Abstract
Objectives. We explored how variance in HIV infection is distributed across multiple geographical scales among people who inject drugs (PWID) in the United States, overall and within racial/ethnic groups.
Methods. People who inject drugs (n = 9077) were recruited via respondent-driven sampling from 19 metropolitan statistical areas (MSAs) for the Centers for Disease Control and Prevention’s 2009 National HIV Behavioral Surveillance system. We used multilevel modeling to determine the percentage of variance in HIV infection explained by zip codes, counties, and MSAs where PWID lived, overall and for specific racial/ethnic groups.
Results. Collectively, zip codes, counties, and MSAs explained 29% of variance in HIV infection. Within specific racial/ethnic groups, all 3 scales explained variance in HIV infection among non-Hispanic/Latino White PWID (4.3%, 0.2%, and 7.5%, respectively), MSAs explained variance among Hispanic/Latino PWID (10.1%), and counties explained variance among non-Hispanic/Latino Black PWID (6.9%).
Conclusions. Exposure to potential determinants of HIV infection at zip codes, counties, and MSAs may vary for different racial/ethnic groups of PWID, and may reveal opportunities to identify and ameliorate intraracial inequities in exposure to determinants of HIV infection at these geographical scales.
Since the mid-1990s, there has been an increase in studies evaluating whether features of the social, economic, physical, and political environment (i.e., place characteristics) affect health. This focus on place characteristics is evident in the development of theories conceptualizing place characteristics as health determinants,1–3 in the use of geospatial and systematic social observation methods to measure place characteristics,4–10 in the application of multilevel modeling to assess the potential impacts of place characteristics,11–18 and in the recognition that interventions should not solely encourage individual behavior change but also modify environmental features.3,16,19
Literature emerging from this field of research demonstrates that place characteristics operationalized at different geographical scales influence psychosocial processes and individual behaviors that increase vulnerability to several health outcomes. With rare exception,20–24 however, studies of place and health typically assess the potential influence of place characteristics at a single geographical scale and do not simultaneously evaluate characteristics of other geographical scales. For example, several studies, including our own,25,26 sample participants from a single metropolitan statistical area (MSA) to assess the relationships of census tract characteristics to health, without sampling participants from multiple MSAs to simultaneously assess the relationships of tract-, county-, and MSA-level characteristics to health.25–32 The decision to focus on characteristics of a single geographical scale may arise because of data availability, cost constraints, or feasibility.
Studies of place and health that focus on a single geographical scale, however, may misspecify relationships and hinder the exploration of causal pathways in 2 ways. First, studies that focus on features measured at a single geographical scale may overlook potential health determinants that are operationalized at other geographical scales. For instance, research assessing the relationships of features of neighborhoods (e.g., economic deprivation, racial/ethnic composition, policing practices, and “crackdowns”) cannot determine the influence of policies, laws, and governmental expenditures that are operationalized at county, MSA, and state levels, and shape neighborhood environments. Second, studies of features of a single geographical scale cannot determine whether relationships between characteristics operating at one geographical scale are confounded, mediated, or modified by characteristics of other geographic scales.3,16,33 The possibility that at least 1 of these mechanisms can occur has been demonstrated in research conducted by Warner and Gomez, which suggests that, among Black women diagnosed with breast cancer, residing in census blocks with high concentrations of Black residents is more protective against mortality in more racially segregated metropolitan areas than less racially segregated metropolitan areas.34
In addition, research assessing the association of place-based factors with health outcomes rarely highlights the extent to which variance in health outcomes is explained by place and place-based factors. Determining whether health outcomes vary geographically can generate hypotheses about inequities in exposure to potential place-based determinants of health, and thereby inform how interventions and social policies are developed and spatially concentrated.35
The present study illustrates the generative possibilities of extending research beyond a single geographical scale by achieving 2 primary aims. The study’s first aim is to determine the share of total variance in HIV infection that is apportioned to zip codes, counties, and MSAs among people who inject drugs (PWID). In the United States, PWID account for 22% of people living with HIV,36 and a growing body of literature demonstrates that features of neighborhoods such as census-tract racial composition and block-level social or physical disorder are associated with HIV-related outcomes among PWID,37,38 as are features of MSAs, including drug-related law enforcement, income inequality, residential segregation, and health service access.39–41 Revealing the geographical scale to which variance in HIV infection is apportioned among PWID can stimulate hypotheses about inequities in exposure to place-based determinants of HIV and inform the development and tailoring of place-based interventions. For example, finding high MSA-level variance in HIV infection may support analyses of whether MSA-level variations in health care service access predict variance in HIV serostatus and, if they do, support interventions to increase health care access in low-access MSAs. In contrast, if little to no variance in HIV infection among PWID is apportioned to MSAs, PWID may encounter a relatively uniform exposure to health care service access.
Previous studies have found that variance in some health outcomes vary across racial/ethnic groups.42,43 The second aim of this study therefore tests the hypothesis that variance in HIV infection will differ within each of 3 racial/ethnic groups of PWID: non-Hispanic/Latino Whites, non-Hispanic/Latino Blacks, and Hispanics/Latinos.
METHODS
We drew data from the Centers for Disease Control and Prevention’s National HIV Behavioral Surveillance (NHBS) system.44 The NHBS system was designed to monitor HIV serostatus, behaviors, and service use in 3 high-risk populations in the United States, including PWID.44 We analyzed data from the 2009 NHBS cycle among PWID.
The 2009 NHBS cycle among PWID was conducted in 20 MSAs (or MSA divisions) in the United States where the AIDS prevalence was highest in 2006.45 We excluded the San Juan–Bayamon MSA from analysis because the sample lacked ethnic diversity (98% were Hispanic/Latino). In total, the 19 MSAs included in analysis accounted for 56% of all cumulative AIDS diagnoses reported by MSAs of all US territories with a population of 500 000 or more at the end of 2010 (San Juan–Bayamon accounted for an additional 2%).46
People who inject drugs in each MSA were recruited by respondent-driven sampling, which is a chain-referral sampling method.47 Recruitment chains began with fewer than 15 “seeds,” who were selected on the basis of recommendations from key informants working closely with PWID, and through community outreach.48 Seeds and subsequent recruiters were asked to recruit up to 5 injectors; recruitment continued until approximately 500 PWID were enrolled in each MSA. Participants were eligible for the study if they were aged 18 years or older, reported injection drug use in the past 12 months, resided in an NHBS-eligible MSA (4 MSAs had 1 county), showed evidence of injection (e.g., track marks), and provided oral consent. A total of 9882 participants met eligibility criteria in the 19 MSAs and 9831 (99.4%) of eligible participants consented to receive HIV testing.
