Abstract
Background: Chemicals inhaled or ingested by mothers can be present in their milk. Our objective was to determine levels of nicotine, cotinine, and caffeine in human milk purchased via the Internet.
Materials and Methods: We purchased human milk (n=102) via the Internet and abstracted seller advertisements for information volunteered about tobacco and caffeine use. Nicotine, cotinine, and caffeine levels in the milk were quantified by mass spectrometry according to published protocols.
Results: No sellers indicated smoking in their advertisement. Many of the milk samples (58%) had detectable nicotine or cotinine; four (4%) of the samples had nicotine or cotinine levels high enough to indicate active smoking. Twelve (12%) sellers said in their advertisements that they specifically limit (4%) or avoid (8%) caffeine entirely. Five (5%) of the samples had caffeine levels consistent with consuming at least 1 cup of coffee 2 hours prior to milk expression. Detectable amounts of caffeine were found in almost all of the samples (97%).
Conclusions: In 102 milk samples, we detected evidence of active smoking, secondhand smoke exposure, and almost ubiquitous caffeine consumption. Buyers of human milk on the Internet should be aware that advertisements do not always include accurate information as to what substances may be present. Sellers may misrepresent their health behaviors or be unaware of lifestyle factors that can lead to exposure to nicotine and caffeine.
Introduction
Human milk is the optimal nutrition for infants.1,2 In the United States, rates of human milk feeding have increased over the past decades, which shows that mothers are trying to adhere to this message.3–6 There are times, however, when a mother cannot breastfeed or has an inadequate supply of her own milk. In those situations, families may seek alternative sources of human milk produced by another mother. Human milk sharing has been documented as far back as in the Koran and the Bible as lactating women used to put other mothers' infants directly to their breasts to suckle.7–11 At present, the majority of women express their milk with an electric pump12 and save it in storage receptacles.13 Those women who produce greater quantities of milk than are needed by their own infants may choose to share their excess. The Internet has pragmatically facilitated the process of sharing and selling milk among women with abundance and families in search of extra milk.14–18
The informal sharing of unpasteurized human milk outside the purview of a recognized milk bank is not supported by health authorities. The U.S. Food and Drug Administration,19 the American Academy of Pediatrics,20 and the Canadian Pediatric Society21 caution against feeding infants raw milk from unfamiliar sources. There was a recent alert in the United Kingdom for “urgent action” to regulate the practice of sharing human milk due to the potential unsafe exposures to the recipient infant, particularly the exposure to potentially harmful bacteria.22 Of human milk samples that we purchased on the Internet, 45% of the samples arrived at greater than 4°C, and 74% contained high counts of aerobic or detectable pathogenic bacteria.23 Moreover, 10% of samples contained quantities of bovine DNA indicative of greater than incidental contamination.24 Although it is unknown how often milk is informally shared or sold outside of nonprofit milk banks, we estimated that in 2011 there were over 13,000 online milk sharers annually.25
Many chemicals inhaled or ingested by lactating women are detectable in human milk.26,27 On popular milk exchanges, sellers of human milk will sometimes offer their smoking and drug use status.28–31 In a previous study, we found that 41% of milk sellers or donors volunteered in their advertisements that they were nonsmokers, none acknowledged being a smoker, and the remainder were silent on the smoking issue.25 Caffeine was less commonly declared; only in 11% of the advertisements did mothers say that they abstained from caffeine ingestion.25 Two percent said that they “limited” caffeine use, and the remaining participants said nothing about caffeine. The odds ratio of providing lifestyle claims was significantly higher among milk sellers compared with milk sharers, suggesting that milk sellers may be more motivated to market their milk.25 Although it is up to the online milk providers and recipients to discuss health behaviors when sharing or selling milk, the information in the initial advertisement is notably incomplete and inconsistent even when discussing practices such as hygiene. Our goals for this study were to assess the accuracy of sellers' statements when advertising their tobacco status and caffeine consumption and to explore the extent of possible exposure for recipient infants to nicotine, its metabolite cotinine, and caffeine.
