Abstract
In Japan in 2013, two cattle in the northwestern part of Kagoshima Prefecture developed fever and swallowing difficulty and were suspected of having Ibaraki disease. The epizootic hemorrhagic virus (EHDV) genome was detected from diseased and asymptomatic cattle by reverse transcription-polymerase chain reaction (RT-PCR). High neutralization antibody titers to Ibaraki virus (IBAV) ranging from 1:128 to 1:1,024 were observed in the RT-PCR-positive cattle, and the virus was isolated in one of the IBAV-positive farms. A pairwise alignment and phylogenetic analysis based on the major outer coat protein VP2 encoded in segment 2 revealed a close relationship between the isolated viruses and previous IBAV isolates. The phylogeny of VP2 also suggested that an IBAV variant isolated in 1997 was distinct from IBAV and sorted into a heterogeneous serotype, EHDV serotype 7. The findings revealed the reemergence of Ibaraki disease in Japan after a 26-year absence. Interestingly, the co-circulation of EHDV serotype 1 with IBAV was observed in the affected region, suggesting the potential reassortment between two heterogeneous serotypes in the field. Sentinel surveillance in Kagoshima Prefecture indicated that the incursion of IBAV occurred in October 2013 and that its spread was limited within the small area. Inadequate environmental temperatures for vector transmission in late autumn might have limited the virus spread to a wider region. The reemergence of Ibaraki disease showed us the importance of continuous vaccination to prevent economic losses.
Keywords: arbovirus, Culicoides-borne, EHDV, epizootic hemorrhagic disease, orbivirus
Ibaraki disease is a noncontagious viral disease of cattle, with clinical manifestations that include fever, anorexia, lachrymation, foamy salivation, nasal discharge, conjunctival injection and swallowing difficulty [23]. The pathognomonic sign of this disease, observed in 20–30% of the affected cattle, is swallowing difficulty, which is caused by severe hemorrhagic, degenerative and necrotic lesions in the musculature of the esophagus, larynx, pharynx and tongue [11]. The etiological agent of Ibaraki disease is Ibaraki virus (IBAV), which is a strain of serotype 2 of epizootic hemorrhagic disease virus (EHDV) belonging to the genus Orbivirus of the family Reoviridae [3, 5]. The EHDV particle is composed of seven structural proteins and 10-segmented, double-stranded RNA genomes [8, 15]. The major outer coat protein, VP2, is encoded by genome segment 2 and is highly variable among the eight serotypes of EHDV. The specificity of the interaction of VP2 with neutralization antibodies is thought to be a major determinant of the virus serotype [3]. It was reported that VP2 of IBAV has 96.8% and 73.6% amino acid (aa) identities with those of Australian and Canadian strains of EHDV serotype 2, respectively, but less than 68.5% aa identity with that of other EHDV serotypes [3]. In contrast, segment 3 encodes the major core protein, VP3, and is highly conserved among EHDV isolates and is used as a target for EHDV group-specific diagnostics [21]. However, a small variation in the core protein is available for typing EHDV isolates based on their geographic origin [4]. IBAV isolates are sorted into an eastern topotype with Australian EHDV strains. As are the other EHDV strains, IBAV is regarded as an arthropod-borne virus (arbovirus) transmitted by Culicoides biting midges [24, 32]. Therefore, the seasonal activity and geographic distribution of Culicoides vectors potentially affect the epidemic period and range of Ibaraki disease. The ability of IBAV to overwinter in Japan is unlikely, and the virus was presumably introduced from lower latitudes with the infected midges carried on seasonal winds in the summer [32].
Ibaraki disease was first recognized in Japan in 1959 as an acute febrile disease resembling bluetongue [23]. During the 1959–60 Ibaraki disease epidemic, 43,793 cases and 4,298 deaths were reported. Smaller outbreaks occurred in southern Japan in 1982, 1987 and 1997–98 [9, 12, 28,29,30]. In addition to the typical Ibaraki disease manifestations, more than 1,000 cases of abortion and still birth were observed in the infected pregnant cows in the 1997–98 outbreak [20]. The virus isolated from the aborted fetuses was serologically related to IBAV but genetically distinct from the previous IBAV isolates, and was thought to be an IBAV variant within EHDV serotype 2 [20, 22]. The prevalence of EHDV serotype 1 was also reported in Japan, but no association between that virus and cattle morbidity has been detected [17].
Kagoshima Prefecture is located at the southern end of Japan’s mainland and includes islands spreading 500 km further to the southwest. Due to its geographic location, the prefecture has been thought of as one of the gates for arbovirus incursions [10, 32]. In October 2013, two cows with foamy salivation and difficulty swallowing were found in the northwestern part of Kagoshima Prefecture and were suspected to be cases of Ibaraki disease. We report here the identification of a causative agent of the disease, and we describe the epidemiological aspects based on sentinel surveillance in Kagoshima Prefecture.
MATERIALS AND METHODS
Blood samples: Heparinized blood samples were collected from cattle with swallowing difficulty and from asymptomatic cattle from the same cowsheds where the affected cattle were raised. The sampling was conducted on the two farms (farms A and B) where the clinical cases were observed, on October 25 and 31, respectively. The blood samples were separated into plasma and blood cells by centrifugation at 2,150 g for 10 min. The blood cells were washed three times with phosphate-buffered saline (PBS) and resuspended in PBS. Serum samples were obtained from the above-mentioned cattle at the same time and 2 weeks later.
