Abstract
Bovine dilated cardiomyopathy (DCM) is an autosomal recessive genetic disorder causing congestive heart failure and subsequent death. Recently, a nonsense mutation c.343C>T in the bovine optic atrophy 3 (OPA3) gene had been reported to cause the DCM in Holstein cattle in Switzerland. However, the mutation has not been confirmed in bovine DCM outside Switzerland. Nine Holstein Friesian cows that were macroscopically and histologically diagnosed with or suspected of DCM and 12 control cows kept in Japan were tested for the mutation. The mutation surrounding OPA3 DNA fragment was amplified by PCR and subjected to direct sequences. The homogeneous c.343C>T mutation was proved to occur in all the affected cows and not in the control cows. The present study is the first report of the mutation in the DCM affected cows outside Switzerland.
Keywords: cattle, dilated cardiomyopathy, nonsense mutation, OPA3 gene
Bovine dilated cardiomyopathy (DCM) is a primary myocardial disorder causing congestive heart failure and subsequent death [3, 7, 13]. It has been reported in Holstein-Friesian cattle worldwide [2, 6, 8,9,10], including Japan [7, 14]. Although pedigree analyses had indicated an autosomal recessive mode of inheritance [6], the causative gene had not been detected for a long while. Recently, the nonsense mutation c.343C>T in the bovine optic atrophy 3 (OPA3) gene has been reported to cause the DCM in Holstein cattle in Switzerland [15]. In brief, the 115th triplet of OPA3 transcript variant 2 cDNA normally consists of cytosine, adenine and guanine, and codes glutamine. When the first base of the triplet, corresponding to 343th base of the mRNA, substitutes thymine, the triplet converts to nonsense codon, which is thought to lead to nonsense mediated decay of OPA3, and finally cause DCM [15]. In Japan, an autosomal recessive mode of inheritance of DCM had been reported [7]; however, the causative gene has not been confirmed. The aim of the present study is to make sure whether the same gene mutation occurs in the cattle affected with DCM in Japan or not.
A total of 9 Holstein Friesian cows that were diagnosed with or suspected of DCM, were selected from the past case collections of our laboratory (Table 1) and tested for the OPA3 gene mutation. According to the following criteria that were described as characteristic lesions of DCM; dilated chambers (Fig. 1A), hypertrophy and occasional vacuolation of cardiac muscle fibers with interstitial fibrosis and edema (Fig. 1B) [7, 12, 13], the case Nos. 3–9 were definitively diagnosed with DCM, and the case Nos. 1 and 2 were suspected of DCM but definitive diagnoses were suspended owing to their age: they were younger than common age at onset between 2–3 to 5 years [2, 7, 9, 13, 15]. All the affected cows were bred in Hokkaido prefecture and admitted to Obihiro University of Agriculture and Veterinary Medicine from June 1995 to April 2012. The detailed histopathological and ultrastructural studies of the 4 cows (Nos. 5, 6, 8 and 9) were previously reported in 2001 [7]. Also, the clinical symptoms and histopathological features of the case No. 1 were reported in 2014 [11]. DNA from 7 frozen myocardial samples (Nos. 3–9) and a formalin fixed paraffin embedded sample (No. 2) were isolated using Nucleospin Tissue (Macherey-Nagel, Düren, Germany). DNA from a blood sample (No. 1) was isolated using Nucleospin Blood (Macherey-Nagel). Exon 2 of OPA 3 gene surrounding c.343C>T mutation was amplified by PCR with OPA3_v2 forward (5′ CTG CAG CTC CTC CAG GTC) and OPA3_v2 reverse (5′ CCT GAA CGC ACA ACC TGA C) primers [15]. The PCR amplifications were performed in 50 µl reactions using 1 µl of DNA template, 5 µl of 10× PrimeSTAR GXL buffer, 0.2 mM of each dNTP, 12.5 pmol of each forward and reverse primer, and 1.25 U of PrimeSTAR GXL DNA polymerase (Takara Bio, Otsu, Japan). The thermal cycling profile consisted of an initial denaturation step at 98°C for 5 min, followed by 30 cycles of 10 sec denaturation at 98°C, 15 sec of annealing at 65°C and a 25 sec extension at 68°C. The presumed length of the PCR product was 354 bp. After confirming the length of the PCR products by agarose gel electrophoresis, they were purified using PCR clean-Up Gel extraction (Macherey-Nagel). Direct sequencing of the purified amplicons was performed in both directions with the ABI prism Big Dye Terminator cycle sequencing Ready Reaction kit v. 3.1 (Applied Biosystems, Foster City, CA, U.S.A.) and run on an ABI Prism 3130 Genetic Analyzer (Applied Biosystems) at a commercial laboratory (Hokkaido System Science Co., Ltd., Sapporo, Japan). The DNA sequences were analyzed using Sequence Scanner Software Version 2.0 (Applied Biosystems). The blood samples of 12 cows without heart failure were also subjected to the test (Table 1).
