Abstract
Tick-borne diseases are often encountered in canine clinical practice. In the present study, a molecular epidemiological survey of dogs in Japan was conducted to understand the prevalence and geographical distribution of Babesia spp., Hepatozoon spp., Ehrlichia spp. and Anaplasma spp. Pathogen-derived DNA in blood samples obtained from 722 dogs with a history of exposure to ticks and/or fleas was examined by PCR. The prevalence of Babesia gibsoni, Babesia odocoilei-like species, Hepatozoon canis and Ehrlichia spp./Anaplasma spp. was 2.4% (16/722), 0.1% (1/722), 2.5% (18/722) and 1.5% (11/722), respectively. While B. gibsoni and Ehrlichia spp./Anaplasma spp. were detected in the western part of Japan, H. canis was detected in Tohoku area in addition to western and central parts of Japan.
Keywords: canine, epidemiological survey, Japan, tick-borne disease
Tick-borne diseases including babesiosis, hepatozoonosis, ehrlichiosis and anaplasmosis are important infectious diseases in canine practice. These pathogens infect blood cells and cause systemic clinical symptoms and severe, sometimes life-threatening, hematopoietic diseases like anemia and thrombocytopenia [6, 13]. In Japan, various tick-borne pathogens, such as Babesia gibsoni, Hepatozoon canis and Anaplasma bovis, were detected in dogs, and previous studies showed the prevalence of tick-borne pathogens and their geographical distribution [4, 12, 14, 16]. However, there are limited data regarding the prevalence of tick-borne pathogens and their geographical distribution in Japan [4, 14]. In addition, the geographical distributions of vector-borne pathogens are likely to change with progressive urban development and human activities. In the present study, a molecular survey of Babesia spp., Hepatozoon spp., Ehrlichia spp. and Anaplasma spp. in dogs presenting to animal hospitals throughout almost entire Japan was conducted.
EDTA-anticoagulated blood samples were collected from dogs that had a history of and/or current infestation with ticks and/or fleas. These dogs presented to animal hospitals located in 42 prefectures in Japan between March 2012 and October 2013. Fighting dogs were excluded from the study population. The collected blood samples were preserved at −80°C until further analysis.
DNA was extracted from 200 µl of each blood sample using QIAamp DNA Blood Mini Kit (QIAGEN, Hilden, Germany) with a final elution volume of 200 µl. Nested PCR was performed to amplify the 18S rRNA gene derived from Babesia spp. and/or Hepatozoon spp. as an initial screening [2, 3, 20]. For the first round PCR, two oligonucleotides, PIRO-F (5′-AGT CAT ATG CTT GTC TTA-3′) and PIRO-R (5′-CCA TCA TTC CAA TTA CAA-3′) were constructed. For the second PCR, BabgenF (5′-GAA ACT GCG AAT GGC TCA TTA-3′) and BabgenR (5′-CGG TAG GCC AAT ACC CTA CCG TC-3′) were used. Babesia spp. and Hepatozoon spp. could be differentiated based on the size of amplified DNA fragments (Babesia spp.: 230 base pairs [bp]; Hepatozoon spp.: 267 bp). If the samples were positive for Babesia in the screening PCR, they were then subjected to semi-nested PCR to amplify a longer region of the 18S rRNA gene [20]. Product length of the semi-nested PCR was approximately 900 bp. Primers Bab F3 (5′-ATG TCT AAG TAC AAG CTT TTT ACG GT-3′) and HEP2P (5′-GAC TTC TCC TTC TTT AAG TGA TAA G-3′) were used for the first round PCR. Bab F3 and BabR3 (5′-AAA GGC GAC GAC CTC CAA TCC CTA GT-3′) were used for the second round PCRs, respectively. An initial screening PCR for Ehrlichia spp. and Anaplasma spp. was conducted based on the nested PCR strategy reported by Sakamoto et al. [16]. In the first round PCR, the primers fD1 (5′- AGA GTT TGA TCC TGG CTC AG-3′) and Rp2 (5′-ACG GCT ACC TTG TTA CGA CTT-3′) were used. In the second round PCR, primers EHR16SD (5′-GGT ACC TAC AGA AGA AGT CC-3′) and EHR16SR (5′-TAG CAC TCA TCG TTT ACA GC-3′) were used. The length of the nested PCR was approximately 340 bp. To determine the nearly complete sequences of the 16S rRNA genes, the 5′ and 3′ regions were separately amplified as described by Inokuma et al. [10]. Primers fD1 and EHR16SR were used to amplify the 5′ region, and primers EHR16SD and Rp2 were used to amplify the 3′ region.
