Abstract
Objectives. We examined the trends and correlates of quadrivalent human papillomavirus vaccine (HPV4) initiation in insured boys during the periods before and after routine use recommendation.
Methods. We grouped data from electronic medical records of boys aged 9 to 17 years from the Kaiser Permanente Southern California prepaid health plan into 3 open cohorts: permissive use: 2009 to 2010; anal cancer indication added: 2010 to 2011; and routine use: 2011 to 2013. We estimated adjusted risk ratios (ARRs) between demographics and vaccination initiation using Poisson regression.
Results. HPV4 initiation increased across cohorts—1.6%, 3.4%, and 18.5%—with the greatest increase among boys aged 11 to 12 years in cohort 3. Initiation was associated with receiving influenza vaccination in the previous year in all cohorts (cohort 3: ARR = 1.48; 95% confidence interval [CI] = 1.46, 1.51) and with non-White race/ethnicity following routine recommendation (cohort 3, non-Hispanic Black: ARR = 1.18; 95% CI = 1.08, 1.30; Hispanic: ARR = 1.23; 95% CI = 1.17, 1.29; Asian/Pacific Islanders: ARR = 1.16; 95% CI = 1.11, 1.20).
Conclusions. Routine use recommendation increased the uptake of HPV4 in boys. System-level interventions to encourage providers to routinely recommend HPV4 vaccination may help increase HPV4 uptake in boys.
Genital human papillomavirus (HPV) infection is the most common sexually transmitted infection in the United States; an estimated 14 million persons are newly infected every year.1 Although most infections cause no symptoms and are self-limited, persistent HPV infection can cause cervical cancer in women as well as other anogenital cancers, oropharyngeal cancer, and genital warts in men and women. Two vaccines that protect against HPV infection are currently available in the United States. Both the quadrivalent (HPV4) and bivalent (HPV2) vaccines protect against HPV types 16 and 18, which cause 70% of cervical cancers; HPV4 also protects against HPV types 6 and 11, which cause 90% of genital warts.2,3
Since the licensure of HPV4 in 2006, the Advisory Committee on Immunization Practices (ACIP) of the Centers for Disease Control and Prevention has recommended the routine vaccination of girls aged 11 and 12 years (and as young as 9 years) and catch-up vaccination for those aged 13 to 26 years not previously vaccinated. However, the recommendation for HPV4 use in males has undergone progressive changes. In 2009, HPV4 was licensed for use in males aged 9 to 26 years, and ACIP recommended “permissive use” for males aged 9 to 26 years for the prevention of genital warts.4 In December 2010, the US Food and Drug Administration added the prevention of anal cancer in males and females as an indication for use. On October 25, 2011, HPV4 was recommended for routine vaccination of males aged 11 or 12 years and for those aged 13 to 21 years not previously vaccinated.5 The vaccination series can be started beginning at age 9 years for both girls and boys.
Because of the high efficacy of HPV4 among males6 and low vaccine coverage among females,7 vaccination of males would provide direct benefits to both males and females. Data from the National Immunization Survey-Teen showed that coverage in adolescent boys has continued to increase since the ACIP routine recommendation for males. The coverage (≥ 1 dose) among boys aged 13 to 17 years increased from 8.3% in 2011 to 20.8% in 2012 and further increased to 34.6% in 2013.7 However, because the National Immunization Survey-Teen reports cumulative coverage and does not capture data among boys aged 9 to 12 years, it is difficult to assess the HPV4 uptake (i.e., initiation of the HPV4 series) after ACIP routine recommendation among age-eligible boys.
Kaiser Permanente Southern California (KPSC) is the largest managed care organization in Southern California and serves more than 4 million racially/ethnically diverse members who are broadly representative of the socioeconomic diversity of the Southern California census population.8 All child and adolescent members of KPSC are fully covered for the cost of childhood vaccines. KPSC adopted and implemented the ACIP recommendation for HPV4 permissive use for males in January 2010 and implemented the routine use recommendation in February 2012. Age-eligible KPSC male members could receive HPV4 at a clinic visit with little or no visit copayment.
We have reported a HPV4 initiation of 1.6% in KPSC male members aged 9 to 17 years in the first year after the permissive use recommendation for males.9 We examined the trends and correlates of HPV4 initiation in insured boys during the periods before and after the routine use recommendation for males.
METHODS
We identified 3 open cohorts of boys aged 9 to 17 years who enrolled in KPSC health plans during the 3 observation periods that corresponded to permissive use recommendation for genital warts indication (cohort 1: October 21, 2009–December 21, 2010), additional anal cancer indication (cohort 2: December 22, 2010–October 24, 2011), and routine use recommendation (cohort 3: October 25, 2011–May 31, 2013). To assess whether the addition of the anal cancer indication increased HPV4 uptake in males during the permissive use recommendation period, we evaluated HPV4 uptake and correlates in this period separately from the permissive use recommendation period when genital warts was the only indication.
We ascertained eligibility for HPV4 initiation analyses through electronic medical records (EMR). We followed boys aged 9 to 17 years at entry into a cohort during each observation period who had no documented HPV4 vaccination on entering into a cohort (i.e., baseline: either at membership enrollment, ninth birthday, or the beginning of the observation period, whichever came last) until initiation of the HPV4 series, membership disenrollment, 18th birthday, or the end of each observation period, whichever came first.
