectroscopy can reliably distinguish pediatric BD from disorders with overlapping symptoms, such as disruptive mood dysregulation disorder, attention deficit/hyperactivity disorder, and, especially, major depressive disorder.121 Comparison with MR spectroscopy studies of normal brain development122 will shed light on the natural history of BD and point investigators toward opportunities for intervention. Longitudinal studies may determine the predictors of continuity with adult BD and whether timely treatment is capable of altering the course of BD in at-risk individuals. Novel study designs combining MR spectroscopy with other neuroimaging methods in a multimodal approach can be used to increase the dimensionality of the information gleaned from a study sample.17,123,124 Finally, future studies would benefit from larger sample sizes because it has been calculated that MR spectroscopy investigations require analyzable data on at least 39 affected subjects and 39 HC to have adequate power to detect a 10% group difference in neurometabolite concentrations.125,126
ABBREVIATIONS
- ACC
anterior cingulate cortex
- ATP
adenosine triphosphate
- BD
bipolar disorder
- GABA
γ-aminobutyric acid
- Gln
glutamine
- Glu
glutamate
- HC
healthy controls
- PCr
phosphocreatine
- Pi
inorganic phosphate
- tChol
total choline
Footnotes
Disclosures: Douglas G. Kondo—RELATED: Grant: National Alliance for Research on Schizophrenia and Depression,* Comments: I received a National Alliance for Research on Schizophrenia and Depression Young Investigator Award, Other: Depressive and Bipolar Alternative Treatment Foundation, Comments: I received a grant from the Depressive and Bipolar Alternative Treatment Foundation,* UNRELATED: Employment: US Department of Veterans Affairs,* Comments: I am employed 0.25 FTE as a VA psychiatrist. Xian-Feng Shi—RELATED: Grant: Utah Science, Technology, and Research Institute,* Veterans Integrated Service Network 19 Mental Illness Research, Education and Clinical Center Pilot Funds.* Perry F. Renshaw—RELATED: Grant: Utah Science, Technology, and Research initiative.* *Money paid to the institution.
REFERENCES
- 1.Merikangas KR, Jin R, He JP, et al. Prevalence and correlates of bipolar spectrum disorder in the world mental health survey initiative. Arch Gen Psychiatry. 2011;68:241–251. doi: 10.1001/archgenpsychiatry.2011.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goldstein BI. Recent progress in understanding pediatric bipolar disorder. Arch Pediatr Adolesc Med. 2012;166:362–371. doi: 10.1001/archpediatrics.2011.832. [DOI] [PubMed] [Google Scholar]
- 3.Goldstein TR, Ha W, Axelson DA, et al. Predictors of prospectively examined suicide attempts among youth with bipolar disorder. Arch Gen Psychiatry. 2012;69:1113–1122. doi: 10.1001/archgenpsychiatry.2012.650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dilsaver SC. An estimate of the minimum economic burden of bipolar I and II disorders in the United States: 2009. J Affect Disord. 2011;129:79–83. doi: 10.1016/j.jad.2010.08.030. [DOI] [PubMed] [Google Scholar]
- 5.Newberg AR, Catapano LA, Zarate CA, et al. Neurobiology of bipolar disorder. Expert Rev Neurother. 2008;8:93–110. doi: 10.1586/14737175.8.1.93. [DOI] [PubMed] [Google Scholar]
- 6.DelBello MP, Adler CM, Strakowski SM. The neurophysiology of childhood and adolescent bipolar disorder. CNS Spectr. 2006;11:298–311. doi: 10.1017/s1092852900020794. [DOI] [PubMed] [Google Scholar]
- 7.Lish JD, Dime-Meenan S, Whybrow PC, et al. The National Depressive and Manic-Depressive Association (DMDA) survey of bipolar members. J Affect Disord. 1994;31:281–294. doi: 10.1016/0165-0327(94)90104-x. [DOI] [PubMed] [Google Scholar]
- 8.Perlis RH, Miyahara S, Marangell LB, et al. Long-term implications of early onset in bipolar disorder: data from the first 1000 participants in the systematic treatment enhancement program for bipolar disorder (STEP-BD) Biol Psychiatry. 2004;55:875–881. doi: 10.1016/j.biopsych.2004.01.022. [DOI] [PubMed] [Google Scholar]