The nucleotide sequences of amplified DNA fragments were inserted into a pCR2.1 plasmid vector (Invitrogen, Carlsbad, CA, U.S.A.), and the nucleotide sequence of the inserted DNA fragments was determined by the dideoxy chain termination method (ABI Prism BigDye Primer Cycle Sequencing Ready Reaction Kit, Applied Biosystems, Foster City, CA, U.S.A.). Nucleotide sequence data from each sample were subjected to Basic Local Alignment Search Tool (BLAST) analysis in the DNA data bank of Japan [1] (DDBJ, http://www.ddbj.nig.ac.jp/Welcome-j.html) to find closely-related species of pathogens. The similarities were also calculated by GENETYX Ver. 10.0.3 software (GENETYX Corp., Tokyo, Japan). Phylogenetic analyses were performed for Babesia spp. to determine the genetic relationship among those pathogens by a neighbor-joining method in the DNADIST program from the PHYLIP software package (http://evolution.genetics.washington.edu/phylip.html), as previously described [5].
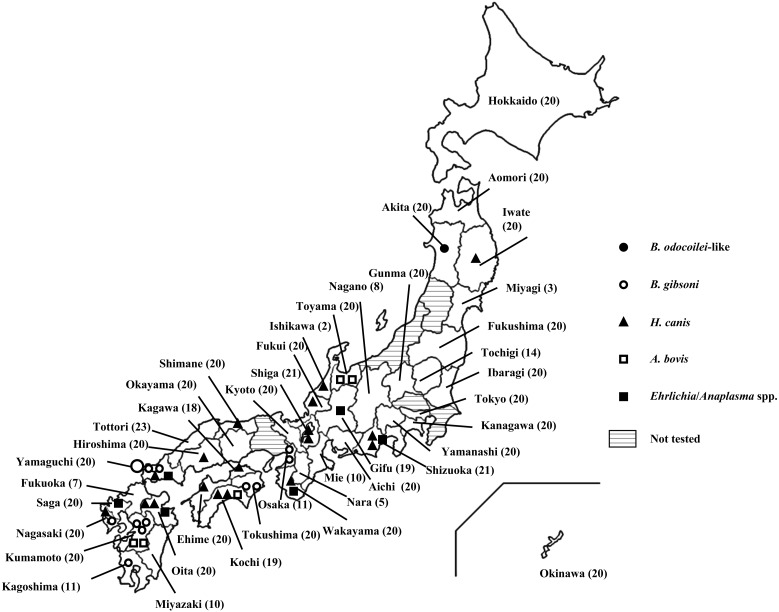
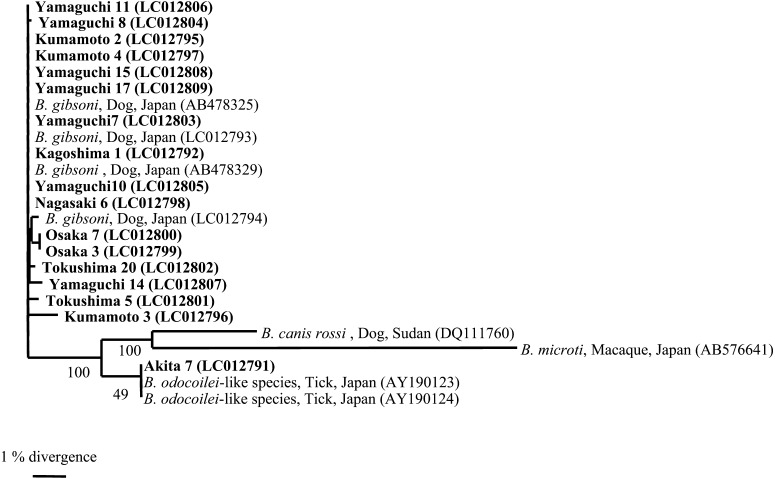
A total of 722 dogs from different prefectures as indicated in Fig. 1 fulfilled the inclusion criteria and were included in this study. Babesia-derived DNA was detected in 17/722 samples in the screening PCR. These blood samples were then analyzed by additional PCR to obtain the longer part of nucleotide sequence, and successful amplifications were obtained from all of 17 samples. A representative clone was obtained from each case and then subjected to phylogenetic analysis. The phylogenetic analysis revealed that 16 samples (LC012792 and LC012795-LC012809) formed a cluster with B. gibsoni, and the remaining sample (LC012791) was grouped with B. odocoilei-like species detected in ticks in Japan (Fig. 2) [11]. The nucleotide sequence of B. odocoilei-like species isolated in this study (LC012791) showed 99.5% identity to those previously isolated from ticks in Akita and Fukui Prefectures (AY190123 and AY190124) [11]. The prevalence of B. gibsoni and B. odocoilei-like species was 2.3% (16/722 dogs) and 0.1% (1/722 dogs), respectively. The geographical distribution of B. gibsoni was limited to the western part of Japan (Fig. 1).
Fig. 1.
Geographical distributions of detected pathogens. Large open circle=five B. gibsoni-infected dogs; small open circle=one B. gibsoni-infected dog; filled circle=one B. odocoilei-infected dog; filled triangle=one H. canis-infected dog; square=one A. bovis-infected dog; filled square=one Ehrlichia/Anaplasma-infected dog. Number of tested samples was indicated in parenthesis after each prefecture name.