Table 1. The cases of the present study and the determined genotype of OPA3 c.>343.
| Case No. | Sex | Age | diagnoses | Genotype |
|---|---|---|---|---|
| 1a) | female | 1y4m | suspected of DCM | TT |
| 2 | female | 2y2m | suspected of DCM | TT |
| 3 | female | 2y8m | DCM | TT |
| 4 | female | 2y9m | DCM | TT |
| 5b) | female | 3y11m | DCM | TT |
| 6b) | female | 4y1m | DCM | TT |
| 7 | female | 4y11m | DCM | TT |
| 8b) | female | 6y2m | DCM | TT |
| 9b) | female | 6y5m | DCM | TT |
| C1 | female | 1y4m | chronic bronchopneumonia | CC |
| C2 | female | 1y8m | – | CC |
| C3 | female | 2y10m | abdominal multiple abscesses | CC |
| C4 | female | 2y2m | chronic suppurative interstitial nephritis | CC |
| C5 | female | 3y10m | chronic cystitis, pulmonary abscess | CC |
| C6 | female | 3y2m | – | CC |
| C7 | female | 4y0m | peritonitis | CC |
| C8 | female | 4y4m | chronic sinusitis | CC |
| C9 | female | 5y4m | actinobacillosis | CC |
| C10 | female | 5y8m | chronic suppurative pyelonephritis | CC |
| C11 | female | 6y0m | peritonitis | CC |
| C12 | female | 6y8m | peritonitis | CC |
Fig. 1.
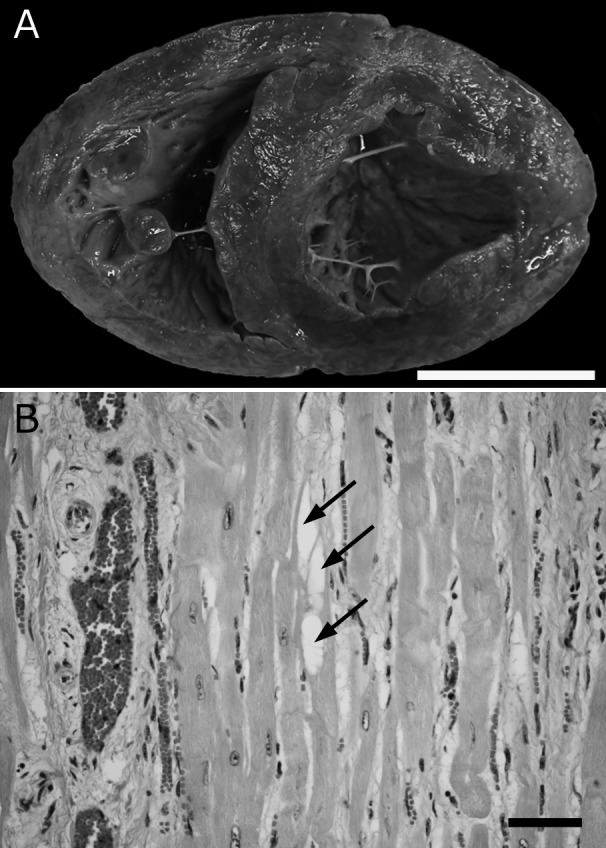
Pathological features of DCM. A) Macroscopic appearance of DCM affected heart. Case No. 1. Both ventricle chambers are distinctively dilated. Bar, 5 cm. B) Histological features of DCM affected heart. Hypertrophy and vacuolation (arrows) of cardiac muscle fibers with interstitial fibrosis are observed. Hematoxylin and eosin. Case No. 1. Bar, 50 µm.
