Abstract
Background
Chemotherapy and radiation therapy provide limited improvement in survival of gastric cancer patients after tumor resection. It is essential to develop a novel therapeutics for gastric cancer. In the recent years, cytokine-induced killer cells (CIKs)-based adoptive immune therapy has been explored in gastric cancer patients. Due to the small number of patients included in each clinical trial and low-power statistical analysis, the effectiveness of this approach is still unclear. To address this issue, we systemically analyzed the relevant clinical trial data published in recent years by powerful statistical meta-analysis.
Material/Methods
Clinical data was searched by multiple electronic databases with a term “gastric cancer” and “cytokine-induced killer cells”. Six relevant clinical trials with case-control studies were extracted for our meta-analysis, including 318 patients receiving CIK cell therapy and 369 patients receiving conventional therapy.
Results
Overall survival (OS) and odds ratio (OR) were analyzed for patients at 1, 2, 3, and 5 years post-CIK cell therapy and post-conventional therapy. Heterogeneity and publication bias were analyzed for included data quality and publication bias. Our meta-analysis from 6 clinical trials suggests that CIK cell therapy significantly increased 5-year OS from 27±2.44% to 49±7.62% (p<0.05) and 5-year OR up to 1.77 (p<0.05). The increased 5-year survival rate was also highly correlated with the increased CD3+ T cell number and ratio of CD4+/CD8+ in the CIK treated patients.
Conclusions
CIK cell therapy significantly increased 5-year survival rate compared to conventional chemotherapy among gastric cancer patients. The study provides powerful statistical evidence for large-scale clinical trials with CIK cell therapy.
MeSH Keywords: Cytokine-Induced Killer Cells, Meta-Analysis, Stomach Neoplasms
Background
Gastric cancer is the second leading cause of death among all cancers, with limited effective therapeutics and poor prognosis around the world [1]. Conventional chemotherapy and radiotherapy is proven effective in improving survival rate, but is still challenged by tumor recurrence and severe adverse effects in the treated patients [2,3]. In the pat 2 decades, a variety of immunotherapeutic approaches were developed for anti-cancer treatment, such as initially developed adoptive transfer of autologous tumor infiltrating lymphocytes (TIL) [4,5]. More recently, tumor cell-targeted T cell therapeutics was explored, such as adoptive transfer of chimeric T cell receptor (TCR)-engineered T cells, in which TCR was coupled with a single-chain variable fragment of tumor antigen antibody. The strategy has been confirmed to be effective in shrinking and diminishing solid tumors in phase I and II clinical trials [6,7], but currently the strategy is still challenged by problems such as the high risk of developing autoimmune diseases, high heterogeneity of tumor antigens, and high cost of T cell engineering. In addition, TCR-engineered T cells have not yet been developed for gastric cancer treatment. Thus, cytokine-induced killer T cells (CIK)-based immune therapy is a feasible approach and has been widely explored in China for the treatment of a variety of cancers such as hepatocellular carcinoma [8], colon cancer [9], and advanced non-small-cell lung cancer [10]. Recent studies in clinical trials also indicated that immunotherapy with CIK cells improved overall recurrence-free survival rate, without evidence of severe adverse effects [11–13]. CIK cells are CD3+CD56+ phenotype cytokine-induced killer T cells, but are not restricted by major histocompatibility complex (MHC) and can be easily grown in a large quantities in vitro [14]. Due to their high proliferative ability, a large number of CIK cells can be produced in 2–3 weeks after in vitro culture of peripheral blood mononuclear cells (PBMCs) stimulated with IL-2, IFN-γ, and anti-CD3 antibody [15]. Studies in vivo showed that PBMC-derived CIK cells can effectively reduce tumor recurrence in association with release of high levels of IFN-γ and TNF-α, but not IL-2 or IL-4 [12,15].