Fig. 2.
Phylogenetic relationship of the partial 18S rRNA gene sequences of Babesia spp. isolated from dogs, ticks and other animals. Sixteen clones of B. gibsoni 18S rRNA gene (LC012792 and LC012795-LC012809) and a clone of B. odocoilei-like species (LC012791) obtained in the present study were analyzed. Pathogen names, host species, country of isolation and the GenBank accession numbers (in parentheses) of compared sequences are shown in the phylogenetic tree: B. gibsoni from four dogs in Japan (AB478325, AB478329, LC012793 and LC012794); B. canis rossi from a dog in Sudan (DQ111760); B. microti from a macaque in Japan (AB576641) and B. odocoilei-like species from two ticks in Japan (AY190123 and AY190124). Numbers under internal nodes indicate the percentages of 1,000 bootstrap replicates that supported the branch. Three-letter codes and numbers represent sample IDs of examined dogs in this study.
Hepatozoon-derived DNA was detected in 18/722 samples (LC012821-LC012824 and LC012826-LC012839). They showed high similarity with H. canis (93–99% homology with EF622096) by sequencing analysis. The prevalence of H. canis was 2.5% (18/722 dogs). H. canis was mainly detected in the central and western parts of Japan, but one sample was obtained from Iwate Prefecture, which is located in the eastern Tohoku area (Fig. 1).
On the screening PCR for Ehrlichia spp. and Anaplasma spp., 11 samples showed high similarity to Ehrlichia spp. and Anaplasma spp. by sequencing analyses of the partial 16S rRNA gene. Nearly complete 16S rRNA genes were successfully amplified in 5 of 11 positive cases, but failed to be amplified in the remaining six cases. DNA sequencing and BLAST analysis of five cases showed that all (LC012811-LC012813, LC012817 and LC012818) were derived from A. bovis (99–100% homology with HM131218). The prevalence of Ehrlichia/Anaplasma and A. bovis was 1.5% (11/722 dogs) and 0.7% (5/722 dogs), respectively. Ehrlichia or Anaplasma infected dogs were also mainly observed in the central and western parts of Japan (Fig. 1). Simultaneous infection with H. canis and A. bovis was observed in a dog, but this dog had no clinical signs associated with hepatozoonosis or anaplasmosis.
