Abstract
Objectives. We investigated the relationships among early childhood caries (ECC), mouth pain, and nutritional status in children aged 1 to 6 years in Southern and Central Vietnam.
Methods. A total of 593 parent–child pairs were recruited from 5 kindergartens or preschools in Ho-Chi Minh City and Da Nang. Parents completed surveys about dietary habits, oral health practices, and children’s mouth pain experience; children received anthropometric assessment and dental examinations.
Results. There was a high prevalence of dental caries (74.4%), mostly untreated, and mouth pain (47.1%). Moderate correlations were found between parents’ and children’s consumption of soda (ρ = 0.361; P < .001) and salty snacks (ρ = 0.292; P < .001). Severity of ECC was associated with decreased weight- and body mass index-for-age z-scores. Presence of pulp-involved caries was associated with strikingly lower height-for-age (mean difference = 0.66; P = .001), weight-for-age (mean difference = 1.17; P < .001), and body mass index-for-age (mean difference = 1.18; P < .001) z-scores. Mouth pain was associated with lower body mass index-for-age z-scores (mean difference = 0.29; P = .013).
Conclusions. ECC might negatively affect children’s nutritional status, which might be mediated by the depth of decay, chronic inflammation, and mouth pain. Family-based and prevention-oriented nutrition and oral health programs are needed and should start during pregnancy and infancy.
Early childhood caries (ECC), the most prevalent chronic disease of childhood, affects 60%–90% of children worldwide and can severely compromise children’s health and well-being.1–6 Children with ECC may experience acute and chronic mouth pain that can adversely affect their quality of life, eating practices, nutritional status, growth, and educational and economic potential.1–3,7–11 The greatest disease burden of ECC may be experienced by children in developing countries where oral health education and access to dental treatment are limited, most tooth decay remains untreated, and where children’s health and educational potential may already be vulnerable because of underlying poverty and malnutrition.3,12–15
Vietnam is a developing country that has experienced a “nutrition transition” over recent decades, with rapid Westernization of the diet and increased consumption of sweetened processed foods and beverages.16 Recent studies in Vietnam documented a “double burden of malnutrition”: persistent undernutrition in rural regions and increasing overweight or obesity in urban areas.16–18 A nationally representative survey in Vietnam indicated that 11.7% of children younger than 5 years experience malnutrition,19 whereas other studies found that 5.3% of children were overweight or obese.20
Although the nutritional transition is also known to increase children’s risk for ECC, there has been limited published research on ECC in Vietnam or its relationship with malnutrition and obesity. The most recent national study we identified was published in 2009. It reported findings from the Vietnam’s National Oral Health Survey in 1999, which examined a representative sample of children aged 6 to 17 years. The survey found high rates of tooth decay (among children aged 6–7 years; the mean number of decayed surfaces was 12.48, and prevalence was 84.9%). This rate was significantly higher than the rate found in a survey conducted 10 years earlier.21 Therefore, there is an urgent need for updated population-level dental information on Vietnamese children, especially those younger than 6 years. This age group may have high rates of ECC based on findings from other Southeast Asian countries.7,22,23 Finally, Vietnam’s oral health surveys have not published a study about the relationship between children’s oral health and their nutritional status. This is particularly important from birth through age 5 years, when children’s nutritional status is most vulnerable and most critical for optimal lifelong development.
Generally, oral health studies that examine associations between dental caries and nutritional status reported mixed results. Some studies found ECC to be associated with underweight1,4,8,24,25; other studies found ECC to be associated with overweight or obesity,26–29 and some found no associations.30–32 Inconsistent relationships between oral health and nutrition status may stem in part from complex interactions among oral health, nutrition, and socioeconomic status. It may also stem from the general documentation of tooth decay by the number of decayed, missing, and filled teeth or surfaces, which count numbers of affected teeth without accounting for depth of tooth decay or the presence of mouth pain. Studies on oral health quality of life have demonstrated associations between the severity of tooth decay and mouth pain in populations from developing countries.2,7 However, few studies have attempted to show the direct connection between mouth pain and children’s nutritional status. There is a need for greater elucidation of the relationship among oral health indexes, mouth pain, and nutritional status.
In this article, we present the baseline results from a longitudinal intervention study. We also present hypothetical conceptual pathways that might link dietary and oral health risk factors with dental and nutritional outcomes for young children.
METHODS
We conducted a cross-sectional analysis of baseline data from a longitudinal intervention study of Vietnamese children aged 1 to 6 years who were recruited in 2011 for a school-based oral health and nutrition study. To minimize regional biases, we recruited a convenience sample of 593 children and their parents from 5 preschools or kindergartens in 2 regions: Ho Chi Minh City (3 schools) and Da Nang (2 schools). Each family could enroll only 1 child in the study; thus, siblings were excluded.
The school sites were selected by our local partners in Vietnam: University of Medicine and Pharmacy in Ho Chi Minh City and East Meets West, a nongovernmental organization working in Central Vietnam (Da Nang area). All of the school sites had existing connections with our local partners and their dentists, and expressed strong support for the program throughout its duration.
We received approval of study protocols and instruments from our institutional review board, whereas our Vietnam-based partners relied on our institutional review board process and approval. On-site coordinators (predominantly school principals) explained the study to parents and sent study packets home with schoolchildren. Packets included an informed consent document (in Vietnamese, for a parent to sign) and surveys to be completed by the mother (or primary caregiver) of the selected child. Children from families supplying consent forms participated in the intervention and data collection. On-site data collection, managed by trained teachers and student volunteers, included anthropometric assessment of the child (weight and height) and dental caries assessment coded according to World Health Organization (WHO) criteria (1997).33 Children were treated on-site, if it was possible, or referred to local dental offices if it was deemed necessary by their dentist examiner.
