Abstract
Background
This study was designed to assay the expression of zinc finger protein X-linked (ZFX) in renal cell carcinoma (RCC) tissues and evaluate the correlation between ZFX expression and prognosis of RCC patients.
Material/Methods
The expressions of ZFX mRNA in 53 RCC tissues and 51 normal tissues were determined by quantitative real-time polymerase chain reaction (qRT-PCR). Immunohistochemistry (IHC) technology was used to measure the expression of ZFX protein. Then chi-square test was conducted to verify the association between ZFX expression and clinical parameters. Next, we explored the overall survival rate of RCC patients with Kaplan-Meier analysis. Finally, the correlation between ZFX expression and the prognosis of RCC patients was evaluated by Cox regression analysis.
Results
The qRT-PCR result showed that the ZFX was significantly up-regulated in RCC tissues. As for the IHC consequence, the positive rate of ZFX expression in RCC specimens was 79.2%, while that in the normal control tissues was only 17.6%. Chi-square test showed that ZFX expression shared no close relationship with age, sex, or smoking (P>0.05), but was tightly associated with TNM stage, tumor size, and lymph node metastasis (P<0.05). Kaplan-Meier analysis showed that patients with ZFX positive expression had higher mortality than those with negative expression (P<0.05). Cox regression analysis revealed that ZFX expression had tight correlation with prognosis of RCC patients (HR=4.997, P=0.045, 95%CI=1.033–24.180).
Conclusions
Our findings show that ZFX could be considered as a predictor for prognosis of RCC patients.
MeSH Keywords: Carcinoma, Renal Cell; Genes, X-Linked; Prognosis
Background
Renal cell carcinoma (RCC) is one of the most frequent malignancies in the urinary system and represents approximately 90% of all kidney cancers [1–3]. It is estimated that about 340 000 patients are diagnosed with RCC annually world-wide [4,5]. In the past 2 decades, the incidence of RCC has increased by 2% world-wide, with a steadily growing mortality rate [6]. After kidney transplantation, the patients are also likely to develop RCC because of the malignant tumor in the donor kidney [7]. At present, 20–30% of RCC patients have distant metastasis at diagnosis [8–11], which contributes to undesirable treatment effects and poor prognosis. In addition, about 40% of human RCC is diagnosed incidentally [12] and 5-year survival rate of RCC patients is only 12.3% [13]. Surgical resection is an effective way to cure RCC, but nearly 30% of patients have tumor recurrence after surgery [1,14]. Therefore, it is necessary to find novel and significant molecular markers for prognosis of RCC patients.
Zinc finger protein X-linked (ZFX) locates at X chromosome [15]. ZFX protein belongs to the zinc finger protein family, members of which are all conserved in vertebrates. The ZFX protein contains an acidic transcriptional activation domain (AD), a nuclear localization sequence (NLS), and a DNA binding domain (DBD) [16–19]. Existing reports have shown that ZFX can act as a transcriptional regulator for self-renewal in embryonic and adult hematopoietic stem cells [20–22]. Currently, emerging evidence indicates that ZFX plays an important role in the initiation and development of several malignancies. Overexpression of ZFX was observed in esophageal carcinoma cell lines [23] and ZFX was upregulated in prostate cancer and glioma [24–26]. Moreover, Fang et al. [18] demonstrated that knockdown of ZFX significantly inhibited renal cell carcinoma cell proliferation and cell cycle progression. However, few studies have investigated the prognostic role of ZFX in RCC.
In this study, we explored ZFX expression in RCC tissues and normal control tissues and evaluated its possible use as a prognostic biomarker for RCC patients. Our findings will contribute to providing timely treatments and improving the survival of RCC patients. Moreover, it is helpful for the use in individualized therapy.