We obtained the HPV4 vaccination records from the EMRs. The EMRs contain health plan members’ unique medical record numbers and detailed immunization history, including all vaccines administered in KPSC facilities and elsewhere before and during the active enrollment period. If a routine vaccine is due at any clinic visit and the member (or the member’s guardian) reported receiving a vaccination outside the KPSC system, a nurse will request documents to ascertain vaccine name, dose, and vaccination date and enter the information into the EMR.
We defined HPV4 recipients as boys who had initiated the HPV4 vaccine series (i.e., received at least 1 dose of HPV4) during each observation period. We ascertained sociodemographic characteristics of the eligible boys, such as age, race/ethnicity, and preferred spoken language through membership files. To mirror the recommended age groups for routine vaccination (11–12 years), we categorized the boys into 3 age groups (at cohort entry): 9 to 10 years, 11 to 12 years, and 13 to 17 years. We categorized White and Black boys as having Hispanic race/ethnicity if Hispanic was reported as their ethnicity. To obtain neighborhood education and income levels as a proxy for the boys’ family socioeconomic status (SES), we mapped the home address to US census block groups. We also used enrollment in a Medicaid program as a proxy indicator for low SES. Because HPV4 was recommended for routine use among girls several years before the permissive use and subsequent routine use recommendations for boys, we hypothesized that parents who had both daughters and sons in the eligible age group might have greater awareness and knowledge of the HPV4 vaccine, which might lead to a higher HPV4 uptake among their sons. We linked boys to their sisters through the health plan membership files.
We defined health care utilization as the number of outpatient visits, emergency department visits, and hospitalizations during the year before baseline. Primary care providers (PCPs) play an important role in childhood vaccine recommendations, and parents consistently cite a health care provider’s recommendation as one of the most important factors in their decision to vaccinate their children.10 Because of the managed care delivery model of KPSC, members can choose a physician as their assigned PCP for routine care or they can receive care from any available PCP without choosing an assigned PCP. Hence, we examined potential associations between HPV4 initiation with whether a boy had an assigned PCP and the specialty of the assigned PCP (i.e., pediatrics, family medicine, or other) at baseline. We ascertained influenza vaccination records of the boys up to 1 year before the baseline through the EMRs.
We computed descriptive statistics including means, SDs, frequencies, and rates for all variables to assess distributions. To examine the trends of HPV4 uptake over time, we plotted initiation among eligible boys in each month during the study period from October 2009 through May 2013. We examined the number of HPV4 recipients and corresponding initiation, along with baseline characteristics, separately for the 3 cohorts during the observation periods. To explore the influence of the ACIP recommendation on the relationship between boys’ characteristics and HPV4 initiation, we employed separate univariate and multivariable regression models for each cohort during the 3 observation periods. In each multivariable model, we evaluated the associations between boys’ age at cohort entry (baseline), race/ethnicity, preferred spoken language, Medicaid program enrollment, neighborhood SES indicators, influenza vaccination in the year before baseline, and PCP’s specialty. We did not include having a sister as a covariate in the multivariable models because we did not find any bivariate associations with HPV4 initiation.
We used SEs, the parameter covariance matrices, and model correlation matrices to assess collinearity of variables for each of the models, and we deemed all acceptable. As strength of physician recommendation for HPV4 vaccination among males might vary by medical center, we used mixed-effects Poisson regression models to adjust for the random cluster effect of the medical centers. We also took into account the length of follow-up time in the analyses. We calculated risk ratios (RRs) and 95% confidence intervals (CIs), and we considered an estimated RR with a 95% CI that covered 1.0 not statistically significant.
We performed all data analyses using SAS software, version 4.3 (SAS Institute, Inc., Cary, NC).
RESULTS
We identified 297 703 boys aged 9 to 17 years in cohort 1 who were eligible for HPV4 series initiation, 357 384 boys in cohort 2, and 345 348 boys in cohort 3. The distribution of age and race/ethnicity was similar across the 3 cohorts. About 25% of the boys were ever enrolled in a Medicaid program and more than 70% of the boys had a pediatrician as their assigned PCP. The average membership length at baseline was 6.7 years among boys in cohort 1 and 5.9 years in cohorts 2 and 3. Across the 3 cohorts, among those who had at least 1 year of membership before baseline, more than 25% of the boys had received influenza vaccine and the majority of the boys had very low rates of documented emergency department visits and hospitalizations.
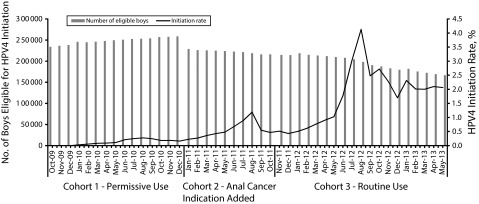
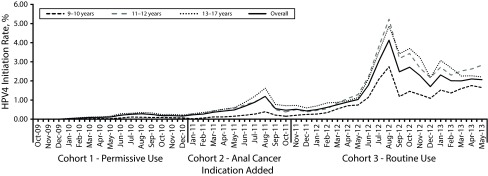
The overall HPV4 series initiation increased over time (1.6%, 3.4%, and 18.5% in cohorts 1, 2, and 3, respectively), with the most substantial increase during the period after ACIP routine use recommendation for boys was issued (Table 1). After the ACIP routine recommendation, the uptake remained low until February 2012, when the regional implementation of the new recommendation started in KPSC (Figure 1). We observed seasonality of uptake, with spikes during the summer season (July–August) every year in the entire study period (Figure 1) and across all age groups (Figurie 2).