In June 2013, 120 calves that had not experienced summer were selected from all over Kagoshima Prefecture for sentinel arbovirus surveillance. Heparinized blood and serum samplings were obtained from the calves in June, August, September and November. The processed plasmas and blood cells were kept at −80°C until the virus isolation and viral genome detection. The serum samples were preserved at −20°C until they were used for the virus neutralization test (VNT).
Virus isolation: Hamster lung (HmLu-1) and baby hamster kidney (BHK-21) cells were grown in Eagle’s minimum essential medium (MEM; Nissui, Tokyo, Japan) supplemented with 0.295% tryptose phosphate broth, 0.015% sodium bicarbonate and 5% bovine serum at 37°C. Virus isolation was conducted with the processed blood samples as described [16]. In brief, tube-cultured cells were washed three times with Earl’s solution, inoculated with the processed samples and incubated for 1 hr at 37°C. After the inocula were replaced with maintenance medium (MEM containing 0.295% tryptose phosphate broth and 0.015% sodium bicarbonate), the inoculated cultures were maintained rolling and observed for cytopathic effects (CPEs) over 7 days. If no CPEs were observed, two further passages were allowed for the supernatant of the primary inoculated culture.
Virus neutralization test: The serum samples were heat-inactivated at 56°C for 30 min. Fifty µl of serial two-fold dilutions of serum, in duplicate rows, were mixed with an equal volume of cultured medium containing 100 TCID50 of the virus in flat-bottomed 96-well plates. The mixtures were incubated at 37°C for 1 hr, and then, 100 µl of the suspension of HmLu-1 cells in serum-free medium (GIT; Wako Pure Chemical Industries, Osaka, Japan) was added to each well. After incubation at 37°C for 7 days under a 5% CO2 atmosphere in a humid chamber, the plates were microscopically observed for the presence of CPEs. The antibody titer is expressed as the reciprocal of the highest serum dilution at which CPEs were inhibited.
Viral RNA extraction and RT-PCR: Viruses were propagated in HmLu-1 and BHK-21 cells. Viral RNA was extracted from cultured fluid and blood cell suspensions with the High Pure Viral RNA Kit (Roche, Mannheim, Germany) per the manufacturer’s instructions. Reverse transcription-polymerase chain reaction (RT-PCR) was conducted with the Titan One tube RT-PCR Kit (Roche) using the following conditions. cDNA synthesis was carried out for 30 min at 50°C, followed by heat inactivation for 2 min at 94°C. The PCR conditions applied were 10 cycles of 30 sec at 94°C, 30 sec at 55°C and 45 sec at 68°C, followed by 25 cycles of 30 sec at 94°C, 30 sec at 55°C and 45 sec at 68°C, with the latter time increased by 5 sec per cycle. For RNA extracted from the blood samples, 10 additional cycles were used in the latter condition.
The partial sequence of segment 3 of EHDV was detected with a group-specific primer set, EP3U1416F and E31931R [21]. For the strain-specific detection directed to genome segment 2, primer sets EHDIBAL2F + EHDIBAL2R-2 and EHD97L2F + EHD97L2R were designed for IBAV and the IBAV 1997 variant, respectively (Table 1). A published primer set, EHDV-1/S2/103-124F and EHDV-1/S2/1021-1001R, was used for specific detection directed to segment 2 of EHDV serotype 1 [13]. IBAVL2-1-17F and EHDIBAL2R-2, and EHDIBAV2F and IBAL2R (Table 1) were applied for the amplification of the entire sequence of segment 2 of IBAV. The annealing temperature on the PCR was changed to 45°C for the former primer set. The RT-PCR products were electrophoresed in 1.5% agarose and TAE (Tris-acetate-EDTA) gel, and ethidium bromide-stained gels were visualized with ultraviolet light.
Table 1. Oligonucleotide sequences for RT-PCR assays and sequencing for segment 2 of EHDVs.
| Oligonucleotidea) | Sequence (5′–3′) | Position | Purpose | References |
|---|---|---|---|---|
| For IBAV | ||||
| IBAVL2-1-17F | GTTAAATTGTCCCCAGA | 1–17 | RT-PCR, sequencing | |
| IBAVL2-552-571F | CTATGCATCGGGTCAGGAAC | 552–571 | sequencing | |
| IBAVL2-1107-1126F | GCCATATGGGGAGATAATAG | 1107–1126 | sequencing | |
| EHDIBAL2F | TACGCAAGGTAAGAGACCAGA | 1667–1687 | RT-PCR, sequencing | |
| IBAVL2-2284-2305F | ATGAGGACCTATTACCAATGTA | 2284–2305 | sequencing | |
| IBAVL2-661-642R | GTTCTTCCTGAAACATCAAC | 661–642 | sequencing | |
| IBAVL2-1212-1193R | GCCACATCATATCTGTTCGC | 1212–1193 | sequencing | |
| IBAVL2-1785-1766R | CAGTCATACAGAACGCTCGG | 1785–1766 | sequencing | |
| EHDIBAL2R-2 | TCTTCTCCACCTCTTGCATT | 2283–2264 | RT-PCR, sequencing | |
| IBAL2R | GTAAGTTTGTTGTTCCCAGTAAACC | 3002–2978 | RT-PCR, sequencing | [22] |
| For EHDV serotype 1 | ||||
| EHDV-1/S2/103-124F | ATATCCTGGCGGAACCATATGG | 103–124 | RT-PCR, sequencing | [13] |
| EHDV-1/S2/462-481F | GCTCGCATACACCTATGAAT | 462–481 | sequencing | |
| EHDV-1/S2/609-590R | ACATCTGGTCGACTATGCCT | 609–590 | sequencing | |
| EHDV-1/S2/1021-1001R | ATCTGCCTGATGTGGTGTTTG | 1021–1001 | RT-PCR, sequencing | [13] |
| For IBAV 1997 variant | ||||
| EHD97L2F | GATGCGATCCTATACAACCATC | 499–520 | RT-PCR | |
| EHD97L2R | AATCGTCAGCACTCTGGTTATC | 868–847 | RT-PCR | |
Sequencing and phylogenetic analysis: The RT-PCR products were processed with the QIAquick PCR Purification Kit (QIAGEN, Hilden, Germany) and then sequenced directly using BigDye Terminator Cycle Chemistry on an ABI 3100-Avanti Genetic Analyzer (Life Technologies, Carlsbad, CA, U.S.A.). The oligonucleotide primers used for the cDNA synthesis and the sequencing for segment 2 of IBAV and EHDV serotype 1 are listed in Table 1. The determined nucleotide (nt) sequences were edited using GENETYX ver. 10.1.3 software (Genetyx, Tokyo, Japan) and compared with the deposited sequences in GenBank by using the GENETYX homology search tools. Neighbor-joining (NJ), maximum likelihood (ML) and maximum parsimony (MP) consensus trees were constructed using MEGA 6 from CLUSTAL sequence alignments of EHDV segment 3 nt sequences and MUSCLE sequence alignments of EHDV VP2 aa sequences (Table 2) with default parameters [25]. The reliability of the inferred consensus tree was tested by a boot-strap resampling of 1,000 pseudo-replicate data sets.