- 9.Merikangas KR, Cui L, Kattan G, et al. Mania with and without depression in a community sample of US adolescents. Arch Gen Psychiatry. 2012;69:943–951. doi: 10.1001/archgenpsychiatry.2012.38. [DOI] [PubMed] [Google Scholar]
- 10.Gore FM, Bloem PJ, Patton GC, et al. Global burden of disease in young people aged 10–24 years: a systematic analysis. Lancet. 2011;377:2093–2102. doi: 10.1016/S0140-6736(11)60512-6. [DOI] [PubMed] [Google Scholar]
- 11.Leverich GS, Post RM, Keck PE, Jr, et al. The poor prognosis of child-hood-onset bipolar disorder. J Pediatr. 2007;150:485–490. doi: 10.1016/j.jpeds.2006.10.070. [DOI] [PubMed] [Google Scholar]
- 12.Carlson GA, Findling RL, Post RM, et al. AACAP 2006 Research Forum-Advancing research in early-onset bipolar disorder: barriers and suggestions. J Child Adolesc Psychopharmacol. 2009;19:3–12. doi: 10.1089/cap.2008.100. [DOI] [PubMed] [Google Scholar]
- 13.Insel TR. The National Institute of Mental Health Strategic Plan. Bethesda, Maryland: US Department of Health and Human Services; 2008. p. 37. [Google Scholar]
- 14.Kupfer DJ, First MB, Regier DA, editors. A Research Agenda for DSM-V. Arlington, Virginia: American Psychiatric Publishing; 2002. [Google Scholar]
- 15.Phillips ML, Vieta E. Identifying functional neuroimaging biomarkers of bipolar disorder: toward DSM-V. Schizophr Bull. 2007;33:893–904. doi: 10.1093/schbul/sbm060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Phillips ML. Coming of age? Neuroimaging biomarkers in youth. Am J Psychiatry. 2010;167:4–7. doi: 10.1176/appi.ajp.2009.09101546. [DOI] [PubMed] [Google Scholar]
- 17.Mayanil T, Wegbreit E, Fitzgerald J, et al. Emerging biosignature of brain function and intervention in pediatric bipolar disorder. Minerva Pediatr. 2011;63:183–200. [PubMed] [Google Scholar]
- 18.Dickstein DP, Reidy BL, Pescosolido MF, et al. Translational neuro-science in pediatric bipolar disorder. Expert Rev Neurother. 2011;11:1699–1701. doi: 10.1586/ern.11.157. [DOI] [PubMed] [Google Scholar]
- 19.Lan MJ, McLoughlin GA, Griffin JL, et al. Metabonomic analysis identifies molecular changes associated with the pathophysiology and drug treatment of bipolar disorder. Mol Psychiatry. 2009;14:269–279. doi: 10.1038/sj.mp.4002130. [DOI] [PubMed] [Google Scholar]
- 20.Sanacora G, Zarate CA, Krystal JH, et al. Targeting the glutamatergic system to develop novel, improved therapeutics for mood disorders. Nat Rev Drug Discov. 2008;7:426–437. doi: 10.1038/nrd2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eastwood SL, Harrison PJ. Markers of glutamate synaptic transmission and plasticity are increased in the anterior cingulate cortex in bipolar disorder. Biol Psychiatry. 2010;67:1010–1016. doi: 10.1016/j.biopsych.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Quiroz J, Gray N, Kato T, et al. Mitochondrially mediated plasticity in the pathophysiology and treatment of bipolar disorder. Neuropsychopharmacology. 2008;33:2551–2565. doi: 10.1038/sj.npp.1301671. [DOI] [PubMed] [Google Scholar]
- 23.Manji H, Kato T, Di Prospero NA, et al. Impaired mitochondrial function in psychiatric disorders. Nat Rev Neurosci. 2012;13:293–307. doi: 10.1038/nrn3229. [DOI] [PubMed] [Google Scholar]
- 24.Konradi C, Eaton M, MacDonald ML, et al. Molecular evidence for mitochondrial dysfunction in bipolar disorder. Arch Gen Psychiatry. 2004;61:300–308. doi: 10.1001/archpsyc.61.3.300. [DOI] [PubMed] [Google Scholar]
- 25.Braitenberg B, Schüz A. Cortex: Statistics and Geometry of Neuronal Connectivity. Berlin: Springer-Verlag; 1998. [Google Scholar]
- 26.Shen J, Petersen KF, Behar KL, et al. Determination of the rate of the glutamate/glutamine cycle in the human brain by in vivo 13C NMR. Proc Natl Acad Sci U S A. 1999;96:8235–8240. doi: 10.1073/pnas.96.14.8235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lebon V, Petersen KF, Cline GW, et al. Astroglial contribution to brain energy metabolism in humans revealed by 13C nuclear magnetic resonance spectroscopy: elucidation of the dominant pathway for neurotransmitter glutamate repletion and measurement of astrocytic oxidative metabolism. J Neurosci. 2002;22:1523–1531. doi: 10.1523/JNEUROSCI.22-05-01523.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rezin GT, Goncalves CL, Daufenbach JF, et al. Acute administration of ketamine reverses the inhibition of mitochondrial respiratory chain induced by chronic mild stress. Brain Res Bull. 2009;79:418–421. doi: 10.1016/j.brainresbull.2009.03.010. [DOI] [PubMed] [Google Scholar]