Materials and Methods
During 2012, we sent a standard e-mail inquiry expressing interest in buying a small amount of human milk to individuals who posted a public advertisement on the Internet searching for buyers. We abstracted information from each advertisement using a standard form. We did not ask mothers specifically about nicotine or caffeine use, nor did we attempt to seek out or exclude mothers who might knowingly or unknowingly use these substances. More detailed methods about sample acquisition have been published previously.15 Samples were analyzed for biomarkers of tobacco and caffeine exposure. For tobacco, we analyzed cotinine and nicotine concentrations, although our primary focus was cotinine because it is the main biomarker used to distinguish smokers from nonsmokers and is less variable than nicotine, which has a short half-life.32 For caffeine use, we measured caffeine levels directly.
Human milk samples were stored at −20°C until needed. A 1-mL aliquot was used for analysis. Nicotine-d4 and caffeine-d9 internal standards were added prior to organic extraction (chloroform:isopropanol 90:10). After drying and reconstitution, the samples were transferred to injection vials for liquid chromatography/tandem mass spectrometery analysis. The samples were analyzed by liquid chromatography/tandem mass spectrometery using multiple reaction monitoring. Milk concentrations of nicotine, cotinine, and caffeine were calculated using experimentally derived standard curves and isotope dilution techniques.
The limit of detection (LOD) was 2 ng/mL for nicotine, 0.05 ng/mL for cotinine, and 5 ng/mL for caffeine. Samples were classified as coming from active smokers if cotinine values were greater than 4.47 ng/mL. This cutoff was established by Benowitz et al.33 based on serum samples from female smokers and nonsmokers in the representative U.S. National Health and Nutrition Examination Study. That study found 4.47 ng/mL to be the optimal cutoff for adult females to distinguish likely active smokers. Although the study of Benowitz et al.33 analyzed serum and not human milk, Luck and Nau34 found serum and milk cotinine levels to be very highly correlated (r=0.89) and with concentrations of similar magnitude, even several hours postexposure. Samples with cotinine values below 4.47 ng/mL but above the LOD were considered to be from nonsmokers exposed to environmental tobacco smoke, and samples below the LOD were considered to be exposed to very low or no environmental tobacco smoke. Any caffeine concentration above the LOD was considered to be positive for maternal caffeine ingestion.
Results
Nicotine/cotinine
No sellers admitted to smoking in their advertisement. Sixty-six (65%) sellers specifically stated that they abstained from smoking; 36 (35%) were silent on tobacco use. Nonetheless, four (4%) samples had cotinine levels high enough to indicate active smoking. One additional sample had high nicotine but low cotinine levels, which we interpreted to suggest a very recent, but isolated, use of tobacco or very heavy secondhand smoke. Overall, 59 (58%) samples had detectable nicotine or cotinine (Table 1).
Table 1.
Human Milk Nicotine and Cotinine Concentrations, Likely Clinical Correlation, and Relevant Advertisement Excerpt
| Seller | Nicotine (ng/mL) | Cotinine (ng/mL) | Likely clinical correlation |
|---|---|---|---|
| 1 | 33.6 | 133.6 | Active smokera |
| 2 | 23.2 | 17.8 | Active smokera |
| 3 | 2.4 | 6.3 | Active smokera |
| 4 | 0.95 | 5.4 | Active smokera |
| 5 | 15.8 | 1.3 | Not a regular smoker, but recently smoked or had heavy secondhand smoke exposurea |
| 6–59 | <5.2 | >0.05 (LOD) and <4.47 | Secondhand smoke exposure |
| 60–98 | Below LOD | Below LOD | No or very low secondhand smoke exposure |
| 99–102 | No detectable peak | Below LOD | No or very low secondhand smoke exposure |
Associated relevant advertisement excerpts: Seller 1, “My milk is from a smoke-free house, it is alcohol-free, drug free”; Seller 2: “Smoke free, drug free and medication free home”; Seller 3, no mention of smoking, caffeine ingestion, or other substances in this ad; Seller 4, “Healthy … With Two Healthy Babies (Both Breast Fed) Smoke and Alcohol Free … ”; and Seller 5, “I am a healthy drug free … woman …. ”
LOD, limit of detection.