We excluded participants from analysis if they had invalid or incomplete surveys, identified as transgender (transgender participants have been excluded from previous NHBS analyses because of their small sample size and lack of data on sexual risk behaviors), did not have a definitive positive or negative NHBS HIV test result, or reported being HIV-positive but had a negative NHBS HIV test result. (Formative research conducted as part of NHBS suggests that self-reported HIV status is not always accurate; therefore, laboratory confirmation is required to classify participants as HIV-positive.) We also excluded participants if they had invalid zip code information or did not identify as non-Hispanic/Latino White (hereafter referred to as White), non-Hispanic/Latino Black (hereafter referred to as Black), or Hispanic/Latino (hereafter referred to as Latino) or identified with 3 or more races. The final analytic sample included 9077 participants.
Measures
Individual characteristics.
Eligible participants were offered anonymous HIV testing. Test results were classified as positive if rapid test results (via blood or saliva) were reactive and confirmed by Western blot or immunofluorescence assay. Nonreactive rapid test results were classified as negative.48
We created 3 mutually exclusive racial/ethnic groups: Latino, White, and Black. We assigned biracial participants who were not Latino to Black or White categories by using Office of Management and Budget guidelines: non-Hispanic White comprised non-Hispanic White and American Indian/Alaska Native or Native Hawaiian/Pacific Islander; and non-Hispanic Black comprised non-Hispanic Black and non-Hispanic White or American Indian/Alaska Native or Native Hawaiian/Pacific Islander.49 We did not categorize Latino participants by their national origin because of small sample size.
We assessed other self-reported sociodemographic characteristics to describe the sample.
Geographical characteristics.
Participants reported the zip code and the county where they lived. Zip codes were the smallest geographical units reported in this study. Participants who reported homelessness at the time of the study interview were also asked to provide the zip code where they most frequently slept.
When participants lived in zip codes that crossed county lines (n = 341), they were assigned to the county where the majority of participants living in that zip code reported residing. We assigned participants to MSAs on the basis of interview site.
Statistical Analysis
We explored sample characteristics by using descriptive statistics. To determine the percentage of variance in HIV infection apportioned to each geographical unit for the entire sample and for each racial/ethnic group, we first conducted multilevel analysis to generate variance estimates for each geographical scale.50–52 We then used these variance estimates to calculate variance components for each geographical scale.50–52
Multilevel analysis.
We used 2 logistic multilevel models to measure the correlation of observations that were nested within zip codes, counties, and MSAs by partitioning variance in the odds of HIV infection to each geographical scale.50–52 The first multilevel model partitioned variance across the entire sample of PWID, without regard to race/ethnicity (equation 1):
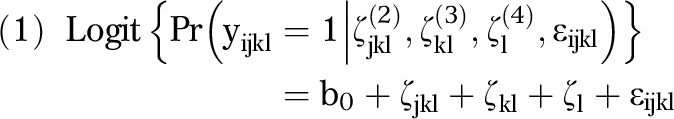 |
where b0 = mean log odds of testing positive for HIV; ζjkl(2) = random intercepts distributed across zip codes with variance Ψ(2); ζkl(3) = random intercepts distributed across counties with variance Ψ(3); ζl(4) = random intercepts distributed across MSAs with variance Ψ(4); and ɛijkl = individual residual error with variance π2/3.
In this model, yijkl denotes the probability of testing positive for HIV for individual i nested in zip code j, which is nested in county k, which is nested in MSA l; the random intercepts at each of the 3 geographical scales are assumed to be independent of the intercepts at the other geographical scales; individual residual error is assumed to be independent of the random intercepts at each geographical scale.52
The second multilevel model partitioned variance across the 3 geographical scales for each of 3 racial/ethnic groups: Black, White, and Latino. We derived random intercepts at each of the 3 geographical scales for each racial/ethnic group in this model.
Variance components.
Using the 4 variance estimates (Ψ(2), Ψ(3),Ψ(4), π2/3) obtained from the first multilevel analysis, we calculated the variance components for each geographical scale:
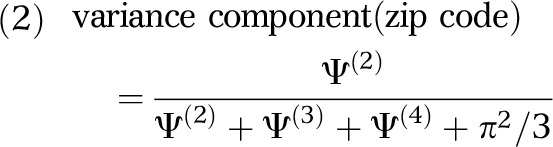 |
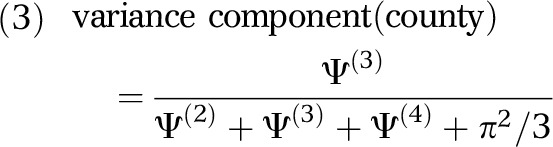 |
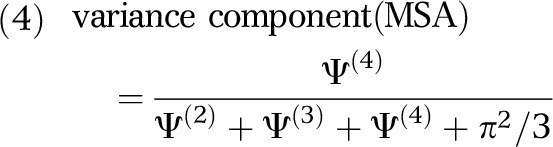 |
Here, each variance component is independent and equals the variance at a specific geographical scale divided by the total variance. Thus, a variance component of 0.05 for zip codes would indicate that 5% of the variance in the odds of HIV infection among PWID was apportioned to that scale.
To determine the proportion of variance apportioned to each geographical scale for each racial/ethnic group, we calculated the variance components by using the variance estimates obtained from the second multilevel model. The variance components equaled the variance of a specific geographical scale for 1 racial/ethnic group divided by the total variance.
Consonant with previous studies that apportioned variance in health outcomes to multiple levels,53,54 we modeled no individual-level or place-based fixed effects to determine the “baseline” distribution of the odds of HIV infection across geographical scales. We conducted sensitivity analysis to explore whether stratifying analyses by age, gender, and geographical region altered the variance component estimates; however, we could not perform estimation because of the limited number of geographical units per stratum. We also partitioned variance across MSAs and respondent-driven sampling recruitment chains. We did not simultaneously apportion variance in HIV infection to MSAs, counties, zip codes, and recruitment chains for each racial/ethnic group because cross-classified multilevel analysis is not possible for the number of random effects. We also did not calculate confidence intervals for variance components because methods to do so are not readily available. We used Stata version 13 (StataCorp LP, College Station, TX).