Survey Instrument and Anthropometric Measures
We distributed a 50-question survey in Vietnamese with multiple choice and open-ended questions in 4 domains: (1) demographics, (2) mother’s health care knowledge and practices, (3) child’s dietary and oral health practices, and (4) reports of child mouth pain, oral health, and general health. This survey was based on questions from global oral health and quality-of-life surveys. We simplified and adapted the questions for a low-literacy population, and its usability and acceptability was demonstrated in 5 countries (data available as a supplement to the online version of this article at http://www.ajph.org). Although the instrument as a whole was not validated, the mouth pain questions were similar to items from validated scales.34,35 We created a score for the child’s composite mouth pain (i.e., pain originating from orofacial structures, such as dental soft and hard tissues) from 3 survey questions (frequency of mouth pain, problems eating because of mouth pain, and problems sleeping because of mouth pain) that were asked of the mother or parent. We assigned children to the highest pain value reported for any of the 3 questions. We converted ordinally scaled junk food consumption items into intervally scaled times per week (conversion key is available as a supplement to the online version of this article at http://www.ajph.org).
Children’s weights and heights were obtained using a professional grade scale and stadiometer (Seca, Chino, CA) by trained student volunteers, and recorded to the nearest 0.1 kilograms and 1 centimeter, respectively. Children wore light clothing and removed their shoes for both measurements.
Clinical Oral Health Examination Measures
Children’s oral health status was recorded during a dental examination by licensed Vietnamese dentists working with dental nurses or dental students. The examinations conducted in South Vietnam involved natural light (or headlight when necessary), a mirror, and a dental probe, with the children lying on a long desk and with the examiner sitting directly behind them. In Central Vietnam, examinations involved the same tools, but the children were seated in a dental chair. Each tooth was recorded as not yet erupted or erupted, primary or deciduous, or secondary or permanent, healthy or decayed, missing, or filled (dmft for primary teeth and DMFT for secondary teeth), according to WHO standards.33 All dentists were trained to use WHO criteria33 at University of Medicine and Pharmacy at Ho Chi Minh City. Examiners from South Vietnam were also calibrated against each other. Dentists from Central Vietnam had additional training in using the WHO criteria in 2007 as part of an oral health survey in Da Nang.
Following procedures from 2 previous studies,2,7 we used the following classification to recode dmfts to rank dental caries severity: dmft = 0 (“no caries”), dmft = 1 to 5 (“low severity caries”), and dmft greater than 5 (“high severity caries”). In the 2 sites in Da Nang (approximately 60% of our sample of children), dentists also recorded the depth of dental caries cavitation for each tooth by visual inspection, which we coded in this subanalysis as: decay with cavitation into only enamel (DE), decay with cavitation into dentin (DD), and decay with cavitation into the pulp (DP). This is a simplification of the PUFA index (presence of severe decayed teeth with Pulpal involvement, Ulcerations, Fistula, or Abscess), which accounts for different types of severely decayed teeth.36
Analytical Methods
Data were entered into 2008 MS Excel (Microsoft, Redmond, WA) by trained students. All data entered were double-checked for accuracy. We used height, weight, gender, and age to calculate standardized anthropometric z-scores according to WHO standards using WHO software: WHO Anthro (v. 3.2.2) for children up to 5 years of age37 and WHO AnthroPlus (v. 1.0.4) for children aged 5 years and older.38 Children whose height-, weight-, or body mass index (BMI)-for-age z-score was −2.00 or less were considered “stunted,” “underweight,” and “wasted,” respectively. Those whose BMI-for-age z-score was 2.00 or greater were considered “overweight or obese.”
Following data checks, we exported data to SPSS version 22.0 (IBM, Armonk, NY) for statistical analysis. We used the Spearman rank-order correlation, the Student t-test, and analysis of variance, as appropriate given the scaling of the variable. In cases in which the response rate for variables used in the statistical tests was less than 90% of the full sample, individuals with missing and nonmissing data for predictive variables were compared on the outcome variable for statistically significant differences using the t-test or the Mann–Whitney U-test (none of these comparisons yielded statistically significant results). We considered all results from individual comparisons with a 2-tailed P value less than .05 to be statistically significant. We used the false discovery rate method to adjust statistical significance thresholds for multiple comparisons when multiple independent analyses tested multiple predictive variables against a single outcome variable.39
RESULTS
Of 593 parent–child pairs, 40.5% were from the southern region of Vietnam (in or around Ho Chi Minh City), and 59.5% were from Central Vietnam (in or around Da Nang). Our sample had 51.2% boys and 48.8% girls. The average child age was 4.1 years (SD = 0.93), with 97.4% in the 2 to 5 years age range. Mothers’ mean educational level was 12.8 (SD = 3.67) years of schooling (Table 1).