TABLE 1—
Baseline Characteristics and HPV4 Series Initiation in Boys Aged 9–17 Years: Kaiser Permanente Southern California, October 2009–May 2013
| Cohort 1 (“Permissive Use”; Oct. 21, 2009–Dec. 21, 2010), No. (%) or Mean ±SD |
Cohort 2 (Anal Cancer Indication; Dec. 22, 2010–Oct. 24, 2011), No. (%) or Mean ±SD |
Cohort 3 (Routine Recommendation; Oct. 25, 2011–May 31, 2013), No. (%) or Mean ±SD |
||||
| Characteristics | Totala | HPV4 Recipients, No. (%)b | Totala | HPV4 Recipients, No. (%)b | Totala | HPV4 Recipients, No. (%)b |
| Overall | 297 703 (100.0) | 4859 (1.6) | 357 384 (100.0) | 12 036 (3.4) | 345 348 (100.0) | 63 905 (18.5) |
| Age, y | 12.8 ±2.7 | 13.7 ±2.1 | 12.4 ±2.8 | 13.7 ±2.1 | 12.4 ±2.8 | 12.3 ±2.2 |
| Age group, y | ||||||
| 9–10 | 81 569 (27.4) | 451 (0.6) | 120 975 (33.9) | 952 (0.8) | 120 023 (34.8) | 16 946 (14.1) |
| 11–12 | 57 908 (19.5) | 969 (1.7) | 66 567 (18.6) | 2807 (4.2) | 63 760 (18.5) | 16 717 (26.2) |
| 13–17 | 158 226 (53.1) | 3439 (2.2) | 169 842 (47.5) | 8277 (4.9) | 161 565 (46.8) | 30 242 (18.7) |
| Race/ethnicity | ||||||
| Non-Hispanic White | 47 115 (15.8) | 664 (1.4) | 72 931 (20.4) | 1840 (2.5) | 71 091 (20.6) | 11 650 (16.4) |
| Non-Hispanic Black | 21 406 (7.2) | 331 (1.5) | 28 631 (8.0) | 1360 (4.8) | 27 271 (7.9) | 5393 (19.8) |
| Hispanic | 14 038 (47.2) | 2786 (2.0) | 172 693 (48.3) | 7183 (4.2) | 165 510 (47.9) | 37 739 (22.8) |
| Asian/Pacific Islanders | 17 308 (5.8) | 263 (1.5) | 25 687 (7.2) | 801 (3.1) | 24 886 (7.2) | 5014 (20.1) |
| Other | 5344 (1.8) | 77 (1.4) | 10 676 (3.0) | 291 (2.7) | 10 385 (3.0) | 1962 (18.9) |
| Unknown | 66 149 (22.2) | 738 (1.1) | 46 766 (13.1) | 561 (1.2) | 46 205 (13.4) | 2147 (4.6) |
| Preferred spoken language | ||||||
| English | 231 106 (77.6) | 3591 (1.6) | 280 931 (78.6) | 9235 (3.3) | 271 696 (78.7) | 48 157 (17.7) |
| Spanish | 50 620 (17.0) | 1190 (2.4) | 57 441 (16.1) | 2645 (4.6) | 54 796 (15.9) | 14 784 (27.0) |
| Other | 15 977 (5.4) | 78 (0.5) | 19 012 (5.3) | 156 (0.8) | 18 856 (5.5) | 964 (5.1) |
| Census block proportion of adults with a high school diploma | ||||||
| Missing | 20 284 (6.8) | 294 (1.4) | 78 586 (22.0) | 1501 (1.9) | 77 085 (22.3) | 10 479 (13.6) |
| > 75% | 143 661 (48.3) | 2061 (1.4) | 143 869 (40.3) | 4278 (3.0) | 139 591 (40.4) | 25 236 (18.1) |
| 50%–75% | 86 265 (29.0) | 1487 (1.7) | 87 453 (24.5) | 3836 (4.4) | 83 617 (24.2) | 17 827 (21.3) |
| < 50% | 47 493 (16.0) | 1017 (2.1) | 47 476 (13.3) | 2421 (5.1) | 45 055 (13.0) | 10 363 (23.0) |
| Census block median annual household income, $ | ||||||
| Missing | 20 284 (6.8) | 294 (1.4) | 78 586 (22.0) | 1 501 (1.9) | 77 085 (22.3) | 10 479 (13.6) |
| 0–25 000 | 7 749 (2.6) | 182 (2.3) | 7 750 (2.2) | 465 (6.0) | 7 285 (2.1) | 1 598 (21.9) |
| 25 001–40 000 | 45 115 (15.2) | 929 (2.1) | 45 708 (12.8) | 2 466 (5.4) | 43 242 (12.5) | 9 491 (21.9) |
| 40 001–60 000 | 84 720 (28.5) | 1 464 (1.7) | 85 547 (23.9) | 3 500 (4.1) | 82 047 (23.8) | 16 992 (20.7) |
| 60 001–80 000 | 69 147 (23.2) | 1 015 (1.5) | 69 662 (19.5) | 2 316 (3.3) | 67 346 (19.5) | 13 092 (19.4) |
| > 80 000 | 70 688 (23.7) | 975 (1.4) | 70 131 (19.6) | 1 788 (2.5) | 68 343 (19.8) | 12 253 (17.9) |
| Membership length at baseline, y | 6.7 ±4.94 | 7.2 ±5.04 | 5.9 ±4.80 | 7.3 ±5.07 | 5.9 ±4.90 | 6.5 ±4.77 |
| Enrolled in Medicaid | ||||||
| No | 230 590 (77.5) | 3 396 (1.5) | 267 882 (75.0) | 8 582 (3.2) | 259 300 (75.1) | 44 184 (17.0) |