The sequencing analysis revealed a G>A mutation at the 117th base of the PCR-amplified DNA fragment in the present DCM-affected cows (accession number LC033513) (Fig. 2) in comparison with the control cows and a normal genomic DNA sequence, which was quoted from Bos taurus breed Hereford chromosome 18, alternate assembly Btau_4.6.1 (NC_007316.5). The 117th locus was a corresponding locus to the 343th base of a bovine OPA3 transcript variant 2 mRNA, and a G>A mutation at the locus was corresponding to C>T mutation on the mRNA. Therefore, the revealed mutation was the same gene mutation in DCM-affected cattle in Switzerland [15]. No overlapping wave forms at the locus. Control cows had no mutations. Thus, the present DCM-cows were the 343TT genotype, and control cows were the 343CC genotype. No other mutation was found in other regions of amplified DNA fragment.
Fig. 2.
Comparison of the part of sequences of registered genomic DNA of normal cow (NC_007316.5) and amplified DNA fragment of the DCM-affected cows of the present study (LC033513) and control cows. Arrow indicates the 117th base of the PCR-amplified DNA fragment. A G>A mutation at the locus in the affected cow was revealed. Bars indicate identical base to that of normal.
The c.343C>T mutation in OPA3 gene has been reported as the causative gene mutation of bovine DCM [15]. However, the mutation was not reported outside the Switzerland. The present study revealed the same mutation occurred in the DCM-affected cows in Japanese herd of Holstein Friesian cattle. Despite that the pathological characteristics of DCM had been well described in previous reports [7, 12, 13], we sometimes encountered a difficult case for definitive diagnosis, especially in the young cases, such as case Nos. 1 and 2, because the histological features are not critically specific to the DCM. Taking into account that all the DCM-affected cattle in Switzerland and Japan had the same point mutation, the causative gene defect of bovine DCM may be the c.343C>T mutation of OPA3, and the present direct sequencing test of the OPA3 mutation locus will be the important diagnostic criteria for the DCM. In addition, the sequencing method is reported to be applicable to carrier detection [15]: the 343CT genotype shows overlapped wave of cytosine and thymine at the locus by the sequencing analysis. A suspected carrier was not found in the present study. On the other hand, it was likely that un-detected carrier might exist in the Japanese herd of cattle and bear the 343TT genotype rather than discontinuous variation happened to make the genotype. The direct genetic test may facilitate surveillance of the mutation within the cattle population in Japan.
OPA3 is one of the genes related to human hereditary optic atrophy and is reported to be the causative gene of the type III 3-methylglutaconic aciduria, which is a neuro-ophthalmologic syndrome in human, also known as Costeff optic atrophy syndrome [1]. In experimental animals, a mutant mouse carrying a missense mutation in the OPA3 gene is generated and reported to develop DCM-like cardiomyopathy [5]. According to the previous reports, the OPA3 protein was mitochondrial membrane associated protein in human [16] and rodent [4], and was indicated to have a crucial role in fission [16]. Ultrastructural abnormality of mitochondria within the myocardium also had been reported in the DCM-affected cows [7]. Furthermore, the 4 cows, which were described in the report [7], were proved to be 343 TT genotypes in the present study. Although the relationship between OPA3 and mitochondria is not clear, the function of OPA3 may be an important key to investigate the bovine DCM.