Material and Methods
Patients and samples
A total of 53 patients with RCC were randomly selected in this study in the Second Hospital of Tianjin Medical University. Among them, 44 cases were males and 9 were females, aged 25–69 years with an average age of 43 years. All patients received no preoperative chemotherapy or radiotherapy. All 53 RCC tissues were included in the case group, and 51 adjacent normal tissues were chosen as a control group. The study was approved by the local ethics committee and all of the patients signed consent forms before surgery.
A 5-year follow-up survey was conducted in all the RCC patients. The information was obtained through a telephone or a questionnaire survey and updated every 3 months. The collected clinical parameters were recorded in a database.
RNA extraction and qRT-PCR
The expression levels of ZFX mRNA were determined with the use of quantitative real-time polymerase chain reaction (qRT-PCR). We extracted the total RNA from RCC tissues and noncancerous tissues by RNeasy Mini Kit (QIAGEN, Germany) according to the manufacturer’s instructions. Then, reverse transcription was conducted with a high-capacity cDNA synthesis kit (Takara, China). After reverse transcription, we used qRT-PCR to evaluate the expression abundance of ZFX mRNA. The reaction was conducted under optimal conditions: 95°C for 3 min, followed by 40 cycles at 95°C for 6 s, and 60°C for 35 s. The relative mRNA expression value was calculated by 2−ddT method. β-actin was utilized as the internal control. The test was done in triplicate.
Immunohistochemistry (IHC) assay
The expression of ZFX protein was measured by IHC test in both RCC tissues and normal tissues. Samples were cut into 4-μm-thick sections and baked at 65°C for 1 h. Deparaffinization and rehydration was performed with alcohols of gradient concentration. The sections were incubated with 0.01 M citric acid buffer (pH 6.0) at 98°C for 10 min and then air dried at room temperature. After that, the sections were mixed with primary antibody at 37°C for 1 h. PBS buffer was used to wash the sections 3 times, each for 3 min. Biotin-labeled second antibody was added to each section at 37°C for 30 min. Staining signaling was conducted with DAB. Samples treated by PBS, rather than primary antibody, were used as negative controls. We also performed positive controls by the sections with ZFX expression. Staining mainly showed brown in cytoplasm. The IHC result was expressed by the staining percentage of cells (0 to 100%). Staining of fewer than 10% of the cells or no staining was considered to be negative expression. Staining of 10–20% of cells was considered to be moderate immunopositivity, and staining of more than 20% of cells was considered to be strong immunopositivity. Both moderate and strong immunopositivity were classified as positive expression. The sections were blocked and preserved for further use.
Statistical analysis
Data collected in this study were analyzed by SPSS18.0 software (SPSS Inc., USA). The relationship between ZFX expression and clinical parameters of RCC patients was evaluated by chi-square test. Kaplan-Meier analysis was performed to detect the overall survival rate of RCC patients with positive ZFX expression and negative ZFX expression. Multivariate analysis was conducted to explore whether there was a correlation between ZFX and prognosis of RCC patients by use of Cox regression analysis. Statistical significance existed when P value was less than 0.05.
Results
Up-regulation of ZFX mRNA in RCC tissues
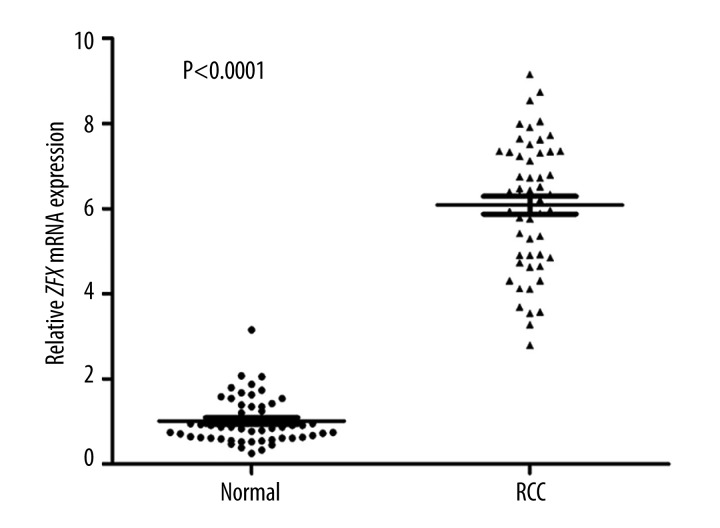
The expression level of ZFX mRNA was significantly higher in RCC tissues compared to normal tissues (Figure 1).