- 29.Diazgranados N, Ibrahim L, Brutsche NE, et al. A randomized add-on trial of an N-methyl-D-aspartate antagonist in treatment-resistant bipolar depression. Arch Gen Psychiatry. 2010;67:793–802. doi: 10.1001/archgenpsychiatry.2010.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zarate CA, Jr, Brutsche NE, Ibrahim L, et al. Replication of ketamine’s antidepressant efficacy in bipolar depression: a randomized controlled add-on trial. Biol Psychiatry. 2012;71:939–946. doi: 10.1016/j.biopsych.2011.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dager SR, Corrigan NM, Richards TL, et al. Research applications of magnetic resonance spectroscopy to investigate psychiatric disorders. Top Magn Reson Imaging. 2008;19:81–96. doi: 10.1097/RMR.0b013e318181e0be. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maddock RJ, Buonocore MH. MR spectroscopic studies of the brain in psychiatric disorders. Curr Top Behav Neurosci. 2012 Feb 1; doi: 10.1007/7854_2011_197. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 33.Yüksel C, Ongur D. Magnetic resonance spectroscopy studies of glutamate-related abnormalities in mood disorders. Biol Psychiatry. 2010;68:785–794. doi: 10.1016/j.biopsych.2010.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yildiz-Yesiloglu A, Ankerst DP. Neurochemical alterations of the brain in bipolar disorder and their implications for pathophysiology: a systematic review of the in vivo proton magnetic resonance spectroscopy findings. Prog Neuropsychopharmacol Biol Psychiatry. 2006;30:969–995. doi: 10.1016/j.pnpbp.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 35.Gigante AD, Bond DJ, Lafer B, et al. Brain glutamate levels measured by magnetic resonance spectroscopy in patients with bipolar disorder: a meta-analysis. Bipolar Disord. 2012;14:478–487. doi: 10.1111/j.1399-5618.2012.01033.x. [DOI] [PubMed] [Google Scholar]
- 36.Stork C, Renshaw PF. Mitochondrial dysfunction in bipolar disorder: evidence from magnetic resonance spectroscopy research. Mol Psychiatry. 2005;10:900–919. doi: 10.1038/sj.mp.4001711. [DOI] [PubMed] [Google Scholar]
- 37.Salvadore G, Zarate CA., Jr Magnetic resonance spectroscopy studies of the glutamatergic system in mood disorders: a pathway to diagnosis, novel therapeutics, and personalized medicine? Biol Psychiatry. 2010;68:780–782. doi: 10.1016/j.biopsych.2010.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Geller B, Tillman R, Bolhofner K, et al. Child bipolar I disorder: prospective continuity with adult bipolar I disorder; characteristics of second and third episodes; predictors of 8-year outcome. Arch Gen Psychiatry. 2008;65:1125–1133. doi: 10.1001/archpsyc.65.10.1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chang K. Adult bipolar disorder is continuous with pediatric bipolar disorder. Can J Psychiatry. 2007;52:418–425. doi: 10.1177/070674370705200703. [DOI] [PubMed] [Google Scholar]
- 40.Axelson DA, Birmaher B, Strober MA, et al. Course of subthreshold bipolar disorder in youth: diagnostic progression from bipolar disorder not otherwise specified. J Am Acad Child Adolesc Psychiatry. 2011;50:1001–1016. doi: 10.1016/j.jaac.2011.07.005. e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kondo DG, Hellem TL, Sung YH, et al. Review: magnetic resonance spectroscopy studies of pediatric major depressive disorder. Depress Res Treat. 2011;2011:650450. doi: 10.1155/2011/650450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.de Graaf RA. In Vivo NMR Spectroscopy: Principles and Techniques. England: John Wiley & Sons; 2007. West Sussex. [Google Scholar]
- 43.Keeler J. Understanding NMR Spectroscopy. Hoboken, New Jersey: Wiley; 2010. [Google Scholar]