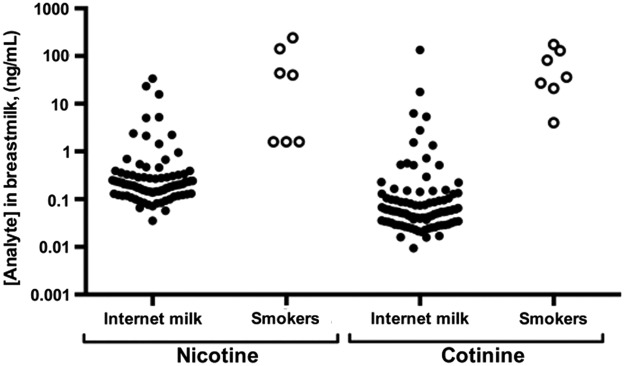
For comparison, we plotted nicotine and cotinine values from the present study alongside values in human milk previously reported by Pelligrini et al.32 for a sample of known active smokers (Fig. 1).
FIG. 1.
Nicotine and cotinine levels in breastmilk purchased on the Internet. Solid dots indicate analyte levels in samples purchased via the Internet for this study. Open dots show analyte values in breastmilk from women who smoke cigarettes. “Smokers” data are extracted from Table 5 of Pelligrini et al.32
Caffeine
Twelve (12%) sellers said in their advertisements that they specifically limit (4%) or avoid (8%) caffeine entirely; the remaining sellers were silent on caffeine use. Five (5%) samples had caffeine levels equivalent to consuming at least 1 cup of coffee 2 hours prior to milk expression; 97 (95%) of samples had lower but detectable levels of caffeine (Table 2).
Table 2.
Human Milk Caffeine Concentrations, Likely Clinical Correlation, and Relevant Advertisement Excerpt
| Seller | Caffeine (ng/mL) | Likely clinical correlation |
|---|---|---|
| 1–5 | >2,000 | Consuming about 1 cup of coffee 2 hours prior to milk expressiona |
| 6–97 | >5 (LOD) and <2,000 | Consumption of variable amounts of caffeine-containing beverages or foods |
| 98–102 | Below LOD | No or very little caffeine consumption around the time of milk expression |
Associated relevant advertisement excerpts: Seller 1, There was no mention of smoking, caffeine ingestion, or other substances in this ad; Seller 2, “Healthy, Non-Smoking Mom …” “I have a very healthy diet …”; Seller 3, “I am a healthy mother of 2, Non-Smoker (never smoked), No Caffeine (coffee or soda), No drug use (never used)”; Seller 4, “I am a non-smoker, non-drinker, and consume a healthy diet of mainly organic proteins, grains, fruits, and vegetables”; and Seller 5, “I don't drink, smoke and have never done any kind of drug.”
LOD, limit of detection.
Discussion
Our goal for this study was to determine in milk samples that we purchased on the Internet the presence of nicotine, its metabolite cotinine, and caffeine and to compare the results with the corresponding Internet advertisement. In the 102 milk samples, we detected evidence of active smoking, secondhand smoke exposure, and almost ubiquitous caffeine consumption. Few of the advertisements associated with these samples reported the use of caffeine, and none reported tobacco use. Outcomes in infants of mothers who smoke have been shown to be cardiovascular dysregulation,35 altered sleep/wake patterns,36 stunted growth,37,38 and an increase in respiratory ailments.38 Caffeine is rapidly transferred into human milk and has been shown to cause irritability and poor sleeping patterns in infants of women who have ingested large amounts.39–41 We previously showed that families seeking milk on the Internet had children with an identified medical condition (21%), intolerance to formula or their mothers' own milk (20%), or a general feeding difficulty (5%).25 Human milk containing these chemicals could have untoward effects on any infant, particularly those most vulnerable.