Figure 1.
The expression of ZFX mRNA in RCC tissues and normal tissues. qRT-PCR was conducted to assay the expression of ZFX mRNA in RCC tissues and normal tissues. The result showed that the level of ZFX mRNA in RCC tissues was significantly higher than in normal tissues (P<0.0001).
High expression of ZFX protein in RCC tissues
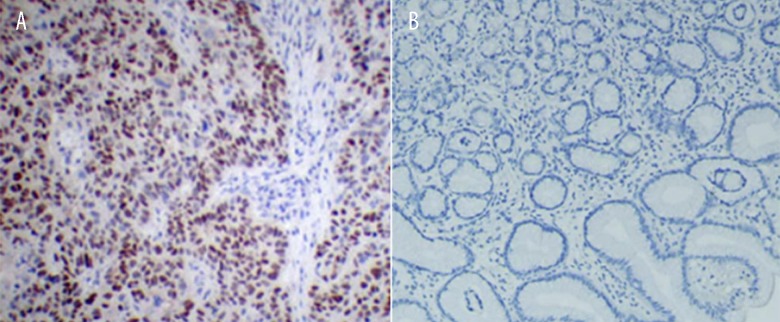
The positive expression rate of ZFX was 79.2% (42 out of 53) in the RCC tissues, but only 17.6% (9 out of 51) in the normal tissues (Table 1, Figure 2). Therefore, the expression level of ZFX was significantly higher in RCC tissues than in normal tissues (P<0.05).
Table 1.
ZFX expression in RCC and normal tissues.
| Tissue | Case No. | Expression | Positive rate | P value | |
|---|---|---|---|---|---|
| Positive | Negative | ||||
| RCC | 53 | 42 | 11 | 79.2% | P<0.05 |
| Normal | 51 | 9 | 42 | 17.6% | |
Figure 2.
IHC analysis of ZFX expression. (A) Positive expression of ZFX in RCC tissues; (B) Negative expression of ZFX in normal tissues. Original magnification: ×200.
Association between ZFX expression and clinical parameters of RCC patients
The result showed that ZFX expression was significantly related with TNM stage, tumor size, and lymph node metastasis (P<0.05). However, no statistical correlation was observed between ZFX expression and age, sex, or smoking (P>0.05) (Table 2).
Table 2.
Relationship of clinical parameters and ZFX expression.
| Characteristics | Case No. | Protein expression | χ2 | P value | |
|---|---|---|---|---|---|
| Positive | Negative | ||||
| Age (years) | 0.279 | 0.597 | |||
| ≤35 | 23 | 19 | 4 | ||
| >35 | 30 | 23 | 7 | ||
| Gender | 0.014 | 0.905 | |||
| Male | 44 | 35 | 9 | ||
| Female | 9 | 7 | 2 | ||
| Smoking | 1.817 | 0.178 | |||
| Yes | 29 | 21 | 8 | ||
| No | 24 | 21 | 3 | ||
| TNM stage | 4.322 | 0.038 | |||
| I,II | 34 | 24 | 10 | ||
| III | 19 | 18 | 1 | ||
| Tumor size (cm) | 4.220 | 0.040 | |||
| ≤5 | 24 | 16 | 8 | ||
| >5 | 29 | 26 | 3 | ||
| Lymph node metastasis | 4.681 | 0.031 | |||
| Yes | 25 | 23 | 2 | ||
| No | 28 | 19 | 9 | ||
Correlation between ZFX and prognosis of RCC patients
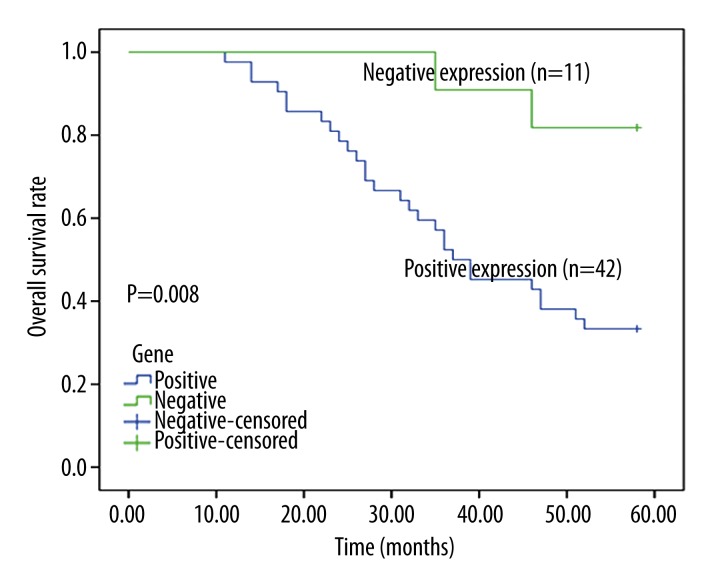