| Yes | 67 073 (22.5) | 1 463 (2.2) | 89 458 (25.0) | 3 454 (3.9) | 86 004 (24.9) | 19 720 (22.9) |
| Has a sister aged 9–17 y | ||||||
| No | 208 677 (70.1) | 3 358 (1.6) | 266 520 (74.6) | 8 891 (3.3) | 257 629 (74.6) | 48 614 (18.9) |
| Yes | 89 026 (29.9) | 1 501 (1.7) | 90 864 (25.4) | 3 145 (3.5) | 87 719 (25.4) | 15 291 (17.4) |
| Assigned PCP | ||||||
| Pediatrics | 226 343 (76.0) | 4 067 (1.8) | 257 635 (72.1) | 9 802 (3.8) | 247 833 (71.8) | 51 004 (20.6) |
| Family or others | 34 157 (11.5) | 347 (1.0) | 34 681 (9.7) | 1 029 (3.0) | 33 652 (9.7) | 4 541 (13.5) |
| None | 37 203 (12.5) | 445 (1.2) | 65 068 (18.2) | 1 205 (1.9) | 63 863 (18.5) | 8 360 (13.1) |
| Health care utilization,c No. | 249 241 | 4145 | 276 091 | 10 376 | 265 715 | 52 315 |
| No. of outpatient visits | 2.8 ±0.95 | 3.5 ±4.87 | 2.6 ±0.81 | 2.8 ±0.12 | 2.6 ±0.80 | 3.0 ±0.95 |
| No. of ED visits | 0.2 ±0.46 | 0.2 ±0.54 | 0.1 ±0.44 | 0.1 ±0.46 | 0.1 ±0.44 | 0.1 ±0.47 |
| No. of hospitalizations | ||||||
| 0 | 245 931 (98.7) | 4079 (1.7) | 272 797 (98.8) | 10 249 (3.8) | 262 548 (98.8) | 51 643 (19.7) |
| 1 | 2757 (1.1) | 58 (2.1) | 2761 (1.0) | 109 (3.9) | 2652 (1.0) | 570 (21.5) |
| ≥ 2 | 553 (0.2) | 8 (1.5) | 533 (0.2) | 18 (3.4) | 515 (0.2) | 102 (19.8) |
| Influenza vaccination in prior yearc | ||||||
| No | 182 004 (73.0) | 2586 (1.4) | 205 672 (74.5) | 7274 (3.5) | 198 398 (74.7) | 33 917 (17.1) |
| Yes | 67 237 (27.0) | 1559 (2.3) | 70 419 (25.5) | 3102 (4.4) | 67 317 (25.3) | 18 398 (27.3) |
Note. ED = emergency department; HPV4 = quadrivalent human papillomavirus vaccine; PCP = primary care provider. All variables are statistically significant at the P < .05 level with the exception of age for cohort 3 (P = .1), and number of hospitalizations for all 3 cohorts (P = .18, .78, .06, respectively). Percentages might not add to 100 because of rounding.
Number and proportion of boys of each subgroup among the total population.
Number of boys who initiated the HPV4 vaccine in each subgroup during the follow-up period and the HPV4 vaccine initiation rate (%) within each subgroup.
Limited to members with at least 1 year membership before baseline (study entry).
FIGURE 1—
Number of eligible boys aged 9–17 years at cohort entry and monthly HPV4 initiation: Kaiser Permanente Southern California, October 2009–May 2013.
Note. HPV4 = quadrivalent human papillomavirus vaccine.
FIGURE 2—
Monthly HPV4 initiation among boys aged 9–17 years, by age group at cohort entry: Kaiser Permanente Southern California, October 2009–May 2013.
Note. HPV4 = quadrivalent human papillomavirus vaccine.
The uptake was higher in boys aged 13 to 17 years than in younger boys before the routine recommendation period (cohorts 1 and 2); however, we saw the highest uptake rate in boys aged 11 to 12 years (i.e., the targeted age group for routine vaccination) after the routine use recommendation (Table 1). The HPV4 initiation varied by boys’ sociodemographic characteristics (Table 1). The HPV4 initiation did not seem to differ between boys who had a sister aged 9 to 17 years and those who did not. However, among a subset of boys with a sister during the permissive use recommendation period (cohort 1), we found that boys were more likely to initiate the HPV4 series if their sisters had initiated the HPV4 vaccine (RR = 2.35; 95% CI = 2.13, 2.59).
