Abstract
Background
Abnormalities of brain energy metabolism are involved in Alzheimer disease (AD). Sirtuin 1 (SIRT1) is a class III histone deacetylase and activates peroxisome proliferator-activated receptor-γ co-activator-1α (PGC-1α), which enhances mitochondrial biogenesis and energy homeostasis. Electroacupuncture (EA) has been reported to improve brain energy metabolism in AD. However, the effect of EA on SIRT1 and PGC-1α in AD remains unclear.
Material/Methods
ATP levels were measured using assay kits in the hippocampus and frontal cortex of senescence-accelerated mouse prone 8 (SAMP8) mice. Western blotting analysis and quantitative real-time RT-PCR were performed to measure the expression of SIRT1 and PGC-1α in the hippocampus of SAMP8 mice. PGC-1α acetylation was analyzed using immunoprecipitation.
Results
Compared with senescence-accelerated resistant mice 1 (SAMR1) mice, SAMP8 mice had a decline in ATP levels and the expression of SIRT1 and PGC-1α. EA treatment improved ATP levels, upregulated the expression of SIRT1 and PGC-1α, and decreased PGC-1α acetylation.
Conclusions
These data suggest that EA improved brain energy metabolism, potentially associated with the upregulation of SIRT1-dependent PGC-1α expression.
MeSH Keywords: Alzheimer Disease, Electroacupuncture, Energy Metabolism, PPAR gamma, Sirtuin 1
Background
Alzheimer disease (AD) is clinically characterized by progressive memory loss and a decline of cognitive functions. Increasing evidence has indicated that global and regional disruptions in brain energy metabolism are critically involved in the pathogenesis of AD [1], including mitochondrial dysfunctions [2], decline in glucose uptake [3], and defects in cholesterol metabolism [4]. The levels of glucose metabolism and ATP are remarkably decreased in AD patients [1,5,6]. In addition, regional metabolic changes were associated with cognitive impairment in AD [7,8]. Improve global energy metabolism may be effective in preventing cognitive impairment [9] associated with brain aging and AD [10,11]. Thus, brain energy metabolism may serve as a therapeutic target in AD [12,13].
Sirtuin 1 (SIRT1) protein is classified as a class III NAD+-dependent histone deacetylase, which plays an important role in metabolic function and longevity in mammals [14,15]. Activation of SIRT1 enhances mitochondrial oxidative function to regulate energy balance. SIRT1 and resveratrol, a SIRT1 activator, both promote neuronal survival in AD [16]. A SIRT1 target is peroxisome proliferator-activated receptor-γ co-activator-1α (PGC-1α). SIRT1 physically interacts with and deacetylates PGC-1α [17]. Additionally, SIRT1 promotes mitochondrial biogenesis through deacetylation and activation of PGC-1α [18,19]. PGC-1α has been shown to be a master regulator of mitochondrial biogenesis and energy metabolism [15,20–22]. A recent study showed that PGC-1α and ATP content were decreased in AD hippocampal tissues and APPswe M17 cells. In addition, PGC-1α overexpression could rescue, whereas knockdown of PGC-1α exacerbated, impaired mitochondrial biogenesis and mitochondrial deficits in APPswe M17 cells [23]. The mRNA and protein expression levels of SIRT1 and PGC-1α in the brains of AD patients were decreased compared with age-matched controls [23–25]. Taken together, increased expression of SIRT1 and PGC-1α would exert beneficial effects in AD.
Electroacupuncture (EA) or acupuncture, a traditional Chinese medicine treatment that stimulates specific acupoints, has been shown to improve cognitive deficits in animal models of AD [26–29]. Acupuncture increased blood perfusion and glycol metabolism in AD model rats [30], which indicated that acupuncture might improve energy metabolism. Our previous study showed that EA increased the activation of AMP-activated protein kinase (AMPK) [31], a master regulator of cellular energy homeostasis. AMPK can activate SIRT1 and PGC-1α [32,33]. Activation of SIRT1 and PGC-1α improved mitochondrial function [34]. In addition, EA improved insulin sensitivity in obese diabetic mice via activation of SIRT1/PGC-1α [35]. However, little is known regarding the effect of EA on the expression SIRT1 and PGC-1α in AD.
In the present study, we investigated the effect of EA on brain energy metabolism in senescence-accelerated mouse prone 8 (SAMP8) mice. Furthermore, we examined the effect of EA on SIRT1 and PGC-1α expression in SAMP8 mice.
Material and Methods
Animals
Male SAMP8 and senescence-accelerated resistant mice 1 (SAMR1) mice (7 months old) were purchased from the Department of Laboratory Animal Science of Peking University. All mice were housed under standard conditions at 22±2°C and a 12 h light/dark cycle with free access to food and water. All procedures followed the “National Institute of Health Guide for the Care and Use of Laboratory Animals” (NIH Publications No. 80-23) and were approved by the Institutional Animal Care and Utilization Committee of Fujian University of Traditional Chinese Medicine.