TABLE 1—
Sample Characteristics: Early Childhood Caries, Mouth Pain, and Nutritional Status; Vietnam; 2011
| Category | Mean ±SD or No. (%) | Valid No. |
| Children’s demographics | ||
| Male | 297 (51.2) | 580 |
| Age, y | 4.10 ±0.93 | 592 |
| 1.00–2.99 | 72 (12.2) | |
| 3.00–4.99 | 408 (68.9) | |
| 5.00–6.99 | 112 (18.9) | |
| Parents’ demographics | ||
| Age, y | 33.04 ±5.08 | 574 |
| Length of formal education, y | 12.82 ±3.67 | 503 |
| Survey respondent is the mother | 561 (95.1) | 590 |
| City | ||
| Ho Chi Minh City (3 sites) | 240 (40.5) | 593 |
| Da Nang (2 sites) | 353 (59.5) | |
| No. of children in the family | 1.6 ±0.6 | 588 |
| Child’s frequency of junk food consumption, times/wk | ||
| Soda | 1.33 ±2.84 | 455 |
| Never or rarely | 289 (63.6) | |
| Weekly or more frequently | 139 (30.5) | |
| Daily or more frequently | 27 (5.9) | |
| Sweets/candy | 1.97 ±3.02 | 476 |
| Never or rarely | 208 (43.7) | |
| Weekly or more frequently | 212 (44.5) | |
| Daily or more frequently | 56 (11.8) | |
| Salty snack | 1.93 ±2.72 | 493 |
| Never or rarely | 199 (40.4) | |
| Weekly or more frequently | 239 (48.5) | |
| Daily or more frequently | 55 (11.1) | |
| Parent’s frequency of junk food consumption, times/wk | ||
| Soda | 0.95 ±1.79 | 568 |
| Never or rarely | 364 (64.1) | |
| Weekly or more frequently | 187 (32.9) | |
| Daily or more frequently | 17 (3.0) | |
| Salty snacks | 0.76 ±1.34 | 567 |
| Never or rarely | 382 (67.4) | |
| Weekly or more frequently | 177 (31.2) | |
| Daily or more frequently | 8 (1.4) | |
| Child oral health outcomes | ||
| Has any tooth decay (yes) | 441 (74.4) | 593 |
| Proportion of d teeth in dmft index (d) | 3320 (95.4) | 3479 |
| Average no. of decayed teeth | 5.60 ±5.24 | 593 |
| Severity of dental caries | 593 | |
| dmft = 0 | 148 (25.0) | |
| dmft = 1–5 | 169 (28.5) | |
| dmft > 5 | 276 (46.5) | |
| Average dmft score | 5.87 ±5.42 | 593 |
| Pulp involved decayed teeth (Da Nang sites only) | 353 | |
| Yes | 110 (31.2) | |
| Teeth | 1.44 ±2.92 | |
| Composite mouth pain experience | 569 | |
| No signs of mouth pain | 301 (52.9) | |
| Occasional mouth pain | 245 (43.1) | |
| Frequent or almost always present mouth pain | 23 (4.0) | |
| Child nutritional outcomes | ||
| Undernourished statusa | ||
| Height for age (stunted), no. | 24 (4.2) | 573 |
| Height for age (stunted), z-score | −0.25 ±1.10 | |
| Weight for age (underweight), no. | 15 (2.6) | 572 |
| Weight for age (underweight), z-score | 0.41 ±1.33 | |
| BMI for age (wasted), no. | 8 (1.4) | 572 |
| BMI for age (wasted), z-score | 0.85 ±1.39 | |
| Overweight statusb | ||
| No. | 113 (19.8) | 572 |
| z-score | 0.85 ±1.39 | |
Note. BMI = body mass index; dmft = decayed, missing, or filled teeth (primary).
Height-, weight-, or BMI-for-age z-score ≤ −2.00.
BMI-for-age z-score ≥ +2.00
Risk Factors
Frequent consumption of junk food was prevalent. Overall, 36.4%, 56.3%, and 59.6% of the children consumed soda, sweets or candy, and salty snacks, respectively, on a weekly or more frequent basis. In addition, mothers reported frequent junk food consumption: approximately one third of them drank soda or ate salty snacks weekly or more frequently (Table 1).
We found statistically significant positive correlations between parents’ and children’s consumption of soda (ρ = 0.361; P < .001) and salty snacks (ρ = 0.292; P < .001; Table 2). We also found positive correlations of children’s dental caries severity with their soda consumption (ρ = 0.152; P = .001) and sweets or candy consumption (ρ = 0.101; P = .027 [P = not significant after adjusting for multiple comparisons]; Table 2). There was no statistically significant difference in frequency of consumption of any type of junk food between age groups of children in the study (data available as a supplement to the online version of this article at http://www.ajph.org).
TABLE 2—
Bivariate Analysis of Dietary Risk Factors, Based on the Spearman’s Rank Correlation Test: Early Childhood Caries, Mouth Pain, and Nutritional Status; Vietnam; 2011
| Predictive Variable | Outcome Variable | Valid No. | Correlation Factor, ρ | P |
| Child’s consumption | Parent’s consumption | |||
| Soda | Soda | 449 | 0.361 | < .001 |
| Salty snack | Salty snack | 479 | 0.292 | < .001 |
| Child’s frequency of junk food consumption | Dental caries severity | |||
| Soda | 455 | 0.152 | .001 | |
| Sweets/candy | 476 | 0.101 | .027a | |
| Salty snack | 493 | 0.013 | .768 | |
| Overall junk food | 437 | 0.095 | .047a |
Not statistically significant after multiple comparison adjustment (false discovery rate39).
Consequences
Three of 4 children in our sample had ECC, with nearly one half having more than 5 teeth with caries (i.e., greater than one fourth of their primary teeth). Nearly all of the tooth decay (95.4%) was untreated. In addition, within Da Nang sites, approximately 1 in 3 children with untreated caries had deep caries into the pulp of the tooth, with an average of 1.44 (SD = 2.92) pulp-involved teeth. Approximately one half of the children in our entire sample were reported to have complained of mouth pain (Table 1).
We found a moderate positive correlation between dental caries severity and composite mouth pain experience (ρ = 0.404; P < .001). Furthermore, we found significant associations between children’s nutritional status (as indicated by weight- and BMI-for-age z-scores) and their dental caries experience. For children in the low-severity caries group, the mean weight- and BMI-for-age z-scores were 0.46 (P = .006; 95% confidence interval [CI] = −0.81, −0.11) and 0.44 (P = .012; 95% CI = −0.81, −0.08) lower, respectively, than those in caries-free children. For children in the high-severity caries group, the mean weight- and BMI-for-age z-scores were 0.59 (P < .001; 95% CI = −0.91, −0.27) and 0.64 (P < .001; 95% CI = −0.97, −0.31) lower, respectively, than those in the caries-free children. Mean height-for-age z-scores did not differ significantly based on dental caries severity (Table 3).
