Abstract
The lack of a functional endothelium on small-diameter vascular grafts leads to intimal hyperplasia and thrombotic occlusion. Shear stress conditioning through controlled hydrodynamics within in vitro perfusion bioreactors has shown promise as a mechanism to drive endothelial cell (EC) phenotype from an activated, pro-inflammatory wound state toward a quiescent functional state that inhibits responses that lead to occlusive failure. As part of an overall design strategy to engineer functional vascular grafts, we present a novel two-phase shear conditioning approach to improve graft endothelialization. Axial rotation was first used to seed uniform EC monolayers onto the lumenal surface of decellularized scaffolds derived from the human umbilical vein. Using computer-controlled perfusion circuits, a flow-ramping paradigm was applied to adapt endothelia to arterial levels of fluid shear stress and pressure without graft denudation. The effects of constant pulse frequencies (CF) on EC quiescence were then compared with pulse frequencies modeled from temporal fluctuations in blood flow observed in vivo, termed physiologically modeled pulse dynamics (PMPD). Constructs exposed to PMPD for 72 h expressed a more functional transcriptional profile, lower metabolic activity (39.8%±8.4% vs. 62.5%±11.5% reduction, p=0.012), and higher nitric oxide production (80.42±23.93 vs. 48.75±6.93 nmol/105 cells, p=0.028) than those exposed to CF. By manipulating in vitro flow conditions to mimic natural physiology, endothelialized vascular grafts can be stimulated to express a nonactivated phenotype that would better inhibit peripheral cell adhesion and smooth muscle cell hyperplasia, conditions that typically lead to occlusive failure. Development of robust, functional endothelia on vascular grafts by modulation of environmental conditions within perfusion bioreactors may ultimately improve clinical outcomes in vascular bypass grafting.
Introduction
Prosthetic small-diameter vascular grafts (SDVG) in current clinical use fail to match the patency rates of autologous vascular grafts1–3 primarily due to occlusive failure brought on via two mechanisms: thrombus formation4 and anastomotic intimal hyperplasia.5 It has been hypothesized that the superior performance of autologous grafts compared with prosthetic materials is largely due to the presence of an inner lining of endothelial cells (EC).1 EC shield the lumenal graft surface from circulating blood (thereby preventing adsorption of thrombogenic plasma proteins) and actively inhibit cellular adhesion through the constitutive production of nitric oxide (NO), prostacyclin, and other biochemical factors.6,7 Furthermore, through paracrine stimulation, EC inhibit smooth muscle cell migration8/proliferation9 and play critical roles in the regulation of vascular tone.10,11 Given the naturally nonthrombogenic properties of nonactivated, quiescent EC, developing a competent endothelium on acellular vascular grafts would theoretically prolong patency.12 For effective vascular regeneration that ultimately includes a functional medial layer, maintaining a functional endothelium is likely to be a defining factor in graft success.
Single-stage endothelialization, in which EC are harvested and seeded onto grafts immediately before implantation, improved graft patency in animal models, but this technique has not been successfully translated to the clinic.13–15 Due to a limited number of EC or endothelial progenitor cells (EPC) that can be extracted from an explanted vessel, biopsy, or blood draw, grafts implanted using a single-stage seeding strategy often have insufficient coverage to manifest therapeutic effects.16,17 It has been suggested that ex vivo expansion of EC before seeding and/or the allotment of additional culture time are necessary to allow maturation of endothelialized grafts before implantation.18 Two-stage endothelialization has previously been shown to reduce thrombosis19 and neointima formation20 in animal models and has also shown promise in the clinic with improvement in patency.21,22 Endothelialized ePTFE grafts had higher patency rates as compared with acellular grafts in infrainguinal21 and coronary artery22 bypass applications. Despite these promising results, this strategy has not achieved widespread success in clinical trials.16 This lack of success is, in part, due to poor retention of seeded EC on the lumenal surface, as well as due to the induction of an inappropriate, activated wound phenotype that promotes inflammatory and thrombotic host responses.23 Various strategies to improve EC retention on the lumenal surface and prolong graft patency after implantation include treatment with surface coatings and shear stress preconditioning to strengthen EC adhesion,23,24 and treatment of grafts with molecules to improve EPC recruitment and in situ endothelialization.25,26 Although recent advances in stem cell technology offer significant potential, controlling differentiation and the subsequent maintenance of a mature, functional EC phenotype—especially for rapid seeding of grafting materials—remains a challenge.
As part of an overall design strategy to develop functional SDVG, we present a novel two-phase approach to improve graft endothelialization in concert with a physiologically modeled shear conditioning paradigm to induce a functional phenotypic state in EC.
Materials and Methods
Additional methods are described in the Supplementary Materials and Methods section and Supplementary Figure S1 (Supplementary Data are available online at www.liebertpub.com/tec).
Cell culture
Human umbilical vein (HUV) EC were extracted from human umbilical cords obtained from Labor & Delivery at Shands Hospital and cultured in VascuLife VEGF EC culture medium as previously described (see Supplementary Materials and Methods section for full medium formulation).27 EC were passaged after reaching approximately 80% confluence every 2–3 days using Accutase (Innovative Cell Technologies, San Diego, CA) and used experimentally at P4.
Dissection and decellularization of HUV
HUV were isolated from the surrounding tissue using an automated dissection procedure that has been previously described.28 Veins were decellularized using 1% sodium dodecyl sulfate in distilled water (dH2O), followed by treatment with 70 U/mL deoxyribonuclease I using a process that has been previously described,29 and stored in phosphate-buffered saline (PBS) at 4°C for a maximum of 2 weeks before use. Representative histological images of hematoxylin and eosin or Masson's Trichrome-stained dissected HUV samples before and after decellularization are shown in Supplementary Figure S2.