Table 2. List of EHDVs used for phylogenetic analysis in this study.
| Strain/isolate | Serotype | Location (year) | Isolation source | GenBank accession No. | ||
|---|---|---|---|---|---|---|
| Segment 2 | Segment 3 | |||||
| Ibaraki-2 | 2 | Japan (1959) | Affected cattle | AB078620 | AB078621 | |
| Y87061 | 2 | Japan (1987) | Cattle | AB078624 | AB078625 | |
| KSB-14/E/97 | Untyped | Japan (1997) | Cattle | AB078628 | AB078629 | |
| KS-7/E/13 | Untyped | Japan (2013) | Cattle | LC018714 | LC042608 | |
| Kawanabe | 1 | Japan (1984) | Cattle | — | LC042606 | |
| KS-1/E/13 | Untyped | Japan (2013) | Cattle | — | LC042607 | |
| New Jersey | 1 | U.S.A. (1955) | White-tailed deer | NC_013397 | AM744979 | |
| Ib Ar 22619 | 1 | Nigeria (1967) | Culicoides spp. | AM745008 | AM745009 | |
| Alberta | 2 | Canada (1962) | White-tailed deer | AM744998 | AM744999 | |
| CSIRO 439 | 2 | Australia (1979) | Cattle | AM744988 | AM744989 | |
| Ib Ar 33853 | 4 | Nigeria (1968) | Culicoides spp. | AM745018 | AM745019 | |
| CSIRO 157 | 5 | Australia (1977) | Cattle | AM745028 | AM745029 | |
| CSIRO 753 | 6 | Australia (1981) | Cattle | AM745038 | AM745039 | |
| 318 | 6 | Bahrain (1983) | Cattle | AM745068 | AM745069 | |
| CSIRO 775 | 7 | Australia (1981) | Cattle | AM745048 | AM745049 | |
| DPP59 | 8 | Australia (1982) | Cattle | AM745058 | AM745059 | |
RESULTS
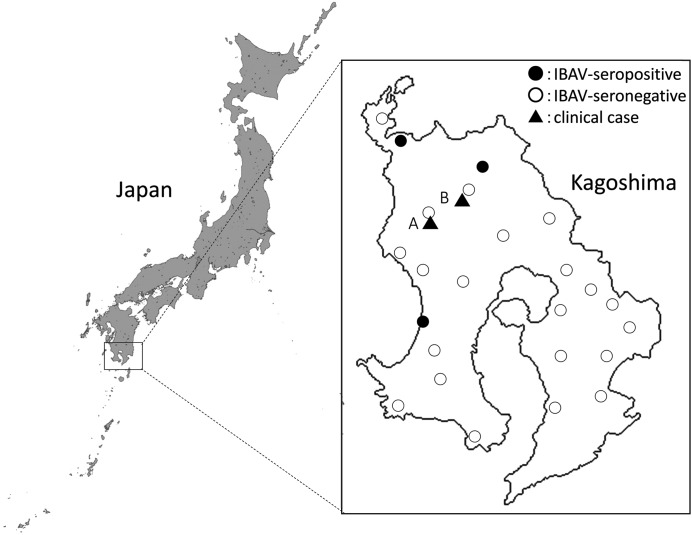
Clinical cases: In October 2013, suspected cases of Ibaraki disease were reported at two cattle breeding farms (farms A and B) located in the northwestern part of the mainland region of Kagoshima Prefecture (Fig. 1). Farms A and B were 10 km apart and maintained 450 and 15 Japanese black beef cattle, respectively. The affected cow at farm A developed a fever over 40°C, foamy salivation, dysphagia, exophthalmos and swelling of the tongue. A cow at farm B showed fever, anorexia, foamy salivation, dysphagia and abdominal distension during the same period. No similar symptoms were observed in any of the other cattle on either farm. The diseased cattle recovered after 22–24 days with supportive care and antibiotic treatment. Vaccination against Ibaraki disease had not been used on either farm recently.
Fig. 1.
Geographical distribution of clinical cases and IBAV seroprevalence in Kagoshima Prefecture, Japan.