EA treatment
Male SAMP8 and male homologous SAMR1 mice were randomly assigned to the following 3 groups: SAMR1 normal control group (Rc), SAMP8 control group (Pc), and SAMP8 electroacupuncture group (Pe). EA treatment was performed as described in our previous study [31]. Briefly, we used nets to fix the mice by an assistant’s hands during the entire treatment. Three stainless steel acupuncture needles were inserted at a depth of 5 mm into the “Dazhui” acupoint (GV14) and the bilateral “Shenshu” acupoint (BL23). We performed continuous-wave stimulation at a frequency of 2 Hz (intensity 1 mA). An individual EA session was administered daily for 20 min, 8 days, and 2 days of rest, for a period of 30 days.
Tissue collection
Biochemical and molecular studies were performed in the mouse hippocampus and frontal cortex excised under ether anesthesia after intra-cardiac perfusion with ice-cold normal saline. We focused on the hippocampus, which is one of the brain areas that is earliest and most severely affected by AD.
Assessment of ATP levels
ATP levels were measured in freshly prepared hippocampal and cortical homogenates samples. Due to the high activities of ATPase in fresh samples, tissue was immediately snap-frozen in liquid nitrogen. All steps for sample preparation were performed at 4°C to avoid the recovery of ATPase activity and subsequent degradation of ATP in the tissue. Assays were performed using a spectrophotometric plate reader (Paradigm, Beckman Coulter, USA). Tissue ATP levels were determined using a luciferin/luciferase ATP Bioluminescent Assay Kit (Sigma, St Louis, MO, USA). Sample preparation and assay procedures were performed according to the manufacturer’s protocols.
Western blotting analysis
Protein samples were isolated from the hippocampus in ice-cold RIPA lysis buffer supplemented with protease inhibitors. After incubation for 40 min at 4°C, homogenates were centrifuged at 14 000 rpm for 15 min at 4°C and the supernatant were collected and stored in aliquots at −80°C until further use.
Equivalent amounts (30 μg) of each sample, calculated using a BCA protein assay kit (Beyotime, Haimen, Jiangsu, China), were separated on 10% SDS-polyacrylamide gels. After electrophoresis, the proteins were transferred onto a nitrocellulose membrane (Millipore) at 100 V for 60 min on ice. The membrane was blocked with 5% w/v nonfat dry milk powder in Tris-buffered saline with 0.05% Tween 20 (TBS-T) for 1 h. The membrane was incubated with primary antibody overnight at 4°C, followed by secondary antibody for 1 h at room temperature. The following primary antibodies were used: rabbit anti-SIRT1 (1:1000, Santa Cruz Biotechnology, Santa Cruz, CA, USA), rabbit anti- PGC-1α (1:1000, Santa Cruz Biotechnology), and anti-β-actin (1:5000, Sigma, St Louis, MO, USA). The secondary antibody was HRP-conjugated goat anti-rabbit IgG (1:1000, Santa Cruz Biotechnology). The membrane was rinsed with TBS-T and the immunocomplex was visualized using an enhanced chemiluminescence detection kit (Thermo Scientific, Rockford, IL, USA). The signals of the membrane were scanned using the FluorChem Scanner and quantified with the NIH Image J software. These results were normalized with β-actin expression levels and confirmed by triplicate measurements of the same sample.
Immunoprecipitation
PGC-1α acetylation was analyzed by immunoprecipitation of PGC-1α followed by Western blotting using an acetyl-lysine antibody according to the immunoprecipitation protocol provided by Abcam. The protein samples were pre-cleared with 20 μl of protein G (Sigma-Aldrich, USA) for 3 h and then centrifuged to obtain supernatant at 15 000g for 10 min. PGC-1α antibody (1:1000, Millipore, USA) and 30 μl of protein G were added to the pre-cleared supernatant then incubated for 12 h at 4°C. The protein G agarose was washed 3 times with cold PBS for 15 min. The immunoprecipitated protein was visualized and blotted using the western blotting method. PGC-1α acetylation was measured using acetyl-lysine antibody (Abcam, USA).