Perfusion circuit design
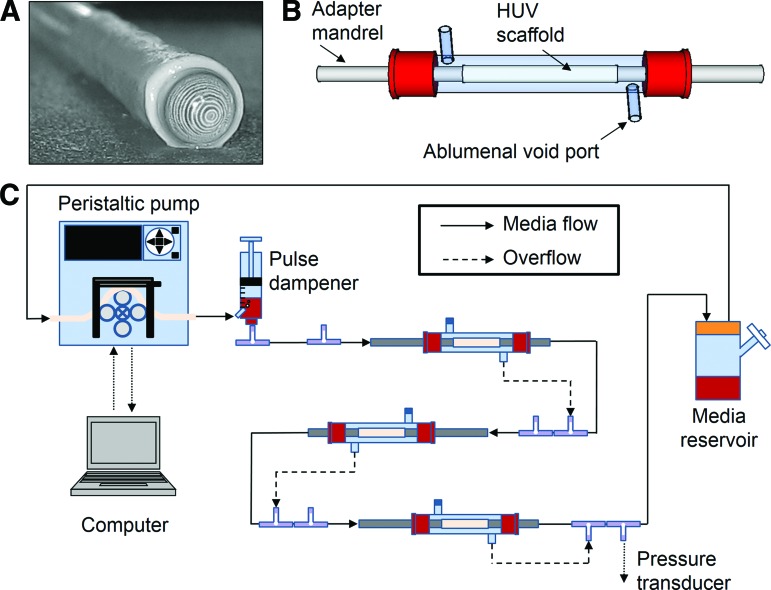
HUV scaffolds (6 cm length) were secured to stainless steel adapter mandrels and inserted into custom-designed glass bioreactors that were integrated into flow circuits as shown in Figure 1.30 Ports fitted onto the body of the bioreactor provided access to the ablumenal void space surrounding each scaffold, and they were used to drive transmural overflow media back into the lumenal circuit as pressure built in the void space (Fig. 1B, C). This ensured an equivalent lumenal flow rate through all three scaffolds connected in series. Medium was drawn from reservoirs fitted with a 0.22 μm filter for gas exchange by peristaltic pumps and driven through the circuit as shown. A pulse dampener was incorporated to reduce pressure spikes and to trap gas bubbles; downstream pressure was monitored using a pressure transducer (Sonometrics TRX-8 CAN). The entire system was placed in an incubator that was maintained at 37°C and 5% CO2.
FIG. 1.
Bioreactor system for development of tissue-engineered vascular grafts. Each human umbilical vein (HUV) scaffold (A) is affixed to adapter mandrels at each end and housed in a custom-designed glass bioreactor (B). (C) A computer-controlled peristaltic pump is used to drive culture medium drawn from a reservoir through the lumen of three scaffolds connected in series. Transmural media overflow is redirected to the lumenal circuit as pressure increases in the ablumenal void space. Color images available online at www.liebertpub.com/tec
The flow rate through the circuit was modulated by the rotational speed of the peristaltic pump, which was driven by Masterflex Linkable Instrument Control Software V3.1. The mean wall SS (τ) to which seeded EC were exposed was estimated according to the Hagen–Poiseuille equation (assuming steady flow and a constant cross-sectional area):
 |
where μ is viscosity, Q is mean volumetric flow rate, and R is the inner vessel radius. At the maximum flow rate, the Reynolds number was calculated to be 299, indicating laminar flow. The entrance length (Le) for fully developed flow, calculated according to
 |
where D is HUV scaffold diameter and Re is the Reynolds number, was determined to be 89.7 mm. Therefore, the tubing upstream of each HUV scaffold was extended so that flow was fully developed before reaching the scaffold.
EC seeding
Perfusion circuits were sterilized by 2 h of perfusion of 0.2% peracetic acid/4% ethanol in dH2O, followed by 2 h of perfusion of PBS (pH 7.4) and 2 h of perfusion of EC medium. Triplicate sets of HUV scaffolds were seeded by injecting EC suspensions (106 cells/mL within ∼20 mL) into the lumenal space, and bioreactors were attached to a rotational seeding apparatus within the incubator. EC were allowed to attach for 5 h during rotation before bioreactors were reconnected to the perfusion circuit, and low flow (0.5 RPM) was initiated for media exchange. Medium was replenished every 3 days, and dextran-supplemented medium was added before flow ramping was initiated.
Adjustment of medium viscosity
To increase media viscosity to 3 mpA*s (approximately that of blood) in an effort to achieve arterial wall SS, 6% (m/v) dextran (MW: 70,000; Sigma-Aldrich, St. Louis, MO) was added to culture media, as determined by the SV-10A tuning fork vibration viscometer. EC growth characteristics in culture media with or without 6% dextran were assessed using the Live/Dead Viability/Cytotoxicity Kit for mammalian cells (Invitrogen, Grand Island, NY) as previously described.27 Supplementation of culture media with 6% dextran had no deleterious effect on growth rate or viability (Supplementary Fig. S3).
Dynamic perfusion culture
A previously designed perfusion regime was adapted for use within this tubular bioreactor system.31 In brief, real-time recordings of human heart rate in a healthy male subject were obtained over a 12-h period and programmed into computer-driven peristaltic pump drives so that the rotational speed corresponded to the observed changes in cardiac output. This regime was termed physiologically modeled pulse dynamics (PMPD). For a comparison, flow was applied at a constant pulse frequency (CF) of 1.45 pascals (Pa) at 80 pulses/min; this was the calculated mean flow rate, pulse frequency, and pressure from the PMPD program averaged over the entire 12 h cycle. Thus, both flow regimes applied the same total magnitude of SS, although at different rates. For a third condition for comparison, a flow program was designed in which the flow rate was held constant, but periodically increased stepwise for 3 min once every hour, termed stepped flow (SF).
Nitrate/nitrite quantification
Total nitrate/nitrite content (stable salt derivatives of NO) was quantified in conditioned media samples using the Nitrate/Nitrite Fluorometric Assay Kit (Cayman Chemical, Ann Arbor, MI).31 Three to four replicate samples (each from an individual circuit consisting of three HUV grafts in series) were analyzed per condition, and results were normalized by subtracting basal levels measured in nonconditioned media and then dividing by cell number.