RT-PCR for EHDV detection: We tested samples from the two diseased cattle (A-1 of farm A and B-1 of farm B) and 13 asymptomatic cattle (A-2 to -6 of farm A and B-2 to -9 of farm B) by RT-PCR using primer sets for the detection of EHDV segment 3 (group-specific) and IBAV segment 2 (strain-specific) (Table 3). The group-specific primer set successfully amplified cDNA of the expected size from both of the diseased cattle and seven of the asymptomatic cattle.
Table 3. RT-PCRs and serological tests to detect the prevalence of EHDV in cattle sampled in the farms where clinical cases were observed.
| Farm | Cattle | RT-PCR | Virus isolationb) | Titer of neutralizing antibody | |||||
|---|---|---|---|---|---|---|---|---|---|
| Ibaraki-2c) | KS-1/E/13 c) | ||||||||
| EHDV segment 3 | IBAV segment 2 | HmLu-1 | BHK-21 | pre | post | pre | post | ||
| A | A-1a) | + | + | – | NT | 1,024 | 1,024 | 2 | 2 |
| A-2 | + | – | – | NT | 2 | 8 | 2 | 256 | |
| A-3 | + | + | – | NT | 2 | 512 | <2 | <2 | |
| A-4 | – | – | – | NT | <2 | 16 | <2 | <2 | |
| A-5 | – | – | – | NT | <2 | <2 | <2 | <2 | |
| A-6 | – | – | – | NT | <2 | <2 | <2 | <2 | |
| B | B-1 a) | + | + | – | – | 128 | 1,024 | 4 | 2 |
| B-2 | – | – | – | – | <2 | 512 | <2 | 2 | |
| B-3 | + | – | – | – | 512 | 256 | 2 | 2 | |
| B-4 | – | – | – | – | <2 | 512 | <2 | 2 | |
| B-5 | + | – | – | – | 2 | 4 | 16 | 128 | |
| B-6 | + | + | – | – | 64 | 128 | <2 | 8 | |
| B-7 | + | + | – | + (KS-7/E/13) | 8 | 256 | <2 | <2 | |
| B-8 | – | – | – | – | <2 | 2 | <2 | <2 | |
| B-9 | + | – | – | + (KS-8/E/13) | <2 | 128 | <2 | 2 | |
a) Cattle showing swallowing difficulty, b) Parenthesis indicates virus isolate, c) EHDV isolates used for VNTs, NT: not tested.
Of the EHDV-positive cattle, the two diseased cattle and three of the asymptomatic cattle (A-1 and -3, and B-1, -6 and -7, respectively) tested positive by the IBAV-specific RT-PCR, but the other four asymptomatic cattle tested negative. Sequences of the cDNA by the group-specific RT-PCR were divided into two groups with 9% nt diversity. The sequence that was detected in the two diseased cattle and five asymptomatic cattle (A-1 and -3, and B-1, -3, -6, -7 and -9) showed high identity with segment 3 of previous IBAV isolates, Ibaraki-2 and Y87061 (97.2–97.8% nt identity). The other sequence amplified from two asymptomatic cattle (A-2 and B-5) had relatively low nt identities (≤ 92%) with the EHDV segment 3 sequence deposited in GenBank. The cDNA from segment 2 in the blood samples had 95.5–95.6% nt identity with that of the previous IBAV isolates.
Virus isolation: Two viruses were isolated in BHK-21 cells from blood cells obtained from two of the asymptomatic cattle (B-7 and B-9) on farm B (Table 3) and designated as KS-7/E/13 and KS-8/E/13, respectively. The group-specific RT-PCR successfully amplified cDNA from these viruses. Six additional viruses were isolated from sentinel cattle blood samples collected between September and November 2013. Of these virus isolates, three were identified as EHDV by the group-specific RT-PCR and designated as KS-1/E13, KS-2/E/13 and KS-5/P/13. The other three isolates were identified as D’Aguilar virus of the genus Orbivirus using a specific RT-PCR assay as described [10] (data not shown).
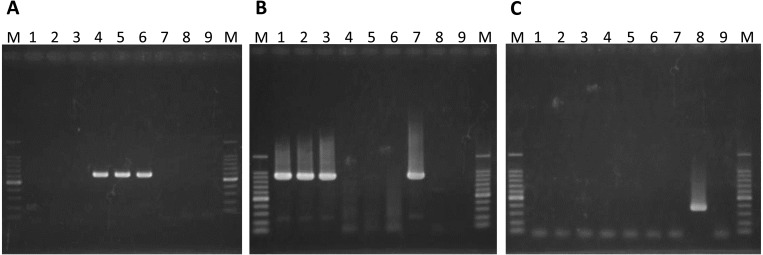
We performed a strain-specific RT-PCR directed to segment 2 of IBAV, the IBAV 1997 variant and EHDV serotype 1 using the EHDV isolates (Fig. 2). The RT-PCR product was amplified with the IBAV-specific primer set from KS-7/E/13 and KS-8/E/13. In contrast, KS-1/E/13, KS-2/E/13 and KS-5/P/13 tested positive by the EHDV serotype 1-specific RT-PCR. No amplification of the EHDV isolates was detected by the IBAV 1997 variant-specific RT-PCR.
Fig. 2.
Electrophoretic analysis of the RT-PCR product from segment 2 of EHDV isolates using IBAV-specific (A), EHDV serotype 1-specific (B) and IBAV 1997 variant-specific (C) primer pairs. Each primer set expectedly generates a specific product with the lengths 617, 919 and 370 bp, respectively. Lanes: 1, KS-1/E/13; 2, KS-2/E/13; 3, KS-5/P/13; 4, KS-7/E/13; 5, KS-8/E/13; 6, Ibaraki-2 (positive control for IBAV); 7, Kawanabe (positive control for EHDV serotype 1); 8, KSB-14/E/97 (positive control for IBAV 1997 variant); 9, negative water control; M, 100 bp DNA ladder.
