Abstract
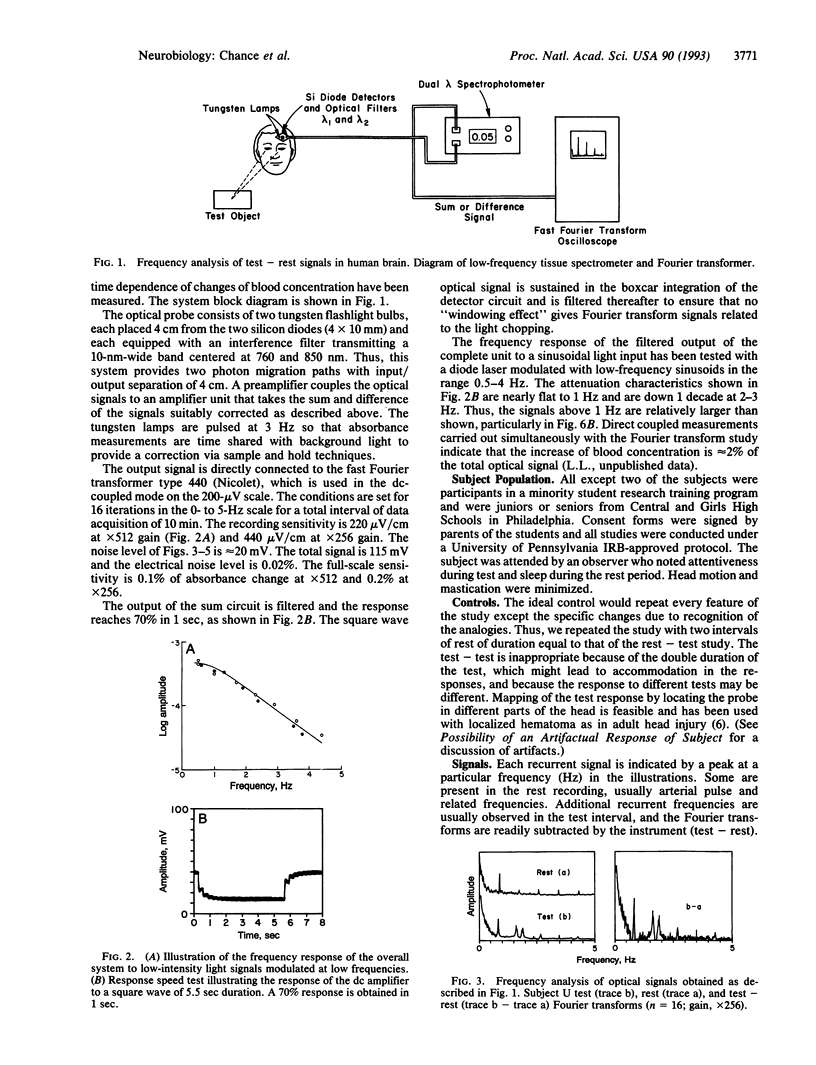
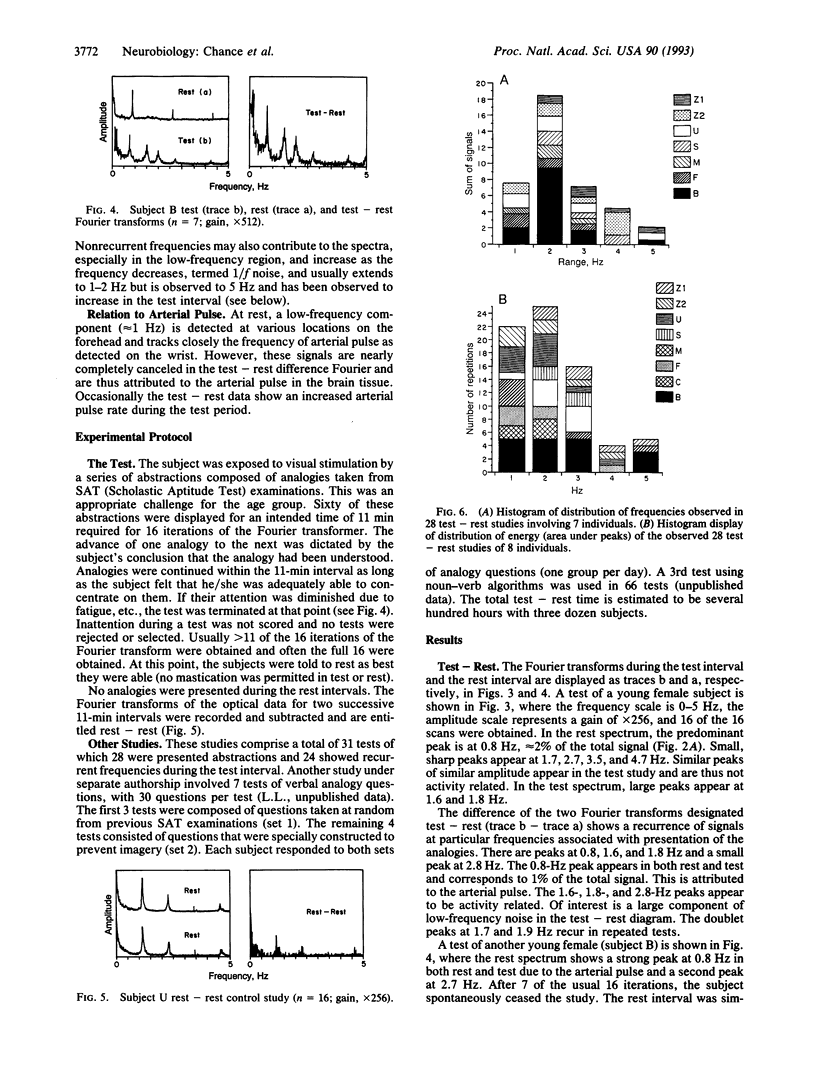
Animal model studies indicate light-absorption changes of the exposed animal brain in response to visual stimulation. Here we report observations of red-light absorbance changes, attributable to repetitive blood concentration changes in response to stimulation in the human brain frontal region by a cognitive process. These responses are observed as low-frequency recurrence of changes by Fourier transform analysis and are attributed to blood concentration change stimulated by the increased metabolic rate of brain tissue in cognitive function. A simple, portable dual wavelength spectrophotometer was attached noninvasively to the human forehead to measure the low frequency and power spectra of fluctuations of absorbances attributed to variations of brain blood concentration in the frontal region. The responses are associated with brain activity in responses to problem solving of analogies presented visually that require an associative function in the frontal region. The method of subtraction of test -rest Fourier transforms minimizes the arterial pulse frequency contributions and identifies specific frequencies--for example, 0.8, 1.6, 1.8 Hz in 24 of 28 tests of nine individuals (85%). Tests in which no increased brain activity was elicited (rest-rest) showed small differences. It is concluded that low-frequency recurrences of brain activity linked to blood concentration increases can be detected in human subjects with an optical device of potentially for simplified tests of cognitive function in the 0- to 3-Hz region and with modifications for wider band recordings in localized tissue volumes by time-resolved spectroscopy.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BRYANT S. H., TOBIAS J. M. Changes in light scattering accompanying activity in nerve. J Cell Physiol. 1952 Oct;40(2):199–219. doi: 10.1002/jcp.1030400204. [DOI] [PubMed] [Google Scholar]
- Barlow C. H., Chance B. Ischemic areas in perfused rat hearts: measurement by NADH fluorescence photography. Science. 1976 Sep 3;193(4256):909–910. doi: 10.1126/science.181843. [DOI] [PubMed] [Google Scholar]
- Belliveau J. W., Kennedy D. N., Jr, McKinstry R. C., Buchbinder B. R., Weisskoff R. M., Cohen M. S., Vevea J. M., Brady T. J., Rosen B. R. Functional mapping of the human visual cortex by magnetic resonance imaging. Science. 1991 Nov 1;254(5032):716–719. doi: 10.1126/science.1948051. [DOI] [PubMed] [Google Scholar]