Quantitative polymerase chain reaction
After 24 h, EC were collected and mRNA was purified using the RNAqueous-4PCR Kit (Ambion, Foster City, CA) according to instructions. Then, 400 ng mRNA per sample was reverse transcribed to cDNA using the RT2 First-Strand kit (SABiosciences, Valencia, CA) according to instructions. Next, cDNA was combined with RT2 SYBR Green qPCR Master Mix and loaded onto human EC biology PCR arrays (SABiosciences). Amplification was performed at 95°C for 10 min, followed by 40 cycles (95°C for 15 s and 60°C for 60 s). The comparative CT method was used to quantify gene expression relative to the housekeeping genes GAPDH, RPL13A, B2M, ACTB, and HRP; EC cultured under static conditions were used as calibrating samples. Gene expression is reported as fold changes relative to calibrating samples (n=3–4 samples per group).
Metabolic activity
Metabolic activity was assessed using the AlamarBlue reagent (Invitrogen). Scaffolds were removed from bioreactors, cut into 1 cm2 squares, and incubated with 10% AlamarBlue reagent in media within 24-well plates at 37°C and 5% CO2 for 4 h, after which time absorbance of each conditioned media sample was read at 570 and 600 nm. Percent medium reduction was calculated according to kit instructions, and results were normalized by cell number for each condition (n=4–6 samples per condition). For comparison, nonseeded decellularized HUV scaffolds were tested and showed no reduction of the AlamarBlue reagent solution.
Statistical analysis
One-way ANOVA followed by Tukey-HSD post hoc testing with the significance level set at 0.05 was used to compare individual means. For cases of unequal variances between groups (determined by Levene's test of homogeneity of variance), Tamhane's T2 test was used instead of Tukey HSD.
Results
Additional maturation time improves surface coverage and EC retention under flow ramping
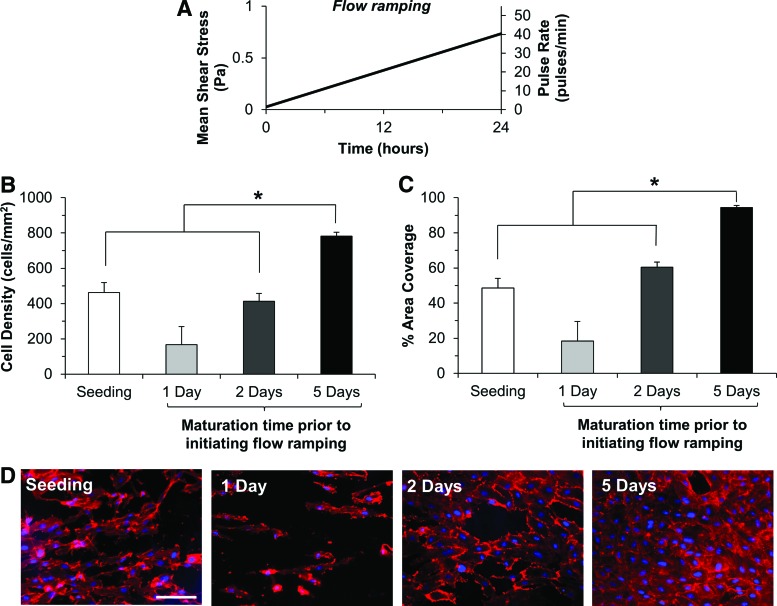
Seeding of HUV grafts with the most efficient rotational seeding method (see Supplementary Results section and Supplementary Fig. S4) resulted in coverage of nearly half of the lumenal surface (462 cells/mm2 at 48.6% area coverage), indicating that additional maturation time was necessary for the adherent EC to grow to confluence. Endothelialized grafts were, therefore, allowed to mature for 1, 2, or 5 days after initial seeding under low shear stress conditions (0.007 Pa). After the specified interval, 6% (m/v) high-molecular-weight (70 kDa) dextran was added to culture media to increase viscosity to 3 mpA*s. This resulted in no significant difference in EC proliferation or viability compared with normal media (Supplementary Fig. S3). The lumenal flow rate was then linearly ramped up to achieve a mean wall SS of 0.734 Pa after 24 h (Fig. 2A).
FIG. 2.
Effects of maturation time on endothelial cell (EC) retention after exposure to ramped flow. EC seeded on HUV scaffolds were allowed to mature on the lumenal surface for 1, 2, or 5 days before 24 h of flow ramping was initiated as described in (A). EC retention was then analyzed as previously described using DAPI/rhodamine phalloidin co-staining. Cell density (B) and percent area coverage (C) were compared between initial seeding and three maturation times; results are presented as mean+standard deviation (SD). Asterisks indicate significant differences in means as specified (p<0.05). Five days of maturation resulted in a significant increase in cell density (B) and percent area coverage (C) when compared with initial seeding or 2 days of maturation. (D) Representative images of EC monolayers cultivated under each condition. Scale bar: 100 μm. Color images available online at www.liebertpub.com/tec
The initiation of flow ramping 1 day after seeding resulted in a loss of cells on the lumenal surface (Fig. 2D), though the differences in cell density/percent coverage were not statistically significant (Fig. 2B, C). Two days of maturation allowed HUV grafts to retain similar cell densities (413±77 vs. 462±125 cells/mm2, p=0.987) and surface coverage (60.4%±5.3% vs. 48.6%±12.2%, p=0.509) as initial seeding. Flow ramping initiated after 5 days of maturation resulted in a significant improvement in cell density (783±49 vs. 462±125 cells/mm2, p=0.016) and surface coverage (94.4%±2.9% vs. 48.6%±12.2%, p=0.005) compared with initial seeding or 2 days of maturation (Fig. 2B, C).
Shear preconditioning facilitates retention of endothelial monolayers on HUV grafts
Decellularized HUV grafts were seeded with HUVEC under an optimal 100 RPH alternating rotational seeding method (Supplementary Fig. S4) and allowed to mature for 5 days under low flow within a perfusion culture system (Fig. 1). After 5 days of maturation, empirically determined to be optimal for strengthening cell-graft binding and preventing early graft denudation (Fig. 2), the lumenal flow rate was progressively ramped according to a previously designed temporal shear regime.29 Over a 48 h period, the mean wall SS applied was steadily increased from 0.03 Pa to 1.45 Pa (Fig. 3A). Onset of flow ramping resulted in a slight, though not statistically significant loss of cells at days 6 (702±95 vs. 738±178 cells/mm2, p=1.000) and 7 (619±172 vs. 738±178 cells/mm2, p=0.713). Despite the decrease in cell density, after onset of ramped flow, the lumenal coverage by EC increased to 97.3%±3.6%, correlating with a 25.7% increase in the mean surface area per cell. This was also apparent morphologically, as cells appeared more spread out after onset of ramped flow compared with days 1 and 5 (Fig. 3D). After 7 days of preconditioning, a confluent and flow-aligned EC monolayer was observed on the lumenal surface of the HUV graft (Fig. 3D).