In a previous study, an epidemiological survey in dogs of Japan was performed using ELISA to detect antibody against B. gibsoni, and the prevalence was reported to be 10.6% [12]. Another survey using ELISA and PCR by Miyama et al. showed that the prevalence of B. gibsoni was 30.4%, but most of the positive dogs were fighting dogs [14]. In the present study, the prevalence of B. gibsoni was 2.4%. This rate was lower than those in the two previous studies [12, 14]. These differences might be owing to differing inclusion criteria and detection methods. Previous studies included only outdoor dogs or dogs with clinical signs suggesting Babesia infection [12, 14]. In addition, fighting dogs were not included in the present study. Although B. gibsoni is generally transmitted by ticks, direct blood contamination during fighting can also be an important transmission route among fighting dogs [14]. Bite wound by other dogs is uncommon event in domestic companion dogs. In addition, serological examination was conducted in the previous studies, but not in this study [12, 14]. Our PCR-based strategy could only detect the circulating pathogen derived DNAs, whereas a serological survey is able to detect the previous infection by finding antibody responses. This would be another reason for the lower B. gibsoni infection rate in this study.
In the present study, the geographical distribution of B. gibsoni was limited to the western part of Japan. A similar trend was observed in a previous study [12]. It is plausible that no remarkable change in geographical distribution of B. gibsoni in domestic dogs has occurred over the last decade in Japan, but it is still critical to carefully consider its possible expansion.
It was noteworthy that B. odocoilei-like species was detected from a dog. The only known hosts of B. odocoilei are deer and musk [18]. This Babesia species causes the disease in immune-compromised animals [7, 15]. In Japan, B. odocoilei-like species have been isolated from ticks collected from dogs in Akita and Fukui Prefectures [11]. The B. odocoilei-like species detected in this study was closely related to the tick-derived isolate. This finding may suggest that B. odocoilei-like species has the potential to infect dogs and can be transmitted by ticks like other Babesia species. The pathogenicity of B. odocoilei–like species in dogs remains unclear, because the dog with B. odocoilei-like species in this study did not exhibit any clinical signs. Further investigation might be necessary to clarify the pathogenicity of B. odocoilei-like species in dogs.
To our knowledge, the present study is the first epidemiological survey of Hepatozoon spp. in dogs throughout Japan. A previous study reported the prevalence of Hepatozoon spp. in hunting dogs, but was conducted in limited areas, such as the islands and peninsulas of Japan [4]. Other information about canine hepatozoonosis in Japan is limited to case reports of dogs with acute onset of hepatozoonosis in Kagoshima and Yamaguchi Prefectures [9, 17, 19]. Our study indicated that H. canis was distributed in Tohoku area in addition to western and central parts of Japan. Our results may serve as a baseline for the prevalence and geographical distribution of H. canis in domestic dogs in Japan.
In the present study, Ehrlichia spp. or Anaplasma spp. was found in 1.5% of examined dogs. A previous epidemiological survey showed that 1.1% of dogs were infected with A. bovis [16]. Another study found Anaplasma platys in 7.5% of dogs in Okinawa Prefecture [8]. In this study, the species of Ehrlichia spp. or Anaplasma could be determined in only 5 out of 11 PCR positive cases, and all of them were A. bovis. No A. platys infected case was found. Although the prevalence of A. bovis in this study was comparable to the previous study at least, its geographical distribution was different [16]. While A. bovis was mainly detected in western part of Japan in the present study, a previous study found it in other areas of Japan as well although more cases were detected in western Japan [16]. This discrepancy might be due to a sample size. While we collected 722 samples from 42 prefectures, a previous study analyzed 1,427 samples from 32 prefectures [16]. Samples used in this study might have been collected from geographically wider regions, however, we should have collected more samples in each prefecture. Furthermore, we have to analyze a possible prevalence of Ehrlichia spp. or other Anaplasma spp. in the future, because we do not know the specific species in remaining 6 PCR positive cases.