A postoperative follow-up was an average of 46.8 months. During the survey, 28 (66.7%) patients with positive expression died, but only 2 (9.1%) died among the RCC patients with negative ZFX expression. Kaplan-Meier survival analysis demonstrated that patients with positive ZFX expression had statistically higher mortality than those with negative expression (Figure 3). Cox regression analysis was conducted to evaluate the correlation between ZFX expression and the prognosis of RCC patients. The result confirmed that ZFX expression had a significant correlation with the prognosis of RCC patients, and positive ZFX expression predicted poor prognosis (HR=4.997, P=0.045, 95% CI=1.033–24.180) (Table 3).
Figure 3.
The overall survival rate of RCC patients was estimated by Kaplan-Meier analysis. The result showed that the overall survival rate of RCC patients with positive ZFX expression was significantly lower than in those with negative expression.
Table 3.
Multivariate analysis of prognostic factors.
| Clinical parameters | P value | HR | 95% CI |
|---|---|---|---|
| Age | 0.093 | 2.107 | 0.883–5.028 |
| Gender | 0.074 | 0.377 | 0.129–1.099 |
| TNM stage | 0.612 | 1.232 | 0.550–2.758 |
| Lymph node metastasis | 0.743 | 0.849 | 0.320–2.258 |
| ZFX expression | 0.045 | 4.997 | 1.033–24.180 |
Discussion
Zinc finger proteins are broadly distributed in eukaryotic genomes and play important roles in genomic regulation by interaction with DNA and proteins (27). Recently, studies have found a close linkage between zinc finger proteins and human tumorigenesis, such as zinc finger 280B protein and prostate cancer, and zinc finger 703 protein and gastric cancer [28,29]. Similar to these proteins, ZFX is a member of the zinc finger protein family. Previous studies have demonstrated that ZFX can function as an oncoprotein in various types of cancers, including gastric cancer [16], prostate cancer [24,25], glioma [26], hepatocellular cancer [30], gallbladder cancer [31], tongue squamous cell carcinoma [32], non-small cell lung cancer [33], and laryngeal squamous cell carcinoma [34]. However, few studies have focused on the prognostic significance of ZFX in RCC.