Nonprofit milk banks permit donation from women who do not smoke; however, caffeine consumption does not prohibit donation.42 A recent analysis of milk donated to a milk bank in Madrid, Spain showed that despite screening attempts using a lifestyle questionnaire, one out of the 400 samples of the Madrid samples contained substantial levels of nicotine/cotinine, and 45.3% (181/400) of the samples had detectable levels of caffeine.43 The authors concluded that although there certainly could have been women who provided misleading information, the screening questionnaires were not specific enough to determine which women might have these substances in their milk. Particularly for caffeine, mothers may not know that they are ingesting the substance, as caffeine is found in numerous drinks, foods, and medicines.
Our chosen cutoff for classifying sellers as active smokers is based on a recent analysis of a representative sample of U.S. women and is designed to maximize sensitivity (96.4%) and specificity (98.4%) given contemporary smoking habits and secondhand smoke levels.33 We found that cotinine levels were high enough to likely correlate to active smoking by four mothers. One sample had a lower cotinine level but a high level of nicotine, which indicates active smoking, nicotine replacement therapy use, or heavy secondhand smoke exposure in the recent period before milk expression. Fifty-eight percent of samples had measurable cotinine. Contemporary general population studies show that 43% of individuals have measurable amounts of cotinine.44 Most women with cotinine levels indicative of active smoking are exposed to nicotine through cigarettes, but it is possible that an individual may be using other nicotine products like a patch or gum. It is unclear at this time what cotinine levels would be for women exposed to the vapor of e-cigarettes. Regardless, infants fed human milk of women who used these products remain exposed to nicotine in the milk throughout, even if they are not additionally exposed through sidestream smoke from cigarettes.
The Madrid study showed a mean caffeine concentration of 496±778 ng/mL (95% confidence interval, 382–609 ng/mL; range, 0–7,564 ng/mL).43 Five samples of mothers' milk in our study had greater than 2,000 ng/mL of caffeine, which is the equivalent of drinking about 1 cup of coffee 2 hours prior to milk expression.32 Two out of these five advertisements stated specifically that the seller did not consume caffeine. Twelve (12%) sellers said in their advertisements that they specifically limit (4%) or avoid (8%) caffeine entirely, yet 95% of samples had measurable amounts of caffeine in their milk. This ubiquitous presence of caffeine is most likely due to caffeine being in many food products. Lactating mothers may not be aware that they were actually ingesting caffeine.
Through this and our other studies,23,24 we have shown that buyers must be cautious of purchasing human milk online. Although the American Academy of Pediatrics does not state that smoking or caffeine consumption by the mother is a contraindication for feeding infants breastmilk,2 we previously reported that 20% of milk samples are bought for premature or medically compromised individuals.25 Although caffeine is routinely used as a respiratory stimulant in premature infants, the use of caffeine in these infants is carefully controlled, and their symptoms are closely monitored.45 The elimination of caffeine is slow in the newborn due to the immaturity of the hepatic N-demethylation metabolic pathway, and the amount of caffeine can accumulate.46 It is unknown if ingestion of additional caffeine from human milk is detrimental.