Due to feasibility of use and potent anti-tumor activity of CIK cells, CIK cells have recently been widely used in clinical trials by several research groups [11,12,16–19]. However, the therapeutic effects of CIK are variable due to numerous factors such as tumor stage, donor cell phenotype, number and purity of donor cells, infusion doses and route, and combined treatment with other adjuvants. It is reported that 5-year overall survival and 5-year disease-free survival (DFS) are greatly improved with CIK therapy, as compared to the conventional chemotherapy, accompanied with increased CD3+ T cell and CD4+/CD8+ T cell ratio; however, multiple doses of CIK cells and combinational treatment with other adjuvants are required for long-term effective anti-tumor effects [13,16,18] such as FOLFOX4 [17], XELOX (Capecitabine and Oxaliplatin) [20], oxaliplatin (L-OHP) [19], and dendritic cells [12]. Age, sex, genetic background, life-style, and tumor stage of patients during CIK therapy may significantly contribute to CIK therapy effectiveness. Thus, it is necessary to establish an international standardized CIK therapy protocol and perform a systemic review of clinical trials by use of powerful statistical analysis to better evaluate CIK therapeutics in patients.
Meta-analysis is a powerful statistical analysis, retrospectively studying pooled data from multiple studies, with much lower random error than other statistical methods. In the present study, we pooled clinical data from 6 relevant CIK-mediated clinical trials involving gastric cancer patients after an extensive online search that included 318 patients [12,13,16–19]. After meta-analysis, we conclude that adoptive transfer of CIK cells significantly increased 5-year survival rate (OS) and odds ratio (OR) (p<0.05), indicating long-term improved therapeutic effects of CIK therapy over the conventional therapeutic approach for patients with gastric cancer.
Material and Methods
Literature research
A comprehensive online literature search was performed to identify relevant studies on CIK therapy for gastric cancer using the NCBI Global Cross-database (PubMed, PMC, Gene, PubChem) and Google Scholar, using search terms for “gastric cancer” and “cytokine-induced killer cells”. No language restriction was imposed in this research. Eligible articles and relevant review articles were also manually reviewed.
Data collection and quality assessment
Articles relevant to comparison between CIK cell therapy and conventional chemotherapy for patients with gastric cancer were included for meta-analysis. Patient condition and courses of treatment were not restricted for data inclusion. Studies without description of recurrence, survival rates, and overall survival rates were excluded. Books, letters, expert opinions, and editorials were also excluded. We also excluded studies published before January 1, 2000. In the second round of selection, we excluded the articles which were not written in English and not aimed at investigating the association between CIK cell therapy and gastric cancers. Articles without comprehensive statistical information and lack of original data were excluded.
Data synthesis and statistical analysis
I2 index was first calculated to assess the heterogeneity. A Mantel-Haenszel (M-H) fixed-effects model (FEM) was used for data without statistically significant heterogeneity and a DerSimonian and Laird (D-L) random-effects model (REM) was used for data with statistically significant heterogeneity. P<0.05 was considered as statistically significant heterogeneity. We used the FEM model in our analysis due to the small size of our dataset. Publication bias was assessed by Begg’s tests using STATA 12.0 software (Stata Statistical Software: Release 12. College Station, TX: StataCorp LP., USA), and data were presented with funnel plot. Overall survival (OS) and odds ratio (OR) with 95% confidence interval (CI) were analyzed for studies after 1, 2, 3, and 5 years of CIK therapy with case controls by using software Revman version 5.2. OR>1 indicates a lower recurrence of gastric cancer and better survival rate.
Statistical analysis of immune responses
For measuring the immune response analysis after CIK therapy, the number of CD3+ and ratio of CD4+/CD8+ among 5-year CIK-treated patients were analyzed and data are presented as mean ±standard deviation. Weighted mean difference (WMD) was calculated by comparing mean and standard deviation in the treatment group to the data acquired in the control group. A positive WMD indicates increase in these 2 parameters, whereas a negative WMD shows a decrease. An increase in these 2 parameters indicates an improved condition. Statistical analysis was performed with STATA 12 (Stata Statistical Software: Release 12. College Station, TX: StataCorp LP).