Sequences of segments 2 and 3 of KS-7/E/13 and KS-8/E/13 were identical to those detected in the two diseased cattle and three of the asymptomatic cattle (A-1 and -3, and B-1, -6 and -7). The amplicons by the group-specific RT-PCR from the two asymptomatic cattle (B-3 and -9) that tested negative in the IBAV-specific RT-PCR were also identical to those of segment 3 of KS-7/E/13 and KS-8/E/13. The RT-PCR product from segment 3 of KS-1/E/13, KS-2/E/13 and KS-5/P/13 matched the sequence from the two asymptomatic cattle (A-2 and B-5) from which the IBAV-related sequences were negative. The RT-PCR product from segment 2 of the isolates shared 97.3% and 96.8% nt identity with the corresponding region of Japanese (Kawanabe) and Australian (DPP2209) strains of EHDV serotype 1, respectively. In our comparative analysis of the aa sequence of VP2, the isolates shared 99.3% and 98.3% identity with the Japanese and Australian strains, respectively.
VNTs for bovine sera: In a VNT using IBAV, high neutralization antibody (NA) titers ranging from 1:128 to 1:1,024 were observed in post-sera of the two diseased cattle and the seven asymptomatic cattle on farms A and B (Table 3). In contrast, the NA titers 1:128 and 1:256 were detected against KS-1/E/13 in post-sera of two of the asymptomatic cattle (A-2 and B-5). A significant rise in the NA titers against both IBAV and KS-1/E/13 was observed between pre- and post-serum collections from the studied farms, indicating that the viruses spread simultaneously in those days. The cross-reactions between the two viruses in the VNT were not significant (≤ 1:8), and none of the serum samples contained high NA titers against both of the viruses at once. The seroconversion of sentinel cattle to IBAV was found in October and November 2013 at three other farms located in the northwestern part of Kagoshima Prefecture (Fig. 1).
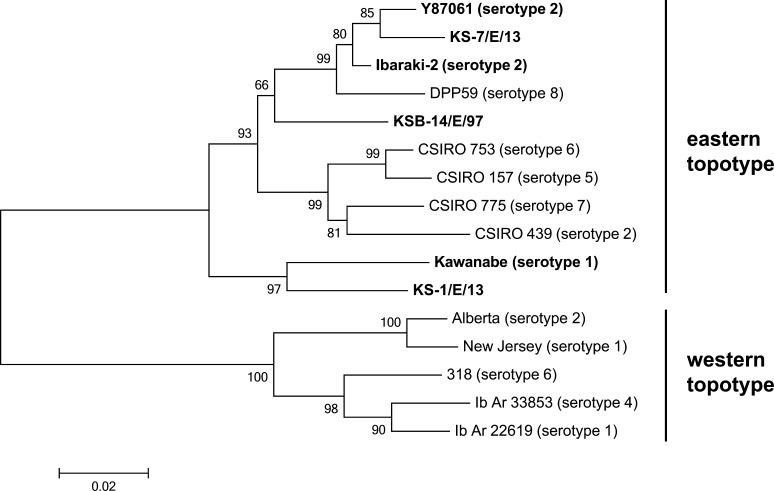
Phylogenetic analysis of EHDV: The phylogenetic tree based on the partial sequence of segment 3 shows that all of the Japanese and Australian isolates/strains composed a single cluster (Fig. 3). However, Kawanabe and KS-1/E/13 formed a single clade and were relatively less related to other members in the cluster. We identified 2,960 nt of segment 2 of KS-7/E/13, and they were shown to code for a deduced 982-aa length of VP2 protein, similar to the previously reported IBAV isolates. The VP2 sequence from KS-7/E/13 shared 95.6% nt and 97.5% aa identities with Ibaraki-2, and 96.1% nt and 98.2% aa identities with Y87061.
Fig. 3.
Phylogenetic comparison of the partial nucleotide sequence of segment 3 of Japanese field isolates and reference strains of EHDV. Detailed information of the viruses used is given in Table 2. All Japanese isolates are highlighted in bold face. Bootstrap values (%) are indicated at the appropriate nodes. The scale presents the number of substitutions per site.
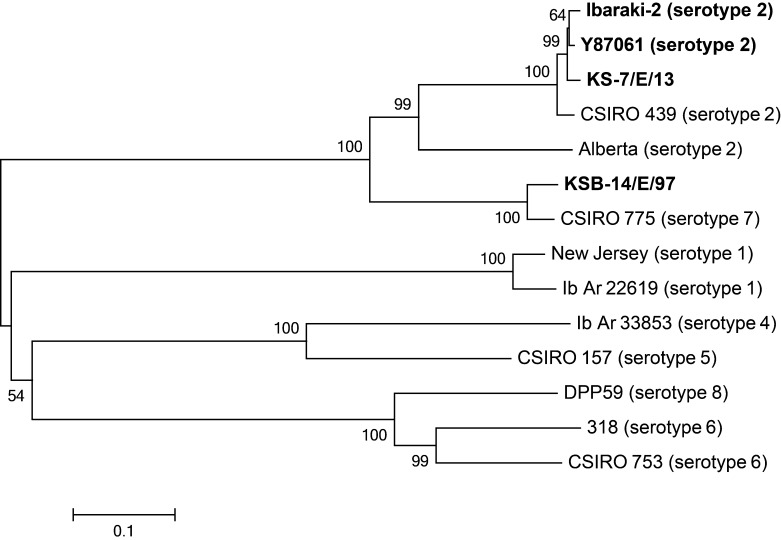
The sequence identity was also high between KS-7/E/13 and an Australian strain (CSIRO 439) of EHDV serotype 2 (93.0% nt and 96.0% aa identities). Relatively low identities were observed with a North American strain (Alberta) of EHDV serotype 2 (71.3% nt and 73.6% aa identities). In contrast, KS-7/E/13 was highly diverged from the IBAV 1997 variant (KSB-14/E/97) (69.7% nt and 67.5% aa identities). The NJ consensus tree based on the aa sequences of EHDV VP2 demonstrated that KS-7/E/13 clustered with previous IBAV isolates (Fig. 4). KSB-14/E/97 was less related to EHDV serotype 2 isolates, but it formed a distinct clade with EHDV serotype 7 (CSIRO 775). The trees constructed by ML and MP supported the NJ consensus tree (data not shown).