Quantitative real-time RT-PCR (qRT-PCR)
Total RNA was extracted from frozen hippocampus using TRIzol (Invitrogen, CA, USA), according to the manufacturer’s instructions. The RNA concentrations were determined using a Nanodrop ND-1000 spectrophotometer (NanoDrop Technologies, Wilmington, DE, USA). All RNA samples had a 260:280 absorbance ratio between 1.9 and 2.1. Next, 0.5 μg of RNA was processed for cDNA synthesis using a High-Capacity cDNA Reverse Transcription kit (Invitrogen, CA, USA). The primers used for qRT-PCR were the following: SIRT1 forward: 5′-TGTGAAGTTACTGCAGGA GTGTAAA-3′, reverse: 5′-GCATAGATACCGTCTCTTGATCTGAA-3′ PGC-1α forward: 5′-AAGTGTGGAACTCTCTGGAACTG-3′, reverse: 5′-GGGTTATCTTGG TTGGCTTTATG-3′; glyceraldehyde-3-phosphate dehydrogenase (GAPDH) forward: 5′-TGGAAAGCTGTGGCGTGAT-3′, reverse: 5′-TGCTTCACCACCTTCTTGAT-3′. Next, qRT-PCR was performed on a 7300 real-time PCR system (Applied Biosystems, CA, USA) using SYBR Green PCR master mix (Applied Biosystems), according to the manufacturer’s instructions. The data were analyzed according to the delta–delta Ct (ΔΔCT) method and were normalized to GAPDH expression in each sample.
Statistical analysis
Data are expressed as the mean ±SEM. The escape latency of mice in the MWM test was analyzed using 2-way analysis of variance (ANOVA) for repeated measurement. Tukey’s test was further used as a post hoc test to detect between-group differences. One-way ANOVA was employed to analyze other data obtained in these experiments followed by LSD (equal variances assumed) or Dunnett’s T3 (equal variances not assumed) for a post hoc test between groups. The statistical significance was established at a level of P<0.05.
Results
EA increased ATP levels in the hippocampus and frontal cortex
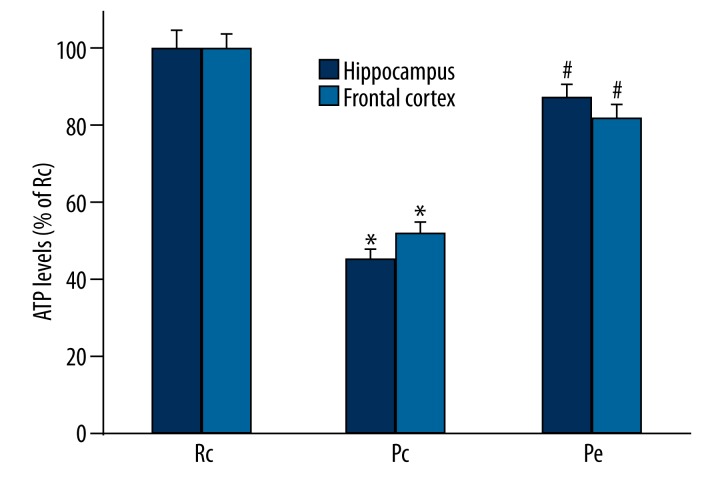
The cellular ATP levels reflect the state of mitochondria function and brain energy metabolism, thus we determined the ATP levels in the hippocampus and frontal cortical tissue to confirm brain energy metabolism. These results showed that the Pc mice showed a significant decrease in ATP levels in the hippocampus and frontal cortex compared with the Rc mice, indicating impaired brain energy metabolism. However, EA significantly reversed the aberrant decrease in ATP levels in the hippocampus and frontal cortex (Figure 1), indicating an improvement in brain energy metabolism.
Figure 1.
Effect of EA on hippocampal and cortical ATP levels in SAMP8 mice. ATP levels were increased in the hippocampus and frontal cortex of Pe mice compared to Pc mice. The data were expressed as the mean ±SEM (n=5). * p<0.05 compared with the Rc group, # p<0.05 compared with the Pc group.