Results
Study selection and description
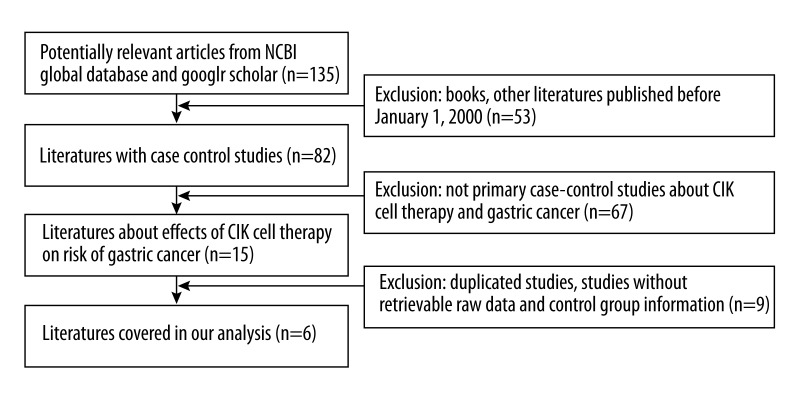
To clarify the significance of CIK treatment on gastric cancer patients, we performed a comprehensive online literature search using the terms “gastric cancer” and “cytokine-induced killer cells”. Initially we found 135 relevant studies. After exclusion of studies that were published before 2000, non-relevant to CIK therapy for gastric cancer, written in other languages, and lacked comprehensive statistical information and original data, we finally identified 6 case-controlled studies for our meta-analysis, which cover 318 patients treated with CIK immunotherapy and 369 patients treated with conventional therapy [12,13,16–20] (Figure 1). The characteristics of included patients are described in Table 1.
Figure 1.
Flow chart of record identification, screening, and study inclusion. We identified 135 studies for first-round exclusion and 6 studies were included for final meta-analysis.
Table 1.
Clinical information of patients in meta-analysis. The table summarizes patients’ basic information regarding the tumor stage, number of patients, cases age, previous treatment, doses of CIK cells, administration route.
| Authors and year | Tumor stage | Number of patients | Median age | Previous treatment | Number of CIK cells per infusion | Administration route |
|---|---|---|---|---|---|---|
| 1-Year | ||||||
| Jiang, 2006 | IV | 57 | 53 | Palliative gastrectomy | 1×109 | Transfusion |
| Shi, 2012 | III, IV | 158 | 57 | 5-FU | 1×109 | Transfusion |
| Liu, 2013 | I, II, III, IV | 98 | 55.7 | 5-HT receptor | 1×109 | Transfusion |
| Gao, 2014 | I, II, III, IV | 27 | 63.02±12.2 | NA | 58.5±22.3×108 | Infusion |
| 2-year | ||||||
| Jiang, 2006 | IV | 57 | 53 | Palliative gastrectomy | 1×109 | Transfusion |
| Jiang, 2010 | I, II, III, IV | 56 | 59.9±10.5 | Chemotherapy | 1×109 | Transfusion |
| Liu, 2013 | I, II, III, IV | 98 | 55.7 | 5-HT receptor | 1×109 | Transfusion |
| 3-year | ||||||
| Jiang, 2006 | IV | 57 | 53 | Palliative gastrectomy | 1×109 | Transfusion |
| Shi, 2012 | III, IV | 158 | 57 | 5-FU | 1×109 | Transfusion |
| Liu, 2013 | ||||||
| Zhao, 2013 | II, III | 165 | 60 | Chemotherapy | 5×109 | Infusion |
| Gao, 2014 | I, II, III, IV | 27 | 63.02±12.2 | NA | 58.5±22.3×108 | Infusion |
| 5-year | ||||||
| Jiang, 2010 | I, II, III, IV | 56 | 59.9±10.5 | Chemotherapy | 1×109 | Transfusion |
| Shi, 2012 | III, IV | 158 | 57 | 5-FU | 1×109 | Transfusion |
| Zhao, 2013 | II, III | 165 | 60 | Chemotherapy | 5×109 | Infusion |
| Gao, 2014 | I, II, III, IV | 27 | 63.02±12.2 | NA | 58.5±22.3×108 | Infusion |
CT – chemotherapy; CIK – cytokine-induced killer cells; IC – intracutaneous; IV – intravenous; IP – intraperitoneal; RT – radiotherapy; SC – subcutaneous; TTP – time to progression; PFS – progression-free.