Fig. 4.
Phylogenetic tree of full-length amino acid sequence of VP2 between reference strains and Japanese field isolates of EHDV listed in Table 2. All Japanese isolates are highlighted in bold face. Bootstrap values (%) are indicated at the appropriate nodes. Scale: the number of substitutions per site.
DISCUSSION
Our observation of typical Ibaraki disease manifestations, such as fever and dysphagia, and the detection of IBAV-related sequences and antibodies in the diseased cattle clearly demonstrated the occurrence of Ibaraki disease in the northwestern part of Kagoshima Prefecture in 2013. The EHDV isolates from one of the studied farms were genetically close to previously reported IBAV isolates and caused the typical clinical signs in the infected cattle. The virus could thus be regarded as IBAV. In contrast, the present phylogenetic comparison showed that KSB-14/E/97 is less related to EHDV serotype 2 (including IBAVs) and should be classified as EHDV serotype 7. A low level of cross-reaction in the VNT between IBAV and EHDV serotype 7 might have led to the misidentification at the time, when the VP2 sequence from all serotypes was not available [3, 20, 22]. Because the 1997–98 epidemic was caused by a different EHDV serotype, it should be distinguished from the other outbreaks of Ibaraki disease.
Of interest is that EHDV serotype 1 also spread in the same area (even on the same farms) during the instances of Ibaraki disease in 2013. EHDV serotype 1 caused hemorrhagic disease of wild deer in North America and may have been involved in the febrile disease of cattle on Reunion Island [6, 19], but no association of ruminant morbidity was reported in other regions [24]. It is likely that EHDV serotype 1 was not involved in the cattle morbidity in 2013, as shown in its previous spread in an overlapping region [17]. The phylogenetic tree based on segment 3 showed that EHDV serotype 1 isolates from Japan were primarily sorted into the eastern topotype, but diverged from other Japanese and Australian isolates. This finding suggested that these isolates had been geographically separated from the other isolates in their evolution for a certain period of time.
Like other segmented viruses, reassortants between heterogeneous serotypes of EHDV were sometimes recovered in the field [1, 2]. Co-circulation of the IBAV and EHDV serotype 1 suggested the potential reassortment between them. A full genome analysis of EHDV isolates in 2013 is needed to determine whether reassortment has occurred. Surprisingly, the co-infection was not found in the EHDV-positive cattle by the genetic and serological tests, although the viruses spread in the studied farms during the same period. Interference between two viruses might restrict the replication of either virus, as shown by an experimental co-infection of calves with bluetongue virus serotypes 1 and 8 [7].
Although seroconversion in cattle to IBAV has been detected sporadically [18], no occurrence of Ibaraki disease has been seen in Japan for 26 years, until the present study. Reports of clinical cases and the sentinel seroconversion to IBAV in the northwestern part of Kagoshima Prefecture are limited. No occurrence of Ibaraki disease was reported in the other 46 prefectures of Japan in 2013 [14], suggesting the local spread of IBAV. The present sentinel surveillance suggested that the incursion of IBAV happened in October 2013. The flight performance of Culicoides vector species is significantly decreased below 20°C [27]. The mean of the average temperatures at four observation points close to the studied and seroconverted farms generally reached 20°C or above during the beginning and middle of October, but rapidly decreased in November (Japan Meteorological Agency). The inadequate temperatures for Culicoides flight behavior might have prevented the transmission cycle and thus restricted the viral spread to within the small area. In the 1959 epizootic outbreak, Ibaraki disease was first recognized in southern Japan in August and swept over the southern half of the nation in the following four months [23]. The history of outbreaks implied that the earlier incursion of the virus may have caused the larger outbreak of the disease in Japan.
The current live attenuated and inactivated vaccines against Ibaraki disease were developed based on Ibaraki-2. The small aa sequence diversity of VP2 between Ibaraki-2 and KS-7/E/13 suggested that the current commercial vaccines are still effective for the prevention of Ibaraki disease. The reemergence of Ibaraki disease reminds us of the importance of continuous vaccination in cattle populations to minimize economic losses. In the meantime, diagnostic and preventive measures should be prepared for other EHDV serotypes that are associated with cattle morbidity in Japan and other countries [22, 26, 31]. Sentinel surveillance will be useful to identify the incursion and circulation of EHDVs in southern Japan with a high risk of arbovirus infections.
Acknowledgments
We thank all of the staff of the livestock hygiene centers of Kagoshima Prefecture for the collection and preparation of the bovine blood samples. We are grateful to Ms. Miyuki Kamimura, Ms. Mai Fujioka and Mr. Zenjiro Sakaguchi for their valuable comments during the preparation of this manuscript. Finally, we would like to offer our heartfelt thanks to Dr. William C. Wilson for his careful and critical reading of this paper.