RESULTS
Among the 9077 PWID included in analysis, the majority were middle-aged (mean = 45.7 years; SD = 10.6) and men (71.7%; Table 1). Most participants were Black (51.6%), followed by White (30.3%) and Latino (18.1%); 67.0% of Latino PWID were US-born; those who were foreign-born reported residing in the United States for an average of 27 years. Overall, participants were impoverished: 84.0% reported earning less than $20 000 a year and 95.6% were not employed full-time. Almost 60% of participants were homeless at some point during the past year. Participants reported injecting drugs for a mean of 23 years (SD = 13.0).
TABLE 1—
Distribution of Individual-Level Characteristics Among 9077 People Who Inject Drugs: 2009 National HIV Behavioral Surveillance System, United States
| Characteristics | Total No. (%) or Mean ±SD (25th, 50th, 75th Percentiles) |
|
Demographic characteristics | |
| Age, y | 45.7 ±10.6 (39, 48, 54) |
| Gender | |
| Male | 6504 (71.7) |
| Female | 2573 (28.3) |
| Race/ethnicity | |
| Hispanic/Latino | 1640 (18.1) |
| Non-Hispanic/Latino Black | 4687 (51.6) |
| Non-Hispanic/Latino White | 2750 (30.3) |
| Married or living as married | 1199 (13.2) |
| High-school graduate (or certificate of high-school equivalency) | 6043 (66.6) |
| Currently employed full-time | 400 (4.4) |
| Yearly income, US$ | |
| < 10 000 | 5503 (60.9) |
| 10 000–19 999 | 2082 (23.1) |
| 20 000–29 999 | 609 (6.7) |
| 30 000–39 999 | 358 (4.0) |
| 40 000–49 999 | 194 (2.2) |
| ≥ 50 000 | 285 (3.2) |
| Homelessnessa | 5436 (59.9) |
| HIV | |
| Positive HIV test resultb | 799 (8.8) |
| Previous knowledge of HIV-positive status | 466 (58.3) |
| Average no. of years infected | 12 ±7.5 (0, 2, 12) |
|
Behavioral characteristicsc | |
| Years injecting drugs | 23.3 ±13.0 (12, 24, 34) |
| Frequency of injection | |
| ≥ daily | 6729 (74.3) |
| < daily | 2329 (25.7) |
| Any vaginal or anal sex | 7773 (85.8) |
Homelessness was defined as reported homelessness; residing on the street; residing in a shelter, single-room occupancy, or car; or temporarily residing with friends or relatives in the past 12 months.
HIV status was classified as positive if rapid test results (via blood or saliva) were reactive and confirmed by Western blot or immunofluorescence assay. Nonreactive rapid test results were classified as negative.
Behavioral characteristics had a reporting period of 12 months.
Approximately 9% of participants tested positive for HIV, with the highest proportion among Black PWID (10.7%), followed by Latino PWID (7.6%) and White PWID (6.3%). Among participants who tested positive, 58.3% reported a previous positive HIV test result and knowledge of their status for a mean of 12 years (SD = 7.5). In comparison with participants who reported receiving their first positive HIV test result at the NHBS visit, participants who reported receiving a previous positive result were more likely (i.e., ≥ 10% difference) to be White, to report stable housing, to inject daily or more often, and to report no unprotected sexual intercourse or sharing of any injection equipment in the past 12 months.
Overall, PWID lived in 968 zip codes, 51 counties, and 19 MSAs (Table 2). An average of 9 participants lived in each zip code, 178 lived in each county, and 478 lived in each MSA. In several MSAs, participants lived in 1 county (11 MSAs for White PWID, 9 MSAs for Black PWID, and 12 MSAs for Latino PWID). One MSA had no Latino participants.
TABLE 2—
Distribution of Geographical Characteristics Among 9077 People Who Inject Drugs: 2009 National HIV Behavioral Surveillance System, United States
| Geographical Characteristics | Total No. Geographical Units | Total No. Metropolitan Statistical Areas | Participants, No. (%) or Mean ±SD (25th, 50th, 75th Percentiles) |
|
Overall | |||
| Zip code | 968 | 9.4 ±19.8 (1, 3, 8) | |
| County | 51 | 178.0 ±225.0 (2, 23, 449) | |
| Metropolitan statistical area | 19 | 477.7 ±93.7 (426, 499, 534) | |
| Region | |||
| Northeast | 5 | 2136 (23.5) | |
| South | 7 | 3644 (40.2) | |
| Midwest | 2 | 937 (10.3) | |
| West | 5 | 2360 (26.0) | |
|
Non-Hispanic Whites | |||
| Zip code | 594 | 4.6 ±9.8 (1, 2, 4) | |
| County | 43 | 64.0 ±87.0 (2, 21, 95) | |
| Metropolitan statistical area | 19 | 144.7 ±93.0 (75, 128, 202) | |
| Region | |||
| Northeast | 5 | 817 (29.7) | |
| South | 7 | 669 (24.3) | |
| Midwest | 2 | 150 (5.5) | |
| West | 5 | 1114 (40.5) | |
|
Non-Hispanic Blacks | |||
| Zip code | 540 | 8.7 ±16.6 (1, 2, 8) | |
| County | 38 | 123.3 ±153.2 (3, 31, 230) | |
| Metropolitan statistical area | 19 | 246.7 ±139.5 (113, 230, 364) | |
| Region | |||
| Northeast | 5 | 729 (15.6) | |
| South | 7 | 2650 (56.5) | |
| Midwest | 2 | 611 (13.0) | |
| West | 5 | 697 (14.9) | |
|
Latinos | |||
| ZIP code | 415 | 4.0 ±7.1 (1, 2, 4) | |
| County | 34 | 48.2 ±63.6 (1, 11, 81) | |
| Metropolitan statistical area | 18 | 91.1 ±79.6 (13, 76, 154) | |
| Region | |||
| Northeast | 5 | 590 (36.0) | |
| South | 7 | 325 (19.8) | |
| Midwest | 1 | 176 (10.7) | |
| West | 5 | 549 (33.5) | |
In the model that apportioned variance to geographical scales without regard to race/ethnicity, 18.6% of the variance in the odds of HIV infection was collectively apportioned to the 3 geographical scales (Table 3); 6.5% was apportioned to zip codes, 2.3% to counties, and 9.8% to MSAs.