TABLE 3—
Bivariate Analysis of Dental Caries, Mouth Pain, and Nutritional Status; Vietnam; 2011
| Predictive Variable | Outcome Variable | Valid No. | Correlation Factor (ρ) or Test Statistic (t or F) | Meana | Mean Difference From Reference (95% CI) | P |
| Dental caries severityb | Composite mouth pain experience | 569 | ρ = 0.404 | < .001 | ||
| Dental caries severityc | Child’s nutritional status | F(2, 570) = 3.12 | .045 | |||
| No caries | Mean height for age | 573 | –0.06 | (Ref) | ||
| Low severity | –0.33 | –0.28 (–0.57, 0.18) | .071 | |||
| High severity | –0.31 | –0.26 (–0.52, 0.12) | .065 | |||
| Dental caries severityc | Child’s nutritional status | F(2, 570) = 9.78 | < .001 | |||
| No caries | Mean weight for age | 573 | 0.82 | (Ref) | ||
| Low severity | 0.36 | –0.46 (–0.81, –0.11) | .006 | |||
| High severity | 0.23 | –0.59 (–0.91, –0.27) | < .001 | |||
| Dental caries severityc | Child’s nutritional status | F(2, 569) = 10.34 | < .001 | |||
| No caries | Mean BMI for age | 572 | 1.27 | (Ref) | ||
| Low severity | 0.82 | –0.44 (–0.81, –0.08) | .012 | |||
| High severity | 0.62 | –0.64 (–0.97, –0.31) | < .001 | |||
| Overweight statusd | Mean no. of decayed teeth | |||||
| Nonoverweight | 572 | 5.80e | (Ref) | |||
| Overweight | t(196.22) = 3.04 | 4.31e | –1.49 (–2.48, –0.52) | .003 | ||
| Composite mouth pain experienced | Child’s nutritional status | |||||
| No pain | Mean height for age | 555 | –0.26 | (Ref) | ||
| Pain | t(553) = –0.13 | –0.24 | –0.02 (–0.17, 0.20) | .896 | ||
| No pain | Mean weight for age | 555 | 0.50 | (Ref) | ||
| Pain | t(553) = 1.71 | 0.30 | –0.20 (–0.42, 0.03) | .088 | ||
| No pain | Mean BMI for age | 554 | 0.97 | (Ref) | ||
| Pain | t(552) = 2.48 | 0.68 | –0.29 (–0.52, –0.06) | .013 | ||
| Dental caries depthc,f,g | Child’s nutritional status | F(3, 336) = 5.07 | .002 | |||
| No caries | Mean height for age | 340 | 0.20 | (Ref) | ||
| DE | –0.09 | –0.29 (–0.77, 0.20) | .423 | |||
| DD | –0.23 | –0.43 (–0.86, 0.02) | .066 | |||
| DP | –0.46 | –0.66 (–1.10, –0.21) | .001 | |||
| Dental caries depthc,f,g | Child’s nutritional status | F(3, 336) = 11.94 | < .001 | |||
| No caries | Mean weight for age | 340 | 1.14 | (Ref) | ||
| DE | 0.71 | –0.43 (–1.02, 0.15) | .227 | |||
| DD | 0.31 | –0.83 (–1.36, –0.29) | < .001 | |||
| DP | –0.03 | –1.17 (–1.71, –0.63) | < .001 | |||
| Dental caries depthc,f,g | Child’s nutritional status | F(3, 335) = 11.16 | < .001 | |||
| No caries | Mean BMI for age | 339 | 1.52 | (Ref) | ||
| DE | 1.14 | –0.38 (–1.00, 0.25) | .41 | |||
| DD | 0.68 | –0.84 (–1.41, –0.27) | .001 | |||
| DP | 0.34 | –1.18 (–1.76, –0.61) | < .001 | |||
| Dental caries depthb,f,g | Dental caries severity | 353 | ρ = 0.807 | < .001 | ||
| Dental caries depthb,f,g | Composite mouth pain experience | 347 | ρ = 0.413 | < .001 |
Note. ANOVA = analysis of variance; BMI = body mass index; CI = confidence interval.
Mean indicates mean z-score, except where noted.
Spearman rank correlation.
All categories within predictive variable compared in a single ANOVA. For clarity, only mean differences with respect to the reference category are shown.
Student t-test.
Mean number of decayed teeth.
Children’s dental status coded according to the deepest level of decay: No caries; DE = decay cavitation only into enamel; DD = any decay cavitation into dentine; DP = any decay cavitation into pulp.
Da Nang sites only.
Surprisingly, we found that overweight children had on average 1.49 (P = .003; 95% CI = −2.48; −0.54) fewer decayed teeth than nonoverweight children (Table 3). However, there was no statistically significant difference in composite mouth pain experience between overweight and nonoverweight children (data not shown).
We also explored the role of mouth pain experience on children’s nutritional status. We found that children who had complained of mouth pain had a mean BMI-for-age z-score that was 0.29 (P = .013; 95% CI = −0.52, −0.06) lower than children who had never complained of mouth pain (Table 3).
In a separate analysis of data from the Da Nang subsample, based on their deepest level of caries (no caries, DE, DD, or DP), we found a strong correlation (ρ = 0.807; P < .001) with dental caries severity. We also found a moderate correlation between the deepest level of caries and composite mouth pain experience (ρ = 0.413; P < .001). For children with a moderate DD, their mean weight- and BMI-for-age z-scores were 0.83 (P < .001; 95% CI = −1.37, −0.29) and 0.84 (P = .001; 95% CI = −1.41, −0.27) lower, respectively, than those in caries-free children. For children with even a single tooth with severe DP, the mean height-, weight-, and BMI-for-age z-scores were 0.65 (P = .001; 95% CI = −1.10, −0.21), 1.17 (P < .001; 95% CI = −1.71, −0.63), and 1.18 (P < .001; 95% CI = −1.76, −0.61) lower, respectively, than those in caries-free children (Table 3).