- 44.Agarwal N, Renshaw PF. Proton MR spectroscopy-detectable major neurotransmitters of the brain: biology and possible clinical applications. AJNR Am J Neuroradiol. 2012;33:595–602. doi: 10.3174/ajnr.A2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kreis R, Ernst T, Ross BD. Development of the human brain: in vivo quantification of metabolite and water content with proton magnetic resonance spectroscopy. Magn Reson Med. 1993;30:424–437. doi: 10.1002/mrm.1910300405. [DOI] [PubMed] [Google Scholar]
- 46.Dager S, Friedman S, Parow A, et al. Brain metabolic alterations in medication-free patients with bipolar disorder. Arch Gen Psychiatry. 2004;61:450–458. doi: 10.1001/archpsyc.61.5.450. [DOI] [PubMed] [Google Scholar]
- 47.Bates TE, Strangward M, Keelan J, et al. Inhibition of N-acetylaspartate production: implications for 1H MRS studies in vivo. Neuroreport. 1996;7:1397–1400. [PubMed] [Google Scholar]
- 48.Pettegrew JW, McClure RJ, Panchalingam K. Spectroscopic imaging of schizophrenia. In: Shenton ME, Turetsky BI, editors. Understanding Neuropsychiatric Disorders: Insights from Neuroimaging. New York: Cambridge University Press; 2011. pp. 48–77. [Google Scholar]
- 49.Friedman S, Dager S, Parow A, et al. Lithium and valproic acid treatment effects on brain chemistry in bipolar disorder. Biol Psychiatry. 2004;56:340–348. doi: 10.1016/j.biopsych.2004.06.012. [DOI] [PubMed] [Google Scholar]
- 50.Moore GJ, Galloway MP. Magnetic resonance spectroscopy: neurochemistry and treatment effects in affective disorders. Psychopharmacol Bull. 2002;36:5–23. [PubMed] [Google Scholar]
- 51.Chaumeil MM, Valette J, Guillermier M, et al. Multimodal neuroimaging provides a highly consistent picture of energy metabolism, validating 31P MRS for measuring brain ATP synthesis. Proc Natl Acad Sci U S A. 2009;106:3988–3993. doi: 10.1073/pnas.0806516106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yildiz A, Sachs GS, Dorer DJ, et al. 31P Nuclear magnetic resonance spectroscopy findings in bipolar illness: a meta-analysis. Psychiatry Res. 2001;106:181–191. doi: 10.1016/s0925-4927(01)00082-8. [DOI] [PubMed] [Google Scholar]
- 53.Shi XF, Kondo DG, Sung YH, et al. Frontal lobe bioenergetic metabolism in depressed adolescents with bipolar disorder: a phosphorus-31 magnetic resonance spectroscopy study. Bipolar Disord. 2012;14:607–617. doi: 10.1111/j.1399-5618.2012.01040.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sikoglu EM, Jensen JE, Vitaliano G, et al. Bioenergetic measurements in children with bipolar disorder: a pilot 31P magnetic resonance spectroscopy study. PLoS One. 2013;8:e54536. doi: 10.1371/journal.pone.0054536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Weber WA, Dudley J, Lee JH, et al. A pilot study of alterations in high energy phosphoryl compounds and intracellular pH in unmedicated adolescents with bipolar disorder. J Affect Disord. 2013;150:1109–1113. doi: 10.1016/j.jad.2013.04.047. [DOI] [PubMed] [Google Scholar]
- 56.Jeong EK, Sung YH, Kim SE, et al. Measurement of creatine kinase reaction rate in human brain using magnetization transfer image-selected in vivo spectroscopy (MT-ISIS) and a volume (31) P/(1) H radiofrequency coil in a clinical 3-T MRI system. NMR Biomed. 2011;24:765–770. doi: 10.1002/nbm.1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Brosnan JT, Brosnan ME. Creatine: endogenous metabolite, dietary, and therapeutic supplement. Annu Rev Nutr. 2007;27:241–261. doi: 10.1146/annurev.nutr.27.061406.093621. [DOI] [PubMed] [Google Scholar]
- 58.Brosnan ME, Edison EE, da Silva R, et al. New insights into creatine function and synthesis. Adv Enzyme Regul. 2007;47:252–260. doi: 10.1016/j.advenzreg.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 59.Schlattner U, Tokarska-Schlattner M, Wallimann T. Mitochondrial creatine kinase in human health and disease. Biochim Biophys Acta. 2006;1762:164–180. doi: 10.1016/j.bbadis.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 60.Andres RH, Ducray AD, Schlattner U, et al. Functions and effects of creatine in the central nervous system. Brain Res Bull. 2008;76:329–343. doi: 10.1016/j.brainresbull.2008.02.035. [DOI] [PubMed] [Google Scholar]