One limitation to our study is that we purchased these samples anonymously from the Internet and that we did not ask any lifestyle questions of the sellers. When sellers requested details about the infants who were going to receive their milk, we did not answer the question and stopped all communication if they insisted on personal identifiers or contact information.14 Thus, the milk samples that we obtained may not generalizable to other milk selling, such as in the cases in which the sellers and buyers developed a personal connection. Perhaps if we asked mothers directly if there were the potential that their milk contained nicotine or caffeine, we would have been able to anticipate which samples contained these substances. The samples from the Madrid milk bank, however, showed that unless the lifestyle questionnaire was extremely detailed and attempted to capture a multitude of food sources, most likely the buyer would not be alerted to factual information.43
It is also possible that individuals' smoking status may be misclassified, especially those with cotinine values near the chosen cutoff. Nevertheless, there were two sellers with cotinine values much higher than the cutoff, providing confidence that there is evidence of active smoking in this study. Additionally, we analyzed only a single sample of milk from each seller, which may not represent typical tobacco use. Reliance on a single sample would tend to result in underestimation of the number of smokers and caffeine users because nicotine and caffeine are rapidly metabolized, and we could not detect the peak level. Thus, the number of positive samples in this study may be an underestimate of the true prevalence.
As more studies are conducted of human milk obtained via the Internet, there will need to be a determination if there is a difference in the milk that was purchased versus given away without payment. This controversy is already ongoing with blood products, for example. At this time the World Health Organization's guidelines suggest that blood should be obtained from unpaid volunteers.47 However, there has been recent studies of economic incentives' impact on blood donation, and it has not been shown that payment leads to a more contaminated blood supply.48 Nonetheless, when blood is donated, it goes through careful examination before being used. When milk is obtained on the Internet, either purchased or given away, there is no testing involved before it is fed to the infant. It is imperative to study the outcomes of infants fed both purchased and shared human milk to determine the associated risks.
Conclusions
In 102 milk samples that we bought via the Internet, we detected evidence of active smoking, secondhand smoke exposure, and almost ubiquitous caffeine consumption. Buyers of human milk on the Internet should be aware that advertisements do not always include information as to what substances may be present. Some sellers may misrepresent their health behaviors or be unaware of lifestyle factors that can lead to exposure to nicotine and caffeine. We showed that infants fed human milk bought from unfamiliar sources may unknowingly be exposed to nicotine and caffeine.
Acknowledgments
This study was supported by grants from The Ohio State University Food Innovation Center and the Cigna Foundation, a gift from the Nicklaus Children's Health Care Foundation, and internal funds of the Research Institute at Nationwide Children's Hospital. We thank Chelsea Dillon and Rachel Ronau for assistance with data collection and Kamma Smith for administrative support.
Disclosure Statement
No competing financial interests exist.
References
- 1.World Health Organization. Global Strategy for Infant and Young Child Feeding. Geneva: World Health Organization, 2003 [Google Scholar]
- 2.Section on Breastfeeding. Breastfeeding and the use of human milk. Pediatrics 2012;129:e827–e841 [DOI] [PubMed] [Google Scholar]