DISCUSSION
Among the preschool- or kindergarten-aged children participating in our study, we found frequent consumption of junk food (soda, sweets or candy, and salty snacks). We noted frequent junk food consumption throughout early childhood, even in infants and toddlers. We found no significant difference between reported rates of junk food consumption by age. This indicated that toddlers were frequently given junk food that could have been given in response to temper tantrums. Junk food consumption might be underreported, especially for older children who were more autonomous and might consume junk food without parental knowledge. We also found a significant association between parents’ junk food consumption and their children’s junk food consumption, underscoring that nutritional practices were likely family based. A few studies established that children’s eating habits, especially consumption of sugary foods and beverages, were determined early in childhood.40–42 Rossow et al. postulated that this pattern was shaped by the family’s diet.42 Our finding that child and parent habits were correlated underscored the importance of family-centered programs to improve nutrition and oral health, and to support making healthier food and beverage choices from birth. Therefore, interventions to improve child nutrition should include parents, family members, and caregivers.
Early Childhood Caries, Mouth Pain, and Impaired Nutrition Status
Our study sample exhibited a high prevalence of ECC (74.4%), and high severity of caries based on the number of decayed teeth (mean = 5.87). These rates were comparable to other countries in Southeast Asia.7,22,23 Furthermore, the majority (95.4%) of children’s tooth decay was untreated, and we found high levels of deeper caries (31.2% into the pulp) for the Da Nang subsample. Moreover, one half of our entire sample complained of mouth pain.
We found a positive association between children’s consumption of soda and sweets or candy and the severity of dental caries (based on dmft status), which was consistent with previous literature.23,41,43,44 The weaker association might reflect the fact that dental caries resulted from sustained consumption of sweets combined with poor oral hygiene practices over time. Frequent consumption of sweets alone might only have a small effect on the risk for dental caries, if oral hygiene practices were adequate. In addition, the effect of dietary consumption on cavitation and nutritional status would be delayed. Toddlers who consumed frequent sugary snacks and beverages might not yet exhibit the severe caries likely to result from their early years’ diet. Following a longitudinal cohort of children for nutrition and oral health practices and outcomes would help elucidate the delays between dietary risk factors and oral health sequelae.
Dental caries severity was associated with deeper caries, mouth pain, and risk for poorer nutritional status. Children with a higher severity of dental caries had significantly lower mean weight- and BMI-for-age z-scores compared with those with lower severity of caries and those without caries. Combined mouth pain experience was positively and moderately correlated with dental caries severity. Furthermore, children who had complained of mouth pain had significantly lower BMI-for-age z-scores. Vania et al. proposed that ECC might shift the distribution of children’s weight to lower values, hypothesizing that mouth pain disrupted children’s eating patterns and sleep.1 Although their study data could not substantiate their hypothesis, our findings supported this relationship.
Although we found a strong association between children’s caries experience and compromised nutritional status by weight- and BMI-for-age z-scores, the association with a lower height-for-age z-score was found only for children with the deepest caries (into the pulp) compared with those without caries (for the Da Nang subsample). This finding was consistent with the generally accepted principle that impairment in children’s height gain (measured by height-for-age z-score, described as stunting or chronic malnutrition) is associated with their mother’s malnutrition and with the child’s malnutrition before age 2 years, whereas impairment in children’s weight gain (measured by weight- and BMI-for-age z-scores, and described as underweight, wasting, and acute malnutrition) is associated with the child’s malnutrition at any age, including after age 2 years.15,45
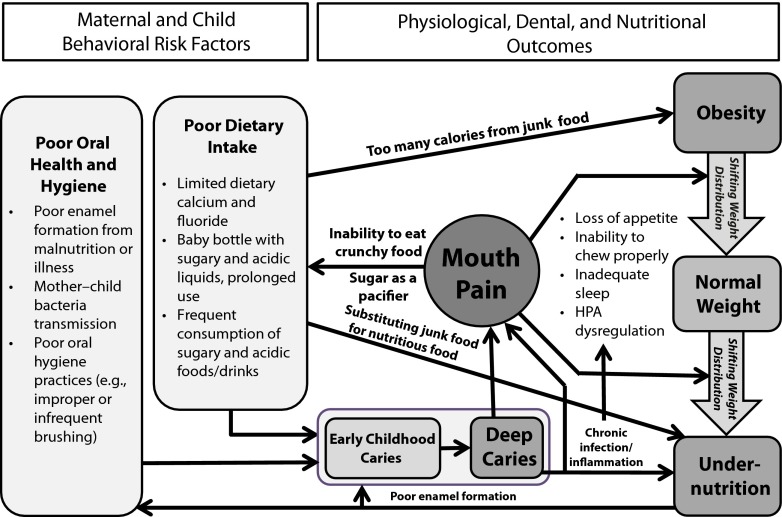
Model Linking Early Childhood Caries, Mouth Pain, and Malnutrition
In our proposed model (Figure 1), untreated deep dental caries commonly leads to chronic inflammation and mouth pain. In turn, chronic inflammation and mouth pain could cause disruption of eating and sleeping, and exacerbate the adverse effects of other risk factors on the child’s nutritional status. In our sample, mouth pain experience was aligned with severity of dental caries by the number of caries and the depth of caries, because the children’s dmft scores consisted primarily of untreated caries.
FIGURE 1—
Conceptual model of behavioral risk factors for early childhood caries and physiological and nutritional outcomes: Early Childhood Caries, Mouth Pain, and Nutritional Status; Vietnam; 2011.