REFERENCES
- 1.Allison A. B., Holmes E. C., Potgieter A. C., Wright I. M., Sailleau C., Breard E., Ruder M. G., Stallknecht D. E.2012. Segmental configuration and putative origin of the reassortant orbivirus, epizootic hemorrhagic disease virus serotype 6, strain Indiana. Virology 424: 67–75. doi: 10.1016/j.virol.2011.12.004 [DOI] [PubMed] [Google Scholar]
- 2.Anbalagan S., Cooper E., Klumper P., Simonson R. R., Hause B. M.2014. Whole genome analysis of epizootic hemorrhagic disease virus identified limited genome constellations and preferential reassortment. J. Gen. Virol. 95: 434–441. doi: 10.1099/vir.0.059659-0 [DOI] [PubMed] [Google Scholar]
- 3.Anthony S. J., Maan S., Maan N., Kgosana L., Bachanek-Bankowska K., Batten C., Darpel K. E., Sutton G., Attoui H., Mertens P. P.2009. Genetic and phylogenetic analysis of the outer-coat proteins VP2 and VP5 of epizootic haemorrhagic disease virus (EHDV): comparison of genetic and serological data to characterise the EHDV serogroup. Virus Res. 145: 200–210. [DOI] [PubMed] [Google Scholar]
- 4.Anthony S. J., Maan N., Maan S., Sutton G., Attoui H., Mertens P. P.2009. Genetic and phylogenetic analysis of the core proteins VP1, VP3, VP4, VP6 and VP7 of epizootic haemorrhagic disease virus (EHDV). Virus Res. 145: 187–199. doi: 10.1016/j.virusres.2009.07.011 [DOI] [PubMed] [Google Scholar]
- 5.Campbell C. H., Breese S. S., Jr, McKercher P. D.1975. Antigenic and morphologic comparisons of Ibaraki and bluetongue viruses. Can. J. Microbiol. 21: 2098–2102. doi: 10.1139/m75-300 [DOI] [PubMed] [Google Scholar]
- 6.Cêtre-Sossah C., Roger M., Sailleau C., Rieau L., Zientara S., Bréard E., Viarouge C., Beral M., Esnault O., Cardinale E.2014. Epizootic haemorrhagic disease virus in Reunion Island: evidence for the circulation of a new serotype and associated risk factors. Vet. Microbiol. 170: 383–390. doi: 10.1016/j.vetmic.2014.02.007 [DOI] [PubMed] [Google Scholar]
- 7.Dal Pozzo F., Martinelle L., Thys C., Sarradin P., De Leeuw I., Van Campe W., De Clercq K., Thiry E., Saegerman C.2013. Experimental co-infections of calves with bluetongue virus serotypes 1 and 8. Vet. Microbiol. 165: 167–172. doi: 10.1016/j.vetmic.2013.01.016 [DOI] [PubMed] [Google Scholar]
- 8.Huismans H., Bremer C. W., Barber T. L.1979. The nucleic acid and proteins of epizootic haemorrhagic disease virus. Onderstepoort J. Vet. Res. 46: 95–104. [PubMed] [Google Scholar]
- 9.Iwasaki S., Goto Y., Miura Y., Nagatomo M., Kono Y.1990. An outbreak of Ibaraki disease in Miyazaki Prefecture. J. Jpn. Vet. Med. Assoc. 43: 244–248(in Japanese with English summary). [Google Scholar]
- 10.Kato T.Shirafuji1, H., Tanaka, S., Sato, M., Yamakawa, M., Tsuda, T. and Yanase, T. 2015. Bovine arboviruses in Culicoides biting midges and sentinel cattle in southern Japan from 2003 to 2013. Transbound. Emerg. Dis. (in press). [DOI] [PubMed] [Google Scholar]
- 11.Kitano Y.2004. Ibaraki disease in cattle. pp. 1221–1226. In: Infectious Diseases of Livestock, 2nd ed. (Coetzer, J. A. W. and Tustin, R. C. eds.), Oxford University Press, Cape Town. [Google Scholar]
- 12.Kitano Y., Yamashita S., Fukuyama T.1988. Pathological observations on cattle that died of Ibaraki disease. J. Jpn. Vet. Med. Assoc. 41: 884–888(in Japanese with English summary). doi: 10.12935/jvma1951.41.884 [DOI] [Google Scholar]
- 13.Maan N. S., Maan S., Nomikou K., Johnson D. J., El Harrak M., Madani H., Yadin H., Incoglu S., Yesilbag K., Allison A. B., Stallknecht D. E., Batten C., Anthony S. J., Mertens P. P.2010. RT-PCR assays for seven serotypes of epizootic haemorrhagic disease virus & their use to type strains from the Mediterranean region and North America. PLoS ONE 5: e12782. doi: 10.1371/journal.pone.0012782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.MAFF2014. Animal Hygiene Weekly. Food Safety and Consumer Bureau, Ministry of Agriculture, Forestry and Fisheries, Tokyo, Japan 3298: 115.(in Japanese). [Google Scholar]
- 15.Mecham J. O., Dean V. C.1988. Protein coding assignment for the genome of epizootic haemorrhagic disease virus. J. Gen. Virol. 69: 1255–1262. doi: 10.1099/0022-1317-69-6-1255 [DOI] [PubMed] [Google Scholar]
- 16.Miura Y., Goto Y., Kubo M., Kono Y.1988. Isolation of Chuzan virus, a new member of the Palyam subgroup of the genus Orbivirus, from cattle and Culicoides oxystoma in Japan. Am. J. Vet. Res. 49: 2022–2025. [PubMed] [Google Scholar]