- Chance B., Leigh J. S., Miyake H., Smith D. S., Nioka S., Greenfeld R., Finander M., Kaufmann K., Levy W., Young M. Comparison of time-resolved and -unresolved measurements of deoxyhemoglobin in brain. Proc Natl Acad Sci U S A. 1988 Jul;85(14):4971–4975. doi: 10.1073/pnas.85.14.4971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delpy D. T., Cope M., van der Zee P., Arridge S., Wray S., Wyatt J. Estimation of optical pathlength through tissue from direct time of flight measurement. Phys Med Biol. 1988 Dec;33(12):1433–1442. doi: 10.1088/0031-9155/33/12/008. [DOI] [PubMed] [Google Scholar]
- Frostig R. D., Lieke E. E., Ts'o D. Y., Grinvald A. Cortical functional architecture and local coupling between neuronal activity and the microcirculation revealed by in vivo high-resolution optical imaging of intrinsic signals. Proc Natl Acad Sci U S A. 1990 Aug;87(16):6082–6086. doi: 10.1073/pnas.87.16.6082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick P. W., Balakrishnan G., Stewart M., Lewis G., Ausman J. I. Cerebral oxygen metabolism measured during hypothermic circulatory arrest: a case report. J Neurosurg Anesthesiol. 1991 Dec;3(4):302–307. doi: 10.1097/00008506-199112000-00011. [DOI] [PubMed] [Google Scholar]
- Mintun M. A., Fox P. T., Raichle M. E. A highly accurate method of localizing regions of neuronal activation in the human brain with positron emission tomography. J Cereb Blood Flow Metab. 1989 Feb;9(1):96–103. doi: 10.1038/jcbfm.1989.13. [DOI] [PubMed] [Google Scholar]
- Nioka S., Chance B., Smith D. S., Mayevsky A., Reilly M. P., Alter C., Asakura T. Cerebral energy metabolism and oxygen state during hypoxia in neonate and adult dogs. Pediatr Res. 1990 Jul;28(1):54–62. doi: 10.1203/00006450-199007000-00013. [DOI] [PubMed] [Google Scholar]
- Salzberg B. M., Obaid A. L., Gainer H. Large and rapid changes in light scattering accompany secretion by nerve terminals in the mammalian neurohypophysis. J Gen Physiol. 1985 Sep;86(3):395–411. doi: 10.1085/jgp.86.3.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sevick E. M., Chance B., Leigh J., Nioka S., Maris M. Quantitation of time- and frequency-resolved optical spectra for the determination of tissue oxygenation. Anal Biochem. 1991 Jun;195(2):330–351. doi: 10.1016/0003-2697(91)90339-u. [DOI] [PubMed] [Google Scholar]
- Stuart B. H., Chance B. NADH brain surface scanning and 3-D computer display. Brain Res. 1974 Aug 23;76(3):473–479. doi: 10.1016/0006-8993(74)90823-3. [DOI] [PubMed] [Google Scholar]