TABLE 3—
Percentage Variance in the Odds of Testing Positive for HIV by Geographical Unit Among 9077 People Who Inject Drugs: 2009 National HIV Behavioral Surveillance System, United States
| Geographical Units | Percentage Variance in HIV Apportioned to Geographical Units |
| Zip code | 6.5 |
| County | 2.3 |
| Metropolitan statistical area | 9.8 |
| Total | 18.6 |
When we added random intercepts for each racial/ethnic group to the model, the percentage of the variance apportioned to the 3 geographical scales increased from 18.6% to 29.0% (Table 4). In this model, the percentage of variance apportioned to counties and MSAs, respectively, increased from 2.3% to 7.1% and from 9.8% to 17.6%; the percentage apportioned to zip codes declined from 6.5% to 4.3%.
TABLE 4—
Percentage Variance in the Odds of Testing Positive for HIV by Geographical Unit and Race/Ethnicity Among 9077 People Who Inject Drugs: 2009 National HIV Behavioral Surveillance System, United States
| Percentage Variance in HIV Apportioned to Geographical Units |
||||
| Geographical Units | Non-Hispanic/Latino White (n = 2750) | Non-Hispanic/Latino Black (n = 4687) | Hispanic/Latino (n = 1640) | All PWID (n = 9077) |
| Zip code | 4.3 | 0.0 | 0.0 | 4.3 |
| County | 0.2 | 6.9 | 0.0 | 7.1 |
| Metropolitan statistical area | 7.5 | 0.0 | 10.1 | 17.6 |
| Total | 12.0 | 6.9 | 10.1 | 29.0 |
Note. PWID = people who inject drugs.
We observed racial/ethnic differences in the variance apportioned to each of the 3 geographical scales. Among White PWID, 7.5% of the variance was apportioned to MSAs, 4.3% to zip codes, and 0.2% to counties. Among Latino PWID, all variance was apportioned to MSAs. Among Black PWID, all variance was apportioned to counties.
When we added random intercepts for recruitment chains to the model that apportioned variance to MSAs without regard to race/ethnicity, 9% was apportioned to recruitment chains and the variance apportioned to MSAs increased from 9% to 14%.
DISCUSSION
This study demonstrates that variance in HIV infection among this sample of PWID was apportioned to 3 geographical scales, and suggests that variance in HIV infection is apportioned to different geographical scales among different racial/ethnic groups. Specifically, a relatively high percentage of variance in HIV infection was apportioned to zip codes, counties, and MSAs among White PWID, whereas variance was apportioned to counties among Black PWID and to MSAs among Latino PWID.
There are several potential explanations for why the geographical variance in HIV infection was entirely apportioned to counties for Black PWID; these potential explanations serve as possible hypotheses to test in future research on place and HIV among PWID in the United States. First, county-level per-capita income, population density, and crime rates have been associated with sexually transmitted infections among Black adults,55,56 and differences in exposure to these county-level factors may partly explain why variance was apportioned to counties among Black PWID in this study. Second, the influence of county-level differences in drug-related law enforcement on variance in HIV risk8,39–41,57,58 may be particularly salient for Black PWID. Although policing strategies are often implemented locally at the neighborhood level,59 decisions about investment in law enforcement are often made at the county level, and past research indicates that counties with higher proportions of Black residents invest more in law enforcement than other counties.60
No variance in HIV infection was apportioned to MSAs for Black PWID. This finding may be an artifact of NHBS’s selection of MSAs with the highest AIDS prevalence. Given disproportionately high rates of HIV infection among Black residents in the states where NHBS sites were selected,46 this sampling method might have produced a uniformly high HIV prevalence among Black PWID across MSAs.
In addition, no variance in HIV was apportioned to zip codes for Black PWID. This finding may partly reflect the influence of racial/ethnic residential segregation in the MSAs in the NHBS. Segregated MSAs tend to restrict Black residents (particularly those who are low-income, as is the case with most NHBS participants) to neighborhoods that are economically deprived and targeted by law enforcement.61–64 These factors have been associated with HIV infection and related outcomes among PWID.38,57,58,65 It is thus likely that homogeneity in exposure to these zip code–level phenomena would foster homogeneity in HIV infection across zip codes among Black PWID in NHBS.66 Residential segregation may also create greater homogeneity in the distribution of HIV risk across zip codes for Black PWID by creating racially/ethnically assortative sexual and injection networks.67–73
Through a similar set of pathways, racial/ethnic residential segregation may explain the lack of variance in HIV infection apportioned to zip codes among Latino PWID. By contrast, 4.5% of variance in HIV infection was apportioned to zip codes among White PWID. White PWID may be less restricted geographically than Black or Latino PWID and reside in zip codes with greater heterogeneity in local features. Future research should assess whether HIV prevalence among Black and Latino PWID is more homogenous across small geographic areas in more segregated MSAs and whether drug and sex network assortativity mediates the relationship between segregation and HIV serostatus.
Geographical variance in HIV infection among Latino PWID was exclusively apportioned to MSAs. This finding may be attributable to MSA-level differences in exposures that create vulnerability to HIV among Latinos, including socioeconomic conditions, health service access and use, immigration policies, and settlement patterns by country of origin.74–79 This last proposition is supported by previous research demonstrating that Latinos of Puerto Rican descent have a higher prevalence of injection-related HIV than other Latinos,80,81 a pattern that may reflect unequal exposure to structural factors across Latino subgroups, such as economic deprivation.74 HIV diagnoses among Latino PWID in NHBS may have been higher in MSAs where a greater proportion of PWID of Puerto Rican descent reside. Future research should determine whether country of origin explains variations in HIV serostatus across MSAs among Latino PWID.
No variance in HIV infection was apportioned to counties for Latino PWID. Previous research suggests that county-level differences in HIV prevalence rates are associated with county-level differences in urbanization.55 Urbanization and other potential county-level determinants of HIV among Latinos may not vary substantially across the counties where this sample of Latino PWID resided. Similar processes may partly explain the relatively low variance in HIV infection apportioned to counties among White PWID (0.2%).