EA upregulated the protein expression of SIRT1 and PGC-1α and decreased PGC-1α acetylation
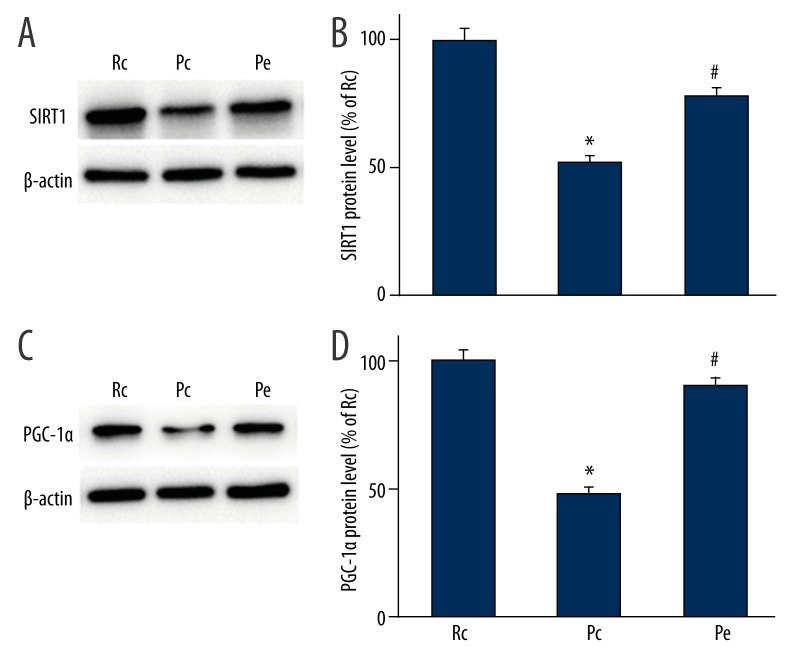
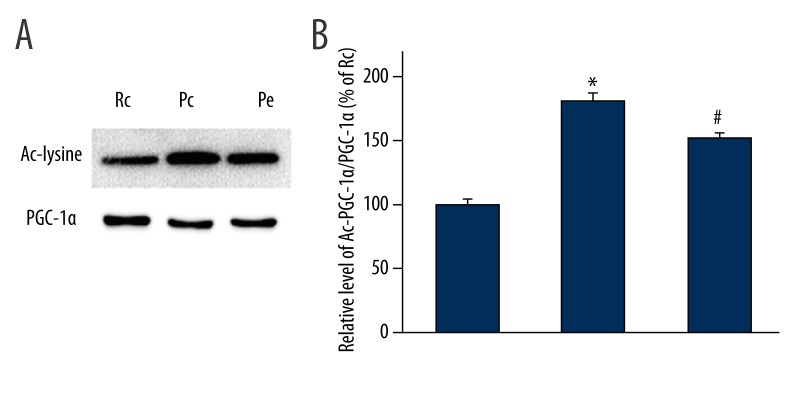
To further explore the underlying neuroprotective mechanisms of EA treatment on AD, the protein levels of SIRT1 and PGC-1α were investigated using Western blotting analyses. PGC-1α acetylation was analyzed using immunoprecipitation. We showed that the expression levels of SIRT1 and PGC-1α were significantly reduced in the Pc group when compared with the Rc group (p<0.05; Figure 2). We also found that the expression levels of both SIRT1 and PGC-1α were significantly increased in the Pe group compared with the Pc group (p<0.05; Figure 2). In accordance with increased protein levels of SIRT1 in the Pe group, there was a decrease in the acetylation state of PGC-1α in the Pe group (p<0.05; Figure 3), suggesting EA increases PGC-1α activity.
Figure 2.
Effect of EA on the protein levels of SIRT1 and PGC-1α in the hippocampus of SAMP8 mice was evaluated using Western blotting analyses. (A) Representative Western blotting analyses for SIRT1. (B) The protein expression of SIRT1 was significantly reduced in the Pc group compared with the Rc group. Compared with the Pc group, the protein expression of SIRT1 was significantly increased in the Pe group. (C) Representative Western blotting analyses for PGC-1α. (D) EA increased PGC-1α protein expression. β-actin was used as a reference protein. Data are expressed as the mean ±SEM (n=5 in each group). * p<0.05 versus Rc group; # p<0.05 versus Pc group.
Figure 3.
EA decreased PGC-1α acetylation in the hippocampus of SAMP8 mice. (A) Lysates of hippocampus were used to immunoprecipitate PGC-1α and Western blotting was performed to detect PGC-1α levels and acetylation. (B) The quantification of the ratio of acetylated to total PGC-1α. The results are expressed as a percentage of the control, which is set at 100%. n=5, * p<0.05 versus Rc group; # p<0.05 versus Pc group.
EA upregulated the gene expression of SIRT1 and PGC-1α
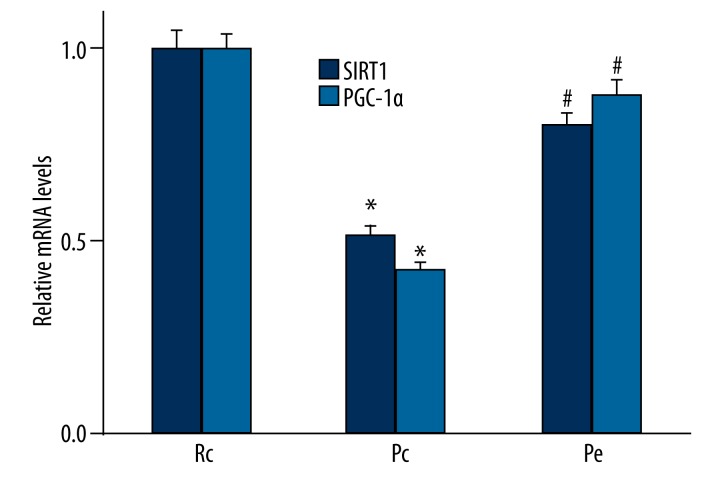
We further analyzed the gene expression of both SIRT1 and PGC-1α with qRT-PCR. Consistent with the results obtained from Western blotting analyses, we also found that the expression of SIRT1 and PGC-1α at the mRNA level were down-regulated in the Pc group compared with the Rc group (p<0.05; Figure 4). However, the expression of SIRT1 and PGC-1α at mRNA level was significantly increased in the Pe group compared with the Pc group (p< 0.05; Figure 4).
