Abstract
Phospholipid hydroperoxide glutathione peroxidase (PhGPx) is an antioxidant enzyme that reduces cellular phospholipid hydroperoxides (PLOOHs) to alcohols. Cellular peroxide tone has been implicated in cell growth and differentiation. By reducing the PLOOH level in the cell membrane, PhGPx regulates the peroxide tone and thereby might be involved in cell growth. We hypothesized that overexpression of PhGPx in human breast cancer cells would decrease their growth rate. We stably transfected MCF-7 cells (Wt) with L-PhGPx and measured cell doubling time, plating efficiency, and cell cycle phase transit times. P-4 cells (8-fold increase in PhGPx activity) showed a 2-fold increase in doubling time; doubling time increased directly with PhGPx activity (r = 0.95). The higher the PhGPx activity, the lower the plating efficiency (r = −0.86). The profile of other antioxidant enzymes was unchanged. Overexpression of PhGPx lowered the steady-state level of PLOOH (by >60%). Results from bromodeoxyuridine pulse-chase experiments and flow cytometry indicate that PhGPx induced a delay in MCF-7 proliferation that was primarily due to a slower progression from G1 to S. These results support the hypothesis that PhGPx plays a regulatory role in the progression of MCF-7 cells from G1 to S possibly by regulating the steady-state levels of PLOOH. These data suggest that PhGPx can lower the peroxide tone, which might change the cellular redox environment resulting in a delay in G1 transit. Thus, PhGPx could be an important factor in cell growth.
Keywords: Glutathione peroxidase, Lipid hydroperoxide, Cell cycle, Antioxidant enzymes
INTRODUCTION
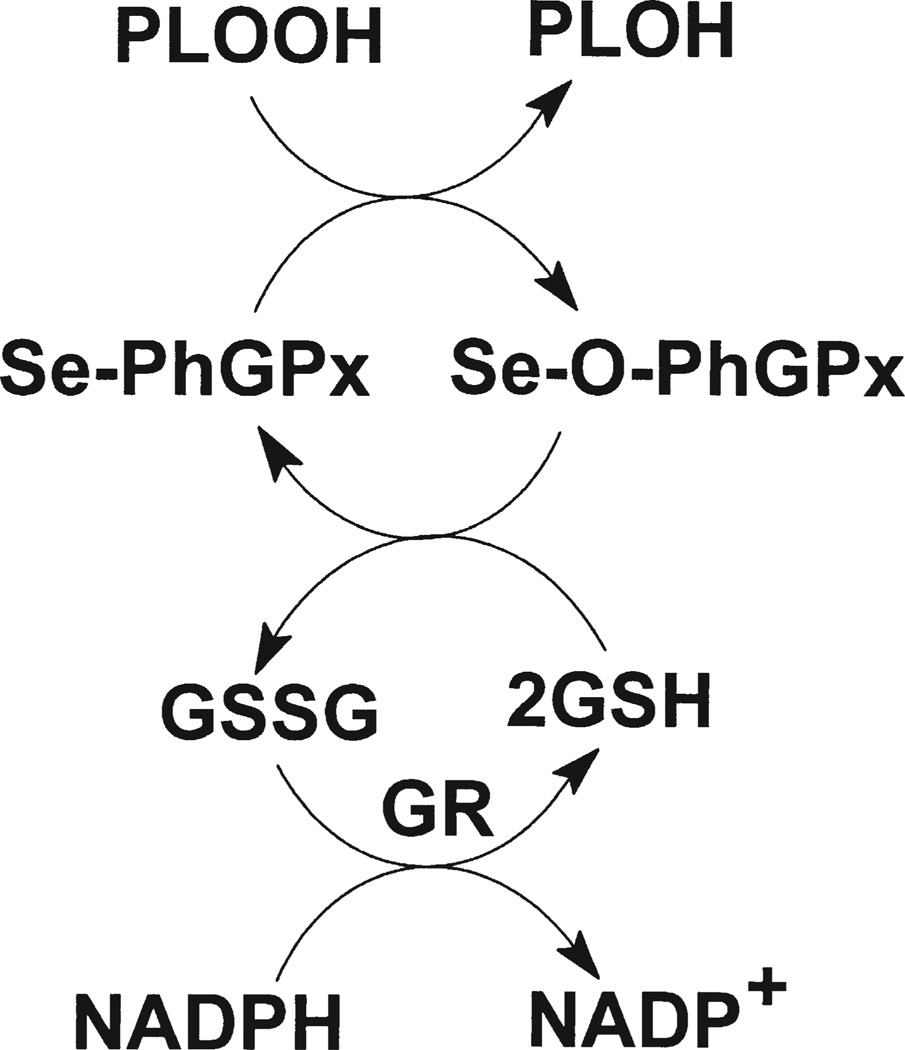
Phospholipid hydroperoxide glutathione peroxidase (PhGPx or GPx-4) is a unique selenium-dependent antioxidant enzyme that reduces membrane-bound phospholipid hydroperoxides (PLOOHs), Scheme 1.[1,2] The human PhGPx gene encodes a cytosolic (S-PhGPx) and mitochondrial (L-PhGPx) form of the protein. L-PhGPx (~23 kDa) contains 27 amino acids at the N-terminus that serve as a mitochondrial-targeting signal. This leader sequence directs the protein to the intermembrane space of the mitochondria, where it is cleaved by a membrane-associated peptidase, yielding a mature protein the same size as S-PhGPx.[3] L-PhGPx is particularly abundant in testis tissue, while S-PhGPx is present in most somatic tissues.
SCHEME 1.
Overview of the mechanism for the conversion of phospholipid hydroperoxides to their corresponding alcohols by PhGPx and the glutathione system.
PhGPx plays an important role in the regulation of cellular hydroperoxides.[4 – 6] Lipid hydroperoxides (LOOHs) have, in the past, been considered toxic compounds. But recent data suggest that they are also signaling molecules. LOOHs have been implicated in a variety of physiological and pathophysiological processes such as cell differentiation, aging, carcinogenesis, inflammation, hypoxia, and atherogenesis.[7 – 10] LOOHs are also involved in NF-κB activation[11,12] and play a role in triggering apoptotic cell death.[13 – 15] Removal of all LOOHs is not necessarily advantageous to cells. Rather, the maintenance of a certain peroxide tone, that is the steady-state level of LOOHs, is important for appropriate functioning of cells. It is of interest to compare the relative abundance of PhGPx and cytosolic glutathione (GPx-1), because they both contribute to the reduction of intracellular hydroperoxides. It has been found that the ratio of PhGPx/GPx-1 is highest in testis tissue and very low in other normal tissues such as liver, kidney, spleen, lung, skeletal muscle, heart, and brain.[16] However, the ratio of PhGPx/GPx-1 is relatively high in tumor tissues derived from liver, kidney, and mammary cells.[16] This suggests that PhGPx might play a role in cell growth.
Glutathione peroxidase,[17 – 19] catalase,[20 – 24] MnSOD[23 – 28] and CuZnSOD[29] have been shown to influence cell growth. To test if PhGPx plays a role in cell growth we stably transfected human mitochondrial PhGPx sense cDNA into MCF-7 cells[30] and studied the impact of PhGPx on cell growth.
MATERIALS AND METHODS
Reagents
The primary polyclonal antibodies against MnSOD, CuZnSOD, and GPx-1 were developed in Dr L.W. Oberley’s laboratory.[31] The primary antibody against catalase (CAT) was obtained from Calbiochem–Novabiochem Corp. (San Diego, CA). Horseradish peroxidase conjugated to goat anti-rabbit IgG was purchased from Boehringer Mannheim (Indianapolis, IN). Primary and secondary antibodies to bromodeoxyuridine were obtained from Beckton Dickinson Immunocytometry Systems (San Jose, CA). Glutathione (GSH), sodium selenite, 5-sulfosalicylic acid (SSA), 5, 5′-dithio-bis-(2-nitrobenzoic acid) (DTNB), dichlorophenol–indophenol, pepsin, bromodeoxyuridine, cytidine, thymidine, porcine pepsin, RNaseA, and 3(4,5-dimethylthiazolyl-2)-2,5-diphenyl tetrazolium acid were purchased from Sigma (St Louis, MO). LipofectAMINE™, minimum essential medium (MEM), fetal bovine serum (FBS), HBSS (without magnesium, calcium and phenol red), and Geneticin™ (G418) were from GIBCO (Grand Island, NY). 2-Vinylpyridine (2-VP) was from Aldrich (Milwaukee, WI). Propidium iodide was from Cal Biochem, (La Jolla, CA).