In this study, a molecular epidemiological survey of tick-borne pathogens in dogs throughout Japan was conducted. This is the first report of infection of B. odocoilei-like species in a dog and the first report on geographical distribution of H. canis covering all of Japan. Our findings may serve as a baseline for the prevalence and geographical distribution of tick-borne pathogens in molecular based survey for domestic dogs in Japan.
Acknowledgments
Kubo and Tateno equally contributed to this paper. This work was supported in part by grants from the Ministry of Education, Culture, Sports, Science and Technology of Japan (Grant number 23580442).
REFERENCES
- 1.Altschul S. F., Gish W., Miller W., Myers E. W., Lipman D. J.1990. Basic local alignment search tool. J. Mol. Biol. 215: 403–410. doi: 10.1016/S0022-2836(05)80360-2 [DOI] [PubMed] [Google Scholar]
- 2.Criado-Fornelio A., Rey-Valeiron C., Buling A., Barba-Carretero J. C., Jefferies R., Irwin P.2007. New advances in molecular epizootiology of canine hematic protozoa from Venezuela, Thailand and Spain. Vet. Parasitol. 144: 261–269. doi: 10.1016/j.vetpar.2006.09.042 [DOI] [PubMed] [Google Scholar]
- 3.Devos J., Geysen D.2004. Epidemiological study of the prevalence of Babesia divergens in a veterinary practice in the mid-east of France. Vet. Parasitol. 125: 237–249. doi: 10.1016/j.vetpar.2004.08.004 [DOI] [PubMed] [Google Scholar]
- 4.El-Dakhly K. M., Goto M., Noishiki K., El-Nahass S., Hirata A., Sakai H., Takashima Y., El-Morsey A., Yanai T.2013. Prevalence and diversity of Hepatozoon canis in naturally infected dogs in Japanese islands and peninsulas. Parasitol. Res. 112: 3267–3274. doi: 10.1007/s00436-013-3505-1 [DOI] [PubMed] [Google Scholar]
- 5.Felsenstein J.1991. PHYLIP Manual Version3.69. University Herbarium, University of California, Berkeley. [Google Scholar]
- 6.Holman P. J., Snowden K. F.2009. Canine hepatozoonosis and babesiosis, and feline cytauxzoonosis. Vet. Clin. North Am. Small Anim. Pract. 39: 1035–1053, v. doi: 10.1016/j.cvsm.2009.08.002 [DOI] [PubMed] [Google Scholar]
- 7.Holman P. J., Madeley J., Craig T. M., Allsopp B. A., Allsopp M. T., Petrini K. R., Waghela S. D., Wagner G. G.2000. Antigenic, phenotypic and molecular characterization confirms Babesia odocoilei isolated from three cervids. J. Wildl. Dis. 36: 518–530. doi: 10.7589/0090-3558-36.3.518 [DOI] [PubMed] [Google Scholar]
- 8.Inokuma H., Fujii K., Matsumoto K., Okuda M., Nakagome K., Kosugi R., Hirakawa M., Onishi T.2002. Demonstration of Anaplasma (Ehrlichia) platys inclusions in peripheral blood platelets of a dog in Japan. Vet. Parasitol. 110: 145–152. doi: 10.1016/S0304-4017(02)00289-3 [DOI] [PubMed] [Google Scholar]
- 9.Inokuma H., Ohno K., Yamamoto S.1999. Serosurvey of Ehrlichia canis and Hepatozoon canis infection in dogs in Yamaguchi Prefecture, Japan. J. Vet. Med. Sci. 61: 1153–1155. doi: 10.1292/jvms.61.1153 [DOI] [PubMed] [Google Scholar]