Note. HPA = hypothalamic-pituitary-adrenal.
However, populations with a significant proportion of children who have filled or extracted teeth will not display chronic inflammation and mouth pain experience entirely consistent with their dmft indexes. In those populations, dmft alone might not accurately reflect the risk to children’s nutritional status, and it would be beneficial to supplement children’s oral health assessments with information about mouth pain experience, for example, with validated instruments like the self-reported scale of oral health outcomes for children aged 5 years34 or the early childhood oral health impact scale.35
Implications of Untreated Early Childhood Carries
Many studies that examined the relationship between malnutrition and ECC might have failed to find associations30–32 or found surprising results1,25,28,29 possibly because depth of caries and mouth pain experience were not accounted for in the study. In our study and other studies, ECC was associated with lower weight in the subset of children with mouth pain because it may adversely affect a child’s physiology through other pathways, including disrupted sleep, eating, chronic inflammation or infection, and possibly other mechanisms24,25,27,46 (Figure 1). We believe that such weight-lowering effects might be temporary because once the child receives adequate dental treatment or loses the carious primary teeth, the chronic inflammation and mouth pain, and their adverse effects, might be gone. Evidence for this exists from studies in which children had extractions for severe dental caries and gained weight following the removal of painful teeth.4,9,11 However, it was also shown that poor dietary habits, and possibly other hygiene behaviors, might persist, perhaps for life.40,41 Both acute and chronic malnutrition might also have life-altering effects.47
Severe ECC that leads to shifted weight distributions has 2 potentially detrimental effects for populations in developing countries. First, severe ECC might exacerbate existing problems with undernutrition, especially among poor populations that might have worse oral health and limited access to treatment.14 Undernutrition in very young children could lead to a vicious cycle; malnutrition could cause worse dentition,8,24 which in turn, could affect the child’s chewing abilities, which are necessary to gain nutrients, thus sustaining an undernourished state. Second, the shifted weight distribution in a study or general population might also mask the observed rate of overweight and obesity, especially among school-aged children, which might obscure the impending double burden (concurrent high prevalence of under- and overnutrition) in countries like Vietnam where prevalence of child overweight is increasing in some regions.16–18
Strengths and Limitations
To date, few studies that examined both oral health and malnutrition accounted for mouth pain in addition to the dmft index to help understand the physiological relationships and nutritional consequences of ECC. Our study involved analysis of early childhood tooth decay, mouth pain, and nutritional indexes from a solid sample size of 593 children from 2 regions of Vietnam. In addition, we partnered with local entities to conduct this baseline study and sustained the partnership to complete an intervention study (2011–2013) to improve children’s oral health and nutrition (these results will be published separately).
We studied a convenience sample of children aged 1 to 6 years, from middle-class families, attending the 5 schools in Da Nang and Ho Chi Minh City in Vietnam. This sample might not be representative of the entire population of the area being sampled. In addition, our cross-sectional analysis from the baseline data limited our ability to determine temporality or causality regarding relationships among ECC, mouth pain, and nutritional indexes. Moreover, we used a self-administered, unvalidated survey, which might contribute to underreporting of food or beverage consumption and child’s mouth pain, which possibly skewed some of our results.
Conclusions
In our sample, we observed a high prevalence of junk food consumption, ECC, and mouth pain, which were associated with lower nutritional status. It is critical that maternal–child health and dental professionals collaborate to implement family-centered, prevention-oriented oral health interventions prenatally and through the first years of life. These efforts could be incorporated into maternal–child health programs and preschools to teach parents and teachers proper nutrition and oral health practices, which would limit junk food consumption and enhance oral hygiene.48 This strategy might help prevent tooth decay, mouth pain, and malnutrition in Vietnam and other developing countries.
Acknowledgments
This research was supported by University of California’s Pacific Rim Research Initiative, the Academic Opportunity Fund of the Associated Students of the University of California, the Big Ideas Competition @ Berkeley, and the University of California Berkeley Undergraduate Research Apprenticeship Program. In addition, generous contributions were received from the Chung Family of Arcadia, CA.
We thank all University of California Berkeley students involved in this project and extend special thanks to the following: Kenny Chung, Wenting Guo, Debbie Huang, Tuyen Nguyen, Long Pham, Michelle Ta, and James Nguyen. Furthermore, we want to thank the staff at East Meets West Foundation and their workers in Vietnam and Oakland, and the University of Medicine and Pharmacy at Ho Chi Minh City. We are also grateful to the participating children and parents.
Human Participant Protection
This study has been fully approved by University of California Berkeley’s Committee for Protection of Human Subjects.