Figure 4.
Effect of EA on gene expressions of SIRT1 and PGC-1α in the hippocampus was measured using qRT-PCR. Consistent with the Western blotting results, we found that the expression of SIRT1 and PGC-1α at the mRNA level was down-regulated in the Pc group compared with the Rc group. Compared with the Pc group, the mRNA expression of SIRT1 and PGC-1α was significantly increased in the Pe group. n=5, * p<0.05 versus Rc group; # p<0.05 versus Pc group.
Discussion
In this study, we showed that EA treatment improved brain energy metabolism in SAMP8 mice. Furthermore, EA treatment increased the protein and mRNA expression of SIRT1 and PGC-1α, and decreased PGC-1α acetylation in SAMP8 mice. These data suggest that the effects of EA treatment on brain energy metabolism in AD might be through the SIRT1-dependent PGC-1α pathway.
Impaired brain metabolism and mitochondrial dysfunction play an important role in the early pathology of AD [1,36,37]. Laminin increases the expression of mitochondrial proteins (cytochrome b and chargerin II) during neurite outgrowth [38]. Cytochrome b and chargerin II are associated with ATP. In the present study, ATP levels in the hippocampus and frontal cortex of SAMP8 mice were significantly reduced compared with SAMR1 mice, which was consistent with previous studies [1,5,6]. In addition, our results showed that EA increased ATP levels in the hippocampus and frontal cortex, which indicated that EA could improve impaired brain energy metabolism. Our previous study showed that EA improves cognitive deficits in SAMP8 mice [31]. Taken together, EA improves cognitive deficits in AD and may be associated with an improvement in brain energy metabolism, which further support the hypothesis that improvement in brain energy metabolism is effective in preventing the cognitive impairment associated with AD [39,40].
SIRT1 regulates fat and glucose metabolism in response to physiological changes in energy levels. Therefore, SIRT1 acts as a crucial regulator of the network that controls energy homeostasis [41]. Moreover, AD shares characteristics and possible origins with diabetes, and metabolic rundown encountered in type 2 diabetes engenders cerebral vascular insufficiencies that are causally associated with long-term neural degenerative processes in AD [42]. Activation of SIRT1 has been shown to have beneficial effects against AD [43,44]. SIRT1 can directly interact and deacetylate PGC-1α [19]. PGC-1α plays an important role as a master regulator of cellular metabolic homeostasis and has been reported to produce beneficial effects on AD [45,46]. PGC-1α has also been suggested to be a potential therapeutic target to increase mitochondrial biogenesis and improve energy metabolism in AD [16,23].
To elucidate the mechanism for the increase in ATP levels following EA treatment, we analyzed the mRNA and protein levels of SIRT1 and PGC-1α in the hippocampus of SAMP8 mice. Our results showed that the mRNA and protein expression levels of SIRT1 and PGC-1α in the hippocampus of SAMP8 mice were reduced compared with that of SAMR1 mice. This finding is consistent with previous studies demonstrating a decrease in the mRNA and protein expression levels of SIRT1 and PGC-1α in postmortem brain tissue in AD patients [23–25]. Additionally, deacetylation and activation of PGC-1α by enhanced SIRT1 activity was involved in neuroprotection in AD [16]. Our results showed that EA decreased PGC-1α acetylation. Together, these results suggest that SIRT1 and PGC-1α play an important role in AD. A decrease in the mRNA and protein expression levels of SIRT1 and PGC-1α may impair brain energy metabolism and may further result in cognitive deficits. Conversely, a recent study reported that PGC-1α overexpression exacerbates Aβ and tau deposition in a transgenic mouse model of AD, which was associated with impairment in proteasome activity [47]. Thus, a more definitive probe of the role of PGC-1α in AD will require further investigation.
It has been reported that Icariin and alpha-lipoic acid protect against brain injury by enhancing SIRT1-dependent PGC-1α expression in ischemic stroke [48,49]. Activating SIRT1 and PGC-1α improved mitochondrial function and protects against metabolic disease [34]. Moreover, EA improved insulin sensitivity via the activation of SIRT1/PGC-1α [35]. We have previously shown that Aβ25–35 suppresses mitochondrial biogenesis and AMPK-SIRT1-PGC-1α pathway in cultured hippocampal neurons [50]. In the present study, EA increased the protein and mRNA expression of SIRT1 and PGC-1α. Increased PGC-1α expression may regulate energy metabolism by improving ATP levels and the production of energy. Improvement in brain energy metabolism may attenuate cognitive deficits. Together, our data suggest that SIRT1 and PGC-1α may be involved in EA treatment for AD. Future studies are planned to determine whether the SIRT1-dependent PGC-1α pathway is a molecular target of EA in the treatment of AD.