CIK treatment increased overall survival rate of patients with gastric cancer
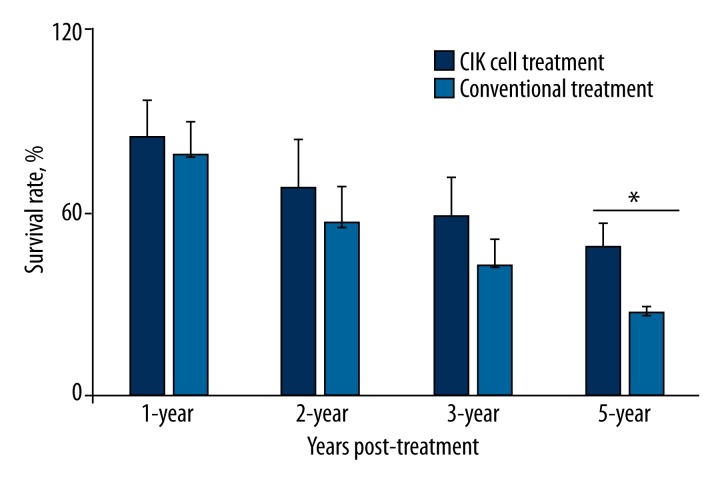
We first analyzed the survival rate of 1-year, 2-year, 3-year, and 5-year post-CIK therapy as compared to the results of conventional controls by using the t test. Patients undergoing conventional treatment have gradual decreases in survival rate from 1-year 79±10.92% to 2-year 56±11.9%, 3-year 43±8.31%, and 5-year 27±2.44%. CIK treatment moderately increased survival rate up to 1-year 85±11.3%, 2-year 69±15.27%, and 3-year 59±12.45%; these differences were not significant compared to conventional control patients (p>0.05, n=4), but we observed significantly improved 5-year survival rate at 49±7.62% in the CIK-treated patients (n=4, p=0.03), indicating long-term therapeutic effects of CIK therapy (Figure 2, Table 2).
Figure 2.
Overall survival rate (OS) of patients at 1-, 2-, 3- and 5-years post-CIK cell treatment and conventional treatment. Data are presented as mean survival rate ±standard error for each group of patients, t test was applied for statistical analysis, p<0.05 is considered significant difference, n=4 per group.
Table 2.
Overall survival rate (OS) in the CIK cell treatment group and conventional treatment group.
| Study | CIK cell treatment OS (%) | Total | Conventional treatment OS (%) | Total |
|---|---|---|---|---|
| 1-Year | ||||
| Jiang, 2006 | 16 (51) | 32 | 12 (48) | 25 |
| Shi, 2012 | 73 (98) | 74 | 73 (95) | 77 |
| Liu, 2013 | 50 (98) | 51 | 44 (93.6) | 47 |
| Gao, 2014 | 25 (93) | 27 | 21 (79) | 27 |
| 2-year | ||||
| Jiang, 2006 | 13 (40) | 32 | 9 (38) | 25 |
| Jiang, 2010 | 55 (73.5) | 75 | 43 (52.6) | 81 |
| Liu, 2013 | 47 (92.2) | 51 | 37 (78.7) | 47 |
| 3-year | ||||
| Jiang, 2006 | 4 (11) | 32 | 3 (12) | 25 |
| Shi, 2012 | 50 (67.7) | 74 | 42 (54.5) | 77 |
| Liu, 2013 | 37 (72.5) | 51 | 28 (59.6) | 47 |
| Zhao, 2013 | 33 (62.3) | 53 | 52 (46.4) | 112 |
| Gao, 2014 | 22 (82) | 27 | 11 (42) | 27 |
| 5-year | ||||
| Jiang, 2010 | 30 (40.4) | 75 | 19 (23.9) | 81 |
| Shi, 2012 | 24 (32.4) | 74 | 18 (23.4) | 77 |
| Zhao, 2013 | 30 (56.6) | 53 | 30 (26.8) | 112 |
| Gao, 2014 | 18 (66) | 27 | 9 (34) | 27 |
To test the heterogeneity (I2) of the datasets included in this study, we analyzed heterogeneity (I2) in the included 1-year, 2-year, 3-year, and 5-year datasets under an M-H fixed-effects model or a D-L random-effects model. The heterogeneity I2 in the 6 included studies was 0.0%, indicating that the datasets included in this study were comparable.