ZFX is best known for its role in regulating the renewal and differentiation of stem cells. Wu et al. [16] reported that knockdown of ZFX inhibited gastric cancer cell growth in vitro and in vivo. Fang et al. [18] concluded that knockdown of ZFX suppressed renal carcinoma cell growth and induced cell apoptosis. Thus, our study focused on investigating the prognostic role of ZFX in RCC. qRT-PCR and IHC assay suggested higher expression level of ZFX in RCC tissues, which indicated that ZFX might play an important role in the pathogenesis of RCC. Further analysis showed the tight relationship between ZFX expression and TNM stage, tumor size, and lymph node metastasis. ZFX was confirmed as a prognostic biomarker in RCC by Kaplan-Meier survival and Cox regression analysis in a coloreccancer study [35]. To the best of our knowledge, the pres talent study is the first to report the correlation between ZFX expression and the prognosis of RCC patients.
According to previous studies, the involvement of ZFX in pathogenesis of RCC might be related to several possible molecular mechanisms. ZFX may impact the development of RCC by regulating cancer-associated signal pathways, such as the ERK-MAPK pathway, which was found in gastric cancer cell growth [16]. Fang et al. suggested that ZFX could regulate RCC cell proliferation and apoptosis through modifying the expression of certain downstream genes, such as Caspase-1, AKT, Survivin, and Ki-67 [36]. Further studies are needed to explore the molecular mechanisms of ZFX in RCC.
Conclusions
Our study showed that ZFX was significantly upregulated in RCC tissues. Kaplan-Meier and Cox regression analysis demonstrated that ZFX might act as a molecular marker for prognosis of RCC patients. However, how ZFX exerts an effect on the prognosis of RCC patients was not explored, and further research is needed to determine this.
Footnotes
Source of support: 1. Specialized Research Fund for Doctoral Program of Higher Education (SRFDP) (20121202120003). 2. Application Base and Frontier Technology Project of Tianjin (13JCQNJC11500)
References
- 1.Wong KW. Renal cell carcinoma with proliferative lupus nephritis. BMJ Case Rep. 2015;2015 doi: 10.1136/bcr-2014-208060. pii: bcr2014208060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hongo F, Takaha N, Oishi M, et al. CDK1 and CDK2 activity is a strong predictor of renal cell carcinoma recurrence. Urol Oncol. 2014;32:1240–46. doi: 10.1016/j.urolonc.2014.05.006. [DOI] [PubMed] [Google Scholar]
- 3.Zhang HM, Yang FQ, Chen SJ, et al. Upregulation of long non-coding RNA MALAT1 correlates with tumor progression and poor prognosis in clear cell renal cell carcinoma. Tumour Biol. 2015;36(4):2947–55. doi: 10.1007/s13277-014-2925-6. [DOI] [PubMed] [Google Scholar]
- 4.Moch H. An overview of renal cell cancer: pathology and genetics. Semin Cancer Biol. 2013;23:3–9. doi: 10.1016/j.semcancer.2012.06.006. [DOI] [PubMed] [Google Scholar]
- 5.Nilsson H, Lindgren D, Mandahl Forsberg A, et al. Primary clear cell renal carcinoma cells display minimal mitochondrial respiratory capacity resulting in pronounced sensitivity to glycolytic inhibition by 3-Bromopyruvate. Cell Death Dis. 2015;6:e1585. doi: 10.1038/cddis.2014.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu HO, Wu Q, Liu WS, et al. ST6Gal-I predicts postoperative clinical outcome for patients with localized clear-cell renal cell carcinoma. Asian Pac J Cancer Prev. 2014;15:10217–23. doi: 10.7314/apjcp.2014.15.23.10217. [DOI] [PubMed] [Google Scholar]