Limitations
Our findings should be interpreted in light of several limitations. Metropolitan statistical areas with high AIDS prevalence were selected to participate in NHBS and recruitment chains may have not extended past geographical boundaries or sociodemographic groups. In addition, PWID recruited through respondent-driven sampling may have been unwilling to participate if they resided far from NHBS sites. These factors may have influenced the magnitude and precision of variance components in this analysis.
Zip code boundaries may not align with subjectively defined neighborhood boundaries. We also could not assess the influence of environments where participants worked, bought or used drugs, or engaged in other activities but did not live. In addition, participants who did not report their counties of residence and lived in zip codes that crossed county boundaries may have been incorrectly assigned to counties where most PWID in that zip code lived. Because zip codes crossed counties in less than 4% (n = 341) of instances, this may have minimally affected our findings.
Because of small sample sizes, we could not determine the geographical distribution of HIV infection among those PWID who were multiracial or were not Latino, Black, or White. We also excluded participants from the analytic sample if they lacked complete information on key characteristics (e.g., confirmatory HIV test result, zip code). Compared with participants excluded from analysis, the analytic sample had larger proportions of PWID who were Black, earned an annual income exceeding $20 000, and who had not been homeless in the past 12 months. Because we excluded only 8% of the study population from the analytic sample, we believe that these differences did not substantially change the results in this study.
On average, HIV-positive participants reported that they had received their first positive HIV result 12 years before participating in NHBS; it is possible some of these participants relocated from the locations where they lived at the time they acquired HIV. Because participants reported residing in the same MSA for an average of 32 years, the MSA-level findings would be less influenced by participant mobility, although zip code and county findings might be affected by this migration.
Conclusions
Despite these limitations, this study revealed that considerable variance in HIV infection among PWID may be apportioned to multiple geographical scales, and that variance in HIV infection may be apportioned to different geographical scales for different racial/ethnic groups. Variance in HIV infection across MSAs for Latino PWID, across counties for Black PWID, and across zip codes, counties, and MSAs for White PWID may relate to differences in exposure to potential modifiable place-based determinants of HIV, including crime, drug-related law enforcement, economic disadvantage, income inequality, and health service access. Future research should test the hypotheses generated by this analysis and evaluate place characteristics operating at multiple geographical scales simultaneously whenever possible. The knowledge attained from such studies can identify where and how social and economic policies, and harm reduction and other HIV/AIDS prevention and treatment strategies should be targeted geographically.
Acknowledgments
This research was supported by grants from the National Institutes of Health: “Place Characteristics and Disparities in HIV in IDUS: A Multilevel Analysis of NHBS” (DA035101; Cooper, PI) and the Emory Center for AIDS Research (P30 AI050409; Curran, PI).
We would like to thank the Centers for Disease Control and Prevention, and members of the National HIV Behavioral Surveillance System Study Group: Atlanta, GA: Jennifer Taussig, Shacara Johnson, Jeff Todd; Baltimore, MD: Colin Flynn, Danielle German; Boston, MA: Debbie Isenberg, Maura Driscoll, Elizabeth Hurwitz; Chicago, IL: Nikhil Prachand, Nanette Benbow; Dallas, TX: Sharon Melville, Richard Yeager, Jim Dyer, Alicia Novoa; Denver, CO: Mark Thrun, Alia Al-Tayyib; Detroit, MI: Emily Higgins, Eve Mokotoff, Vivian Griffin; Houston, TX: Aaron Sayegh, Jan Risser, Hafeez Rehman; Los Angeles, CA: Trista Bingham, Ekow Kwa Sey; Miami, FL: Lisa Metsch, David Forrest, Dano Beck, Gabriel Cardenas; Nassau-Suffolk, NY: Chris Nemeth, Lou Smith, Carol-Ann Watson; New Orleans, LA: William T. Robinson, DeAnn Gruber, Narquis Barak; New York, NY: Alan Neaigus, Samuel Jenness, Travis Wendel, Camila Gelpi-Acosta, Holly Hagan; Newark, NJ: Henry Godette, Barbara Bolden, Sally D’Errico; Philadelphia, PA: Kathleen A. Brady, Althea Kirkland, Mark Shpaner; San Diego, CA: Vanessa Miguelino-Keasling, Al Velasco; San Francisco, CA: H. Fisher Raymond; San Juan, PR: Sandra Miranda De León, Yadira Rolón-Colón; Seattle, WA: Maria Courogen, Hanne Thiede, Richard Burt; St Louis, MO: Michael Herbert, Yelena Friedberg, Dale Wrigley, Jacob Fisher; Washington, DC: Marie Sansone, Tiffany West-Ojo, Manya Magnus, Irene Kuo; Behavioral Surveillance Team. We also thank the men and women who participated in NHBS and the staff at all NHBS sites.
Human Participant Protection
The institutional review boards of Emory University and each NHBS site and the Centers for Disease Control and Prevention approved study protocols.