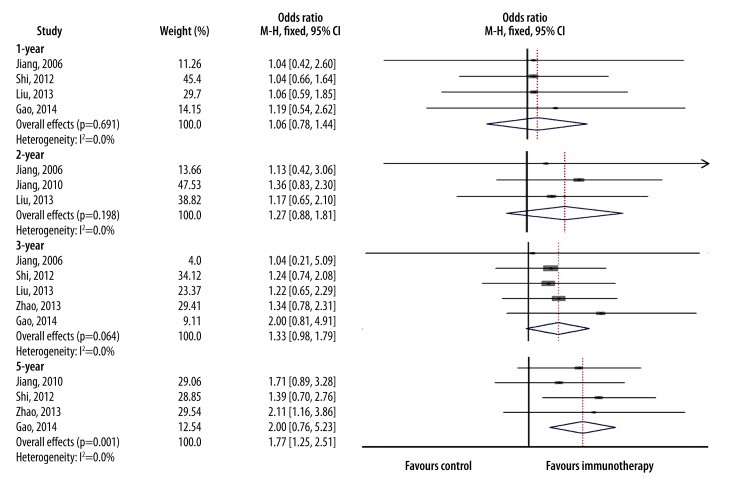
The M-H fixed-effects model was applied for our meta-analysis among 318 patients who received CIK therapy in combination with conventional chemotherapy and 369 patients who received conventional therapy alone. Survival rates between these 2 groups is represented as OR with 95% CI. A larger OR value indicates a better therapeutic effect and p<0.05 indicates significance. After analysis, we obtained a 1-year overall OR of 1.06 (95% CI=0.78–1.44, p=0.691); 2-year overall OR of 1.27 (95% CI=0.88–1.81, p=0.198); 3-year overall OR of 1.33 (95% CI=0.98–1.79, p=0.064), and 5-year overall OR of 1.77 (95% CI=1.25–2.51, p = 0.001) (Figure 3). Thus CIK treatment significantly increased 5-year survival rate, and moderately increased 1-year, 2-year, and 3-year survival rates, but not significantly, consistent with the results obtained by using t tests.
Figure 3.
Forest plot for odds ratio (OR). Overall survival rate of patients in each study at 1-, 2-, 3-, and 5-years post-CIK cell treatment was compared to the overall survival rate of patients with post-conventional chemotherapy by meta-analysis. The Mantel-Haenszel (M-H) fixed-effects model (FEM) was used. OR is represented by a square with size proportional to the provided information. The ends of the horizontal bars denote the 95% confidence interval (CI). The blue diamond represent overall OR of each study.
Assessment of publication bias
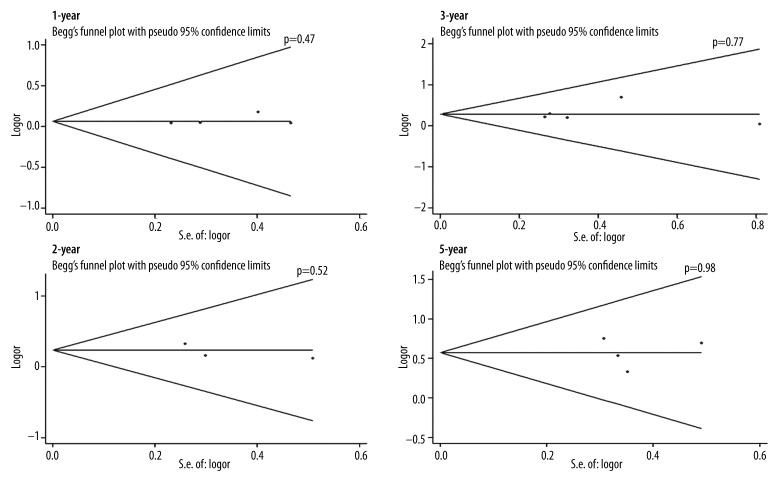
To determine whether there was publication bias for the included studies, we performed both Begg’s and Egger’s tests for the 6 included studies, and data is presented by Funnel plot. As shown in Figure 4, there was no significant publication bias in 1-year, 2-year, 3-year, and 5-year post-CIK and conventional treatments (p=0.47, p=0.52, and p=77, p=0.98, respectively) (Figure 4).
Figure 4.
Funnel plot for publication bias. 1-, 2-, 3- and 5-year OS were compared between CIK cell immunotherapy and conventional chemotherapy in Mantel-Haenszel (M-H) fixed-effects model (FEM). Data was analyzed by Begg’s test, and presented as Funnel plots (with pseudo 95% confidence intervals). The p value indicates the results of Begg’s test.