- 7.Eccher A, Boschiero L, Fior F, et al. Donor kidneys with miliary papillary renal cell neoplasia: the role of the pathologist in determining suitability for transplantation. Ann Transplant. 2014;19:362–66. doi: 10.12659/AOT.890620. [DOI] [PubMed] [Google Scholar]
- 8.Coppin C, Kollmannsberger C, Le L, et al. Targeted therapy for advanced renal cell cancer (RCC): a Cochrane systematic review of published randomised trials. BJU Int. 2011;108:1556–63. doi: 10.1111/j.1464-410X.2011.10629.x. [DOI] [PubMed] [Google Scholar]
- 9.Jayson M, Sanders H. Increased incidence of serendipitously discovered renal cell carcinoma. Urology. 1998;51:203–5. doi: 10.1016/s0090-4295(97)00506-2. [DOI] [PubMed] [Google Scholar]
- 10.Godwin JL, Zibelman M, Plimack ER, Geynisman DM. Immune checkpoint blockade as a novel immunotherapeutic strategy for renal cell carcinoma: a review of clinical trials. Discov Med. 2014;18:341–50. [PubMed] [Google Scholar]
- 11.Zhao J, Huang X, Sun F, et al. Prognostic factors for overall survival with targeted therapy in Chinese patients with metastatic renal cell carcinoma. Can Urol Assoc J. 2014;8:E821–27. doi: 10.5489/cuaj.2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Iacovelli R, Massari F, Albiges L, et al. Evidence and clinical relevance of tumor flare in patients who discontinue tyrosine kinase inhibitors for treatment of metastatic renal cell carcinoma. Eur Urol. 2015;68(1):154–60. doi: 10.1016/j.eururo.2014.10.034. [DOI] [PubMed] [Google Scholar]
- 13.Delea TE, Amdahl J, Diaz J, et al. Cost-effectiveness of pazopanib versus sunitinib for renal cancer in the United States. J Manag Care Spec Pharm. 2015;21:46–54a–b. doi: 10.18553/jmcp.2015.21.1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Araujo WF, Naves MA, Ravanini JN, et al. Renin-angiotensin system (RAS) blockade attenuates growth and metastatic potential of renal cell carcinoma in mice. Urol Oncol. 2015 doi: 10.1016/j.urolonc.2014.11.022. pii: S1078-1439(14)00446-3. [DOI] [PubMed] [Google Scholar]
- 15.Zha W, Cao L, Shen Y, Huang M. Roles of Mir-144-ZFX pathway in growth regulation of non-small-cell lung cancer. PLoS One. 2013;8:e74175. doi: 10.1371/journal.pone.0074175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu S, Lao XY, Sun TT, et al. Knockdown of ZFX inhibits gastric cancer cell growth in vitro and in vivo via downregulating the ERK-MAPK pathway. Cancer Lett. 2013;337:293–300. doi: 10.1016/j.canlet.2013.04.003. [DOI] [PubMed] [Google Scholar]
- 17.Schneider-Gadicke A, Beer-Romero P, Brown LG, et al. Putative transcription activator with alternative isoforms encoded by human ZFX gene. Nature. 1989;342:708–11. doi: 10.1038/342708a0. [DOI] [PubMed] [Google Scholar]
- 18.Fang Q, Fu WH, Yang J, et al. Knockdown of ZFX suppresses renal carcinoma cell growth and induces apoptosis. Cancer Genet. 2014;207:461–66. doi: 10.1016/j.cancergen.2014.08.007. [DOI] [PubMed] [Google Scholar]
- 19.Palmer CJ, Galan-Caridad JM, Weisberg SP, et al. Zfx facilitates tumorigenesis caused by activation of the Hedgehog pathway. Cancer Res. 2014;74:5914–24. doi: 10.1158/0008-5472.CAN-14-0834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Galan-Caridad JM, Harel S, Arenzana TL, et al. Zfx controls the self-renewal of embryonic and hematopoietic stem cells. Cell. 2007;129:345–57. doi: 10.1016/j.cell.2007.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cellot S, Sauvageau G. Zfx: at the crossroads of survival and self-renewal. Cell. 2007;129:239–41. doi: 10.1016/j.cell.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 22.Harel S, Tu EY, Weisberg S, et al. ZFX controls the self-renewal of human embryonic stem cells. PLoS One. 2012;7:e42302. doi: 10.1371/journal.pone.0042302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang D, Gao Q, Guo L, et al. Isolation and identification of cancer stem-like cells in esophageal carcinoma cell lines. Stem Cells Dev. 2009;18:465–73. doi: 10.1089/scd.2008.0033. [DOI] [PubMed] [Google Scholar]