- 10.Inokuma H., Terada Y., Kamio T., Raoult D., Brouqui P.2001. Analysis of the 16S rRNA gene sequence of Anaplasma centrale and its phylogenetic relatedness to other ehrlichiae. Clin. Diagn. Lab. Immunol. 8: 241–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Inokuma H., Yoshizaki Y., Shimada Y., Sakata Y., Okuda M., Onishi T.2003. Epidemiological survey of Babesia species in Japan performed with specimens from ticks collected from dogs and detection of new Babesia DNA closely related to Babesia odocoilei and Babesia divergens DNA. J. Clin. Microbiol. 41: 3494–3498. doi: 10.1128/JCM.41.8.3494-3498.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Konishi K., Sakata Y., Miyazaki N., Jia H., Goo Y. K., Xuan X., Inokuma H.2008. Epidemiological survey of Babesia gibsoni infection in dogs in Japan by enzyme-linked immunosorbent assay using B. gibsoni thrombospondin-related adhesive protein antigen. Vet. Parasitol. 155: 204–208. doi: 10.1016/j.vetpar.2008.04.025 [DOI] [PubMed] [Google Scholar]
- 13.Little S. E.2010. Ehrlichiosis and anaplasmosis in dogs and cats. Vet. Clin. North Am. Small Anim. Pract. 40: 1121–1140. doi: 10.1016/j.cvsm.2010.07.004 [DOI] [PubMed] [Google Scholar]
- 14.Miyama T., Sakata Y., Shimada Y., Ogino S., Watanabe M., Itamoto K., Okuda M., Verdida R. A., Xuan X., Nagasawa H., Inokuma H.2005. Epidemiological survey of Babesia gibsoni infection in dogs in eastern Japan. J. Vet. Med. Sci. 67: 467–471. doi: 10.1292/jvms.67.467 [DOI] [PubMed] [Google Scholar]
- 15.Pattullo K. M., Wobeser G., Lockerbie B. P., Burgess H. J.2013. Babesia odocoilei infection in a Saskatchewan elk (Cervus elaphus canadensis) herd. J. Vet. Diagn. Invest. 25: 535–540. doi: 10.1177/1040638713491746 [DOI] [PubMed] [Google Scholar]
- 16.Sakamoto L., Ichikawa Y., Sakata Y., Matsumoto K., Inokuma H.2010. Detection of Anaplasma bovis DNA in the peripheral blood of domestic dogs in Japan. Jpn. J. Infect. Dis. 63: 349–352. [PubMed] [Google Scholar]
- 17.Sakuma M., Nakahara Y., Suzuki H., Uchimura M., Sekiya Z., Setoguchi A., Endo Y.2009. A case report: a dog with acute onset of Hepatozoon canis infection. J. Vet. Med. Sci. 71: 835–838. doi: 10.1292/jvms.71.835 [DOI] [PubMed] [Google Scholar]
- 18.Schoelkopf L., Hutchinson C. E., Bendele K. G., Goff W. L., Willette M., Rasmussen J. M., Holman P. J.2005. New ruminant hosts and wider geographic range identified for Babesia odocoilei (Emerson and Wright 1970). J. Wildl. Dis. 41: 683–690. doi: 10.7589/0090-3558-41.4.683 [DOI] [PubMed] [Google Scholar]
- 19.Shimokawa Miyama T., Umeki S., Baba K., Sada K., Hiraoka H., Endo Y., Inokuma H., Hisasue M., Okuda M., Mizuno T.2011. Neutropenia associated with osteomyelitis due to Hepatozoon canis infection in a dog. J. Vet. Med. Sci. 73: 1389–1393. doi: 10.1292/jvms.11-0202 [DOI] [PubMed] [Google Scholar]
- 20.Tateno M., Nishio T., Matsuo T., Sakuma M., Nakanishi N., Izawa M., Asari Y., Okamura M., Shimokawa Miyama T., Setoguchi A., Endo Y.2013. Epidemiological survey of tick-borne protozoal infection in iriomote cats and tsushima leopard cats in Japan. J. Vet. Med. Sci. 75: 985–989. doi: 10.1292/jvms.13-0015 [DOI] [PubMed] [Google Scholar]