- 61.Hilsted J, Jensen KE, Thomsen C, et al. Maintenance of high-energy brain phosphorous compounds during insulin-inducedhypoglycemia in men: 31P nuclear magnetic resonance spectroscopy study. Diabetes. 1988;37:760–762. doi: 10.2337/diab.37.6.760. [DOI] [PubMed] [Google Scholar]
- 62.Petroff OA, Prichard JW, Behar KL, et al. Cerebral intracellular pH by 31P nuclear magnetic resonance spectroscopy. Neurology. 1985;35:781–788. doi: 10.1212/wnl.35.6.781. [DOI] [PubMed] [Google Scholar]
- 63.Leibenluft E. Severe mood dysregulation, irritability, and the diagnostic boundaries of bipolar disorder in youths. Am J Psychiatry. 2011;168:129–142. doi: 10.1176/appi.ajp.2010.10050766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zepf FD, Holtmann M. Disruptive mood dysregulation disorder. In: Rey JM, editor. IACAPAP e-Textbook of Child and Adolescent Mental Health. Geneva, Switzerland: International Association for Child and Adolescent Psychiatry and Allied Professions; 2012. [Acessed December 29, 2013]. pp. 1–11. Available at: http://iacapap.org/wp-content/uploads/E.3-MOOD-DYSREGULATION-072012.pdf. [Google Scholar]
- 65.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th Edition (DSM-5) Arlington, Virginia: American Psychiatric Publishing; 2013. [Google Scholar]
- 66.Castillo M, Kwock L, Courvoisie H, et al. Proton MR spectroscopy in children with bipolar affective disorder: preliminary observations. AJNR Am J Neuroradiol. 2000;21:832–838. [PMC free article] [PubMed] [Google Scholar]
- 67.Moore CM, Biederman J, Wozniak J, et al. Mania, glutamate/glutamine and risperidone in pediatric bipolar disorder: a proton magnetic resonance spectroscopy study of the anterior cingulate cortex. J Affect Disord. 2007;99:19–25. doi: 10.1016/j.jad.2006.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Moore CM, Frazier JA, Glod CA, et al. Glutamine and glutamate levels in children and adolescents with bipolar disorder: a 4.0-T proton magnetic resonance spectroscopy study of the anterior cingulate cortex. J Am Acad Child Adolesc Psychiatry. 2007;46:524–534. doi: 10.1097/chi.0b013e31802f5f2c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cecil KM, DelBello MP, Sellars MC, et al. Proton magnetic resonance spectroscopy of the frontal lobe and cerebellar vermis in children with a mood disorder and a familial risk for bipolar disorders. J Child Adolesc Psychopharmacol. 2003;13:545–555. doi: 10.1089/104454603322724931. [DOI] [PubMed] [Google Scholar]
- 70.Chang K, Adleman N, Dienes K, et al. Decreased N-acetylaspartate in children with familial bipolar disorder. Biol Psychiatry. 2003;53:1059–1065. doi: 10.1016/s0006-3223(02)01744-4. [DOI] [PubMed] [Google Scholar]
- 71.Sassi RB, Stanley JA, Axelson D, et al. Reduced NAA levels in the dorsolateral prefrontal cortex of young bipolar patients. Am J Psychiatry. 2005;162:2109–2115. doi: 10.1176/appi.ajp.162.11.2109. [DOI] [PubMed] [Google Scholar]
- 72.Olvera RL, Caetano SC, Fonseca M, et al. Low levels of N-acetyl aspartate in the left dorsolateral prefrontal cortex of pediatric bipolar patients. J Child Adolesc Psychopharmacol. 2007;17:461–473. doi: 10.1089/cap.2007.0102. [DOI] [PubMed] [Google Scholar]
- 73.Caetano SC, Olvera RL, Hatch JP, et al. Lower N-acetyl-aspartate levels in prefrontal cortices in pediatric bipolar disorder: a (1)H magnetic resonance spectroscopy study. J Am Acad Child Adolesc Psychiatry. 2011;50:85–94. doi: 10.1016/j.jaac.2010.10.007. [DOI] [PubMed] [Google Scholar]
- 74.Patel NC, Cecil KM, Strakowski SM, et al. Neurochemical alterations in adolescent bipolar depression: a proton magnetic resonance spectroscopy pilot study of the prefrontal cortex. J Child Adolesc Psychopharmacol. 2008;18:623–627. doi: 10.1089/cap.2007.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Davanzo P, Yue K, Thomas MA, et al. Proton magnetic resonance spectroscopy of bipolar disorder versus intermittent explosive disorder in children and adolescents. Am J Psychiatry. 2003;160:1442–1452. doi: 10.1176/appi.ajp.160.8.1442. [DOI] [PubMed] [Google Scholar]