Boys with a pediatrician as their assigned PCP had a higher uptake than did those who either had a PCP from other specialties or had no assigned PCP in all 3 cohorts. Boys who had a longer membership before baseline and had more outpatient visits during the year before baseline were more likely to initiate the HPV4 series (Table 1). Boys who received an influenza vaccination within 1 year before baseline had a much higher rate of HPV4 initiation in all 3 cohorts; furthermore, this difference in initiation was more prominent during the routine use recommendation period (27.3% in boys who received influenza vaccine vs 17.1% in those who did not).
In multivariable analyses, receiving influenza vaccination within 1 year before baseline was one of the strongest predictors of HPV4 initiation in all 3 cohorts (Table 2). Boys who received influenza vaccination were 48% more likely to initiate the HPV4 series following the routine use recommendation for HPV4 among males. Boys aged 9 to 10 years in all 3 cohorts were much less likely to initiate the HPV4 vaccine series (adjusted RR [ARR] = 0.39; 95% CI = 0.22, 0.67) across the 3 assessment periods. Before the routine use recommendation era, boys aged 13–17 years were more likely than those aged 11 to 12 years to initiate HPV4 (ARR = 1.58; 95% CI = 1.32, 1.88 in cohort 1; and adjusted RR = 1.55; 95% CI = 1.33, 1.80 in cohort 2), but following the routine use recommendation there was no significant difference (ARR = 0.97; 95% CI = 0.94, 1.00 in cohort 3). Compared with non-Hispanic White boys, Hispanic, non-Hispanic Black, and Asian boys were generally more likely to initiate the HPV4 series after the routine recommendation (Table 2).
TABLE 2—
Correlates for Initiation of HPV4 in Boys Aged 9–17 Years: Kaiser Permanente Southern California, October 2009–May 2013
| Cohort 1, “Permissive Use”; Oct. 21, 2009–Dec. 21, 2010 |
Cohort 2, Anal Cancer Indication; Dec. 22, 2010–Oct. 24, 2011 |
Cohort 3, Routine Recommendation; Oct 25, 2011–May 31, 2013 |
||||
| Covariate | Crude RR (95% CI) | Adjusted RR (95% CI) | Crude RR (95% CI) | Adjusted RR (95% CI) | Crude RR (95% CI) | Adjusted RR (95% CI) |
| Age group, y | ||||||
| 9–10 | 0.39 (0.35, 0.44) | 0.39 (0.34, 0.45) | 0.22 (0.20, 0.23) | 0.22 (0.19, 0.25) | 0.64 (0.63, 0.65) | 0.67 (0.64, 0.69) |
| 11–12 (Ref) | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| 13–17 | 1.46 (1.36, 1.57) | 1.58 (1.32, 1.88) | 1.46 (1.40, 1.53) | 1.55 (1.33, 1.80) | 0.90 (0.89, 0.92) | 0.97 (0.94, 1.00) |
| Race/ethnicity | ||||||
| Non-Hispanic White (Ref) | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Non-Hispanic Black | 1.10 (0.96, 1.25) | 0.99 (0.69, 1.41) | 1.95 (1.81, 2.09) | 1.52 (1.12, 2.06) | 1.23 (1.20, 1.27) | 1.18 (1.08, 1.30) |
| Hispanic | 1.44 (1.33, 1.57) | 1.20 (0.86, 1.70) | 1.79 (1.70, 1.88) | 1.36 (1.08, 1.71) | 1.49 (1.46, 1.52) | 1.23 (1.17, 1.29) |
| Asian/Pacific Islanders | 1.08 (0.94, 1.25) | 1.09 (0.87, 1.37) | 1.24 (1.14, 1.35) | 1.19 (0.84, 1.69) | 1.23 (1.19, 1.27) | 1.16 (1.11, 1.20) |
| Other | 1.03 (0.81, 1.30) | 0.96 (0.63, 1.46) | 1.11 (0.98, 1.26) | 1.11 (0.88, 1.41) | 1.19 (1.13, 1.24) | 1.16 (1.10, 1.22) |
| Unknown | 0.91 (0.82, 1.02) | 0.96 (0.73, 1.26) | 0.74 (0.67, 0.82) | 0.79 (0.68, 0.93) | 0.45 (0.43, 0.47) | 0.53 (0.48, 0.59) |
| Preferred spoken language | ||||||
| English (Ref) | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Spanish | 1.52 (1.42, 1.62) | 1.10 (0.93, 1.30) | 1.10 (0.93, 1.30) | 1.47 (1.41, 1.54) | 1.03 (1.88, 1.20) | 1.59 (1.56, 1.61) |
| Other | 0.41 (0.32, 0.51) | 0.45 (0.34, 0.61) | 0.45 (0.34, 0.61) | 0.39 (0.33, 0.45) | 0.53 (0.42, 0.67) | 0.46 (0.43, 0.48) |
| Census block median annual household income, $ | ||||||
| 0–25 000 | 1.38 (1.19, 1.61) | 1.33 (0.68, 2.61) | 1.54 (1.40, 1.70) | 1.46 (0.95, 2.25) | 1.10 (1.05, 1.15) | 1.07 (0.97, 1.17) |
| 25 001–40 000 | 1.20 (1.11, 1.30) | 1.14 (0.83, 1.56) | 1.36 (1.29, 1.44) | 1.30 (1.03, 1.64) | 1.09 (1.06, 1.11) | 1.03 (0.98, 1.09) |
| 40 001–60 000 (Ref) | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| 60 001–80 000 | 0.84 (0.78, 0.91) | 0.89 (0.73, 1.07) | 0.79 (0.75, 0.83) | 0.85 (0.74, 0.98) | 0.91 (0.89, 0.93) | 0.98 (0.96, 1.00) |
| > 80 000 | 0.78 (0.72, 0.85) | 0.88 (0.71, 1.10) | 0.58 (0.55, 0.62) | 0.70 (0.56, 0.87) | 0.82 (0.80, 0.83) | 0.96 (0.92, 1.00) |
| Census block proportion of adults with a high school diploma,% | ||||||
| > 75 (Ref) | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| 50–75 | 1.21 (1.13, 1.30) | 0.99 (0.70, 1.39) | 1.55 (1.49, 1.62) | 1.13 (0.92, 1.38) | 1.23 (1.21, 1.25) | 1.07 (1.01, 1.13) |