Cell Culture and Stable Transfection of PhGPx into MCF-7 Cells
MCF-7 cells, from American Type Culture Collection (ATCC), were cultured routinely in MEM containing 10% FBS, 1% non-essential amino acids, and 30nM sodium selenite. Cells were transfected with L-PhGPx (cDNA of L-PhGPx inserted into pcDNA3.1) using LipofectAMINE (LifeTech Inc., NY) as described previously.[30] After stable transfection, the selected clones were grown in medium with the selection antibiotic G418 (350 µg/mL). Wt (wild type), Neo (vector only control), and stably transfected cell lines (P1, P2, P3, and P4) were utilized. These transfected cells have significantly elevated levels of PhGPx, both at the mRNA and protein level. The activity of PhGPx is 8-fold higher in P-4 cells compared to Wt and Neo. The antibiotic was removed one cell passage before an experiment and cells were grown in the same medium as Wt. Cells were incubated at 37°C in a humidified atmosphere with 5% CO2.
Glutathione Measurement
The GSH/GSSG assay was adapted from Anderson[32] with minor modifications. Total intracellular GSH (GSH + GSSG) was determined by the colorimetric reaction of DTNB (5,5′-dithio-bis(2-nitrobenzoic acid)) with GSH to form TNB (5-thio-2-nitrobenzoic acid). The rate of TNB formation, which is proportional to the total GSH concentration, was measured spectrophotometrically at 412 nm. The cellular GSSG level was determined by the same DTNB assay after GSH was masked with 2-VP. GSH was determined by subtracting the GSSG content from the total GSH content. Glutathione disulfide levels were below the detection limit of this assay.
Cell Doubling Time
The cell growth rate was determined by counting the number of cells as a function of time. Cells were seeded in 60mm dishes at ~5 × 104 cells per dish containing 4mL medium. Triplicates for each clone were plated for each time point. MCF-7 cells tend to aggregate after trypsinization; we found that detaching the cells in calcium- and magnesium-free solution with EDTA overcomes this problem. Thus, for detaching cells for counting they were incubated with 5mM EDTA in HBSS (without calcium and magnesium) for 20 min. Cells were diluted with HBSS and counted every day for 9 days with a Coulter Counter®. Doubling time (Td) was calculated from the logarithmic phase on the growth curve using Td = 0.693*t/ln(Nt/N0), where t is the time in days, Nt is the cell number at time t, N0 is the cell number at initial time.
Plating Efficiency
A single cell suspension was plated into 60-mm dishes. The cell number plated was calculated to yield ideally about 100 colonies per dish. The cells were kept in culture medium and incubated at 37°C for 14 days to allow colony formation. Cells were fixed with methanol/acetic acid (3:1, v/v) and stained with 0.1% crystal violet. Colonies containing 50 cells or more were counted. The plating efficiency (PE) was calculated as PE = (Colonies formed/Cells seeded) × 100%.
Lipid Hydroperoxide Assay
The steady-state level of cellular PLOOHs was determined using the Lipid Hydroperoxide Kit (Cayman, Ann Arbor, MI) as described in detail previously.[30]
Protein Assay
Cell pellets were harvested by gently scraping the cells from the culture dishes. The pellets were lysed using sonication. The protein concentration of cell lysates was determined by BioRad protein assay according to the manufacturer’s protocol (Bio-Rad, Hercules, CA).
Western Analysis for MnSOD, CuZnSOD and CAT
Western analysis was used for the examination of the protein levels of MnSOD, CuZnSOD, and CAT in the cells after PhGPx transfection. Briefly, cell homogenate (30 µg) was exposed to SDS-PAGE and transferred to nitrocellulose membranes (Schleicher and Schuell, Keene, NH). The membranes were probed with specific antibodies, MnSOD (1:1000 dilution), CuZnSOD (1:250 dilution), and CAT (1:1000 dilution) overnight at 4°C and exposed to X-ray film using an enhanced hemiluminescence (ECL) kit (Amersham Pharmacia Biotech, Piscataway, NJ).
Activity Assay for MnSOD, CuZnSOD, Catalase and Glutathione Reductase
Native activity gel assay[33] was used to examine the activity of SOD enzymes in the cells after PhGPx transfection. Cells were harvested by scraping from 60mm dishes. The cell pellet was homogenized and the protein was quantified. Total cellular protein (200 µg for SOD, 30 µg for CAT, and 500 µg for GR) was separated in a 12% native polyacrylamide gel with 5% stacking gel. The protein was electrophoresed in pre-electrophoresis buffer for 3 h at 4°C, followed by 4 h in electrophoresis buffer (Tris 6.06 g/L, glycine 22.5 g/L, disodium EDTA 0.68 g/L, pH 8.3) at 4°C. For SOD activities, the gel was stained with 2.34mM NBT, 28 µM riboflavin, and 28mM TEMED for 20 min in the dark, followed by exposure to a bright fluorescent light. The achromatic bands indicate the presence of the corresponding SOD activities. For catalase (CAT) activity, the gel was incubated with 0.003% H2O2 for 10 min, followed by incubation with 2% ferric chloride and 2% potassium ferricyanide. The achromatic bands indicate the presence of CAT activity. For glutathione reductase (GR), the gel was placed in a freshly made dye solution (3.4mM GSSG, 0.36mM NADPH, 0.052mM dichlorophenol–indophenol, 1.1mM 3(4,5-dimethyl thiazolyl-2)-2,5-diphenyltetrazolium acid prepared in 250mM Tris, pH 8.0). GR activity is indicated by the presence of a purple precipitate in the gel.
Native Immunoblotting Analysis for GPx-1
The protein level of GPx-1 was examined using a native immunoblotting method.[17] Total cellular protein (700 µg) was separated on a 12% native polyacrylamide gel with 5% stacking gel that had been pre-electrophoresed. The native protein was transferred to a nitrocellulose membrane, and probed with GPx-1 primary antibody to human GPx-1 (1:300 dilution) overnight at 4°C. After that, the membrane was incubated with horseradish peroxidase conjugated with goat-anti-rabbit IgG (1:5,000) for 1 h at room temperature. The visualization of the antigen/antibody complex was done using an enhanced chemiluminescence (ECL) kit (Amersham).
BrdU Labeling and Flow Cytometric Assay
Asynchronously growing cell cultures in 100mm tissue culture dishes were pulse-labeled with bromodeoxyuridine (BrdU, 10 µM final concentration) for 30 min at 37°C. At the end of the pulse labeling, monolayer cultures were washed with sterile and warm phosphate-buffered saline (PBS) to remove any unincorporated BrdU from the medium. Fresh media with cytidine and thymidine (10 µM final concentration) were added and BrdU-labeled cells were continued in culture and harvested at regular intervals by trypsinization. Cell pellets, collected by centrifugation, were washed once with PBS and fixed in 70% ethanol. Fixed cells were stored at 4°C and analyzed by flow cytometry.
Flow cytometric analysis of BrdU-labeled cells was performed following previously published procedures.[34,35] Flow cytometric analysis was carried out on a FACSCalibur (Becton Dickinson, San Jose, CA) equipped with 15mW and 488nm argon-ion laser. FITC fluorescence was detected through a 535nm band-pass filter and PI-fluorescence detected through a 640nm long-pass filter. Data from a minimum of 20,000 cells were acquired.
The acquired data were displayed as dual-parameter PI vs log-FITC histograms and three compartments (BrdU-positive S-phase cells, BrdU-negative G1 and G2 phases) identified using CellQuest-software (San Jose, CA). Fractions of cells in G1 and G2 were used as a measurement of G1 and G2 transit time. Transit through S-phase was measured by determining relative movement (RM) as described earlier by Begg et al.[35] RM was calculated from the mean DNA content of undivided BrdU-positive cell population normalized to mean DNA content of the G1 and G2 populations.
Statistics
Mean values, standard errors, and linear regression were determined using Microsoft Excel program (Excel 97 SR-1). ANOVA–Tukey test was used to compare the differences between groups. In all cases, the statistical significance of differences between the two variants was determined at the level of P < 0.05. All the data presented were from the average of at least three independent experiments. In the case of immuno blot analyses, representative results are presented.