References
- 1.Krieger N. Epidemiology and the web of causation: has anyone seen the spider? Soc Sci Med. 1994;39(7):887–903. doi: 10.1016/0277-9536(94)90202-x. [DOI] [PubMed] [Google Scholar]
- 2.Krieger N. Theories for social epidemiology in the 21st century: an ecosocial perspective. Int J Epidemiol. 2001;30(4):668–677. doi: 10.1093/ije/30.4.668. [DOI] [PubMed] [Google Scholar]
- 3.Rhodes T. The “risk environment”: a framework for understanding and reducing drug-related harm. Int J Drug Policy. 2002;13:85–94. [Google Scholar]
- 4.Bernstein KT, Curriero FC, Jennings JM, Olthoff G, Erbelding EJ, Zenilman J. Defining core gonorrhea transmission utilizing spatial data. Am J Epidemiol. 2004;160(1):51–58. doi: 10.1093/aje/kwh178. [DOI] [PubMed] [Google Scholar]
- 5.Jennings JM, Curriero FC, Celentano D, Ellen JM. Geographic identification of high gonorrhea transmission areas in Baltimore, Maryland. Am J Epidemiol. 2005;161(1):73–80. doi: 10.1093/aje/kwi012. [DOI] [PubMed] [Google Scholar]
- 6.Cooper HL, Wodarski S, Cummings J et al. Public housing relocations in Atlanta, Georgia, and declines in spatial access to safety net primary care. Health Place. 2012;18(6):1255–1260. doi: 10.1016/j.healthplace.2012.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Russell E, Kramer MR, Cooper HL, Thompson WW, Arriola KR. Residential racial composition, spatial access to care, and breast cancer mortality among women in Georgia. J Urban Health. 2011;88(6):1117–1129. doi: 10.1007/s11524-011-9612-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cooper H, Des Jarlais D, Ross Z, Tempalski B, Bossak BH, Friedman SR. Spatial access to sterile syringes and the odds of injecting with an unsterile syringe among injectors: a longitudinal multilevel study. J Urban Health. 2012;89(4):678–696. doi: 10.1007/s11524-012-9673-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sampson RJ, Raudenbush SW. Systematic social observation of public spaces: a new look at disorder in urban neighborhoods. Am J Sociol. 1999;105:603–651. [Google Scholar]
- 10.Furr-Holden CD, Smart MJ, Pokorni JL et al. The NIfETy method for environmental assessment of neighborhood-level indicators of violence, alcohol, and other drug exposure. Prev Sci. 2008;9(4):245–255. doi: 10.1007/s11121-008-0107-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Diez-Roux AV. Bringing context back into epidemiology: variables and fallacies in multilevel analysis. Am J Public Health. 1998;88(2):216–222. doi: 10.2105/ajph.88.2.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Diez Roux A. Investigating neighborhood and area effects on health. Am J Public Health. 2001;91(11):1783–1789. doi: 10.2105/ajph.91.11.1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Diez Roux A. Invited commentary: places, people, and health. Am J Epidemiol. 2002;155(6):516–519. doi: 10.1093/aje/155.6.516. [DOI] [PubMed] [Google Scholar]
- 14.Diez Roux AV, Mair C. Neighborhoods and health. Ann N Y Acad Sci. 2010;1186:125–145. doi: 10.1111/j.1749-6632.2009.05333.x. [DOI] [PubMed] [Google Scholar]
- 15.Macintyre S, Ellaway A. Neighborhoods and health: an overview. In: Kawachi I, Berkman LF, editors. Neighborhoods and Health. New York, NY: Oxford University Press; 2003. pp. 20–44. [Google Scholar]
- 16.Glass TA, McAtee MJ. Behavioral science at the crossroads in public health: extending horizons, envisioning the future. Soc Sci Med. 2006;62(7):1650–1671. doi: 10.1016/j.socscimed.2005.08.044. [DOI] [PubMed] [Google Scholar]
- 17.Kaplan GA. What’s wrong with social epidemiology, and how can we make it better? Epidemiol Rev. 2004;26:124–135. doi: 10.1093/epirev/mxh010. [DOI] [PubMed] [Google Scholar]
- 18.Celeste RK, Bastos JL, Faerstein E. Trends in the investigation of social determinants of health: selected themes and methods. Cad Saude Publica. 2011;27(1):183–189. doi: 10.1590/s0102-311x2011000100019. [DOI] [PubMed] [Google Scholar]
- 19.Link BG, Phelan J. Social conditions as fundamental causes of disease. J Health Soc Behav. 1995 Spec no:80–94. [PubMed] [Google Scholar]
- 20.Fan JX, Hanson HA, Zick CD, Brown BB, Kowaleski-Jones L, Smith KR. Geographic scale matters in detecting the relationship between neighbourhood food environments and obesity risk: an analysis of driver license records in Salt Lake County, Utah. BMJ Open. 2014;4(8):e005458. doi: 10.1136/bmjopen-2014-005458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Duncan C, Jones K, Moon G. Context, composition and heterogeneity: using multilevel models in health research. Soc Sci Med. 1998;46(1):97–117. doi: 10.1016/s0277-9536(97)00148-2. [DOI] [PubMed] [Google Scholar]
- 22.Duncan C, Jones K, Moon G. Do places matter? A multi-level analysis of regional variations in health-related behaviour in Britain. Soc Sci Med. 1993;37(6):725–733. doi: 10.1016/0277-9536(93)90366-c. [DOI] [PubMed] [Google Scholar]
- 23.Duncan C, Jones K, Moon G. Psychiatric morbidity: a multilevel approach to regional variations in the UK. J Epidemiol Community Health. 1995;49(3):290–295. doi: 10.1136/jech.49.3.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Congdon P. The impact of area context on long term illness and premature mortality: an illustration of multi-level analysis. Reg Stud. 1995;29:327–344. [Google Scholar]
- 25.Cooper HL, Hunter-Jones J, Kelley M et al. The aftermath of public housing relocations: relationships between changes in local socioeconomic conditions and depressive symptoms in a cohort of adult relocaters. J Urban Health. 2014;91:223–241. doi: 10.1007/s11524-013-9844-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cooper HL, Haley DF, Linton S et al. Impact of public housing relocations: are changes in neighborhood conditions related to STIs among relocaters? Sex Transm Dis. 2014;41(10):573–579. doi: 10.1097/OLQ.0000000000000172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shimotsu ST, Jones-Webb RJ, MacLehose RF, Nelson TF, Forster JL, Lytle LA. Neighborhood socioeconomic characteristics, the retail environment, and alcohol consumption: a multilevel analysis. Drug Alcohol Depend. 2013;132(3):449–456. doi: 10.1016/j.drugalcdep.2013.03.010. [DOI] [PubMed] [Google Scholar]