| < 50 | 1.52 (1.41, 1.64) | 1.08 (0.57, 2.06) | 1.85 (1.76, 1.94) | 1.13 (0.74, 1.74) | 1.35 (1.32, 1.38) | 1.07 (0.99, 1.15) |
| Medicaid | 1.56 (1.46, 1.66) | 1.33 (1.16, 1.53) | 1.38 (1.33, 1.44) | 1.17 (1.06, 1.29) | 1.54 (1.52, 1.57) | 1.26 (1.22, 1.31) |
| Assigned PCP | ||||||
| Pediatrics (Ref) | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Family and others | 0.61 (0.55, 0.69) | 0.58 (0.47, 0.72) | 0.86 (0.80, 0.92) | 0.75 (0.63, 0.88) | 0.73 (0.71, 0.75) | 0.77 (0.72, 0.82) |
| None | 0.95 (0.86, 1.05) | 0.80 (0.64, 0.99) | 0.77 (0.73, 0.82) | 0.85 (0.69, 1.06) | 1.03 (1.00, 1.05) | 0.77 (0.73, 0.81) |
| Influenza vaccination in prior year | 1.50 (1.41, 1.59) | 1.56 (1.43, 1.70) | 1.23 (1.18, 1.28) | 1.31 (1.24, 1.39) | 1.44 (1.42, 1.46) | 1.48 (1.46, 1.51) |
Note. CI = confidence interval; HPV4 = quadrivalent human papillomavirus vaccine; PCP = primary care provider; RR = risk ratio. All variables are included in the multivariable regression models.
Consistent with the highest HPV4 initiation observed in Hispanic boys, boys who chose Spanish as their preferred spoken language were also more likely to initiate the HPV4 series during all observation periods (Table 2), and the association was even more prominent after routine vaccination was implemented (cohort 3: ARR = 1.59; 95% CI = 1.56, 1.61). A small number of boys (< 6%) who chose languages other than English or Spanish as their preferred spoken language were much less likely to initiate the vaccine series in all 3 cohorts (Table 2). The SES indicators determined by neighborhood levels of household income and education attainment were only marginally associated with HPV4 initiation, but enrollment in a Medicaid program was statistically significantly associated with increased HPV4 initiation in all 3 cohorts (Table 2). Boys who did not have an assigned PCP were less likely to initiate the HPV4 series across the 3 cohorts; and the observed reverse association seemed more prominent following the routine use recommendation period (ARR = 0.77; 95% CI = 0.73, 0.81).
DISCUSSION
Increasing HPV vaccine uptake in the United States has been challenging, particularly among males, possibly as a result of the variable strength of the recommendation by the ACIP since the licensure of HPV vaccine for males. Our results showed that the addition of the anal cancer indication did not have a significant impact on the HPV4 initiation in boys aged 9 to 17 years when HPV4 was recommend for permissive use. However, the uptake of HPV4 increased significantly among boys after the routine use recommendation. We also observed a delay in HPV4 uptake among boys in the initial few months following the permissive use recommendation and some delay in the increase in HPV4 uptake following the routine use recommendation from ACIP. These delays may be partially caused by the lag time for adoption and implementation of ACIP recommendations at KPSC, as well as the gradual change in practice during the initial period following a new recommendation.
Even though HPV4 would be most effective when given before exposure to HPV through sexual contact,4 when the vaccine was recommended for permissive use for boys, uptake was higher among older boys (13–17 years) than among younger boys (9–12 years). The observed significant shift of the HPV4 initiation age from 13 to 17 years to a younger age after the routine use recommendation and the significant increase in HPV4 uptake in boys aged 11 to 12 years coincides with the guideline of routine HPV4 vaccination among boys aged 11 to 12 years. It is plausible that health care providers and parents may feel more comfortable vaccinating younger boys following the routine recommendation.
The higher uptake among older adolescents before the routine use recommendation was consistent with the findings from previous survey studies. Several earlier survey studies revealed a parental preference for vaccinating children at an older age,11,12 which may stem from a perceived lower risk of HPV infection at a younger age and concerns that the HPV4 vaccination encourages sexual behaviors among younger adolescents.10,13,14 Some studies also suggested that health care providers were more likely to recommend HPV4 to older adolescents.13
The observed peak of vaccination uptake in summer months is likely because of an increase in visits during the summer for well-child and well-teen visits, with the administration of other vaccines (i.e., Tdap, meningococcal vaccine) that are also required for school and sports physicals.