RESULTS
PhGPx Inhibits Cell Growth in MCF-7 Cells
Excessive generation of ROS can cause cell death, while moderate ROS production may stimulate signal transduction and gene activation.[36,37] Hydroperoxides have been reported to play a role in cell proliferation and differentiation.[10,38] The stage-specific expression pattern of PhGPx in spermatogenesis suggests that LOOH’s are involved in the maturation of sperm.[39] We hypothesized that PhGPx may modulate cell growth by regulating cellular hydroperoxide tone. Cell proliferation was examined with growth curves and cell doubling time. The ability of cells to undergo attachment and division was measured with plating efficiency.
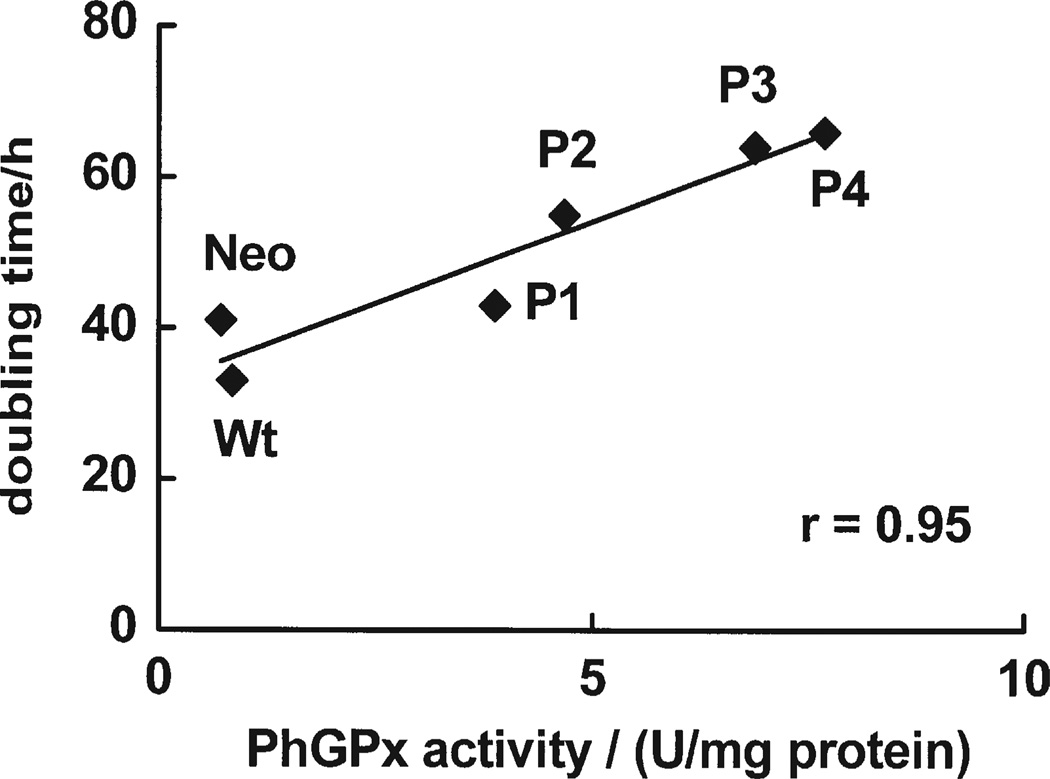
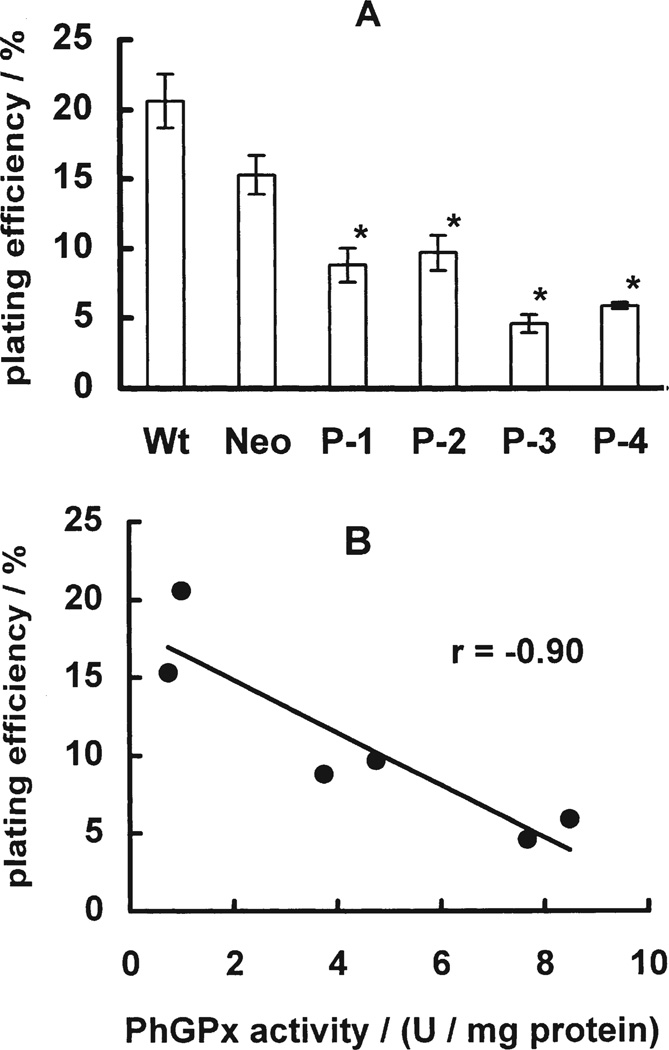
Growth curves are a measure of the balance between cell proliferation and cell death. Cells transfected with different levels of PhGPx (P-1, P-2, P-3, P-4)[30] exhibited different growth rates compared to controls (Wt and Neo), (data not shown). The cell doubling time ranged from 66 h in the cells with the highest PhGPx activity (P-4), to 33 h in Wt, Fig. 1. Cell doubling time correlated directly with cellular PhGPx activity (r = 0.95). PhGPx also significantly influenced plating efficiency. Control cells (Wt, Neo) had higher plating efficiency, 20.5% and 15.3%, compared to P-4, 4.6%, Fig. 2A. Correlation analysis showed that plating efficiency correlates inversely with PhGPx activity. These results indicate that PhGPx can influence cell growth. Increased expression of PhGPx can increase cell-doubling time and significantly decrease plating efficiency.
FIGURE 1.
PhGPx inhibits cell growth. The cell doubling time was calculated from the growth curves during exponential growth phase.
FIGURE 2.
PhGPx decreases plating efficiency. (A) Plating efficiency. Cells were seeded into 60-mm dishes at a certain cell number. After 2 weeks incubation at 37°C, cells were fixed and stained. Colonies containing more than 50 cells were counted. The plating efficiency (PE) was calculated as: PE = (Colonies formed/number of cells seeded) × 100%. Data are mean ± SE, n = 3; *p < 0.05 compared to Wt. (B) Correlation of plating efficiency and PhGPx activity. Data are derived from Fig. 2A and Table I.