- 3.Jacknowitz A. Increasing breastfeeding rates: Do changing demographics explain them? Womens Health Issues 2007;17:84–92 [DOI] [PubMed] [Google Scholar]
- 4.Li R, Zhao Z, Mokdad A, et al. Prevalence of breastfeeding in the United States: The 2001 National Immunization Survey. Pediatrics 2003;111:1198–1201 [PubMed] [Google Scholar]
- 5.Ryan AS, Wenjun Z, Acosta A. Breastfeeding continues to increase into the new millennium. Pediatrics 2002;110:1103–1109 [DOI] [PubMed] [Google Scholar]
- 6.Wright A, Schanler R. The resurgence of breastfeeding at the end of the second millennium. J Nutr 2001;131:421S–425S [DOI] [PubMed] [Google Scholar]
- 7.Jones F, Human Milk Banking Association of North America. History of North American donor milk banking: One hundred years of progress. J Hum Lact 2003;19:313–318 [DOI] [PubMed] [Google Scholar]
- 8.Thorley V. Breasts for hire and shared breastfeeding: Wet nursing and cross feeding in Australia, 1900–2000. Health Hist 2008;10:88–109 [PubMed] [Google Scholar]
- 9.Wolf J. Mercenary hirelings” or “a great blessing?” Doctors' and mothers' conflicted perceptions of wet nursing and the ramifications for infant feeding in Chicago, 1871–1961. J Soc Hist 1999;33:97–120 [Google Scholar]
- 10.Dunn PM. The Holy Bible: Insights into perinatal practice in ancient times. Arch Dis Child Fetal Neonatal Ed 1996;75:F219–F220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moran L, Gilad J. From folklore to scientific evidence: Breast-feeding and wet-nursing in islam and the case of non-puerperal lactation. Int J Biomed Sci 2007;3:251–257 [PMC free article] [PubMed] [Google Scholar]
- 12.Labiner-Wolfe J, Fein SB, Shealy KR, et al. Prevalence of breast milk expression and associated factors. Pediatrics 2008;122(Suppl 2):S63–S68 [DOI] [PubMed] [Google Scholar]
- 13.Geraghty SR. Photo album of pumped breastmilk. Breastfeed Med 2011;6:433–434 [DOI] [PubMed] [Google Scholar]
- 14.Geraghty SR, Heier JE, Rasmussen KM. Got milk? Sharing human milk via the Internet. Public Health Rep 2011;126:161–164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Geraghty SR, McNamara KA, Dillon CE, et al. Buying human milk via the Internet: Just a click away. Breastfeed Med 2013;8:474–478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gribble KD. “I'm happy to be able to help:” Why women donate milk to a peer via Internet-based milk sharing networks. Breastfeed Med 2014;9:251–256 [DOI] [PubMed] [Google Scholar]
- 17.Palmquist AE, Doehler K. Contextualizing online human milk sharing: Structural factors and lactation disparity among middle income women in the U.S. Soc Sci Med 2014;122:140–147 [DOI] [PubMed] [Google Scholar]
- 18.Perrin MT, Goodell LS, Allen JC, et al. A mixed-methods observational study of human milk sharing communities on Facebook. Breastfeed Med 2014;9:128–134 [DOI] [PubMed] [Google Scholar]
- 19.U.S. Food and Drug Administration. Use of Donor Human Milk. 2010. Available at www.fda.gov/ScienceResearch/SpecialTopics/PediatricTherapeuticsResearch/ucm235203.htm (accessed June1, 2012)
- 20.Gartner LM, Morton J, Lawrence RA, et al. Breastfeeding and the use of human milk. Pediatrics 2005;115:496–506 [DOI] [PubMed] [Google Scholar]
- 21.Kim J, Unger S. Human milk banking. Paediatr Child Health 2010;15:595–602 [PMC free article] [PubMed] [Google Scholar]
- 22.Steele S, Martyn J, Foell J. Risks of the unregulated market in human breast milk. BMJ 2015;350:h1485. [DOI] [PubMed] [Google Scholar]
- 23.Keim SA, Hogan JS, McNamara KA, et al. Microbial contamination of human milk purchased via the Internet. Pediatrics 2013;132:e1227–e1235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Keim SA, Kulkarni MM, McNamara K, et al. Cow's milk contamination of human milk purchased via the Internet. Pediatrics 2015;135:e1157–e1162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Keim SA, McNamara KA, Jayadeva CM, et al. Breast milk sharing via the internet: the practice and health and safety considerations. Matern Child Health J 2014;18:1471–1479 [DOI] [PubMed] [Google Scholar]