- 76.Strawn JR, Patel NC, Chu WJ, et al. Glutamatergic effects of divalproex in adolescents with mania: a proton magnetic resonance spectroscopy study. J Am Acad Child Adolesc Psychiatry. 2012;51:642–651. doi: 10.1016/j.jaac.2012.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Young RC, Biggs JT, Ziegler VE, et al. A rating scale for mania: reliability, validity and sensitivity. Br J Psychiatry. 1978;133:429–435. doi: 10.1192/bjp.133.5.429. [DOI] [PubMed] [Google Scholar]
- 78.Davanzo P, Thomas MA, Yue K, et al. Decreased anterior cingulate myo-inositol/creatine spectroscopy resonance with lithium treatment in children with bipolar disorder. Neuropsychopharmacology. 2001;24:359–369. doi: 10.1016/S0893-133X(00)00207-4. [DOI] [PubMed] [Google Scholar]
- 79.Patel NC, DelBello MP, Cecil KM, et al. Lithium treatment effects on myoinositol in adolescents with bipolar depression. Biol Psychiatry. 2006;60:998–1004. doi: 10.1016/j.biopsych.2006.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Patel NC, DelBello MP, Cecil KM, et al. Temporal change in N-acetyl-aspartate concentrations in adolescents with bipolar depression treated with lithium. J Child Adolesc Psychopharmacol. 2008;18:132–139. doi: 10.1089/cap.2007.0088. [DOI] [PubMed] [Google Scholar]
- 81.DelBello MP, Cecil KM, Adler CM, et al. Neurochemical effects of olanzapine in first-hospitalization manic adolescents: a proton magnetic resonance spectroscopy study. Neuropsychopharmacology. 2006;31:1264–1273. doi: 10.1038/sj.npp.1300950. [DOI] [PubMed] [Google Scholar]
- 82.Chang K, Delbello M, Chu WJ, et al. Neurometabolite effects of response to quetiapine and placebo in adolescents with bipolar depression. J Child Adolesc Psychopharmacol. 2012;22:261–268. doi: 10.1089/cap.2011.0153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chang K, Karchemskiy A, Kelley R, et al. Effect of divalproex on brain morphometry, chemistry, and function in youth at high-risk for bipolar disorder: a pilot study. J Child Adolesc Psychopharmacol. 2009;19:51–59. doi: 10.1089/cap.2008.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gallelli KA, Wagner CM, Karchemskiy A, et al. N-acetylaspartate levels in bipolar offspring with and at high-risk for bipolar disorder. Bipolar Disord. 2005;7:589–597. doi: 10.1111/j.1399-5618.2005.00266.x. [DOI] [PubMed] [Google Scholar]
- 85.Singh M, Spielman D, Adleman N, et al. Brain glutamatergic characteristics of pediatric offspring of parents with bipolar disorder. Psychiatry Res. 2010;182:165–171. doi: 10.1016/j.pscychresns.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Singh MK, Spielman D, Libby A, et al. Neurochemical deficits in the cerebellar vermis in child offspring of parents with bipolar disorder. Bipolar Disord. 2011;13:189–197. doi: 10.1111/j.1399-5618.2011.00902.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wozniak J, Gonenc A, Biederman J, et al. A magnetic resonance spectroscopy study of the anterior cingulate cortex in youth with emotional dysregulation. Isr J Psychiatry Relat Sci. 2012;49:62–69. [PMC free article] [PubMed] [Google Scholar]
- 88.Achenbach TM, Edelbrock CS. Manual for the Child Behavior Checklist and Revised Child Behavior Profile. Burlington, Vermont: Department of Psychiatry, University of Vermont; 1983. p. 230. [Google Scholar]
- 89.Mick E, Biederman J, Pandina G, et al. A preliminary meta-analysis of the child behavior checklist in pediatric bipolar disorder. Biol Psychiatry. 2003;53:1021–1027. doi: 10.1016/s0006-3223(03)00234-8. [DOI] [PubMed] [Google Scholar]
- 90.Biederman J, Wozniak J, Kiely K, et al. CBCL clinical scales discriminate prepubertal children with structured interview-derived diagnosis of mania from those with ADHD. J Am Acad Child Adolesc Psychiatry. 1995;34:464–471. [PubMed] [Google Scholar]
- 91.American Psychiatric Association. DSM-5 Childhood and Adolescent Disorders Work Group. Justification for Temper Dysregulation Disorder with Dysphoria: Introduction of a Diagnostic Category for Temper Dysregulation with Dysphoria (TDD) into the Mood Disorders Section of DSM-V. Arlington, Virginia: American Psychiatric Association; 2010. [Google Scholar]