PhGPx does not Alter the Antioxidant Enzyme Profile
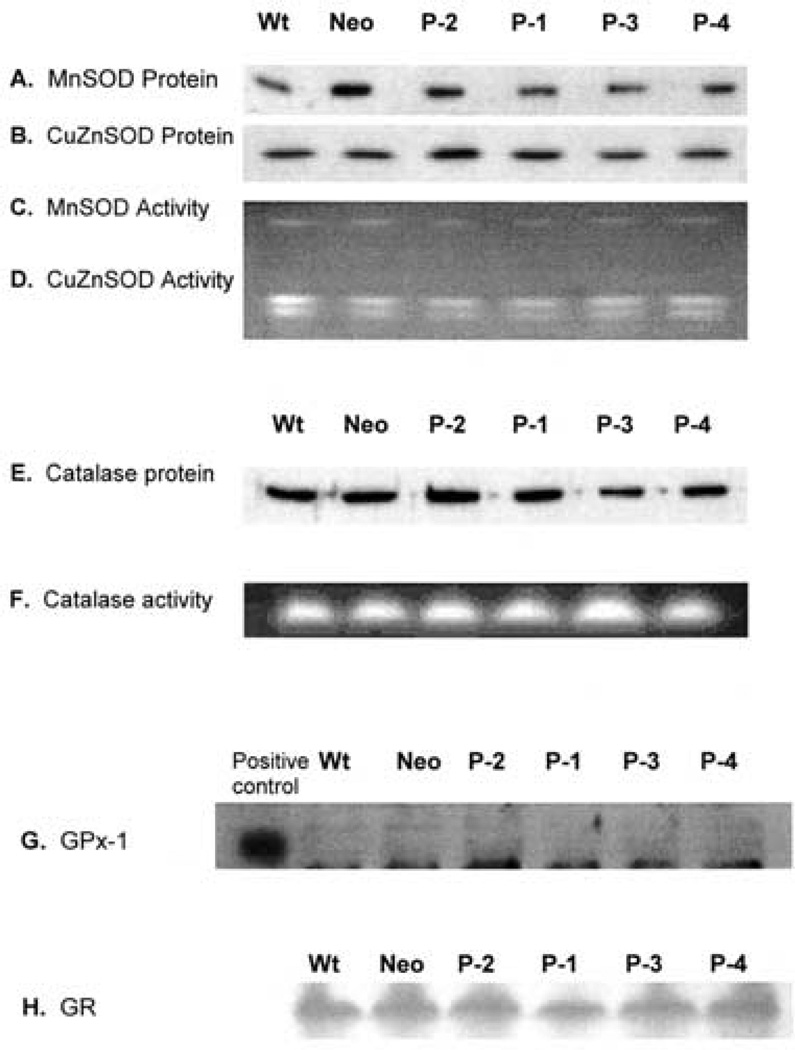
The antioxidant system is important for cells in the defense against endogenous and exogenous oxidative stress. The antioxidant enzymes work coordinately to achieve an equilibrium between pro-oxidant and antioxidant systems. Tumor cells appear to have an altered antioxidant system compared to normal cells. For example, MnSOD and GPx-1 in general are lower in tumor cells compared to their normal counterparts.[25,40] Experimentally altering expression of a particular antioxidant enzyme in cells leads to changes in the levels of other antioxidant enzyme.[41] To test if overexpression of PhGPx influences other antioxidant enzymes, the protein levels and activity of MnSOD, CuZnSOD, CAT, GR and GPx-1 were examined in cells transfected with different levels of L-PhGPx (P-1, P-2, P-3, P-4). The protein levels and activities of MnSOD, CuZnSOD, GR and CAT did not change significantly after PhGPx transfection, Fig. 3. The protein level of GPx-1 in MCF-7 cells was below the detection limit before and after the transfection. These observations indicate that overexpression of PhGPx does not significantly change the other measured cellular antioxidant enzyme levels.
FIGURE 3.
PhGPx does not alter the levels of MnSOD, CuZnSOD, CAT or GPx-1 in MCF-7 cells. (A) Western blot for MnSOD. Total cellular protein (30µg) was electrophoresed on a 12.5% SDS-polyacrylamide gel and detected using anti-human MnSOD polyclonal antibody. (B) Western blot for CuZnSOD. Total cellular protein (30 µg) was electrophoresed on a 12.5% SDS-polyacrylamide gel and detected using anti-human CuZnSOD polyclonal antibody. (C and D) In gel activity assay for MnSOD and CuZnSOD. Total cellular protein (200µg) was electrophoresed on a 12% native gel, followed by incubation in nitroblue tetrazolium (NBT) and riboflavin-TEMED solution. The gel was washed with water and illuminated under a bright fluorescent light. Achromatic bands indicate the presence of SOD.(E).Western blot for catalase. Total cellular protein (30µg) was electrophoresed on a 12.5% SDS-polyacrylamide gel, and detected by the anti-human catalase polyclonal antibody. (F) In gel activity assay for catalase. Total cellular protein (30µg) was electrophoresed on a 12% native gel, followed by incubation with 0.003% H2O2. The activity of catalase was determined by staining the gel with ferric chloride and potassium ferricyanide. (G) The native immunoblotting assay for GPx-1. Total cellular protein (700µg) was separated on a 12% native gel. After transferring onto a nitrocellulose membrane, GPx-1 was detected by a polyclonal antibody against human GPx-1. (H) In gel activity assay for GR. Cellular protein (500µg) was separated onto a 12% native gel. GR activity was detected by staining the gel with 3.4 mM GSSG, 0.36 mM NADPH, 0.052mM dichlorophenol–indophenol, and 1.1mM 3(4,5-dimethythiazolyl-2)-2,5-diphenyl tetrazolium. Data are representative of three independent experiments.
PhGPx has a Minor Effect on Cellular GSH Pool
PhGPx is a seleno-dependent glutathione peroxidase that requires GSH as a cofactor for the reduction of PLOOH, Scheme 1. Thus, PhGPx may contribute to the redox state of the GSSG/2GSH couple and the overall redox environment of the cell.[42]
Transfection of PhGPx resulted in only a minor change of GSH in MCF-7 cells, Table I. Total GSH levels in cells after PhGPx transfection were slightly decreased compared to Wt and Neo controls, ranging from 5 to 9µmol GSH per mg protein. The Neo cells had the highest level of GSH (10 µmol/mg protein) compared to Wt and other transfected cells. However, there were no statistical differences in these variations. The GSSG levels in all the cells examined were below the detection limit (<1.8 nmol/mg protein). The level of GR after PhGPx transfection was also studied and no change in its activity was observed, Fig. 3.
TABLE I.
PhGPx activity
| Cell lines | GSH (µmol/mg protein) |
PhGPx activity* (U/mg protein) |
|---|---|---|
| Wt | 8.2 ± 2.3 | 0.84 ± 0.22 |
| Neo | 10.1 ± 3.2 | 0.71 ± 0.09 |
| P-1 | 5.4 ± 1.5 | 3.87 ± 0.43† |
| P-2 | 8.8 ± 3.6 | 4.67 ± 0.97† |
| P-3 | 6.9 ± 1.5 | 6.89 ± 0.84‡ |
| P-4 | 7.8 ± 2.3 | 7.70 ± 0.80‡ |
The PhGPx activity is as reported in Ref. [30].
P < 0.05 compared to Wt.
P < 0.001 compared to Wt.
In contrast to GPx-1 transfection,[17] overexpression of PhGPx appears not to influence the cellular GSH system. This difference might be explained by the different location of GPx-1 and L-PhGPx in cells. GPx-1 is present in the cytosol, as well as in the mitochondria.[43] L-PhGPx is located in the intermembrane space of the mitochondria.[44] The main source of GSH for GPx-1 and L-PhGPx differs. GPx-1 utilizes dominantly the cytosolic GSH, whereas L-PhGPx uses mitochondrial GSH. Typically, only 10% of the cellular GSH is found in the mitochondria.[45] Thus, changes in mitochondrial GSH might not be observed in a measurement of total cellular GSH.