References
- 1.Vania A, Parisella V, Capasso F et al. Early childhood caries underweight or overweight, that is the question. Eur J Paediatr Dent. 2011;12(4):231–235. [PubMed] [Google Scholar]
- 2.Abanto J, Carvalho TS, Mendes FM, Wanderley MT, Bönecker M, Raggio DP. Impact of oral diseases and disorders on oral health‐related quality of life of preschool children. Community Dent Oral Epidemiol. 2011;39(2):105–114. doi: 10.1111/j.1600-0528.2010.00580.x. [DOI] [PubMed] [Google Scholar]
- 3.Casamassimo PS, Thikkurissy S, Edelstein BL, Maiorini E. Beyond the dmft: the human and economic cost of early childhood caries. J Am Dent Assoc. 2009;140(6):650–657. doi: 10.14219/jada.archive.2009.0250. [DOI] [PubMed] [Google Scholar]
- 4.Acs G, Shulman R, Chussid S, Ng M. The effect of dental rehabilitation on the body weight of children with early childhood caries. Pediatr Dent. 1999;21(2):109–113. [PubMed] [Google Scholar]
- 5.Tang RS, Huang M, Huang S. Relationship between dental caries status and anemia in children with severe early childhood caries. Kaohsiung J Med Sci. 2013;29(6):330–336. doi: 10.1016/j.kjms.2012.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marcenes W, Kassebaum NJ, Bernabe E et al. Global burden of oral conditions in 1990-2010: a systematic analysis. J Dent Res. 2013;92(7):592–597. doi: 10.1177/0022034513490168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Krisdapong S, Somkotra T, Kueakulpipat W. Disparities in early childhood caries and its impact on oral health-related quality of life of preschool children. Asia Pac J Public Health. 2014;26(3):285–294. doi: 10.1177/1010539512438608. [DOI] [PubMed] [Google Scholar]
- 8.Delgado‐Angulo EK, Hobdell MH, Bernabe E. Childhood stunting and caries increment in permanent teeth: a three and a half year longitudinal study in Peru. Int J Paediatr Dent. 2013;23(2):101–109. doi: 10.1111/j.1365-263X.2012.01229.x. [DOI] [PubMed] [Google Scholar]
- 9.Monse B, Duijster D, Sheiham A, Grijalva-Eternod CS, van Palenstein Helderman W, Hobdell MH. The effects of extraction of pulpally involved primary teeth on weight, height and BMI in underweight Filipino children. A cluster randomized clinical trial. BMC Public Health. 2012;12:725. doi: 10.1186/1471-2458-12-725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Plata-Salamán CR. Anorexia during acute and chronic disease. Nutrition. 1996;12(2):69–78. doi: 10.1016/s0899-9007(96)90702-9. [DOI] [PubMed] [Google Scholar]
- 11.Duijster D, Sheiham A, Hobdell MH, Itchon G, Monse B. Associations between oral health-related impacts and rate of weight gain after extraction of pulpally involved teeth in underweight preschool Filipino children. BMC Public Health. 2013;13:533. doi: 10.1186/1471-2458-13-533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kathmandu RY. The burden of restorative dental treatment for children in Third World countries. Int Dent J. 2002;52(1):1–9. [PubMed] [Google Scholar]
- 13.van Palenstein Helderman W, Mikx F, Nijmegen GJT, Hung HT, Luc PH. Workforce requirements for a primary oral health care system. Int Dent J. 2000;50(6):371–377. [PubMed] [Google Scholar]
- 14.Uetani M, Jimba M, Kaku T, Ota K, Wakai S. Oral health status of vulnerable groups in a village of the central highlands, southern Vietnam. Int J Dent Hyg. 2006;4(2):72–76. doi: 10.1111/j.1601-5037.2006.00165.x. [DOI] [PubMed] [Google Scholar]
- 15.Black RE, Victora CG, Walker SP et al. Maternal and child undernutrition and overweight in low-income and middle-income countries. Lancet. 2013;382(9890):427–451. doi: 10.1016/S0140-6736(13)60937-X. [DOI] [PubMed] [Google Scholar]
- 16.Khan NC, Hoan PV. Vietnam recommended dietary allowances 2007. Asia Pac J Clin Nutr. 2008;17(suppl 2):409–415. [PubMed] [Google Scholar]
- 17.Khan NC, Khoi HH. Double burden of malnutrition: the Vietnamese perspective. Asia Pac J Clin Nutr. 2008;17(suppl 1):116–118. [PubMed] [Google Scholar]
- 18.Cuong TQ, Dibley M, Bowe S, Hanh T, Loan T. Obesity in adults: an emerging problem in urban areas of Ho Chi Minh City, Vietnam. Eur J Clin Nutr. 2007;61(5):673–681. doi: 10.1038/sj.ejcn.1602563. [DOI] [PubMed] [Google Scholar]
- 19.General Statistical Office of Vietnam. Vietnam–Multiple Indicator Cluster Survey 2011, Final Report. Hanoi, Vietnam: : General Statistical Office of Vietnam; 2011. [Google Scholar]
- 20.World Health Organization. Global nutrition database 2014–Vietnam. Available at: http://www.who.int/nutgrowthdb/database/countries/who_standards/vnm_dat.pdf?ua=1. Accessed October 21, 2014.