CIK treatment increased immune responses among patients with gastric cancer
Helper CD4+ T cells and cytotoxic CD8+ T cells are major cell types fighting against primary tumor cells and tumor metastasis. It was reported that the number of CD3+ T cells and CD4+/CD8+ ratio are significantly lower in the most gastric cancer patients compared to those in healthy controls after chemotherapy [16]. To determine whether the improved therapeutic effects of CIK treatment is associated with increased number of CD3+ T cells and elevated CD4+/CD8+ ratio in our included study, we pooled the CD3+, CD4+ and CD8+ T cell data from 4 included studies. The results showed that the number of CD3+ T cells and the CD4+/CD8+ ratio were both moderately increased in the 5-year CIK-treated group as compared to the control group (Table 3). The increased immune responses were correlated to increased overall weighted mean difference (WMD), at 15.43 (95% CI: 5.45–25.41, p=0.002) for CD3+ T cell number and 0.44 (95% CI: 0.32–0.56, p < 0.001) for CD4+/CD8+ ratio. The random-effects model was applied for analysis of immune responses due to high heterogeneity (I2=95.7%) and small database (Table 4).
Table 3.
Pooled data for immune responses in CIK cell treatment group and conventional chemotherapy.
| Study | Patient | CD3+ | CD4+ | CD8+ | CD4+/CD8+ | |
|---|---|---|---|---|---|---|
| Jiang, 2006 | 32 | Pre-therapy | 49.6±11.7 | 31.5±8.4 | 32.7±9.5 | 0.9±0.1 |
| Post-therapy | 58.2±11.7 | 38.5±9.7 | 28.6±5.1 | 1.4±0.2 | ||
| Liu, 2013 | 51 | Pre-therapy | 57.21±9.44 | 29.54±7.82 | 26.32±6.48 | 1.06±0.25 |
| Post-therapy | 66.75±9.81 | 38.12±8.02 | 19.62±5.92 | 1.37±0.29 | ||
| Shi, 2012 | 74 | Pre-therapy | 50.8±8.5 | 26.5±6.1 | NA | 0.9±0.4 |
| Post-therapy | 62.6±11.3 | 36.0±6.6 | NA | 1.4±0.3 | ||
| Zhao, 2013 | 25 | Pre-therapy | 48.95±6.89 | NA | NA | NA |
| Post-therapy | 80.7±9.21 | NA | NA | NA |
Table 4.
Meta-analysis for immune responses.
| Period | Analysis method | Heterogeneity | WMD | Publication bias | |||||
|---|---|---|---|---|---|---|---|---|---|
| I2 (%) | p-value | Overall | Lower | Upper | p-value | Begg | Egger | ||
| CD3+ | Random | 95.7 | 0.002 | 15.43 | 5.45 | 25.41 | 0.002 | 0.734 | 0.758 |
| CD4+/CD8+ | Random | 78.2 | 0.01 | 0.44 | 0.32 | 0.56 | <0.001 | 1.000 | 0.708 |
Discussion
Before cellular immunotherapy is explored for anti-tumor therapy, cancer patients usually receive conventional therapy after tumor resection. However, the adjunct conventional therapy did not significantly prolong patient survival without significant improvement of 5-year survival rate, whereas the conventional therapy induced severe adverse effects in most patients [3,16,21]. Recent extensive studies in animal models and clinical trials have confirmed that the immunotherapy approach is more effective in anti-tumor activity than the conventional therapy, but the results are variable among reports. Clinical trials for gastric cancer by cellular immunotherapy have been explored in China in recent years [12,13,16]. However, due to variability in sample sizes and lack of standardized clinical trial protocol and statistical methods, there is still no firm conclusion about the effects of CIK-mediated therapy in gastric cancer. To address this issue, we performed an extensive online search, finally obtaining 6 clinical trials relevant to CIK-mediated immunotherapy for gastric cancer in China. We pooled datasets from 4 clinical trials covering 318 patients receiving CIK immunotherapy and 369 patients receiving conventional therapy. After analysis using the t test, we observed a greatly improved 5-year survival rate of up to 49±7.6% in patients receiving CIK immunotherapy as compared to those receiving conventional therapy with a survival rate of 27±2.4% (p<0.05). Further meta-analysis showed that CIK immunotherapy significantly increased OR, up to 1.77 (p<0.005). In addition, we observed moderately increased OR (1.06, 1.27, and 1.33) among patients receiving 1-year and 3-year post CIK-therapy, as compared to the control groups, respectively. We speculate that the short duration of follow-up after CIK treatment accounts for the moderate improvement of OR as compared to that of 5-year post-CIK therapy. Unfortunately, the studies did not provide detailed information about symptom control, tumor histology, or computed tomography (CT) scan imaging during the short period of time after surgery. We expect a greater symptom relief, tumor shrinkage, and slower tumor growth among the patients at 1 and 3 years post-CIK therapy than in the patients who received conventional therapy only. Overall, our meta-analysis showed that CIKs therapy significantly improved 5-year survival of gastric cancer patients and that the significance is not caused by publication bias.