FIG. 3.
Shear preconditioning of endothelialized HUV grafts. Five days after seeding HUV scaffolds with EC, the flow rate was progressively increased as shown in (A) until physiological wall SS levels were reached. The table (B) shows the lumenal pressure (mean±SD) at various time points throughout the preconditioning period. Scaffolds were co-stained with DAPI/rhodamine phallodin and analyzed in ImageJ over the course of the preconditioning phase. Cell density (mean−SD) and percent area coverage (mean+SD) over the 7 day culture period are shown in (C). Representative images (D) at high magnification (40×) show EC morphology at various time points. Scale bar: 50 μm. Color images available online at www.liebertpub.com/tec
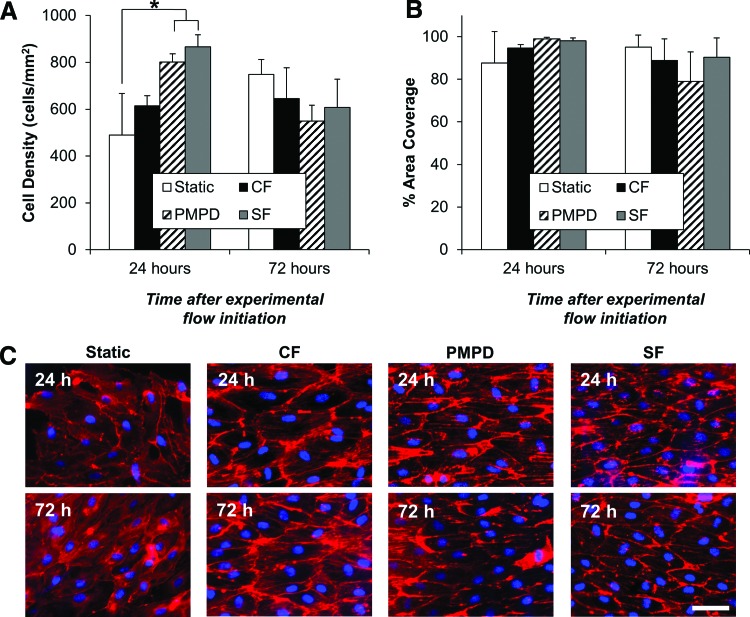
Endothelial monolayer integrity is preserved after exposure to experimental perfusion regimes
For comparison to CF stimulation, the traditional method of in vitro perfusion culture for vascular grafts, two additional perfusion programs were designed to stimulate EC under a dynamic range of conditions (Fig. 4). PMPD and SF each resulted in a significantly higher EC density than static-cultured grafts after 24 h of culture (Fig. 5A). After 72 h of exposure to PMPD or SF, a slight decrease in cell density was observed, though area coverage remained stable, indicating increased EC spreading under these higher shear and pressure conditions. After 72 h, however, no significant difference in cell density or surface area coverage by EC was observed among any of the experimental conditions (Fig. 5A, B). EC cultured under any of the three perfusion conditions maintained similar cytoskeletal alignment in the flow direction at 24 and 72 h of perfusion (Fig. 5C).
FIG. 4.
Experimental perfusion conditions for endothelialized HUV grafts. After applying the 7-day preconditioning regime shown in Figure 3, EC-seeded HUV grafts were exposed to one of three experimental perfusion conditions: constant pulse frequency (CF) (A), physiologically modeled pulse dynamics (PMPD) (B), or stepped flow (SF) (C). Shown at the left are the instantaneous mean wall SS and pulse rates over the 12-h flow cycle. Shown in the tables at the right are the minimum, maximum, and average pressure and mean SS values applied to the grafts during each flow regime.
FIG. 5.
Morphological characterization of flow-conditioned, endothelialized HUV grafts. EC-seeded HUV grafts were cultured under one of four experimental conditions: static culture, CF, PMPD, or SF. After 24 or 72 h, scaffolds were stained with DAPI/rhodamine phalloidin and analyzed as previously described. Mean cell density and percent area coverage are presented in panels (A) and (B), respectively, as mean+SD Asterisks indicate significant differences between individual means (p<0.05). Representative images of EC morphology under each experimental condition after 24 or 72 h are shown in (C). Scale bar: 50 μm. Color images available online at www.liebertpub.com/tec
Perfusion culture stimulates a more functional transcriptional profile in EC compared with static culture conditions
After 72 h of stimulation, EC mRNA was collected for gene expression analysis. Several genes of interest were analyzed, and results were grouped according to gene function (Figs. 6–7).
FIG. 6.
Hemostasis and inflammation gene expression in flow conditioned, endothelialized HUV grafts. Shown is fold mRNA expression (with respect to static cultured EC) of genes involved in hemostasis (A) and inflammation (B). Results are presented as mean+S.E.M. (n=3–4 samples per group). Each asterisk above a data bar indicates a significant difference with respect to static controls; other asterisks indicate a significant difference between flow groups as specified. #indicates significant differences in means relative to all other experimental groups (p<0.05). Experimental perfusion groups: CF: constant pulse frequency, PMPD; SF. Gene abbreviations: ANXA5: annexin V; FN1: fibronectin-1; PLAT: tissue plasminogen activator; PLAU: urokinase plasminogen activator; TFPI: tissue factor pathway inhibitor; THBD: thrombomodulin; vWF: von Willebrand Factor; SELE: E-selectin; SELPLG: selectin P ligand; ICAM-1: intercellular adhesion molecule-1; PECAM-1: platelet-endothelial cell adhesion molecule-1; TNF: tumor necrosis factor; TNFSF10: TNF superfamily member 10; MCP-1: monocyte chemoattractant protein-1.
FIG. 7.