- 17.Miura Y., Miyazato S., Kubo M., Goto Y., Kono Y.1988. Kawanabe virus, an isolate from a calf in Japan: a new virus belonging to the New Jersey serotype of the epizootic hemorrhagic disease serogroup of genus Orbivirus. Jpn. J. Vet. Sci. 50: 942–945. doi: 10.1292/jvms1939.50.942 [DOI] [PubMed] [Google Scholar]
- 18.Motokawa M., Seimiya Y., Yaegashi E., Tsunemitsu E.2002. First detection of the Ibaraki virus antibody in cattle in the Tohoku district Japan. J. Jpn. Vet. Med. Assoc. 55: 658–660(in Japanese with English summary). doi: 10.12935/jvma1951.55.658 [DOI] [Google Scholar]
- 19.Murphy M. D., Hanson B. A., Howerth E. W., Stallknecht D. E.2006. Molecular characterization of epizootic hemorrhagic disease virus serotype 1 associated with a 1999 epizootic in white-tailed deer in the eastern United States. J. Wildl. Dis. 42: 616–624. doi: 10.7589/0090-3558-42.3.616 [DOI] [PubMed] [Google Scholar]
- 20.Ohashi S., Yoshida K., Watanabe Y., Tsuda T.1999. Identification and PCR-restriction fragment length polymorphism analysis of a variant of the Ibaraki virus from naturally infected cattle and aborted fetuses in Japan. J. Clin. Microbiol. 37: 3800–3803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ohashi S., Yoshida K., Yanase T., Kato T., Tsuda T.2004. Simultaneous detection of bovine arboviruses using single-tube multiplex reverse transcription-polymerase chain reaction. J. Virol. Methods 120: 79–85. doi: 10.1016/j.jviromet.2004.04.006 [DOI] [PubMed] [Google Scholar]
- 22.Ohashi S., Yoshida K., Yanase T., Tsuda T.2002. Analysis of intratypic variation evident in an Ibaraki virus strain and its epizootic hemorrhagic disease virus serogroup. J. Clin. Microbiol. 40: 3684–3688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Omori T., Inaba Y., Morimoto T., Tanaka Y., Ishitani R.1969. Ibaraki virus, an agent of epizootic disease of cattle resembling bluetongue. I. Epidemiologic, clinical and pathologic observations and experimental transmission to calves. Jpn. J. Microbiol. 13: 139–157. doi: 10.1111/j.1348-0421.1969.tb00447.x [DOI] [PubMed] [Google Scholar]
- 24.Savini G., Afonso A., Mellor P., Aradaib I., Yadin H., Sanaa M., Wilson W., Monaco F., Domingo M.2011. Epizootic heamorragic disease. Res. Vet. Sci. 91: 1–17. doi: 10.1016/j.rvsc.2011.05.004 [DOI] [PubMed] [Google Scholar]
- 25.Tamura K., Stecher G., Peterson D., Filipski A., Kumar S.2013. MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Mol. Biol. Evol. 30: 2725–2729. doi: 10.1093/molbev/mst197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Temizel E. M., Yesilbag K., Batten C., Senturk S., Maan N. S., Clement-Mertens P. P., Batmaz H.2009. Epizootic hemorrhagic disease in cattle, Western Turkey. Emerg. Infect. Dis. 15: 317–319. doi: 10.3201/eid1502.080572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tsutsui T., Hayama Y., Yamakawa M., Shirafuji H., Yanase T.2011. Flight behavior of adult Culicoides oxystoma and Culicoides maculatus under different temperatures in the laboratory. Parasitol. Res. 108: 1575–1578. [DOI] [PubMed] [Google Scholar]
- 28.Uchikoshi N., Enaga N., Shimohira H., Aoki Y., Minamikawa F., Tokunaga M., Imamura S., Hara T., Miyachi M., Kajiwara T.1983. An outbreak of Ibaraki disease in Kyushu, Japan in 1982. J. Jpn. Vet. Med. Assoc. 36: 648–652(in Japanese with English summary). doi: 10.12935/jvma1951.36.648 [DOI] [Google Scholar]
- 29.Uchinuno Y., Ishibashi K., Yokoyama A., Kawanabe M., Takagi E.2000. Occurrence of Ibaraki disease in two successive years: possibly caused by virus overwintering. J. Jpn. Vet. Med. Assoc. 53: 372–376(in Japanese with English summary). [Google Scholar]
- 30.Watanabe Y., Makiuchi H., Imafuji T., Yamasaki K., Onitsuka T., Ohashi S.2000. An outbreak of Ibaraki disease in Kagoshima prefecture in 1997 and properties of the virus isolated from bovine aborted and still birth fetus. J. Jpn. Vet. Med. Assoc. 53: 302–306(in Japanese with English summary). [Google Scholar]
- 31.Yadin H., Brenner J., Bumbrov V., Oved Z., Stram Y., Klement E., Perl S., Anthony S., Maan S., Batten C., Mertens P. P.2008. Epizootic haemorrhagic disease virus type 7 infection in cattle in Israel. Vet. Rec. 162: 53–56. doi: 10.1136/vr.162.2.53 [DOI] [PubMed] [Google Scholar]
- 32.Yanase T., Kato T., Kubo T., Yoshida K., Ohashi S., Yamakawa M., Miura Y., Tsuda T.2005. Isolation of bovine arboviruses from Culicoides biting midges (Diptera: Ceratopogonidae) in southern Japan: 1985–2002. J. Med. Entomol. 42: 63–67. [DOI] [PubMed] [Google Scholar]