- 21.Do LG, Spencer AJ, Roberts-Thomson KF, Trinh HD, Nguyen TT. Oral health status of Vietnamese children: findings from the National Oral Health Survey of Vietnam 1999. Asia Pac J Public Health. 2011;23(2):217–227. doi: 10.1177/1010539509340047. [DOI] [PubMed] [Google Scholar]
- 22.Cariño KMG, Shinada K, Kawaguchi Y. Early childhood caries in northern Philippines. Community Dent Oral Epidemiol. 2003;31(2):81–89. doi: 10.1034/j.1600-0528.2003.00010.x. [DOI] [PubMed] [Google Scholar]
- 23.Senesombath S, Nakornchai S, Banditsing P, Lexomboon D. Early childhood caries and related factors in Vientiane, Lao PDR. Southeast Asian J Trop Med Public Health. 2010;41(3):717–725. [PubMed] [Google Scholar]
- 24.Oliveira LB, Sheiham A, Bönecker M. Exploring the association of dental caries with social factors and nutritional status in Brazilian preschool children. Eur J Oral Sci. 2008;116(1):37–43. doi: 10.1111/j.1600-0722.2007.00507.x. [DOI] [PubMed] [Google Scholar]
- 25.Sharma A, Hegde AM. Relationship between body mass index, caries experience and dietary preferences in children. J Clin Pediatr Dent. 2009;34(1):49–52. doi: 10.17796/jcpd.34.1.17364206hqm0477h. [DOI] [PubMed] [Google Scholar]
- 26.Willerhausen B, Blettner M, Kasaj A, Hohenfellner K. Association between body mass index and dental health in 1,290 children of elementary schools in a German city. Clin Oral Investig. 2007;11(3):195–200. doi: 10.1007/s00784-007-0103-6. [DOI] [PubMed] [Google Scholar]
- 27.Hooley M, Skouteris H, Millar L. The relationship between childhood weight, dental caries and eating practices in children aged 4–8 years in Australia, 2004–2008. Pediatr Obes. 2012;7(6):461–470. doi: 10.1111/j.2047-6310.2012.00072.x. [DOI] [PubMed] [Google Scholar]
- 28.Hayden C, Bowler JO, Chambers S et al. Obesity and dental caries in children: a systematic review and meta‐analysis. Community Dent Oral Epidemiol. 2013;41(4):289–308. doi: 10.1111/cdoe.12014. [DOI] [PubMed] [Google Scholar]
- 29.Gerdin EW, Angbratt M, Aronsson K, Eriksson E, Johansson I. Dental caries and body mass index by socio‐economic status in Swedish children. Community Dent Oral Epidemiol. 2008;36(5):459–465. doi: 10.1111/j.1600-0528.2007.00421.x. [DOI] [PubMed] [Google Scholar]
- 30.Werner SL, Phillips C, Koroluk LD. Association between childhood obesity and dental caries. Pediatr Dent. 2012;34(1):23–27. [PubMed] [Google Scholar]
- 31.Pinto A, Kim S, Wadenya R, Rosenberg H. Is there an association between weight and dental caries among pediatric patients in an urban dental school? A correlation study. J Dent Educ. 2007;71(11):1435–1440. [PubMed] [Google Scholar]
- 32.Sheller B, Churchill SS, Williams BJ, Davidson B. Body mass index of children with severe early childhood caries. Pediatr Dent. 2009;31(3):216–221. [PubMed] [Google Scholar]
- 33.World Health Organization. Oral Health Surveys: Basic Methods. Geneva, Switzerland: World Health Organization; 1997. [Google Scholar]
- 34.Tsakos G, Blair YI, Yusuf H, Wright W, Watt RG, Macpherson LM. Developing a new self-reported scale of oral health outcomes for 5-year-old children (SOHO-5) Health Qual Life Outcomes. 2012;10:62. doi: 10.1186/1477-7525-10-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pahel BT, Rozier RG, Slade GD. Parental perceptions of children’s oral health: the Early Childhood Oral Health Impact Scale (ECOHIS) Health Qual Life Outcomes. 2007;5:6. doi: 10.1186/1477-7525-5-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Monse B, Heinrich‐Weltzien R, Benzian H, Holmgren C, van Palenstein Helderman W. PUFA–an index of clinical consequences of untreated dental caries. Community Dent Oral Epidemiol. 2010;38(1):77–82. doi: 10.1111/j.1600-0528.2009.00514.x. [DOI] [PubMed] [Google Scholar]
- 37.WHO Multicentre Growth Reference Study Group. WHO Child Growth Standards: Length/Height-for-Age, Weight-for-Age, Weight-for-Length, Weight-for-Height and Body Mass Index-for-Age: Methods and Development. Geneva, Switzerland: World Health Organization; 2006. p. 312. [Google Scholar]
- 38.de Onis M, Onyango AW, Borghi E, Siyam A, Nishida C, Siekmann J. Development of a WHO growth reference for school-aged children and adolescents. Bull World Health Organ. 2007;85(9):660–667. doi: 10.2471/BLT.07.043497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Series B Stat Methodol. 1995;57(1):289–300. [Google Scholar]
- 40.Alm A, Wendt L, Koch G, Birkhed D, Nilsson M. Caries in adolescence–influence from early childhood. Community Dent Oral Epidemiol. 2012;40(2):125–133. doi: 10.1111/j.1600-0528.2011.00647.x. [DOI] [PubMed] [Google Scholar]
- 41.Ruottinen S, Karjalainen S, Pienihakkinen K et al. Sucrose intake since infancy and dental health in 10-year-old children. Caries Res. 2004;38(2):142–148. doi: 10.1159/000075938. [DOI] [PubMed] [Google Scholar]
- 42.Rossow I, Kjaernes U, Holst D. Patterns of sugar consumption in early childhood. Community Dent Oral Epidemiol. 1990;18(1):12–16. doi: 10.1111/j.1600-0528.1990.tb00654.x. [DOI] [PubMed] [Google Scholar]
- 43.Slabšinskienė E, Milčiuvienė S, Narbutaitė J et al. Severe early childhood caries and behavioral risk factors among 3-year-old children in Lithuania. Medicina (Kaunas) 2010;46(2):135–141. [PubMed] [Google Scholar]
- 44.Fisher-Owens SA, Gansky SA, Platt LJ et al. Influences on children’s oral health: a conceptual model. Pediatrics. 2007;120(3):e510–e520. doi: 10.1542/peds.2006-3084. [DOI] [PubMed] [Google Scholar]
- 45.Bhutta ZA, Ahmed T, Black RE et al. What works? Interventions for maternal and child undernutrition and survival. Lancet. 2008;371(9610):417–440. doi: 10.1016/S0140-6736(07)61693-6. [DOI] [PubMed] [Google Scholar]
- 46.Alkarimi HA, Watt RG, Pikhart H, Sheiham A, Tsakos G. Dental caries and growth in school-age children. Pediatrics. 2014;133(3):e616–e623. doi: 10.1542/peds.2013-0846. [DOI] [PubMed] [Google Scholar]
- 47.Victora CG, Adair L, Fall C et al. Maternal and child undernutrition: consequences for adult health and human capital. Lancet. 2008;371(9609):340–357. doi: 10.1016/S0140-6736(07)61692-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Donoff B, McDonough JE, Riedy CA. Integrating oral and general health care. N Engl J Med. 2014;371(24):2247–2249. doi: 10.1056/NEJMp1410824. [DOI] [PubMed] [Google Scholar]