- 28.Gibbons J, Yang T-C. Self-rated health and residential segregation: how does race/ethnicity matter? J Urban Health. 2014;91(4):648–660. doi: 10.1007/s11524-013-9863-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brown AF, Liang LJ, Vassar SD et al. Neighborhood socioeconomic disadvantage and mortality after stroke. Neurology. 2013;80(6):520–527. doi: 10.1212/WNL.0b013e31828154ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brown AF, Liang L-J, Vassar SD et al. Neighborhood disadvantage and ischemic stroke: the cardiovascular health study (CHS) Stroke. 2011;42(12):3363–3368. doi: 10.1161/STROKEAHA.111.622134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nepomnyaschy L, Reichman NE. Low birthweight and asthma among young urban children. Am J Public Health. 2006;96(9):1604–1610. doi: 10.2105/AJPH.2005.079400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Unger E, Diez-Roux AV, Lloyd-Jones DM et al. Association of neighborhood characteristics with cardiovascular health in the multi-ethnic study of atherosclerosis. Circ Cardiovasc Qual Outcomes. 2014;7(4):524–531. doi: 10.1161/CIRCOUTCOMES.113.000698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Subramanian SV, Duncan C, Jones K. Multilevel perspectives on modeling census data. Environ Plann A. 2001;33:399–417. [Google Scholar]
- 34.Warner ET, Gomez SL. Impact of neighborhood racial composition and metropolitan residential segregation on disparities in breast cancer stage at diagnosis and survival between Black and White women in California. J Community Health. 2010;35(4):398–408. doi: 10.1007/s10900-010-9265-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Centers for Disease Control and Prevention. Integrated prevention services for HIV infection, viral hepatitis, sexually transmitted diseases, and tuberculosis for persons who use drugs illicitly: summary guidance from CDC and the US Department of Health and Human Services. MMWR Recomm Rep. 2012;61(RR-5):1–40. [PubMed] [Google Scholar]
- 36.Atlanta, GA: Centers for Disease Control and Prevention; 2012. Monitoring selected national HIV prevention and care objectives by using HIV surveillance data—United States and 6 US dependent areas—2010. [Google Scholar]
- 37.Bluthenthal RN, Do DP, Finch B, Martinez A, Edlin BR, Kral AH. Community characteristics associated with HIV risk among injection drug users in the San Francisco Bay area: a multilevel analysis. J Urban Health. 2007;84(5):653–666. doi: 10.1007/s11524-007-9213-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Latkin CA, Curry AD, Hua W, Davey MA. Direct and indirect associations of neighborhood disorder with drug use and high-risk sexual partners. Am J Prev Med. 2007;32(6 suppl):S234–S241. doi: 10.1016/j.amepre.2007.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Friedman SR, Perlis T, Des Jarlais DC. Laws prohibiting over-the-counter syringe sales to injection drug users: relations to population density, HIV prevalence, and HIV incidence. Am J Public Health. 2001;91(5):791–793. doi: 10.2105/ajph.91.5.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Friedman S, Tempalski B, Cooper H . Metropolitan area characteristics, injection drug use and HIV among injectors. In: Thomas YF, Richardon D, Cheung I, editors. Geography and Drug Addiction. Berlin, Germany: Springer-Verlag; 2008. [Google Scholar]
- 41.Friedman SR, Cooper HLF, Tempalski B et al. Relationships of deterrence and law enforcement to drug-related harms among drug injectors in US metropolitan areas. AIDS. 2006;20(1):93–99. doi: 10.1097/01.aids.0000196176.65551.a3. [DOI] [PubMed] [Google Scholar]
- 42.Kramer MR, Hogue CR. Place matters: variation in the Black/White very preterm birth rate across US metropolitan areas, 2002–2004. Public Health Rep. 2008;123:576–585. doi: 10.1177/003335490812300507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chesson HW, Sternberg M, Leichliter JS, Aral SO. The distribution of chlamydia, gonorrhoea and syphilis cases across states and counties in the USA, 2007. Sex Transm Infect. 2010;86(suppl 3):iii52–iii57. doi: 10.1136/sti.2009.040873. [DOI] [PubMed] [Google Scholar]
- 44.Gallagher KM, Sullivan PS, Lansky A, Onorato IM. Behavioral surveillance among people at risk for HIV infection in the US: the National HIV Behavioral Surveillance System. Public Health Rep. 2007;122(suppl 1):32–38. doi: 10.1177/00333549071220S106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Centers for Disease Control and Prevention. HIV/AIDS Surveillance Report, 2006. 2008;Vol 18 Available at: http://www.cdc.gov/hiv/topics/surveillance/resources/reports. Accessed August 24, 2015. [Google Scholar]
- 46.Diagnoses of HIV infection and AIDS in the United States and dependent areas. Atlanta, GA: Centers for Disease Control and Prevention; 2012. HIV Surveillance Report, 2010. [Google Scholar]
- 47.Lansky A, Abdul-Quader AS, Cribbin M et al. Developing an HIV behavioral surveillance system for injecting drug users: the National HIV Behavioral Surveillance System. Public Health Rep. 2007;122(suppl 1):48–55. doi: 10.1177/00333549071220S108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Broz D, Wejnert C, Pham HT et al. HIV infection and risk, prevention, and testing behaviors among injecting drug users—National HIV Behavioral Surveillance System, 20 US cities, 2009. MMWR Surveill Summ. 2014;63(suppl 6):1–51. [PubMed] [Google Scholar]
- 49.Washington, DC: US Office of Management and Budget; 2000. Provisional guidance on the implementation of the 1997 standards for federal data on race and ethnicity, Executive Office of the President, December 15. [Google Scholar]
- 50.Goldstein H, Browne W, Rasbash J. Partitioning variation in multilevel models. Underst Stat. 2002;1:223–231. [Google Scholar]
- 51.Browne WJ, Subramanian SV, Jones K, Goldstein H. Variance partitioning in multilevel logistic models that exhibit overdispersion. J R Stat Soc Ser A Stat Soc. 2005;168:599–613. [Google Scholar]
- 52.Rabe-Hesketh S, Skrondal A. Multilevel and Longitudinal Modeling Using Stata. 2nd ed. College Station, TX: Stata Press; 2008. [Google Scholar]
- 53.Wright D, Bobashev GV, Novak SP. Decomposing the total variation in a nested random effects model of neighborhood, household, and individual components when the dependent variable is dichotomous: implications for adolescent marijuana use. Drug Alcohol Depend. 2005;78(2):195–204. doi: 10.1016/j.drugalcdep.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 54.Wright DA, Bobashev G, Folsom R. Understanding the relative influence of neighborhood, family, and youth on adolescent drug use. Subst Use Misuse. 2007;42(14):2159–2171. doi: 10.1080/10826080701212675. [DOI] [PubMed] [Google Scholar]