Conclusions
The present study indicates that EA can improve brain energy metabolism in SAMP8 mice. The mechanism may be associated with the enhancement of SIRT1-dependent PGC-1α expression. The SIRT1-dependent PGC-1α pathway may be a target for EA treatment or other therapeutic interventions in AD.
Footnotes
Conflict of interest
The authors declare that they have no conflicts of interest.
Source of support: This work was funded by the National Natural Science Foundation of China (grant no. 81102625), the Natural Science Foundation of Fujian Province Grants (grant no. 2012J05154), and study abroad scholarships of Fujian Province
References
- 1.Kapogiannis D, Mattson MP. Disrupted energy metabolism and neuronal circuit dysfunction in cognitive impairment and Alzheimer’s disease. Lancet Neurol. 2011;10:187–98. doi: 10.1016/S1474-4422(10)70277-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Galindo MF, Ikuta I, Zhu X, et al. Mitochondrial biology in Alzheimer’s disease pathogenesis. J Neurochem. 2010;114:933–45. doi: 10.1111/j.1471-4159.2010.06814.x. [DOI] [PubMed] [Google Scholar]
- 3.Mosconi L, Pupi A, De Leon MJ. Brain glucose hypometabolism and oxidative stress in preclinical Alzheimer’s disease. Ann NY Acad Sci. 2008;1147:180–95. doi: 10.1196/annals.1427.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Martins IJ, Berger T, Sharman MJ, et al. Cholesterol metabolism and transport in the pathogenesis of Alzheimer’s disease. J Neurochem. 2009;111:1275–308. doi: 10.1111/j.1471-4159.2009.06408.x. [DOI] [PubMed] [Google Scholar]
- 5.Hoyer S. Glucose metabolism and insulin receptor signal transduction in Alzheimer disease. Eur J Pharmacol. 2004;490:115–25. doi: 10.1016/j.ejphar.2004.02.049. [DOI] [PubMed] [Google Scholar]
- 6.Hoyer S. Oxidative metabolism deficiencies in brains of patients with Alzheimer’s disease. Acta Neurol Scand Suppl. 1996;165:18–24. doi: 10.1111/j.1600-0404.1996.tb05868.x. [DOI] [PubMed] [Google Scholar]
- 7.Sperling RA, Dickerson BC, Pihlajamaki M, et al. Functional alterations in memory networks in early Alzheimer’s disease. Neuromolecular Med. 2010;12:27–43. doi: 10.1007/s12017-009-8109-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fouquet M, Desgranges B, Landeau B, et al. Longitudinal brain metabolic changes from amnestic mild cognitive impairment to Alzheimer’s disease. Brain. 2009;132:2058–67. doi: 10.1093/brain/awp132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Etgen T, Sander D, Huntgeburth U, et al. Physical activity and incident cognitive impairment in elderly persons: the INVADE study. Arch Intern Med. 2010;170:186–93. doi: 10.1001/archinternmed.2009.498. [DOI] [PubMed] [Google Scholar]
- 10.Nichol K, Deeny SP, Seif J, et al. Exercise improves cognition and hippocampal plasticity in APOE epsilon4 mice. Alzheimers Dement. 2009;5:287–94. doi: 10.1016/j.jalz.2009.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kannangara TS, Lucero MJ, Gil-Mohapel J, et al. Running reduces stress and enhances cell genesis in aged mice. Neurobiol Aging. 2011;32:2279–86. doi: 10.1016/j.neurobiolaging.2009.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mosconi L, De Santi S, Li J, et al. Hippocampal hypometabolism predicts cognitive decline from normal aging. Neurobiol Aging. 2008;29:676–92. doi: 10.1016/j.neurobiolaging.2006.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mosconi L, Mistur R, Switalski R, et al. FDG-PET changes in brain glucose metabolism from normal cognition to pathologically verified Alzheimer’s disease. Eur J Nucl Med Mol Imaging. 2009;36:811–22. doi: 10.1007/s00259-008-1039-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bordone L, Guarente L. Calorie restriction, SIRT1 and metabolism: understanding longevity. Nat Rev Mol Cell Biol. 2005;6:298–305. doi: 10.1038/nrm1616. [DOI] [PubMed] [Google Scholar]