Increased Expression of PhGPx Decreases Steady-state Levels of PLOOH
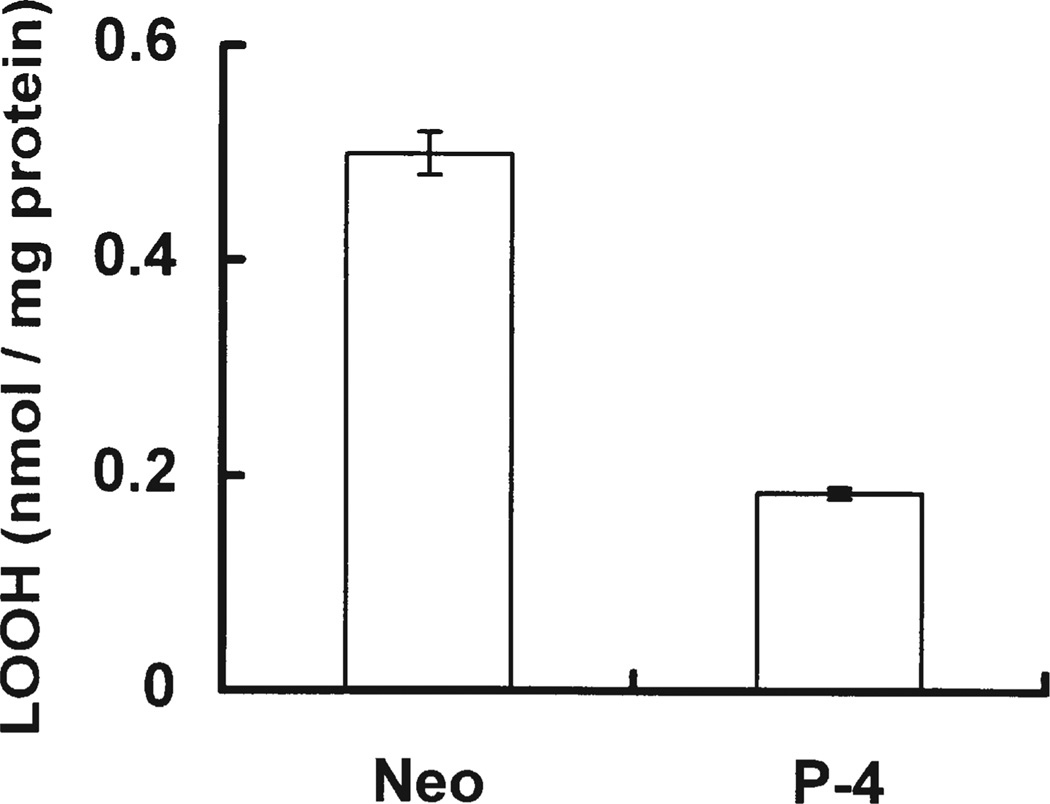
The glutathione peroxidases (GPxs) and the non-selenium glutathione S-transferases (GSTs)[46–48] play an important role in lowering the steady-state level of hydroperoxides. To examine whether transfection of PhGPx changes the steady-state level of PLOOHs, we analyzed Neo and P-4 cell lines for lipid hydroperoxides. Steady-state levels of cellular PLOOHs were measured without any designed exposure to an oxidative stress. PLOOH steady-state level in P-4 cells was found to be only 37% of that of Neo, Fig. 4. Thus, PhGPx can lower the steady-state level of PLOOHs and thereby alters cellular peroxide tone.
FIGURE 4.
PhGPx influences the steady-state level of cellular PLOOHs. Neo and P-4 cells were grown on 100-mm dishes to 80% confluence. Cellular lipids were extracted with chloroform/methanol (2:1, v/v). The chloroform layer was used for PLOOH determination using the Cayman Lipid Hydroperoxide Assay Kit. Total PLOOHs were normalized to total cellular protein. Data are mean, n = 2, the bars represent the range.
Overexpression of PhGPx Delays G1 Transit
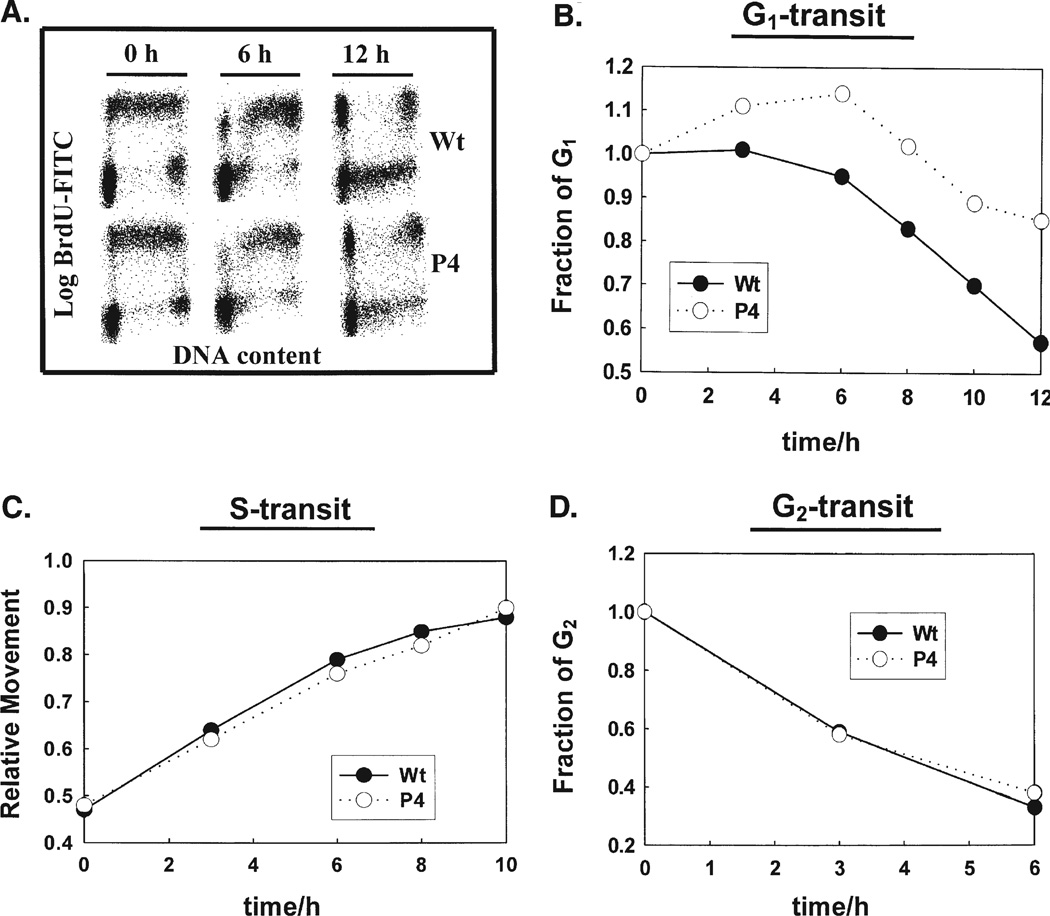
The slowing of the rate of cell proliferation in P-4 cells compared to control cells in Fig. 1 indicates that there must be changes in the cell cycle. To determine if there is a delay in transit through a specific cell cycle phase we did a BrdU-pulse-chase experiment using flow cytometry. This allows us to determine the time needed to move through a specific phase of the cell cycle. The changes in DNA content were used as a measure of movement of cells throughout the cell cycle. The DNA content can be determined using BrdU and FITC fluorescence. Cells were exposed to BrdU for 30 min and fluorescence measured at different time points, Fig. 5A. BrdU-positive cells are in S phase, while BrdU negative cells are in G1 or G2 phase, depending on their DNA content. The fraction of cells in G1 and G2 were evaluated to quantitatively assess changes in cell cycle progression in MCF-7 Wt and P-4 cells. In Wt cells, the fraction of cells in the BrdU-negative G1 compartment decreased gradually as cells entered S-phase, Fig. 5B. After 12 h approximately 40% of the BrdU-negative G1 cells were synthesizing DNA. In contrast, progression of BrdU-negative G1 cells into S-phase was slower in PhGPx overexpressing P-4 cells, Fig. 5B. Initially, the fraction of BrdU-negative G1 cells increased from 1 to 1.15 after 6 h in P-4 cells followed by a slow exit. The initial increase in the G1 fraction was due to a combination of entry of two daughter cells into G1 for each cell completing mitosis. As the G2-cells completed mitosis and G1-cells continued entering S, the fraction of G1-cells decreased continually, but at a slower rate then Wt cells. The fraction of P-4 BrdU-negative G1 cells decreased approximately 10% at 10 h and 20% at 12 h, while the Wt decrease 30% at 10 h and 40% at 12 h.
FIGURE 5.
PhGPx overexpressing MCF-7 cells show a G1-delay : Asynchronously growing, monolayer cultures of MCF-7 and P-4 cells were pulse-labeled with BrdU. At representative times, cells were harvested by trypsinization and fixed in 70% ethanol. Cell cycle progression was measured by indirect immunostaining of BrdU-labeled cells and flow cytometry. (A) BrdU-positive (S phase) and BrdU-negative (G1 and G2 phases) cells were distinguished by density plots of cell cycle phase distributions at 0, 6 and 12 h after BrdU-labeling. PI vs BrdU-FITC flow cytometer histograms were determined. Changes in DNA content were used as a measure of movement of cells through the cell cycle. (B) Progression through G1. In Wt cells, the fraction of cells in G1 continuously decreased as these cells moved into S. In contrast, the fraction of P-4 cells in G1 increased slightly 2–6 h after BrdU labeling, followed by a gradual drop as cells entered S-phase. (C) Progression through S into G2 was evaluated by measuring the relative movement (RM) parameter. (D) The exit of cells from G2 in wild type and PhGPx overexpressing cell lines.