- 24.Jiang H, Zhang L, Liu J, et al. Knockdown of zinc finger protein X-linked inhibits prostate cancer cell proliferation and induces apoptosis by activating caspase-3 and caspase-9. Cancer Gene Ther. 2012;19:684–89. doi: 10.1038/cgt.2012.53. [DOI] [PubMed] [Google Scholar]
- 25.Tricoli JV, Bracken RB. ZFY gene expression and retention in human prostate adenocarcinoma. Genes Chromosomes Cancer. 1993;6:65–72. doi: 10.1002/gcc.2870060202. [DOI] [PubMed] [Google Scholar]
- 26.Zhou Y, Su Z, Huang Y, et al. The Zfx gene is expressed in human gliomas and is important in the proliferation and apoptosis of the human malignant glioma cell line U251. J Exp Clin Cancer Res. 2011;30:114. doi: 10.1186/1756-9966-30-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brayer KJ, Segal DJ. Keep your fingers off my DNA: protein-protein interactions mediated by C2H2 zinc finger domains. Cell Biochem Biophys. 2008;50:111–31. doi: 10.1007/s12013-008-9008-5. [DOI] [PubMed] [Google Scholar]
- 28.Gao S, Hsieh CL, Zhou J, Shemshedini L. Zinc Finger 280B regulates sGCalpha1 and p53 in prostate cancer cells. PLoS One. 2013;8:e78766. doi: 10.1371/journal.pone.0078766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang G, Ma F, Zhong M, et al. ZNF703 acts as an oncogene that promotes progression in gastric cancer. Oncol Rep. 2014;31:1877–82. doi: 10.3892/or.2014.2997. [DOI] [PubMed] [Google Scholar]
- 30.Lai KP, Chen J, He M, et al. Overexpression of ZFX confers self-renewal and chemoresistance properties in hepatocellular carcinoma. Int J Cancer. 2014;135:1790–99. doi: 10.1002/ijc.28819. [DOI] [PubMed] [Google Scholar]
- 31.Tan Z, Zhang S, Li M, et al. Regulation of cell proliferation and migration in gallbladder cancer by zinc finger X-chromosomal protein. Gene. 2013;528:261–66. doi: 10.1016/j.gene.2013.06.064. [DOI] [PubMed] [Google Scholar]
- 32.Yin J, Jiang Y, Wu H, et al. Overexpression of ZFX and its involvement in squamous cell carcinoma of the tongue. Oncol Rep. 2015;33:141–48. doi: 10.3892/or.2014.3572. [DOI] [PubMed] [Google Scholar]
- 33.Jiang M, Xu S, Yue W, et al. The role of ZFX in non-small cell lung cancer development. Oncol Res. 2012;20:171–78. doi: 10.3727/096504012x13548165987493. [DOI] [PubMed] [Google Scholar]
- 34.Fang J, Yu Z, Lian M, et al. Knockdown of zinc finger protein, X-linked (ZFX) inhibits cell proliferation and induces apoptosis in human laryngeal squamous cell carcinoma. Mol Cell Biochem. 2012;360:301–7. doi: 10.1007/s11010-011-1069-x. [DOI] [PubMed] [Google Scholar]
- 35.Yan X, Yan L, Su Z, et al. Zinc-finger protein X-linked is a novel predictor of prognosis in patients with colorectal cancer. Int J Clin Exp Pathol. 2014;7:3150–57. [PMC free article] [PubMed] [Google Scholar]
- 36.Fang J, Yu Z, Lian M, et al. Knockdown of zinc finger protein, X-linked (ZFX) inhibits cell proliferation and induces apoptosis in human laryngeal squamous cell carcinoma. Mol Cell Biochem. 2012;360:301–7. doi: 10.1007/s11010-011-1069-x. [DOI] [PubMed] [Google Scholar]