- 15.Rodgers JT, Lerin C, Gerhart-Hines Z, et al. Metabolic adaptations through the PGC-1 alpha and SIRT1 pathways. FEBS Lett. 2008;582:46–53. doi: 10.1016/j.febslet.2007.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim D, Nguyen MD, Dobbin MM, et al. SIRT1 deacetylase protects against neurodegeneration in models for Alzheimer’s disease and amyotrophic lateral sclerosis. Embo J. 2007;26:3169–79. doi: 10.1038/sj.emboj.7601758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nemoto S, Fergusson MM, Finkel T. SIRT1 functionally interacts with the metabolic regulator and transcriptional coactivator PGC-1{alpha} J Biol Chem. 2005;280:16456–60. doi: 10.1074/jbc.M501485200. [DOI] [PubMed] [Google Scholar]
- 18.Gerhart-Hines Z, Rodgers JT, Bare O, et al. Metabolic control of muscle mitochondrial function and fatty acid oxidation through SIRT1/PGC-1alpha. Embo J. 2007;26:1913–23. doi: 10.1038/sj.emboj.7601633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rodgers JT, Lerin C, Haas W, et al. Nutrient control of glucose homeostasis through a complex of PGC-1alpha and SIRT1. Nature. 2005;434:113–18. doi: 10.1038/nature03354. [DOI] [PubMed] [Google Scholar]
- 20.Wareski P, Vaarmann A, Choubey V, et al. PGC-1{alpha} and PGC-1{beta} regulate mitochondrial density in neurons. J Biol Chem. 2009;284:21379–85. doi: 10.1074/jbc.M109.018911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Puigserver P, Spiegelman BM. Peroxisome proliferator-activated receptor-gamma coactivator 1 alpha (PGC-1 alpha): transcriptional coactivator and metabolic regulator. Endocr Rev. 2003;24:78–90. doi: 10.1210/er.2002-0012. [DOI] [PubMed] [Google Scholar]
- 22.Scarpulla RC. Metabolic control of mitochondrial biogenesis through the PGC-1 family regulatory network. Biochim Biophys Acta. 2011;1813:1269–78. doi: 10.1016/j.bbamcr.2010.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sheng B, Wang X, Su B, et al. Impaired mitochondrial biogenesis contributes to mitochondrial dysfunction in Alzheimer’s disease. J Neurochem. 2012;120:419–29. doi: 10.1111/j.1471-4159.2011.07581.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Qin W, Haroutunian V, Katsel P, et al. PGC-1alpha expression decreases in the Alzheimer disease brain as a function of dementia. Arch Neurol. 2009;66:352–61. doi: 10.1001/archneurol.2008.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Julien C, Tremblay C, Emond V, et al. Sirtuin 1 reduction parallels the accumulation of tau in Alzheimer disease. J Neuropathol Exp Neurol. 2009;68:48–58. doi: 10.1097/NEN.0b013e3181922348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li G, Zhang X, Cheng H, et al. Acupuncture improves cognitive deficits and increases neuron density of the hippocampus in middle-aged SAMP8 mice. Acupunct Med. 2012;30:339–45. doi: 10.1136/acupmed-2012-010180. [DOI] [PubMed] [Google Scholar]
- 27.Cheng H, Yu J, Jiang Z, et al. Acupuncture improves cognitive deficits and regulates the brain cell proliferation of SAMP8 mice. Neurosci Lett. 2008;432:111–16. doi: 10.1016/j.neulet.2007.12.009. [DOI] [PubMed] [Google Scholar]
- 28.Wang F, Zhong H, Li X, et al. Electroacupuncture attenuates reference memory impairment associated with astrocytic NDRG2 suppression in APP/PS1 transgenic mice. Mol Neurobiol. 2014;50:305–13. doi: 10.1007/s12035-013-8609-1. [DOI] [PubMed] [Google Scholar]
- 29.Li X, Guo F, Zhang Q, et al. Electroacupuncture decreases cognitive impairment and promotes neurogenesis in the APP/PS1 transgenic mice. BMC Complement Altern Med. 2014;14:37–46. doi: 10.1186/1472-6882-14-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lu Y, Huang Y, Tang C, et al. Brain areas involved in the acupuncture treatment of AD model rats: a PET study. BMC Complement Altern Med. 2014;14:178–85. doi: 10.1186/1472-6882-14-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dong W, Guo W, Zheng X, et al. Electroacupuncture improves cognitive deficits associated with AMPK activation in SAMP8 mice. Metab Brain Dis. 2015;30:777–84. doi: 10.1007/s11011-014-9641-1. [DOI] [PubMed] [Google Scholar]