Because it was reported that the number of CD3+ T cells and the CD4+/CD8+ ratio are significantly lower in most gastric cancer patients than those in healthy controls after chemotherapy [9], we performed additional statistical analysis of immune responses in the 4 pooled studies. The results indicated that CD3+ T cells number and ratio of CD4+/CD8+ T cells were moderately increased after CIK treatment as compared to the results prior to CIK treatment. The WMD of CD3+ T cell number was increased up to 15.43 [95% CI: 5.45–25.41, p=0.002], and overall WMD of CD4+/CD8+ ratio was increased up to 0.44 [95% CI: 0.32–0.56, p<0.001]. Thus, the improved therapeutic effects of CIK are correlated to the increased immune responses, providing strong evidence of the role of CIK cells in the defense against gastric tumors.
Conclusions
It should be noted that there were only 6 relevant clinical trials extracted for our meta-analysis, and a larger sample size may be required for further meta-analysis to obtain more conclusive results. In addition, all 6 clinical trials were reported in China, and data from other countries is still lacking. Therefore, establishing an international registry on CIK cell-mediated cancer therapy is a critical step in standardization of clinical trial protocol [22]. Further investigation of donor CIK cell tissue distribution, survival and biological function, and host cellular and humoral immune responses to CIK cells would greatly strengthen the conclusions drawn in this study.
Footnotes
Conflict of interest
The authors declare that they have no conflict of interest.
Compliance with ethical standards
The authors declare that this study complies with ethical standards.
Source of support: 1) Science & Technology Department of Sichuan Province (No. 2015JY0005); 2) The Technical Program of Nanchong (No. 14A0021); 3) Educational Department of Sichuan Province (No. 12ZB0426, No. 13ZB0237); 4) The Bureau of Science and Techonogy of Nanchong (No. 2010SF07)
References
- 1.Chen W, Zheng R, Zhang S, et al. Annual report on status of cancer in China, 2010. Chin J Cancer Res. 2014;26(1):48–58. doi: 10.3978/j.issn.1000-9604.2014.01.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sampieri CL, Leon-Cordoba K, Remes-Troche JM. Matrix metalloproteinases and their tissue inhibitors in gastric cancer as molecular markers. J Cancer Res Ther. 2013;9(3):356–63. doi: 10.4103/0973-1482.119302. [DOI] [PubMed] [Google Scholar]
- 3.Ottoman RE, Langdon EA, Rochlin DB, Smart CR. Side-effects of combined radiation and chemotherapy in the treatment of malignant tumors. Radiology. 1963;81:1014–17. doi: 10.1148/81.6.1014. [DOI] [PubMed] [Google Scholar]
- 4.Khammari A, Knol AC, Nguyen JM, et al. Adoptive TIL transfer in the adjuvant setting for melanoma: long-term patient survival. J Immunol Res. 2014;2014:186212. doi: 10.1155/2014/186212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Svane IM, Verdegaal EM. Achievements and challenges of adoptive T cell therapy with tumor-infiltrating or blood-derived lymphocytes for metastatic melanoma: what is needed to achieve standard of care? Cancer Immunol Immunother. 2014;63(10):1081–91. doi: 10.1007/s00262-014-1580-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lamers CH, van Steenbergen-Langeveld S, van Brakel M, et al. T cell receptor-engineered T cells to treat solid tumors: T cell processing toward optimal T cell fitness. Hum Gene Ther Methods. 2014;25(6):345–57. doi: 10.1089/hgtb.2014.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leuci V, Mesiano G, Gammaitoni L, et al. Genetically redirected T lymphocytes for adoptive immunotherapy of solid tumors. Curr Gene Ther. 2014;14(1):52–62. doi: 10.2174/1566523213666131223130353. [DOI] [PubMed] [Google Scholar]