- 55.Vaughan AS, Rosenberg E, Shouse RL, Sullivan PS. Connecting race and place: a county-level analysis of White, Black, and Hispanic HIV prevalence, poverty, and level of urbanization. Am J Public Health. 2014;104(7):e77–e84. doi: 10.2105/AJPH.2014.301997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chesson HW, Kent CK, Owusu-Edusei K, II, Leichliter JS, Aral SO. Disparities in sexually transmitted disease rates across the “eight Americas”. Sex Transm Dis. 2012;39(6):458–464. doi: 10.1097/OLQ.0b013e318248e3eb. [DOI] [PubMed] [Google Scholar]
- 57.Wood E, Spittal PM, Small W et al. Displacement of Canada’s largest public illicit drug market in response to a police crackdown. CMAJ. 2004;170(10):1551–1556. doi: 10.1503/cmaj.1031928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Werb D, Wood E, Small W et al. Effects of police confiscation of illicit drugs and syringes among injection drug users in Vancouver. Int J Drug Policy. 2008;19(4):332–338. doi: 10.1016/j.drugpo.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Alexander M. The New Jim Crow: Mass Incarceration in the Age of Colorblindness. New York, NY: The New Press; 2012. [Google Scholar]
- 60.Ruddell R, Thomas MO. Minority threat and police strength: an examination of the Golden State. Police Pract Res. 2009;11:256–273. [Google Scholar]
- 61.Massey D, Denton N. American Apartheid: Segregation and the Making of the Underclass. Cambridge, MA: Harvard University Press; 1993. [Google Scholar]
- 62.Denton N. Are African Americans still hypersegregated? In: Grisby JE III, Bullard RD, Lee C, Feagin JR, editors. Residential Apartheid: The American Legacy. Los Angeles, CA: CAAS Publications, University of California; 1994. [Google Scholar]
- 63.Logan JR. The persistence of segregation in the 21st century metropolis. City Community. 2013;12(2) doi: 10.1111/cico.12021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Galster GC, Mikelsons M. The geography of metropolitan opportunity—a case-study of neighborhood conditions confronting youth in Washington, DC. Hous Policy Debate. 1995;6:73–102. [Google Scholar]
- 65.Genberg BL, Gange SJ, Go VF et al. The effect of neighborhood deprivation and residential relocation on long-term injection cessation among injection drug users (IDUs) in Baltimore, Maryland. Addiction. 2011;106(11):1966–1974. doi: 10.1111/j.1360-0443.2011.03501.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nunn A, Yolken A, Cutler B et al. Geography should not be destiny: focusing HIV/AIDS implementation research and programs on microepidemics in US neighborhoods. Am J Public Health. 2014;104(5):775–780. doi: 10.2105/AJPH.2013.301864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Laumann EO, Youm Y. Racial/ethnic group differences in the prevalence of sexually transmitted diseases in the United States: a network explanation. Sex Transm Dis. 1999;26(5):250–261. doi: 10.1097/00007435-199905000-00003. [DOI] [PubMed] [Google Scholar]
- 68.Nunn A, Dickman S, Cornwall A et al. Concurrent sexual partnerships among African American women in Philadelphia: results from a qualitative study. Sex Health. 2012;9(3):288–296. doi: 10.1071/SH11099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Burt RD, Hagan H, Sabin K, Thiede H. Evaluating respondent-driven sampling in a major metropolitan area: comparing injection drug users in the 2005 Seattle area National HIV Behavioral Surveillance System survey with participants in the RAVEN and Kiwi Studies. Ann Epidemiol. 2010;20(2):159–167. doi: 10.1016/j.annepidem.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pierce TG. Gen-X junkie: ethnographic research with young White heroin users in Washington, DC. Subst Use Misuse. 1999;34(14):2095–2114. doi: 10.3109/10826089909039440. [DOI] [PubMed] [Google Scholar]
- 71.Braine N, Acker C, Goldblatt C, Yi H, Friedman S, Des Jarlais DC. Neighborhood history as a factor shaping syringe distribution networks among drug users at a US syringe exchange. Soc Networks. 2008;30(3):235–246. doi: 10.1016/j.socnet.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Curtis R, Friedman SR, Neaigus A, Jose B, Goldstein M, Ildefonso G. Street-level drug markets: network structure and HIV risk. Soc Networks. 1995;17:229–249. [Google Scholar]
- 73.Abdul‐Quader AS, Heckathorn D, McKnight C et al. Effectiveness of respondent-driven sampling for recruiting drug users in New York City: findings from a pilot study. J Urban Health. 2006;83(3):459–476. doi: 10.1007/s11524-006-9052-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Munoz EA, Ortega ST. Regional socioeconomic and sociocultural differences among US Latinos: the effects of historical and contemporary Latino immigration/migration streams. Great Plains Res. 1997;7:289–314. [Google Scholar]
- 75.Foulkes M, Newbold KB. Migration propensities, patterns, and the role of human capital: comparing Mexican, Cuban, and Puerto Rican interstate migration, 1985–1990. Prof Geogr. 2000;52:133–145. [Google Scholar]
- 76.Weinick RM, Jacobs EA, Stone LC, Ortega AN, Burstin H. Hispanic healthcare disparities: challenging the myth of a monolithic Hispanic population. Med Care. 2004;42(4):313–320. doi: 10.1097/01.mlr.0000118705.27241.7c. [DOI] [PubMed] [Google Scholar]
- 77.Diaz T, Klevens M. Differences by ancestry in sociodemographics and risk behaviors among Latinos with AIDS. The Supplement to HIV and AIDS Surveillance Project Group. Ethn Dis. 1997;7(3):200–206. [PubMed] [Google Scholar]
- 78.Hacker K, Chu J, Leung C et al. The impact of immigration and customs enforcement on immigrant health: perceptions of immigrants in Everett, Massachusetts, USA. Soc Sci Med. 2011;73(4):586–594. doi: 10.1016/j.socscimed.2011.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hardy LJ, Getrich CM, Quezada JC, Guay A, Michalowski RJ, Henley E. A call for further research on the impact of state-level immigration policies on public health. Am J Public Health. 2012;102(7):1250–1254. doi: 10.2105/AJPH.2011.300541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Centers for Disease Control and Prevention. Geographic differences in HIV infection among Hispanics or Latinos—46 states and Puerto Rico, 2010. MMWR Morb Mortal Wkly Rep. 2012;61(40):805–810. [PubMed] [Google Scholar]
- 81.Centers for Disease Control and Prevention. HIV/AIDS among Hispanics—United States, 2001–2005. MMWR Morb Mortal Wkly Rep. 2007;56(40):1052–1057. [PubMed] [Google Scholar]