The transit of BrdU-negative G2 cells in control and P-4 cells was not significantly different compared to Wt, Fig. 5D. The fraction of BrdU-negative G2 cells decreased approximately 40% at 3 h in both cell lines and continued to decrease, 70% at 6 h. The G2-transit time, calculated at 50% level, was found to be approximately 4 h for both cell lines.
Increases in RM values with time were used as a measure of the transit of cells through S-phase. The initial RM values, 30 min after BrdU labeling, were 0.47 for control and 0.48 for P-4 cell lines, respectively, Fig. 5C. The RM value increased linearly in both cell lines reaching approximately 0.8 at 6 h and 0.9 at 10 h. The S-transit time calculated from these RM values was approximately 12 h for both cell lines. These results show that overexpression of PhGPx had no significant effect in cells transit through S-phase. Thus the increased doubling time observed for cells with increased PhGPx activity can be attributed to a delay in G1.
DISCUSSION
Transfection or transduction of antioxidant enzymes such as MnSOD, CuZnSOD, catalase, and GPx-1 into tumor cells has been successful and the changes in cell growth, tumor growth, and tumor phenotype have been documented.[17 – 23 – 29,31,41] Overexpression of either MnSOD or CuZnSOD, superoxide-removing enzymes, decreased the rate of tumor cell growth and plating efficiency in vitro and also inhibited tumor formation in nude mice. It is thought that alteration of antioxidant enzymes changes the cellular redox environment, thereby modulating redox-sensitive signals that influence the cell cycle. Hydrogen peroxide, a second messenger molecule, could be a trigger for these signal changes. This hypothesis is supported by the finding that double transfection of MnSOD and GPx-1[17] or MnSOD and catalase[23,24] (GPx-1 and catalase remove hydrogen peroxide) reversed the growth inhibitory effects of MnSOD. In addition because of the high diffusability of H2O2, extracellular catalase has also been shown to alter cell growth.[20 – 22] It has recently been suggested that cyclin dependent kinases, that are involved in cell cycle regulation, are inhibited by H2O2.[49,50] Thus, modulation of the levels of peroxides (peroxide tone) may be a key aspect of cell signaling.
PhGPx has received extensive interest because of its major antioxidant function in the defense against LOOH-mediated oxidative damage. However, in addition to the damage of cellular membranes at high levels of LOOHs, low levels of LOOHs may be involved in signal transduction pathways.[7,12] Therefore, PhGPx, as an enzyme that regulates the level of PLOOH, may play a role in cell growth. To test this hypothesis, MCF-7 cells were stably transfected with PhGPx (L-PhGPx sense cDNA).[30]
Here we have demonstrated that overexpression of L-PhGPx indeed has an impact on tumor cell proliferation. Increases in PhGPx activity increase cell-doubling time and significantly decreases plating efficiency, Figs. 1 and 2. There can be various reasons for the increase in doubling time. One possible reason could be an imbalance in antioxidants vs pro-oxidants. It has been shown that changing the balance in the antioxidant network by increasing the activity of MnSOD results in changes in expression of other antioxidant enzymes.[26,40] To determine if overexpression of PhGPx modulates other antioxidant enzymes, we measured protein levels and enzymatic activities of MnSOD, CuZnSOD, CAT, GPx-1, and GR. No significant changes in these antioxidant enzymes were observed in cells transfected with PhGPx compared to WT, Fig. 3.
A second possibility for the changes in proliferation could be a change in the cellular peroxide tone. Double transfection of MnSOD and GPx-1[17] or MnSOD and catalase[23,24] reverses the inhibition of cell growth that MnSOD causes, suggesting that hydroperoxide levels are critical in governing cell differentiation and proliferation. Increased PhGPx can decrease the intracellular PLOOHs, thereby disturbing the cellular peroxide tone. We found a decrease in the steady-state level of PLOOH in the MCF-7 cells overexpressing PhGPx, Fig. 4. This result is consistent with a role for PLOOH in cell proliferation.
A third possibility for the increased doubling time could be that PhGPx and PLOOH affect the cell cycle. Increasing evidence suggests that the production of reactive oxygen species is tightly regulated and serves a physiological function during mitogenic stimulation of cultured cells. Reactive oxygen species have been implicated in the regulation of several physiologic processes including cell proliferation,[51] senescence,[52] differentiation,[53] and apoptosis;[54] very low levels (EC50 ~ pM) of oxygenated metabolites of docosahexaenoate and arachidonate have potent vasodilating properties.[55,56] Results from the present study show that PhGPx has a regulatory role in proliferation of tumor cells. PhGPx-mediated regulation occurs predominantly during the cell’s progression from G1 to S suggesting that changes in steady-state levels of PLOOH or oxygenated products derived from PLOOH could specifically affect G1 cell cycle regulatory processes, Fig. 5. The specific effects of PhGPx on G1 cell cycle regulatory proteins remains to be determined.
As seen with other antioxidant enzymes, overexpression of PhGPx in breast cancer cells can slow cell growth. However, the mechanism of signaling might be different. Hydrogen peroxide is a mobile messenger that can diffuse over quite a distance, thereby initiating a signal at a different location than its origin. In contrast, the lipid hydroperoxides that are substrates for PhGPx are tethered in the membrane. Thus, as signaling species their sphere of influence will be much smaller, i.e. very near the site of origin. Our results support the idea that intracellular antioxidant enzymes can have a regulatory role in progression of cells through specific phases of the cell cycle thereby influencing cell proliferation. The detailed mechanisms are yet to be understood.
Acknowledgements
We thank Ms Mary Sturm for assisting in the cell cycle work. This work was supported by NIH grants CA66081, CA81090, and CA69593.
Abbreviations
- BrdU
bromodeoxyuridine
- CAT
catalase
- CuZnSOD
copper zinc superoxide dismutase
- EDTA
disodium ethylenediaminetetraacetic acid
- EtOH
ethanol
- GSH
glutathione
- GSSG
glutathione disulfide
- GPx-1
cytosolic glutathione peroxidase
- GR
glutathione reductase
- H2O2
hydrogen peroxide
- MCF-7
human breast carcinoma cells
- MnSOD
manganese superoxide dismutase
- Neo
MCF-7 cells with empty vector
- L-PhGPx
mitochondrial phospholipid hydroperoxide glutathione peroxidase
- LOH
lipid alcohol
- LOOHs
lipid hydroperoxides
- P-1, P-2, P-3, P-4
are MCF-7 clones stably transfected with PhGPx
- PBS
phosphate buffered saline
- PE
plating efficiency
- PLOOH
phosphatidylcholine hydroperoxide
- PI
propidium iodide
- RM
relative movement parameter
- Se-PhGPx
represents the reduced enzyme
- Se-O-PhGPx
represents the oxidized enzyme
- SOD
superoxide dismutase
- TEMED
N,N,N′,N′-tetramethylethylene diamine
- Wt
parental MCF-7 cells
References