Proliferation and vascular signaling gene expression in flow conditioned, endothelialized HUV grafts. Shown is fold mRNA expression (with respect to static cultured EC) of genes involved in proliferation (A) and vascular signaling (B). Results are presented as mean+S.E.M. (n=3–4 samples per group). Each asterisk above a data bar indicates a significant difference with respect to static controls; other asterisks indicate a significant difference between flow groups as specified. #indicates significant differences in means relative to all other experimental groups (p<0.05). Experimental perfusion groups: CF: constant pulse frequency, PMPD; SF. Gene abbreviations: TYMP: thymidine phosphorylase; FGF1: fibroblast growth factor 1; FLT1: fms-related tyrosine kinase-1; KDR: kinase insert domain receptor; SPHK1: sphingosine kinase 1; TEK: tyrosine kinase, endothelial; VEGFA: vascular endothelial growth factor A; ACE: angiotensin 1 converting enzyme; EDN1: endothelin 1; eNOS: endothelial nitric oxide synthase; SOD1: superoxide dismutase 1; ALOX5: arachidonate 5-lipoxygenase; IL7: interleukin 7; IL11: interleukin 11.
Transcriptional profiles for hemostasis-related genes were mixed. All three perfusion regimes induced upregulation of fibronectin 1 (FN1), but no significant differences were found for other genes (Fig. 6A). von Willebrand Factor (vWF) was upregulated in CF-conditioned EC only (1.73±0.18-fold, p=0.018).
Results were more consistent in genes associated with inflammation. Selectin P ligand (SELPLG) and tumor necrosis factor (TNF) were upregulated in EC conditioned by all three perfusion groups, though the latter was significantly lower under PMPD than SF (1.39±0.03 vs. 1.58±0.02-fold, p=0.022). CF stimulation induced significantly higher expression of PECAM-1 (2.70±0.19-fold) and TNFSF10 (2.02±0.12-fold) than all other groups (Fig. 6B).
The pro-angiogenic growth factors FGF1 and VEGFA were found to be upregulated in EC exposed to CF and PMPD (Fig. 7A). SF-conditioned EC also showed upregulation of FGF1, but no upregulation of VEGFA (2.26±0.06-fold, p=0.178) and significantly lower expression than under CF (4.87±0.52-fold, p=0.004) and PMPD (5.34±0.34-fold, p=0.002). The VEGF receptor FLT1 (aka VEGFR1) was downregulated under all three perfusion regimes, while KDR (aka VEGFR2) was upregulated by only CF and PMPD. The angiopoietin-1 receptor TEK (aka TIE-2) was also upregulated under all three perfusion regimes, though expression was significantly lower under PMPD than CF (1.62±0.03 vs. 2.26±0.16-fold, p=0.008).
PMPD-conditioned EC had significant upregulation of superoxide dismutase (SOD1, 2.47±0.29-fold, p=0.005) and arachidonate 5-lipoxygenase (ALOX5, 4.38±0.20-fold, p=0.009), and showed the highest expression of all the perfusion groups profiled (Fig. 7B). However, SF conditioning induced significantly higher expression of endothelial nitric oxide synthase (eNOS; 1.42±0.13-fold) than SC (p=0.042), CF (p=0.048), or PMPD (p=0.011). All three perfusion regimes resulted in downregulation of angiotensin-converting enzyme (ACE) and upregulation of IL11 (Fig. 7B).
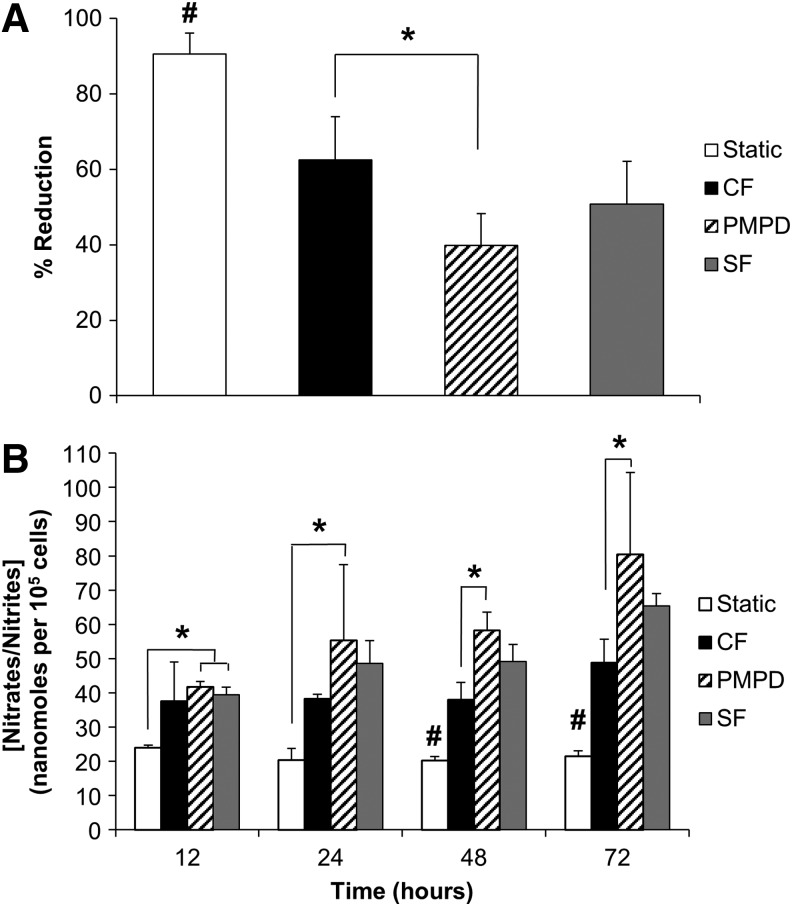
PMPD reduces metabolic activity in endothelialized HUV grafts
After 72 h of perfusion culture, a significant decrease in metabolic activity occurred in EC-seeded grafts exposed to perfusion conditions compared with static-cultured grafts (Fig. 8A). PMPD-conditioned EC had the lowest metabolic activity of all groups, which was also significantly lower than that of CF-conditioned cells (39.8%±8.4% vs. 62.5%±11.5% reduction, p=0.012).