REFERENCES
- 1.Anikster Y., Kleta R., Shaag A., Gahl W. A., Elpeleg O.2001. Type III 3-methylglutaconic aciduria (optic atrophy plus syndrome, or Costeff optic atrophy syndrome): identification of the OPA3 gene and its founder mutation in Iraqi Jews. Am. J. Hum. Genet. 69: 1218–1224. doi: 10.1086/324651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bradley R., Jefferies A. R., Jackson P. G., Wijeratne W. V.1991. Cardiomyopathy in adult Holstein Friesian cattle in Britain. J. Comp. Pathol. 104: 101–112. doi: 10.1016/S0021-9975(08)80092-8 [DOI] [PubMed] [Google Scholar]
- 3.Buczinski S., Rezakhani A., Boerboom D.2010. Heart disease in cattle: diagnosis, therapeutic approaches and prognosis. Vet. J. 184: 258–263. doi: 10.1016/j.tvjl.2009.05.005 [DOI] [PubMed] [Google Scholar]
- 4.Da Cruz S., Xenarios I., Langridge J., Vilbois F., Parone P. A., Martinou J. C.2003. Proteomic analysis of the mouse liver mitochondrial inner membrane. J. Biol. Chem. 278: 41566–41571. doi: 10.1074/jbc.M304940200 [DOI] [PubMed] [Google Scholar]
- 5.Davies V. J., Powell K. A., White K. E., Yip W., Hogan V., Hollins A. J., Davies J. R., Piechota M., Brownstein D. G., Moat S. J., Nichols P. P., Wride M. A., Boulton M. E., Votruba M.2008. A missense mutation in the murine Opa3 gene models human Costeff syndrome. Brain 131: 368–380. doi: 10.1093/brain/awm333 [DOI] [PubMed] [Google Scholar]
- 6.Dolf G., Stricker C., Tontis A., Martig J., Gaillard C.1998. Evidence for autosomal recessive inheritance of a major gene for bovine dilated cardiomyopathy. J. Anim. Sci. 76: 1824–1829. [DOI] [PubMed] [Google Scholar]
- 7.Furuoka H., Yagi S., Murakami A., Honma A., Kobayashi Y., Matsui T., Miyahara K., Taniyama H.2001. Hereditary dilated cardiomyopathy in Holstein-Friesian cattle in Japan: association with hereditary myopathy of the diaphragmatic muscles. J. Comp. Pathol. 125: 159–165. doi: 10.1053/jcpa.2001.0494 [DOI] [PubMed] [Google Scholar]
- 8.Kümper H., Bahnemann R.1992. [Myocardial fibrosis of cattle in Hesse]. Tierarztl. Prax. 20: 254–258(in German). [PubMed] [Google Scholar]
- 9.Leifsson P. S., Agerholm J. S.2004. Familial occurrence of bovine dilated cardiomyopathy in Denmark. J. Vet. Med. A Physiol. Pathol. Clin. Med. 51: 332–335. doi: 10.1111/j.1439-0442.2004.00652.x [DOI] [PubMed] [Google Scholar]
- 10.McLennan M. W., Kelly W. R.1990. Dilated (congestive) cardiomyopathy in a Friesian heifer. Aust. Vet. J. 67: 75–76. doi: 10.1111/j.1751-0813.1990.tb07705.x [DOI] [PubMed] [Google Scholar]
- 11.Miura S., Kumagai D., Horiuchi N., Shitamura K., Kobayashi Y., Inokuma H.2014. Cardiomyopathy with muscular degeneration of tongue and diaphragm in a Holstein cow. Jui-chikusan Shinpo 67: 445–448(in Japanese). [Google Scholar]
- 12.Nart P., Williams A., Thompson H., Innocent G. T.2004. Morphometry of bovine dilated cardiomyopathy. J. Comp. Pathol. 130: 235–245. doi: 10.1016/j.jcpa.2003.11.002 [DOI] [PubMed] [Google Scholar]
- 13.Nart P., Thompson H., Barrett D. C., Armstrong S. C., McPhaden A. R.2004. Clinical and pathological features of dilated cardiomyopathy in Holstein-Friesian cattle. Vet. Rec. 155: 355–361. doi: 10.1136/vr.155.12.355 [DOI] [PubMed] [Google Scholar]
- 14.Nomura Y., Une Y., Shirota K., Ishikawa S., Watanabe M.1985. Dilated cardiomyopathy in Holstein-Friesian cattle. Heart Vessels 1: 127–129. doi: 10.1007/BF02072378 [DOI] [Google Scholar]
- 15.Owczarek-Lipska M., Plattet P., Zipperle L., Drögemüller C., Posthaus H., Dolf G., Braunschweig M. H.2011. A nonsense mutation in the optic atrophy 3 gene (OPA3) causes dilated cardiomyopathy in Red Holstein cattle. Genomics 97: 51–57. doi: 10.1016/j.ygeno.2010.09.005 [DOI] [PubMed] [Google Scholar]
- 16.Ryu S. W., Jeong H. J., Choi M., Karbowski M., Choi C.2010. Optic atrophy 3 as a protein of the mitochondrial outer membrane induces mitochondrial fragmentation. Cell. Mol. Life Sci. 67: 2839–2850. doi: 10.1007/s00018-010-0365-z [DOI] [PMC free article] [PubMed] [Google Scholar]