- 92.Leibenluft E, Charney DS, Towbin KE, et al. Defining clinical phenotypes of juvenile mania. Am J Psychiatry. 2003;160:430–437. doi: 10.1176/appi.ajp.160.3.430. [DOI] [PubMed] [Google Scholar]
- 93.Dickstein DP, van der Veen JW, Knopf L, et al. Proton magnetic resonance spectroscopy in youth with severe mood dysregulation. Psychiatry Res. 2008;163:30–39. doi: 10.1016/j.pscychresns.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 94.Dickstein DP, Towbin KE, Van Der Veen JW, et al. Randomized double-blind placebo-controlled trial of lithium in youths with severe mood dysregulation. J Child Adolesc Psychopharmacol. 2009;19:61–73. doi: 10.1089/cap.2008.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Singh I, Rose N. Biomarkers in psychiatry. Nature. 2009;460:202–207. doi: 10.1038/460202a. [DOI] [PubMed] [Google Scholar]
- 96.Goodman WK for the Research on Biomarkers for Mental Disorders. Concept Clearance: National Advisory Mental Health Council. Bethesda, Maryland: National Institute of Mental Health; 2009. [Google Scholar]
- 97.Strakowski SM, Adler CM, Almeida J, et al. The functional neuroanatomy of bipolar disorder: a consensus model. Bipolar Disord. 2012;14:313–325. doi: 10.1111/j.1399-5618.2012.01022.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Luykx JJ, Laban KG, van den Heuvel MP, et al. Region and state specific glutamate downregulation in major depressive disorder:a meta-analysis of (1)H-MRS findings. Neurosci Biobehav Rev. 2012;36:198–205. doi: 10.1016/j.neubiorev.2011.05.014. [DOI] [PubMed] [Google Scholar]
- 99.Brady RO, Jr, Cooper A, Jensen JE, et al. A longitudinal pilot proton MRS investigation of the manic and euthymic states of bipolar disorder. Transl Psychiatry. 2012;2:e160. doi: 10.1038/tp.2012.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Brennan BP, Hudson JI, Jensen JE, et al. Rapid enhancement of glutamatergic neurotransmission in bipolar depression following treatment with riluzole. Neuropsychopharmacology. 2010;35:834–846. doi: 10.1038/npp.2009.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Roybal DJ, Singh MK, Cosgrove VE, et al. Biological evidence for a neurodevelopmental model of pediatric bipolar disorder. Isr J Psychiatry Relat Sci. 2012;49:28–43. [PubMed] [Google Scholar]
- 102.Williams RS, Cheng L, Mudge AW, et al. A common mechanism of action for three mood-stabilizing drugs. Nature. 2002;417:292–295. doi: 10.1038/417292a. [DOI] [PubMed] [Google Scholar]
- 103.Bose S, French S, Evans FJ, et al. Metabolic network control of oxidative phosphorylation:multiple roles of inorganic phosphate. J Biol Chem. 2003;278:39155–39165. doi: 10.1074/jbc.M306409200. [DOI] [PubMed] [Google Scholar]
- 104.Brazy PC, Mandel LJ. Does availability of inorganic phosphate regulate cellular oxidative metabolism? News Physiol Sci. 1986;1:100–103. [Google Scholar]
- 105.Freeman D, Bartlett S, Radda G, et al. Energetics of sodium transport in the kidney: saturation transfer 31P-NMR. Biochim Biophys Acta. 1983;762:325–336. doi: 10.1016/0167-4889(83)90087-3. [DOI] [PubMed] [Google Scholar]
- 106.Iotti S, Lodi R, Gottardi G, et al. Inorganic phosphate is transported into mitochondria in the absence of ATP biosynthesis: an in vivo 31P NMR study in the human skeletal muscle. Biochem Biophys Res Commun. 1996;225:191–194. doi: 10.1006/bbrc.1996.1152. [DOI] [PubMed] [Google Scholar]
- 107.Brown GC. Control of respiration and ATP synthesis in mammalian mitochondria and cells. Biochem J. 1992;284(pt 1):1–13. doi: 10.1042/bj2840001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Balaban RS. Regulation of oxidative phosphorylation in the mammalian cell. Am J Physiol. 1990;258:C377–C389. doi: 10.1152/ajpcell.1990.258.3.C377. [DOI] [PubMed] [Google Scholar]
- 109.Glinn M, Ni B, Paul SM. Inorganic phosphate enhances phospho- nucleotide concentrations in cultured fetal rat cortical neurons. Brain Res. 1997;757:85–92. doi: 10.1016/s0006-8993(97)00162-5. [DOI] [PubMed] [Google Scholar]