FIG. 8.
Metabolic activity and nitric oxide synthesis in flow conditioned, endothelialized HUV grafts. (A) After 72 h of conditioning, metabolic activity of EC-seeded grafts was assessed using AlamarBlue reagent. Percent reduction was normalized by the number of cells under each condition (n=4–6 samples per condition). (B) Conditioned media samples were collected at 12, 24, 48, and 72 h of variable perfusion culture. Total nitrate/nitrite content in conditioned media samples was quantified and normalized by the number of cells under each perfusion condition (n=3–4 samples per condition). Results are presented as mean+SD Asterisks indicate significant differences in means as specified; #indicates significant differences in means relative to all other experimental groups (p<0.05). Experimental perfusion groups: CF; PMPD; SF.
PMPD induces increased endothelial NO production compared with CF stimulation
EC production of NO was assessed via periodic quantification of total nitrate/nitrite content in conditioned culture media samples. PMPD induced the highest NO production of all experimental groups, and it was the only perfusion condition with a significantly higher nitrate/nitrite formation than static cultured cells at all time points assessed (Fig. 8B). Furthermore, the amount of NO produced by PMPD-conditioned EC was significantly higher than the amount produced by EC exposed to CF stimulation at 48 (58.2±5.3 vs. 38.0±5.2 nmol/105 cells, p=0.003) and 72 h (80.4±23.9 vs. 48.8±6.9 nmol/105 cells, p=0.028).
Discussion
In these investigations, the HUV was chosen as a model conduit for in vitro endothelialization due to its demonstrated clinical relevance,32 applicability as a tissue engineering scaffold,27,28,33 and mechanical compliance similar to natural vessels, which allows the scaffold to deform under physiological pressure conditions.34,35 Compared with the saphenous vein, the HUV is a more widely available vascular tissue source and can be obtained noninvasively, providing a vessel of appropriate length and microstructural properties that is free of bifurcations and can be used for vascular reconstructive therapies.36 Using this model, our aim was to address issues that have limited the success of graft endothelialization in both tissue engineering applications and direct clinical use. Specifically, EC denudation at reperfusion, and an inappropriate “wound” state are characterized by EC upregulation of pro-inflammatory or thrombotic genes, leading to an increased potential for graft occlusion.37–39 Our working hypothesis is that through exposure to appropriate fluid pulse and shear conditioning in vitro, EC will adopt a functional state that is more resilient to arterial hemodynamics and with reduced potential for early inflammatory and thrombotic events.31
Whether engineering fully cellular constructs or seeding EC alone, denudation of EC-seeded grafts due to shear exposure has been a significant problem limiting the success of in vitro endothelialization, and the outcomes of the two-stage seeding method would likely be improved by increasing the resistance of seeded cells to hemodynamic stresses. In this study, a shear ramping technique previously developed using a custom parallel-plate flow chamber with the HUV utilized as a substrate was used to temporally condition EC-seeded HUV scaffolds to arterial SS.29 As the applied SS was increased over the preconditioning period, EC aligned in the flow direction and increased their surface area. These adaptations are consistent with the flattened morphology previously observed,29 which has been shown to reduce SS gradients across the cell membrane.40 The present results are in agreement with previous investigations showing improved EC retention when gradual flow ramping was employed compared with sudden onset at reperfusion.20,30,41,42 Use of a more efficient, rotational seeding method in concert with the application of a substrate-tailored mechanical shear ramping program allowed a confluent, shear-resistant endothelium to be developed on the entire interior lumenal surface of HUV grafts within 1 week of seeding.
An important consideration that has not been addressed is early phenotypic expression of EC seeded onto vascular grafts. Explanted EC cultured in the absence of laminar SS have a more rapid turnover,43 while sustained exposure to growth factors and other culture medium additives used to stimulate EC proliferation concurrently induce stress, eventually leading to a pro-inflammatory and thrombotic senescent state.44 This activated state is significantly different than the quiescent phenotype usually expressed in vivo by arterial EC under healthy conditions.12,45 Implantation of EC that have undergone this phenotypic shift may therefore not elicit desired therapeutic effects, and approaches toward minimizing wound responses are highly sought after. We have previously shown that relative to static or constant pulse frequencies, the application of a temporally variable physiological flow regime induced a more functional, in vivo-like phenotype.31 This regime was thus adapted for use in fully biomimetic tubular perfusion culture to revert EC seeded on vascular allografts to a more appropriate phenotypic state. NO production, a hallmark of endothelial quiescence, was significantly higher in endothelia exposed to PMPD relative to endothelia under constant pulsation, which has to date been the predominant standard for graft assessment. Decreased metabolic activity under PMPD was in agreement with a transcriptional profile that showed a less inflammatory, low-turnover state. The present results confirm that variations in shear mechanics applied to EC in vitro result in similar phenotypic adaptations when applied to endothelialized biomaterials, and the changes evoked are therefore not restricted to EC cultured on inert/synthetic substrates.
For additional comparison, an SF regime was designed in which the pulse frequency/applied SS was temporarily increased in a step change for a brief period each hour. The SF was intended to distinguish whether the pro-functional phenotypic changes elicited by PMPD were strictly due to periodic variations in applied SS, as opposed to continuous change. EC exposed to SF expressed a phenotype that was somewhat between the two extremes observed under CF or PMPD conditions. Thus, the authors concluded that the continuous changes in fluid mechanical stresses applied during PMPD, which mirror the constant flux in hemodynamics observed in vivo, contribute to the marked improvements in EC quiescence. Application of ramped flow followed by similar PMPD flow variations would likely drive EC toward a more favorable state on prosthetic grafts.