- 1.Ursini F, Maiorino M, Gregolin C. The selenoenzyme phospholipid hydroperoxide glutathione peroxidase. Biochim. Biophys. Acta. 1985;839:62–70. doi: 10.1016/0304-4165(85)90182-5. [DOI] [PubMed] [Google Scholar]
- 2.Thomas JP, Maiorino M, Ursini F, Girotti AW. Protective action of phospholipid hydroperoxide glutathione peroxidase against membrane-damaging lipid peroxidation in situ reduction of phospholipid and cholesterol hydroperoxides. J. Biol. Chem. 1990;265:454–461. [PubMed] [Google Scholar]
- 3.Pushpa-Rekha TR, Burdsall AL, Oleksa LM, Chisolm GM, Driscoll DM. Rat phospholipid-hydroperoxide glutathione peroxidase. cDNA cloning and identification of multiple transcription and translation start sites. J. Biol. Chem. 1995;270:26993–26999. doi: 10.1074/jbc.270.45.26993. [DOI] [PubMed] [Google Scholar]
- 4.Weitzel F, Wendel A. Selenoenzymes regulate the activity of leukocyte 5-lipoxygenase via the peroxide tone. J. Biol. Chem. 1993;268:6288–6292. [PubMed] [Google Scholar]
- 5.Schnurr K, Belkner J, Ursini F, Schewe T, Kuhn H. The selenoenzyme phospholipid hydroperoxide glutathione peroxidase controls the activity of the 15-lipoxygenase with complex substrates and preserves the specificity of the oxygenation products. J. Biol. Chem. 1996;271:4653–4658. doi: 10.1074/jbc.271.9.4653. [DOI] [PubMed] [Google Scholar]
- 6.Imai H, Narashima K, Arai M, Sakamoto H, Chiba N, Nakagawa Y. Suppression of leukotriene formation in RBL-2H3 cells that overexpressed phospholipid hydroperoxide glutathione peroxidase. J. Biol. Chem. 1998;273:1990–1997. doi: 10.1074/jbc.273.4.1990. [DOI] [PubMed] [Google Scholar]
- 7.Bindoli A. Lipid peroxidation in mitochondria. Free Radic. Biol. Med. 1988;5:247–261. doi: 10.1016/0891-5849(88)90018-4. [DOI] [PubMed] [Google Scholar]
- 8.Gutteridge JM. Free radicals in disease processes: a compilation of cause and consequence. Free Radic. Res. Commun. 1993;19:141–158. doi: 10.3109/10715769309111598. [DOI] [PubMed] [Google Scholar]
- 9.Shigenaga MK, Hagen TM, Ames BN. Oxidative damage and mitochondrial decay in aging. Proc. Natl Acad. Sci. 1994;91:10771–10778. doi: 10.1073/pnas.91.23.10771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Steinbeck MJ, Kim JK, Trudeau MJ, Hauschka PV, Karnovsky MJ. Involvement of hydrogen peroxide in the differentiation of clonal HD-11EM cells into osteoclast-like cells. J. Cell. Physiol. 1998;176:574–587. doi: 10.1002/(SICI)1097-4652(199809)176:3<574::AID-JCP14>3.0.CO;2-#. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kretz-Remy C, Mehlen P, Mirault ME, Arrigo AP. Inhibition of IκB-a phosphorylation and degradation and subsequent NF-κB activation by glutathione peroxidase overexpression. J. Cell Biol. 1996;133:1083–1093. doi: 10.1083/jcb.133.5.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brigelius-Flohe R, Friedrichs B, Maurer S, Schultz M, Streicher R. Interleukin-1-induced human nuclear factor kappa B activation is inhibited by overexpression of phospholipid hydroperoxide glutathione peroxidase in a human endothelial cell line. Biochem. J. 1997;328:199–203. doi: 10.1042/bj3280199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sandstrom PA, Tebbey PW, Cleave SV, Buttke TM. Lipid hydroperoxides induce apoptosis in T cells displaying a HIV-associated glutathione peroxidase deficiency. J. Biol. Chem. 1994;269:798–801. [PubMed] [Google Scholar]
- 14.Nomura K, Imai H, Koumura T, Arai M, Nakagawa Y. Mitochondrial phospholipid hydroperoxide glutathione peroxidase suppresses apoptosis mediated by a mitochondrial death pathway. J. Biol. Chem. 1999;274:29294–29302. doi: 10.1074/jbc.274.41.29294. [DOI] [PubMed] [Google Scholar]
- 15.Nomura K, Imai H, Koumura T, Kobayashi T, Nakagawa Y. Mitochondrial phospholipid hydroperoxide glutathione peroxidase inhibits the release of cytochrome c from mitochondria by suppressing the peroxidation of cardiolipin in hypoglycaemia-induced apoptosis. Biochem. J. 2000;351:183–193. doi: 10.1042/0264-6021:3510183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Imai H, Sumi D, Hanamoto A, Arai M, Sugiyama A, Chiba N, Kuchino Y, Nakagawa Y. Molecular cloning and functional expression of a cDNA for rat phospholipid hydroperoxide glutathione peroxidase: 3′-untranslated region of the gene is necessary for functional expression. J. Biochem. 1995;118:1061–1067. doi: 10.1093/jb/118.5.1061. [DOI] [PubMed] [Google Scholar]
- 17.Li S, Yan T, Yang JQ, Oberley TD, Oberley LW. The role of cellular glutathione peroxidase redox regulation in the suppression of tumor cell growth by manganese superoxide dismutase. Cancer Res. 2000;60:3927–3939. [PubMed] [Google Scholar]
- 18.Li N, Oberley TD, Oberley LW, Zhong W. Inhibition of cell growth in NIH/3T3 fibroblasts by overexpression of manganese superoxide dismutase: mechanistic studies. J. Cell. Physiol. 1998;175:359–369. doi: 10.1002/(SICI)1097-4652(199806)175:3<359::AID-JCP14>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 19.Yang JQ, Buettner GR, Domann FE, Li Q, Engelhardt JF, Weydert CD, Oberley LW. v-Ha-ras mitogenic signaling through superoxide and derived reactive oxygen species. Mol. Carcinog. 2002;33:206–218. doi: 10.1002/mc.10037. [DOI] [PubMed] [Google Scholar]
- 20.Kim H, You S, Kong BW, Foster LK, Farris J, Foster DN. Necrotic cell death by hydrogen peroxide in immortal DF-1 chicken embryo fibroblast cells expressing deregulated MnSOD and catalase. Biochim. Biophys. Acta. 2001;1540:137–146. doi: 10.1016/s0167-4889(01)00131-8. [DOI] [PubMed] [Google Scholar]
- 21.Preston TJ, Muller WJ, Singh G. Scavenging of extracellular H2O2 by catalase inhibits the proliferation of HER-2/Neu-transformed rat-1 fibroblasts through the induction of a stress response. J. Biol. Chem. 2001;276:9558–9564. doi: 10.1074/jbc.M004617200. [DOI] [PubMed] [Google Scholar]
- 22.Brar SS, Kennedy TP, Whorton AR, Murphy TM, Chitano P, Hoidal JR. Requirement for reactive oxygen species in serum-induced and platelet-derived growth factor-induced growth of airway smooth muscle. J. Biol. Chem. 2001;274:20017–20026. doi: 10.1074/jbc.274.28.20017. [DOI] [PubMed] [Google Scholar]
- 23.Rodriguez AM, Carrico PM, Mazurkiewicz JE, Melendez JA. Mitochondrial or cytosolic catalase reverses the MnSOD-dependent inhibition of proliferation by enhancing respiratory chain activity, net ATP production, decreasing the steady state levels of H2O2 . Free Radic. Biol. Med. 2000;29:801–813. doi: 10.1016/s0891-5849(00)00362-2. [DOI] [PubMed] [Google Scholar]
- 24.Kim KH, Rodriguez AM, Carrico PM, Melendez JA. Potential mechanisms for the inhibition of tumor cell growth by manganese superoxide dismutase. Antioxd. Redox Signal. 2001;3:361–373. doi: 10.1089/15230860152409013. [DOI] [PubMed] [Google Scholar]
- 25.Oberley LW, Buettner GR. The role of superoxide dismutase in cancer: A review. Cancer Res. 1979;39:1141–1149. [PubMed] [Google Scholar]
- 26.Zhang HJ, Yan T, Oberley TD, Oberley LW. Comparison of effects of two polymorphic variants of manganese superoxide dismutase on human breast MCF-7 cancer cell phenotype. Cancer Res. 1999;59:6276–6283. [PubMed] [Google Scholar]
- 27.Oberley LW. Anticancer therapy by overexpression of superoxide dismutase. Antioxd. Redox Signal. 2001;3:461–472. doi: 10.1089/15230860152409095. [DOI] [PubMed] [Google Scholar]