The initiation of the HPV4 series among boys was significantly associated with boys’ history of influenza vaccination and was correlated with boys’ sociodemographic characteristics. Boys from racial/ethnic minority (Hispanic, Black, and Asian) backgrounds and from a neighborhood with lower incomes and lower education attainment levels and those enrolled in a Medicaid program were more likely to initiate the HPV4 vaccine. Our findings are similar to the observed higher initiation in Hispanic boys and boys who were eligible for the Vaccines for Children program from a national sample of adolescents.15 This pattern of initiation may be owing to a greater acceptance of the HPV vaccine in these populations.16,17
We did not observe an association between having a sister aged 9 to 17 years and HPV4 series initiation in our study population during either the pre- or postroutine use recommendation periods. But during the permissive use recommendation period, those in a subset of boys who had 1 or more sisters were more likely to initiate the HPV4 series if their sisters had initiated the HPV4.
Many survey studies suggest that most parents report that health care providers’ recommendation of the HPV4 vaccine had a substantial influence on their decision to vaccinate their children.10,11 However health care providers rarely recommended HPV4 for boys during the permissive use recommendation period, and parents often report that their insurance would not cover the HPV4 vaccine or that they did not know whether their health insurance covered the HPV4 vaccine.18 The HPV4 initiation among boys following the permissive use recommendation in our study population of insured boys was extremely low (1.6%); it was similar to that in a national sample of adolescent boys (1.4%) in 2010 and that in an insured population of the Vaccine Safety Datalink through June 2011 (1.3%).19,20 The observed low uptake among the insured population of this study during the permissive use recommendation period is likely because of the lack of providers’ recommendation rather than the concerns for the cost, because all child and adolescent members of KPSC are fully covered for the cost of childhood vaccines.
Even though we did not directly measure the providers’ recommendation of HPV4, it is plausible that the providers were more likely to recommend HPV4 vaccination for boys when the vaccine was recommended as a routine childhood vaccine, which may have partially driven the substantial increase in overall HPV4 initiation among boys in cohort 3. Moreover, we found that those who did not have an assigned PCP or had a PCP in family medicine or other specialties were much less likely to initiate the HPV4 series than were boys who had a pediatrician as their assigned PCP; we observed a similar association in all 3 cohorts. This association suggested that pediatricians may be more likely than other PCPs to recommend the HPV4 vaccine for boys, and boys without an assigned PCP are less likely to receive the provider recommendation for HPV4 vaccination.
Strengths and Limitations
There are some potential limitations to be noted when interpreting our findings. First, we used census data as a proxy for family SES, which may not accurately reflect the actual individual SES. However, we included enrollment in Medicaid programs as an individual-level SES indicator, and our findings suggested that enrollment in Medicaid programs was a stronger correlate for HPV4 initiation than was the neighborhood-level SES indicators.
Second, we were not able to collect data on providers’ recommendation using automated EMR data, and provider recommendation has been reported as having a strong association with HPV vaccine uptake in previous studies. Because how strongly providers recommend the HPV4 vaccine may vary by medical center, we adjusted for the random cluster effect by medical center in regression models.
Third, there are unmeasured factors in the EMRs that potentially bias our estimated association between the correlates and HPV4 initiation, such as parental and family factors, peer influence, and a perceived risk of sexually transmitted infections. Lastly, we were not able to ascertain vaccination status after the disenrollment; therefore, the observed HPV4 initiation in the study cohorts might be a conservative estimate of the true uptake if we could follow all boys until they turned 18. However, as we have taken into account the follow-up time in the Poisson regression models, this potential misclassification of the HPV4 initiation status because of censoring after loss to follow-up is unlikely to bias the relative risk estimates for the correlates for HPV4 initiation.
Our study also has numerous strengths. We took advantage of detailed EMR data to examine the trends of the HPV4 initiation among cohorts of insured boys aged 9 to 17 years from a large racially/ethnically diverse population. Because of KPSC’s integrated health care delivery system, we were able to use the comprehensive EMR data to ascertain immunization records, which minimized recall bias inherent in most observational studies that rely on self-reported data. We were also able to link boys with their providers to examine PCPs’ characteristics associated with boys’ HPV4 uptake.
Conclusions
Our findings highlight the association between the routine use recommendation with not only the overall uptake of HPV4 among boys but also the shift to an earlier HPV4 series initiation age. However, the overall HPV4 initiation remained low (18.5%) in this insured population. The findings of the correlates for HPV4 initiation during the routine HPV4 vaccination period underscore the importance of improving HPV4 acceptance among parents of non-Hispanic White boys and those from high-income families. System-level interventions (e.g., electronic provider alerts integrated into EMRs) to encourage providers to routinely recommend HPV4 vaccination may help increase HPV4 uptake in boys, and targeted education and intervention programs for nonpediatrician PCPs are warranted.
Human Participant Protection
The Kaiser Permanente Southern California institution review board approved the study protocol and waived informed consent.