- 26.Hale TW, Rowe HE. Medications & Mother's Milk, 16th ed. Plano, Texas: Hale Publishing, 2014 [Google Scholar]
- 27.Medicine USNLo. LactMed: A Toxnet Database. Available at http://toxnet.nlm.nih.gov/newtoxnet/lactmed.htm (accessed May1, 2015)
- 28.Human Milk 4 Human Babies. Available at www.hm4hb.net/ (accessed May1, 2015)
- 29.Only the Breast. Available at http://onlythebreast.com/ (accessed May1, 2015)
- 30.Faulkner K. Milk Share. Available at http://milkshare.birthingforlife.com/ (accessed May1, 2015)
- 31.Walker S. Eats on Feets. Available at www.eatsonfeets.org/ (accessed May1, 2015)
- 32.Pellegrini M, Marchei E, Rossi S, et al. Liquid chromatography/electrospray ionization tandem mass spectrometry assay for determination of nicotine and metabolites, caffeine and arecoline in breast milk. Rapid Commun Mass Spectrom 2007;21:2693–2703 [DOI] [PubMed] [Google Scholar]
- 33.Benowitz NL, Bernert JT, Caraballo RS, et al. Optimal serum cotinine levels for distinguishing cigarette smokers and nonsmokers within different racial/ethnic groups in the United States between 1999 and 2004. Am J Epidemiol 2009;169:236–248 [DOI] [PubMed] [Google Scholar]
- 34.Luck W, Nau H. Nicotine and cotinine concentrations in serum and urine of infants exposed via passive smoking or milk from smoking mothers. J Pediatr 1985;107:816–820 [DOI] [PubMed] [Google Scholar]
- 35.Dahlström A, Ebersjö C, Lundell B. Nicotine in breast milk influences heart rate variability in the infant. Acta Paediatr 2008;97:1075–1079 [DOI] [PubMed] [Google Scholar]
- 36.Mennella JA, Yourshaw LM, Morgan LK. Breastfeeding and smoking: Short-term effects on infant feeding and sleep. Pediatrics 2007;120:497–502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Berlanga Mdel R, Salazar G, Garcia C, et al. Maternal smoking effects on infant growth. Food Nutr Bull 2002;23(3 Suppl):142–145 [PubMed] [Google Scholar]
- 38.Yilmaz G, Isik Agras P, Hizli S, et al. The effect of passive smoking and breast feeding on serum antioxidant vitamin (A, C, E) levels in infants. Acta Paediatr 2009;98:531–536 [DOI] [PubMed] [Google Scholar]
- 39.Bailey DN, Weibert RT, Naylor AJ, et al. A study of salicylate and caffeine excretion in the breast milk of two nursing mothers. J Anal Toxicol 1982;6:64–68 [DOI] [PubMed] [Google Scholar]
- 40.Martin I, Lopez-Vilchez MA, Mur A, et al. Neonatal withdrawal syndrome after chronic maternal drinking of mate. Ther Drug Monit 2007;29:127–129 [DOI] [PubMed] [Google Scholar]
- 41.Rivera-Calimlim L. Drugs in breast milk. Drug Ther (NY) 1977;7:59–63 [PubMed] [Google Scholar]
- 42.Human Milk Banking Association of North America. Guidelines for the Establishment and Operation of a Donor Human Milk Bank. Fort Worth, TX: Human Milk Banking Association of North America, 2013 [Google Scholar]
- 43.Escuder-Vieco D, Garcia-Algar O, Pichini S, et al. Validation of a screening questionnaire for a human milk bank to determine the presence of illegal drugs, nicotine, and caffeine. J Pediatr 2014;164:811–814 [DOI] [PubMed] [Google Scholar]
- 44.Pirkle JL, Bernert JT, Caudill SP, et al. Trends in the exposure of nonsmokers in the U.S. population to secondhand smoke: 1988–2002. Environ Health Perspect 2006;114:853–858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schmidt B, Roberts RS, Davis P, et al. Caffeine therapy for apnea of prematurity. N Engl J Med 2006;354:2112–2121 [DOI] [PubMed] [Google Scholar]
- 46.Friguls B, Joya X, García-Algar O, et al. A comprehensive review of assay methods to determine drugs in breast milk and the safety of breastfeeding when taking drugs. Anal Bioanal Chem 2010;397:1157–1179 [DOI] [PubMed] [Google Scholar]
- 47.World Health Organization. Universal Access to Safe Blood Transfusion. Geneva: World Health Organization, 2008 [Google Scholar]
- 48.Lacetera N, Macis M, Slonim R. Public health. Economic rewards to motivate blood donations. Science 2013;340:927–928 [DOI] [PubMed] [Google Scholar]