- 8.Li X, Dai D, Song X, et al. A meta-analysis of cytokine-induced killer cells therapy in combination with minimally invasive treatment for hepatocellular carcinoma. Clin Res Hepatol Gastroenterol. 2014;38(5):583–91. doi: 10.1016/j.clinre.2014.04.010. [DOI] [PubMed] [Google Scholar]
- 9.Wang ZX, Cao JX, Liu ZP, et al. Combination of chemotherapy and immunotherapy for colon cancer in China: a meta-analysis. World J Gastroenterol. 2014;20(4):1095–106. doi: 10.3748/wjg.v20.i4.1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Han RX, Liu X, Pan P, et al. Effectiveness and safety of chemotherapy combined with dendritic cells co-cultured with cytokine-induced killer cells in the treatment of advanced non-small-cell lung cancer: a systematic review and meta-analysis. PloS One. 2014;9(9):e108958. doi: 10.1371/journal.pone.0108958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Du X, Jin R, Ning N, et al. In vivo distribution and antitumor effect of infused immune cells in a gastric cancer model. Oncol Rep. 2012;28(5):1743–49. doi: 10.3892/or.2012.2013. [DOI] [PubMed] [Google Scholar]
- 12.Gao D, Li C, Xie X, et al. Autologous tumor lysate-pulsed dendritic cell immunotherapy with cytokine-induced killer cells improves survival in gastric and colorectal cancer patients. PloS One. 2014;9(4):e93886. doi: 10.1371/journal.pone.0093886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jiang JT, Shen YP, Wu CP, et al. Increasing the frequency of CIK cells adoptive immunotherapy may decrease risk of death in gastric cancer patients. World J Gastroenterol. 2010;16(48):6155–62. doi: 10.3748/wjg.v16.i48.6155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cui Y, Yang X, Zhu W, et al. Immune response, clinical outcome and safety of dendritic cell vaccine in combination with cytokine-induced killer cell therapy in cancer patients. Oncol Lett. 2013;6(2):537–41. doi: 10.3892/ol.2013.1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim YJ, Lim J, Kang JS, et al. Adoptive immunotherapy of human gastric cancer with ex vivo expanded T cells. Arch Pharm Res. 2010;33(11):1789–95. doi: 10.1007/s12272-010-1111-7. [DOI] [PubMed] [Google Scholar]
- 16.Jiang J, Xu N, Wu C, et al. Treatment of advanced gastric cancer by chemotherapy combined with autologous cytokine-induced killer cells. Anticancer Res. 2006;26(3B):2237–42. [PubMed] [Google Scholar]
- 17.Liu H, Song J, Yang Z, Zhang X. Effects of cytokine-induced killer cell treatment combined with FOLFOX4 on the recurrence and survival rates for gastric cancer following surgery. Exp Ther Med. 2013;6(4):953–56. doi: 10.3892/etm.2013.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shi L, Zhou Q, Wu J, et al. Efficacy of adjuvant immunotherapy with cytokine-induced killer cells in patients with locally advanced gastric cancer. Cancer Immunol Immunother. 2012;61(12):2251–59. doi: 10.1007/s00262-012-1289-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhao Q, Zhang H, Li Y, et al. Anti-tumor effects of CIK combined with oxaliplatin in human oxaliplatin-resistant gastric cancer cells in vivo and in vitro. J Exp Clin Cancer Res. 2010;29:118. doi: 10.1186/1756-9966-29-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang ZM, Zhuang RY, Chen Y, et al. [A pilot study of chemotherapy combined with intraperitoneal perfusion of cytokine-induced killer cells for advanced gastric cancer patients with ascites]. Zhonghua wei chang wai ke za zhi. 2013;16(1):28–31. [PubMed] [Google Scholar]
- 21.Palesty JA, Wang W, Javle MM, Yang GY. Side effects of therapy: case 3. Gastric cancer after radiotherapy of pediatric Hodgkin’s disease. J Clin Oncol. 2004;22(12):2507–9. doi: 10.1200/JCO.2004.09.168. [DOI] [PubMed] [Google Scholar]
- 22.Hontscha C, Borck Y, Zhou H, et al. Clinical trials on CIK cells: first report of the international registry on CIK cells (IRCC) J Cancer Res Clin Oncol. 2011;137(2):305–10. doi: 10.1007/s00432-010-0887-7. [DOI] [PMC free article] [PubMed] [Google Scholar]