- 32.Canto C, Gerhart-Hines Z, Feige JN, et al. AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity. Nature. 2009;458:1056–60. doi: 10.1038/nature07813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jager S, Handschin C, St-Pierre J, et al. AMP-activated protein kinase (AMPK) action in skeletal muscle via direct phosphorylation of PGC-1alpha. Proc Natl Acad Sci USA. 2007;104:12017–22. doi: 10.1073/pnas.0705070104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lagouge M, Argmann C, Gerhart-Hines Z, et al. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha. Cell. 2006;127:1109–22. doi: 10.1016/j.cell.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 35.Liang F, Chen R, Nakagawa A, et al. Low-frequency electroacupuncture improves insulin sensitivity in obese diabetic mice through activation of SIRT1/PGC-1alpha in skeletal muscle. Evid Based Complement Alternat Med. 2011;2011:735297–305. doi: 10.1155/2011/735297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ferreira IL, Resende R, Ferreiro E, et al. Multiple defects in energy metabolism in Alzheimer’s disease. Curr Drug Targets. 2010;11:1193–206. doi: 10.2174/1389450111007011193. [DOI] [PubMed] [Google Scholar]
- 37.Moreira PI, Cardoso SM, Santos MS, et al. The key role of mitochondria in Alzheimer’s disease. J Alzheimers Dis. 2006;9:101–10. doi: 10.3233/jad-2006-9202. [DOI] [PubMed] [Google Scholar]
- 38.Weeks BS, Burbelo P, Jucker M, et al. Laminin stimulates expression of two mitochondrial proteins during neurite outgrowth. Int J Dev Neurosci. 1996;14:365–74. doi: 10.1016/0736-5748(96)00019-6. [DOI] [PubMed] [Google Scholar]
- 39.D’Amico M, Di Filippo C, Marfella R, et al. Long-term inhibition of dipeptidyl peptidase-4 in Alzheimer’s prone mice. Exp Gerontol. 2010;45:202–7. doi: 10.1016/j.exger.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 40.Longo FM, Yang T, Knowles JK, et al. Small molecule neurotrophin receptor ligands: novel strategies for targeting Alzheimer’s disease mechanisms. Curr Alzheimer Res. 2007;4:503–6. doi: 10.2174/156720507783018316. [DOI] [PubMed] [Google Scholar]
- 41.Houtkooper RH, Pirinen E, Auwerx J. Sirtuins as regulators of metabolism and healthspan. Nat Rev Mol Cell Biol. 2012;13:225–38. doi: 10.1038/nrm3293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang F, Guo X, Shen X, et al. Vascular dysfunction associated with type 2 diabetes and Alzheimer’s disease: a potential etiological linkage. Med Sci Monit Basic Res. 2014;20:118–29. doi: 10.12659/MSMBR.891278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Qin W, Yang T, Ho L, et al. Neuronal SIRT1 activation as a novel mechanism underlying the prevention of Alzheimer disease amyloid neuropathology by calorie restriction. J Biol Chem. 2006;281:21745–54. doi: 10.1074/jbc.M602909200. [DOI] [PubMed] [Google Scholar]
- 44.Lalla R, Donmez G. The role of sirtuins in Alzheimer’s disease. Front Aging Neurosci. 2013;5:16. doi: 10.3389/fnagi.2013.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Katsouri L, Parr C, Bogdanovic N, et al. PPARgamma co-activator-1alpha (PGC-1alpha) reduces amyloid-beta generation through a PPARgamma-dependent mechanism. J Alzheimers Dis. 2011;25:151–62. doi: 10.3233/JAD-2011-101356. [DOI] [PubMed] [Google Scholar]
- 46.Handschin C, Spiegelman BM. Peroxisome proliferator-activated receptor gamma coactivator 1 coactivators, energy homeostasis, and metabolism. Endocr Rev. 2006;27:728–35. doi: 10.1210/er.2006-0037. [DOI] [PubMed] [Google Scholar]
- 47.Dumont M, Stack C, Elipenahli C, et al. PGC-1alpha overexpression exacerbates beta-amyloid and tau deposition in a transgenic mouse model of Alzheimer’s disease. Faseb J. 2014;28:1745–55. doi: 10.1096/fj.13-236331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhu HR, Wang ZY, Zhu XL, et al. Icariin protects against brain injury by enhancing SIRT1-dependent PGC-1alpha expression in experimental stroke. Neuropharmacology. 2010;59:70–76. doi: 10.1016/j.neuropharm.2010.03.017. [DOI] [PubMed] [Google Scholar]
- 49.Fu B, Zhang J, Zhang X, et al. Alpha-lipoic acid upregulates SIRT1-dependent PGC-1alpha expression and protects mouse brain against focal ischemia. Neuroscience. 2014;281:251–57. doi: 10.1016/j.neuroscience.2014.09.058. [DOI] [PubMed] [Google Scholar]
- 50.Dong W, Wang F, Guo W, et al. Abeta25-35 Suppresses Mitochondrial Biogenesis in Primary Hippocampal Neurons. Cell Mol Neurobiol. 2015 doi: 10.1007/s10571-015-0222-6. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]