References
- 1.Satterwhite CL, Torrone E, Meites E et al. Sexually transmitted infections among US women and men: prevalence and incidence estimates, 2008. Sex Transm Dis. 2013;40(3):187–193. doi: 10.1097/OLQ.0b013e318286bb53. [DOI] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. Quadrivalent human papillomavirus vaccine: recommendations of the advisory committee on immunization practices (ACIP) MMWR Recomm Rep. 2007;56(RR–2):1–24. [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. FDA licensure of bivalent human papillomavirus vaccine (HPV2, Cervarix) for use in females and updated HPV vaccination recommendations from the Advisory Committee on Immunization Practices (ACIP) MMWR Morb Mortal Wkly Rep. 2010;59(20):626–629. [Erratum in MMWR Morb Mortal Wkly Rep. 2010;59(36):1184] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. FDA licensure of quadrivalent human papillomavirus vaccine (HPV4, Gardasil) for use in males and guidance from the Advisory Committee on Immunization Practices (ACIP) MMWR Morb Mortal Wkly Rep. 2010;59(20):630–632. [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention. Recommendations on the use of quadrivalent human papillomavirus vaccine in males–Advisory Committee on Immunization Practices (ACIP), 2011. MMWR Morb Mortal Wkly Rep. 2011;60(50):1705–1708. [PubMed] [Google Scholar]
- 6.Hillman RJ, Giuliano AR, Palefsky JM et al. Immunogenicity of the quadrivalent human papillomavirus (type 6/11/16/18) vaccine in males 16 to 26 years old. Clin Vaccine Immunol. 2012;19(2):261–267. doi: 10.1128/CVI.05208-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Centers for Disease Control and Prevention. Human papillomavirus vaccination coverage among adolescents, 2007–2013, and postlicensure vaccine safety monitoring, 2006–2014—United States. MMWR Morb Mortal Wkly Rep. 2014;63(29):620–624. [PMC free article] [PubMed] [Google Scholar]
- 8.Koebnick C, Langer-Gould AM, Gould MK et al. Sociodemographic characteristics of members of a large, integrated health care system: comparison with US Census Bureau data. Perm J. 2012;16(3):37–41. doi: 10.7812/tpp/12-031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hechter RC, Chao C, Sy LS et al. Quadrivalent human papillomavirus vaccine uptake in adolescent boys and maternal utilization of preventive care and history of sexually transmitted infections. Am J Public Health. 2013;103(9):e63–e68. doi: 10.2105/AJPH.2013.301495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Holman DM, Benard V, Roland KB, Watson M, Liddon N, Stokley S. Barriers to human papillomavirus vaccination among US adolescents: a systematic review of the literature. JAMA Pediatr. 2014;168(1):76–82. doi: 10.1001/jamapediatrics.2013.2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Perkins RB, Apte G, Marquez C et al. Factors affecting human papillomavirus vaccine use among White, Black, and Latino parents of sons. Pediatr Infect Dis J. 2013;32(1):e38–e44. doi: 10.1097/INF.0b013e31826f53e3. [DOI] [PubMed] [Google Scholar]
- 12.Dempsey AF, Zimet GD, Davis RL, Koutsky L. Factors that are associated with parental acceptance of human papillomavirus vaccines: a randomized intervention study of written information about HPV. Pediatrics. 2006;117(5):1486–1493. doi: 10.1542/peds.2005-1381. [DOI] [PubMed] [Google Scholar]
- 13.Liddon N, Hood J, Wynn BA, Markowitz LE. Acceptability of human papillomavirus vaccine for males: a review of the literature. J Adolesc Health. 2010;46(2):113–123. doi: 10.1016/j.jadohealth.2009.11.199. [DOI] [PubMed] [Google Scholar]
- 14.Liddon NC, Hood JE, Leichliter JS. Intent to receive HPV vaccine and reasons for not vaccinating among unvaccinated adolescent and young women: findings from the 2006–2008 National Survey of Family Growth. Vaccine. 2012;30(16):2676–2682. doi: 10.1016/j.vaccine.2012.02.007. [DOI] [PubMed] [Google Scholar]
- 15.Reiter PL, Gilkey MB, Brewer NT. HPV vaccination among adolescent males: results from the National Immunization Survey-Teen. Vaccine. 2013;31(26):2816–2821. doi: 10.1016/j.vaccine.2013.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Watts LA, Joseph N, Wallace M et al. HPV vaccine: a comparison of attitudes and behavioral perspectives between Latino and non-Latino women. Gynecol Oncol. 2009;112(3):577–582. doi: 10.1016/j.ygyno.2008.12.010. [DOI] [PubMed] [Google Scholar]
- 17.Constantine NA, Jerman P. Acceptance of human papillomavirus vaccination among Californian parents of daughters: a representative statewide analysis. J Adolesc Health. 2007;40(2):108–115. doi: 10.1016/j.jadohealth.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 18.Reiter PL, McRee AL, Kadis JA, Brewer NT. HPV vaccine and adolescent males. Vaccine. 2011;29(34):5595–5602. doi: 10.1016/j.vaccine.2011.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Centers for Disease Control and Prevention. National and state vaccination coverage among adolescents aged 13 through 17 years—United States, 2010. MMWR Morb Mortal Wkly Rep. 2011;60(33):1117–1123. [PubMed] [Google Scholar]
- 20.Schmidt MA, Gold R, Kurosky SK et al. Uptake, coverage, and completion of quadrivalent human papillomavirus vaccine in the vaccine safety Datalink, July 2006–June 2011. J Adolesc Health. 2013;53(5):637–641. doi: 10.1016/j.jadohealth.2013.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]