- 110.From AH, Zimmer SD, Michurski SP, et al. Regulation of the oxidative phosphorylation rate in the intact cell. Biochemistry. 1990;29:3731–3743. doi: 10.1021/bi00467a020. [DOI] [PubMed] [Google Scholar]
- 111.Zhang J, Shorr L, Yoshiyama M, et al. Hyperperfusion and cardioplegia effects on myocardial high-energy phosphate distribution and energy expenditure. Am J Physiol. 1994;267:H894–H904. doi: 10.1152/ajpheart.1994.267.3.H894. [DOI] [PubMed] [Google Scholar]
- 112.Pedemonte CH, Beauge L. Inhibition of (Na+, K+)-ATPase by magnesium ions and inorganic phosphate and release of these ligands in the cycles of ATP hydrolysis. Biochim Biophys Acta. 1983;748:245–253. doi: 10.1016/0167-4838(83)90301-1. [DOI] [PubMed] [Google Scholar]
- 113.Apell HJ, Roudna M, Corrie JE, et al. Kinetics of the phosphorylation of Na,K-ATPase by inorganic phosphate detected by a fluorescence method. Biochemistry. 1996;35:10922–10930. doi: 10.1021/bi960238t. [DOI] [PubMed] [Google Scholar]
- 114.Gatto C, Helms JB, Prasse MC, et al. Kinetic characterization of tetrapropylammonium inhibition reveals how ATP and Pi alter access to the Na+-K+-ATPase transport site. Am J Physiol Cell Physiol. 2005;289:C302–C311. doi: 10.1152/ajpcell.00043.2005. [DOI] [PubMed] [Google Scholar]
- 115.Looney SW, el-Mallakh RS. Meta-analysis of erythrocyte Na,K-ATPase activity in bipolar illness. Depress Anxiety. 1997;5:53–65. doi: 10.1002/(sici)1520-6394(1997)5:2<53::aid-da1>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 116.Chance B, Leigh JS, Jr, Kent J, et al. Multiple controls of oxidative metabolism in living tissues as studied by phosphorus magnetic resonance. Proc Natl Acad Sci USA. 1986;83:9458–9462. doi: 10.1073/pnas.83.24.9458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Erecińska M, Silver IA. Metabolism and role of glutamate in mammalian brain. Prog Neurobiol. 1990;35:245–296. doi: 10.1016/0301-0082(90)90013-7. [DOI] [PubMed] [Google Scholar]
- 118.Hertz L. Intercellular metabolic compartmentation in the brain: past, present and future. Neurochem Int. 2004;45:285–296. doi: 10.1016/j.neuint.2003.08.016. [DOI] [PubMed] [Google Scholar]
- 119.Rothman DL, De Feyter HM, de Graaf RA, et al. 13C MRS studies of neuroenergetics and neurotransmitter cycling in humans. NMR Biomed. 2011;24:943–957. doi: 10.1002/nbm.1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Mason GF, Krystal JH. MR spectroscopy: its potential role for drug development for the treatment of psychiatric diseases. NMR Biomed. 2006;19:690–701. doi: 10.1002/nbm.1080. [DOI] [PubMed] [Google Scholar]
- 121.Cardoso de Almeida JR, Phillips ML. Distinguishing between unipolar depression and bipolar depression: current and future clinical and neuroimaging perspectives. Biol Psychiatry. 2013;73:111–118. doi: 10.1016/j.biopsych.2012.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Marsh R, Gerber AJ, Peterson BS. Neuroimaging studies of normal brain development and their relevance for understanding childhood neuropsychiatric disorders. J Am Acad Child Adolesc Psychiatry. 2008;47:1233–1251. doi: 10.1097/CHI.0b013e318185e703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Enzi B, Duncan NW, Kaufmann J, et al. Glutamate modulates resting state activity in the perigenual anterior cingulate cortex: a combined fMRI-MRS study. Neuroscience. 2012;227:102–109. doi: 10.1016/j.neuroscience.2012.09.039. [DOI] [PubMed] [Google Scholar]
- 124.Hyder F, Rothman DL. Quantitative fMRI and oxidative neuroenergetics. Neuroimage. 2012;62:985–994. doi: 10.1016/j.neuroimage.2012.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Steen RG, Hamer RM, Lieberman JA. Measurement of brain metabolites by 1H magnetic resonance spectroscopy in patients with schizophrenia: a systematic review and meta-analysis. Neuropsychopharmacology. 2005;30:1949–1962. doi: 10.1038/sj.npp.1300850. [DOI] [PubMed] [Google Scholar]
- 126.Kraguljac NV, Reid M, White D, et al. Neurometabolites in schizophrenia and bipolar disorder: a systematic review and meta-analysis. Psychiatry Res. 2012;203:111–125. doi: 10.1016/j.pscychresns.2012.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]