- 28.Manna SK, Zhang HJ, Yan T, Oberley LW, Aggarwal BB. Overexpression of manganese super-oxide dismutase suppresses tumor necrosis factor-induced apoptosis and activation of nuclear transcription factor-kappaB and activated protein-1. J. Biol. Chem. 1998;273:13245–13254. doi: 10.1074/jbc.273.21.13245. [DOI] [PubMed] [Google Scholar]
- 29.Zhang Y, Zhao W, Zhang HJ, Domann FE, Oberley LW. Overexpression of copper zinc superoxide dismutase suppresses human glioma cell growth. Cancer Res. 2002;62:1205–1212. [PubMed] [Google Scholar]
- 30.Wang HP, Qian SY, Schafer FQ, Domann FE, Oberley LW, Buettner GR. Phospholipid hydroperoxide glutathione peroxidase protects against the singlet oxygen-induced cell damage of photodynamic therapy. Free Radic. Biol. Med. 2001;30:825–835. doi: 10.1016/s0891-5849(01)00469-5. [DOI] [PubMed] [Google Scholar]
- 31.Oberley LW, McCormick ML, Sierra E, StClair DK. Manganese superoxide dismutase in normal and transformed human embryonic lung fibroblasts. Free Radic. Biol. Med. 1989;6:379–384. doi: 10.1016/0891-5849(89)90083-x. [DOI] [PubMed] [Google Scholar]
- 32.Anderson ME. Tissue glutathione. In: Greenwald RA, editor. Handbook of Methods for Oxygen Radical Research. Boca Raton: CRC Press; 1985. [Google Scholar]
- 33.Beauchamp C, Fridovich I. Superoxide dismutase: improved assays and assay applicable to polyacrylamide gels. Anal. Biochem. 1971;44:276–287. doi: 10.1016/0003-2697(71)90370-8. [DOI] [PubMed] [Google Scholar]
- 34.Goswami PC, He W, Higashikubo R, Roti Roti JL. Accelerated G1-transit following transient inhibition of DNA replication is dependent on two processes. Exp. Cell Res. 1994;214:198–208. doi: 10.1006/excr.1994.1249. [DOI] [PubMed] [Google Scholar]
- 35.Begg AC, McNally NJ, Shrieve DC, Karcher H. A method to measure the duration of DNA synthesis and the potential doubling time from a single sample. Cytometry. 1985;6:620–625. doi: 10.1002/cyto.990060618. [DOI] [PubMed] [Google Scholar]
- 36.Sun Y, Oberley LW. Redox regulation of transcriptional activators. Free Radic. Biol. Med. 1996;21:335–348. doi: 10.1016/0891-5849(96)00109-8. [DOI] [PubMed] [Google Scholar]
- 37.Dypbukt JM, Ankarcrona M, Burkitt M, Sioholm A, Strom K, Orrenius S, Nicotera P. Different prooxidant levels stimulate growth, trigger apoptosis, or produce necrosis of insulin-secreting RINm5F cells. The role of intracellular polyamines. J. Biol. Chem. 1994;269:30553–30560. [PubMed] [Google Scholar]
- 38.Kuhn H, Brash AR. Occurrence of lipoxygenase products in membranes of rabbit reticulocytes. Evidence for a role of the reticulocyte lipoxygenase in the maturation of red cells. J. Biol. Chem. 1990;265:1454–1458. [PubMed] [Google Scholar]
- 39.Maiorino M, Wissing JB, Brigelius-Flohe R, Calabrese F, Roveri A, Steinert P, Ursini F, Flohe L. Testosterone mediates expression of the selenoprotein PhGPx by induction of spermatogenesis and not by direct transcriptional gene activation. FASEB J. 1998;12:1359–1370. doi: 10.1096/fasebj.12.13.1359. [DOI] [PubMed] [Google Scholar]
- 40.Yan T, Jiang X, Zhang HJ, Li S, Oberley LW. Use of commercial antibodies detection of the primary antioxidant enzymes. Free Radic. Biol. Med. 1998;25:688–693. doi: 10.1016/s0891-5849(98)00112-9. [DOI] [PubMed] [Google Scholar]
- 41.Zhong W, Oberley LW, Oberley TD, Yan T, Domann FE, St Clair DK. Inhibition of cell growth and sensitization to oxidative damage by overexpression of manganese superoxide dismutase in rat glioma cells. Cell Growth Different. 1996;7:1175–1186. [PubMed] [Google Scholar]
- 42.Schafer FQ, Buettner GR. Redox state of the cell as viewed though the glutathione disulfide/glutathione couple. Free Radic. Biol. Med. 2001;30:1191–1212. doi: 10.1016/s0891-5849(01)00480-4. [DOI] [PubMed] [Google Scholar]
- 43.Esworthy RS, Ho YS, Chu FF. The GPx1 gene encodes mitochondrial glutathione peroxidase in the mouse liver. Arch. Biochem. Biophys. 1997;340:59–63. doi: 10.1006/abbi.1997.9901. [DOI] [PubMed] [Google Scholar]
- 44.Godeas C, Sandri G, Panfile E. Distribution of phospholipid hydroperoxide glutathione peroxidase (PhGPx) in rat testis mitochondria. Biochim. Biophys. Acta. 1994;1191:147–150. doi: 10.1016/0005-2736(94)90242-9. [DOI] [PubMed] [Google Scholar]
- 45.Meredith MJ, Reed DJ. Status of the mitochondrial pool of glutathione in the isolated hepatocyte. J. Biol. Chem. 1982;257:3747–3753. [PubMed] [Google Scholar]
- 46.Leyland-Jones BR, Townsend AJ, Tu CPD, Cowan KH, Goldsmith ME. Antineoplastic drug sensitivity of human MCF-7 breast cancer cells stably transfected with a human α class glutathione S-transferase gene. Cancer Res. 1991;51:587–594. [PubMed] [Google Scholar]
- 47.Hurst R, Bao Y, Jemth P, Mannervik B, Williamson G. Phospholipid hydroperoxide glutathione peroxidase activity of human glutathione transferases. Biochem. J. 1998;332:97–100. doi: 10.1042/bj3320097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fisher AB, Dodia C, Manevich Y, Chen JW, Feinstein S. Phospholipid hydroperoxides are substrates for non-selenium glutathione peroxidase. J. Biol. Chem. 1999;274:21326–21334. doi: 10.1074/jbc.274.30.21326. [DOI] [PubMed] [Google Scholar]
- 49.Chang TS, Jeong W, Choi SY, Yu S, Kang SW, Rhee SG. Regulation of peroxiredoxin I activity by Cdc2-mediated phosphorylation. J. Biol. Chem. 2002;277:25370–25376. doi: 10.1074/jbc.M110432200. [DOI] [PubMed] [Google Scholar]
- 50.Savitsky PA, Finkel T. Redox regulation of Cdc25C. J. Biol. Chem. 2002;277:20535–20540. doi: 10.1074/jbc.M201589200. [DOI] [PubMed] [Google Scholar]
- 51.Shibanuma M, Kuroki T, Nose K. Induction of DNA replication and expression of proto-oncogenes c-myc and c-fos in quiescent Balb/3T3 cells by xanthine–xanthine oxidase. Oncogene. 1998;3:14–21. [Google Scholar]
- 52.DeHaan JB, Cristiano F, Lanello R, Bladier C, Kelner MJ, Kola I. Elevation in the ratio of Cu/Zn superoxide dismutase to glutathione peroxidase activity induces features of cellular senescence and this effect is mediated by hydrogen peroxide. Hum. Mol. Genet. 1996;5:283–292. doi: 10.1093/hmg/5.2.283. [DOI] [PubMed] [Google Scholar]
- 53.Allen RG, Balin AK. Oxidative influence in development and differentiation: an overview of a free radical theory of development. Free Radic. Biol. Med. 1989;6:631–661. doi: 10.1016/0891-5849(89)90071-3. [DOI] [PubMed] [Google Scholar]
- 54.Hockenbery DM, Oltvai ZN, Yin YM, Milliman CL, Korsmeyer SJ. Bcl-2 function in an antioxidant pathway to prevent apoptosis. Cell. 1993;75:241–251. doi: 10.1016/0092-8674(93)80066-n. [DOI] [PubMed] [Google Scholar]
- 55.Zhang Y, Oltman CL, Lu T, Lee HC, Dellsperger KC, VanRollins M. EET homologs potently dilate coronary microvessels and activate BK(Ca) channels. Am. J. Physiol.—Heart Circul. Physiol. 2001;280(6):H2430–H2440. doi: 10.1152/ajpheart.2001.280.6.H2430. [DOI] [PubMed] [Google Scholar]
- 56.Ye D, Zhang D, Oltman C, Dellsperger KC, Lee H-C, VanRollins M. Cytochrome P-450 expoxygenase metabolites of docosahexaenoate potentially dilate coronary arterioles by activating large conductance, calcium activated potassium channels. J. Pharmacol. Exp. Therap. 2002;303:768–776. doi: 10.1124/jpet.303.2.768. [DOI] [PubMed] [Google Scholar]