In conclusion, the described improvements in seeding technique and perfusion dynamics resulted in the development of a confluent, shear-resistant neo-endothelium within a clinically relevant time frame. The use of fluid shear preconditioning was shown to strengthen EC adhesion in a natural adaptive fashion without the necessity of potentially thrombogenic adhesive coatings. Similarly, the implementation of perfusion conditions that more accurately replicate hemodynamic variability to evoke EC quiescence circumvents the more expensive and laborious gene delivery methods that to date have had limited success in improving graft patency. This work represents important strides in the translation of in vitro endothelialization and graft regeneration as a whole toward a viable clinical strategy. In addition, these observations indicate that a broader perspective is needed in the application of mechanical stimuli to cellularized tissues, where the constant frequencies currently studied in many areas of regenerative medicine may lead to undesirable results. The variability associated with in vivo pulse dynamics and its modulating effects on cell phenotype is likely to translate to other organ systems where biological variability in tissue mechanics is pronounced, and this may lead to more clinically relevant tissue regeneration.
Supplementary Material
Acknowledgment
This work was supported by the National Institutes of Health (grant numbers R01-HL088207, R01-HL088207-03S1).
Disclosure Statement
No competing financial interests exist.
References
- 1.Johnson W.C., and Lee K.K. A comparative evaluation of polytetrafluoroethylene, umbilical vein, and saphenous vein bypass grafts for femoral-popliteal above-knee revascularization: a prospective randomized Department of Veterans Affairs cooperative study. J Vasc Surg 32, 268, 2000 [DOI] [PubMed] [Google Scholar]
- 2.Archie J.P. Femoropopliteal bypass with either adequate ipsilateral reversed saphenous vein or obligatory polytetrafluoroethylene. Ann Vasc Surg 8, 475, 1994 [DOI] [PubMed] [Google Scholar]
- 3.Debus E.S., Larena-Avellaneda A., Heimlich F., Goertz J., and Fein M. Alloplastic bypass material below the knee: actual rationale. J Cardiovasc Surg (Torino) 54, 159, 2013 [PubMed] [Google Scholar]
- 4.Sarkar S., Sales K.M., Hamilton G., and Seifalian A.M. Addressing thrombogenicity in vascular graft construction. J Biomed Mater Res B Appl Biomater 82, 100, 2007 [DOI] [PubMed] [Google Scholar]
- 5.Haruguchi H., and Teraoka S. Intimal hyperplasia and hemodynamic factors in arterial bypass and arteriovenous grafts: a review. J Artif Organs 6, 227, 2003 [DOI] [PubMed] [Google Scholar]
- 6.Wu K.K., and Thiagarajan P. Role of endothelium in thrombosis and hemostasis. Annu Rev Med 47, 315, 1996 [DOI] [PubMed] [Google Scholar]
- 7.Watson S.P. Platelet activation by extracellular matrix proteins in haemostasis and thrombosis. Curr Pharm Des 15, 1358, 2009 [DOI] [PubMed] [Google Scholar]
- 8.Garg U.C., and Hassid A. Nitric oxide-generating vasodilators and 8-bromo-cyclic guanosine monophosphate inhibit mitogenesis and proliferation of cultured rat vascular smooth muscle cells. J Clin Invest 83, 1774, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garanich J.S., Pahakis M., and Tarbell J.M. Shear stress inhibits smooth muscle cell migration via nitric oxide-mediated downregulation of matrix metalloproteinase-2 activity. Am J Physiol Heart Circ Physiol 288, H2244, 2005 [DOI] [PubMed] [Google Scholar]
- 10.Davies P.F. Flow-mediated endothelial mechanotransduction. Physiol Rev 75, 519, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Triggle C.R., Samuel S.M., Ravishankar S., Marei I., Arunachalam G., and Ding H. The endothelium: influencing vascular smooth muscle in many ways. Can J Physiol Pharmacol 90, 713, 2012 [DOI] [PubMed] [Google Scholar]
- 12.Aird W.C. Phenotypic heterogeneity of the endothelium: I. Structure, function, and mechanisms. Circ Res 100, 158, 2007 [DOI] [PubMed] [Google Scholar]
- 13.Alobaid N., Salacinski H.J., Sales K.M., Hamilton G., and Seifalian A.M. Single stage cell seeding of small diameter prosthetic cardiovascular grafts. Clin Hemorheol Microcirc 33, 209, 2005 [PubMed] [Google Scholar]
- 14.Herring M., Gardner A., and Glover J. A single-staged technique for seeding vascular grafts with autogenous endothelium. Surgery 84, 498, 1978 [PubMed] [Google Scholar]
- 15.Herring M.B., Dilley R., Jersild R.A., Boxer L., Gardner A., and Glover J. Seeding arterial prostheses with vascular endothelium. The nature of the lining. Ann Surg 190, 84, 1979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heyligers J.M., Arts C.H., Verhagen H.J., de Groot P.G., and Moll F.L. Improving small-diameter vascular grafts: from the application of an endothelial cell lining to the construction of a tissue-engineered blood vessel. Ann Vasc Surg 19, 448, 2005 [DOI] [PubMed] [Google Scholar]
- 17.Herring M., Smith J., Dalsing M., Glover J., Compton R., Etchberger K., et al. Endothelial seeding of polytetrafluoroethylene femoral popliteal bypasses: the failure of low-density seeding to improve patency. J Vasc Surg 20, 650, 1994 [DOI] [PubMed] [Google Scholar]
- 18.Bordenave L., Fernandez P., Rémy-Zolghadri M., Villars S., Daculsi R., and Midy D. In vitro endothelialized ePTFE prostheses: clinical update 20 years after the first realization. Clin Hemorheol Microcirc 33, 227, 2005 [PubMed] [Google Scholar]
- 19.Zilla P., Preiss P., Groscurth P., Rösemeier F., Deutsch M., Odell J., et al. In vitro-lined endothelium: initial integrity and ultrastructural events. Surgery 116, 524, 1994 [PubMed] [Google Scholar]
- 20.Dardik A., Liu A., and Ballermann B.J. Chronic in vitro shear stress stimulates endothelial cell retention on prosthetic vascular grafts and reduces subsequent in vivo neointimal thickness. J Vasc Surg 29, 157, 1999 [DOI] [PubMed] [Google Scholar]
- 21.Deutsch M., Meinhart J., Zilla P., Howanietz N., Gorlitzer M., Froeschl A., et al. Long-term experience in autologous in vitro endothelialization of infrainguinal ePTFE grafts. J Vasc Surg 49, 352; discussion 62, 2009 [DOI] [PubMed] [Google Scholar]
- 22.Laube H.R., Duwe J., Rutsch W., and Konertz W. Clinical experience with autologous endothelial cell-seeded polytetrafluoroethylene coronary artery bypass grafts. J Thorac Cardiovasc Surg 120, 134, 2000 [DOI] [PubMed] [Google Scholar]
- 23.Menu P., Stoltz J.F., and Kerdjoudj H. Progress in vascular graft substitute. Clin Hemorheol Microcirc 53, 117, 2013 [DOI] [PubMed] [Google Scholar]
- 24.Paternotte E., Kerdjoudj H., Kokten T., Stoltz J.F., Kearney-Schwartz A., Voegel J.C., et al. Endothelialized and preconditioned natural umbilical arteries with long term patency open the route for future human uses. Clin Hemorheol Microcirc 54, 223, 2013 [DOI] [PubMed] [Google Scholar]
- 25.Wu Y., Li L., Chen W., Zeng W., Zeng L., Wen C., et al. Maintaining moderate platelet aggregation and improving metabolism of endothelial progenitor cell increase the patency rate of tissue engineered blood vessels. Tissue Eng Part A, 2015. [Epub ahead of print]; DOI: 10.1089/ten.tea.2015.0013 [DOI] [PubMed] [Google Scholar]
- 26.Goh E.T., Wong E., Farhatnia Y., Tan A., and Seifalian A.M. Accelerating in situ endothelialisation of cardiovascular bypass grafts. Int J Mol Sci 16, 597, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Uzarski J.S., Van De Walle A.B., and McFetridge P.S. Preimplantation processing of ex vivo-derived vascular biomaterials: effects on peripheral cell adhesion. J Biomed Mater Res A 101, 123, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Daniel J., Abe K., and McFetridge P.S. Development of the human umbilical vein scaffold for cardiovascular tissue engineering applications. ASAIO J 51, 252, 2005 [DOI] [PubMed] [Google Scholar]
- 29.Uzarski J.S., Van de Walle A.B., and McFetridge P.S. In vitro method for real-time, direct observation of cell-vascular graft interactions under simulated blood flow. Tissue Eng Part C Methods 20, 116, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McFetridge P.S., Bodamyali T., Horrocks M., and Chaudhuri J.B. Endothelial and smooth muscle cell seeding onto processed ex vivo arterial scaffolds using 3D vascular bioreactors. ASAIO J 50, 591, 2004 [DOI] [PubMed] [Google Scholar]
- 31.Uzarski J.S., Scott E.W., and McFetridge P.S. Adaptation of endothelial cells to physiologically-modeled, variable shear stress. PLoS One 8, e57004, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dardik H. A 30-year odyssey with the umbilical vein graft. J Am Coll Surg 203, 582, 2006 [DOI] [PubMed] [Google Scholar]
- 33.Hoenicka M., Lehle K., Jacobs V.R., Schmid F.X., and Birnbaum D.E. Properties of the human umbilical vein as a living scaffold for a tissue-engineered vessel graft. Tissue Eng 13, 219, 2007 [DOI] [PubMed] [Google Scholar]
- 34.Li W., Huang T., Zeng Y., and Yao Z. The relationship between gestational age and compliance in human umbilical vein and its possible application in vascular grafting. Ann Vasc Surg 20, 237, 2006 [DOI] [PubMed] [Google Scholar]
- 35.Reinhart-King C.A., Fujiwara K., and Berk B.C. Physiologic stress-mediated signaling in the endothelium. Methods Enzymol 443, 25, 2008 [DOI] [PubMed] [Google Scholar]
- 36.Li W.C., Zhang H.M., Wang P.J., Xi G.M., Wang H.Q., Chen Y., et al. Quantitative analysis of the microstructure of human umbilical vein for assessing feasibility as vessel substitute. Ann Vasc Surg 22, 417, 2008 [DOI] [PubMed] [Google Scholar]
- 37.Rotmans J.I., Heyligers J.M., Stroes E.S., and Pasterkamp G. Endothelial progenitor cell-seeded grafts: rash and risky. Can J Cardiol 22, 1113, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rotmans J.I., Heyligers J.M., Verhagen H.J., Velema E., Nagtegaal M.M., de Kleijn D.P., et al. In vivo cell seeding with anti-CD34 antibodies successfully accelerates endothelialization but stimulates intimal hyperplasia in porcine arteriovenous expanded polytetrafluoroethylene grafts. Circulation 112, 12, 2005 [DOI] [PubMed] [Google Scholar]
- 39.Baguneid M., de Mel A., Yildirimer L., Fuller B.J., Hamilton G., and Seifalian A.M. In vivo study of a model tissue-engineered small-diameter vascular bypass graft. Biotechnol Appl Biochem 58, 14, 2011 [DOI] [PubMed] [Google Scholar]
- 40.Barbee K.A., Davies P.F., and Lal R. Shear stress-induced reorganization of the surface topography of living endothelial cells imaged by atomic force microscopy. Circ Res 74, 163, 1994 [DOI] [PubMed] [Google Scholar]
- 41.Yazdani S.K., Tillman B.W., Berry J.L., Soker S., and Geary R.L. The fate of an endothelium layer after preconditioning. J Vasc Surg 51, 174, 2010 [DOI] [PubMed] [Google Scholar]
- 42.Inoguchi H., Tanaka T., Maehara Y., and Matsuda T. The effect of gradually graded shear stress on the morphological integrity of a huvec-seeded compliant small-diameter vascular graft. Biomaterials 28, 486, 2007 [DOI] [PubMed] [Google Scholar]
- 43.Davies P.F., Remuzzi A., Gordon E.J., Dewey C.F., and Gimbrone M.A. Turbulent fluid shear stress induces vascular endothelial cell turnover in vitro. Proc Natl Acad Sci U S A 83, 2114, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Erusalimsky J.D. Vascular endothelial senescence: from mechanisms to pathophysiology. J Appl Physiol 106, 326, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Durr E., Yu J., Krasinska K.M., Carver L.A., Yates J.R., Testa J.E., et al. Direct proteomic mapping of the lung microvascular endothelial cell surface in vivo and in cell culture. Nat Biotechnol 22, 985, 2004 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.