Abstract
A 7-year-old female boxer dog died suddenly without any clinical signs. It was suspected that the dog had arrhythmogenic right ventricular cardiomyopathy (ARVC) due to ventricular premature complexes and ventricular tachycardia at 3 years of age. The final diagnosis of ARVC was confirmed by histological characteristics, such as loss of cardiocytes and fibrofatty replacement, occurring in the right and left ventricular walls. In the cardiocytes, non-lipid vacuoles were observed. Cardiac fibrosis and intimal thickening of the small arteries occurred without fatty replacement in the inner muscle layer including the papillary muscles of the left ventricular wall. This paper describes the pathomorphological details of an ARVC case with coincidental cardiac fibrosis in the inner muscle layer of the left ventricular wall.
Keywords: arrhythmogenic right ventricular cardiomyopathy, boxer dog, fibrosis, intimal thickening of small arteries
Arrhythmogenic right ventricular cardiomyopathy (ARVC) is a type of cardiomyopathy. ARVC has been reported in boxer dogs and resembles human ARVC [1, 5, 11]. ARVC in dogs has been reported in some breeds [12], and among them, the condition in boxer dogs is particularly known to be familial [1, 5, 6, 11]. The symptomatic features of ARVC in boxer dogs are ventricular arrhythmias originating from the right ventricle, syncope and sudden death. In order for boxer dogs to be diagnosed with ARVC, it is necessary to carry out various examinations including electrocardiogram, a cardiac echo, observation of clinical signs and histopathology [1, 5]. A histopathological examination is helpful for postmortem diagnosis of this disease [5]. ARVC in boxer dogs is characterized by loss of cardiocytes (cardiac myocytes) and fatty or fibrofatty replacement in the right and sometimes left ventricular walls [1, 5]. The mutations of some desmosomal proteins or non-desmosomal proteins in humans [4, 16] and some non-desmosomal proteins in boxer dogs [6, 7, 15] have been reported as the cause of ARVC. Here, we report an interesting case of boxer ARVC with the features of non-lipid vacuoles in the cardiocytes and cardiac fibrosis accompanied by the intimal thickening of small arteries in the inner muscle layer including papillary muscles of the left ventricular wall.
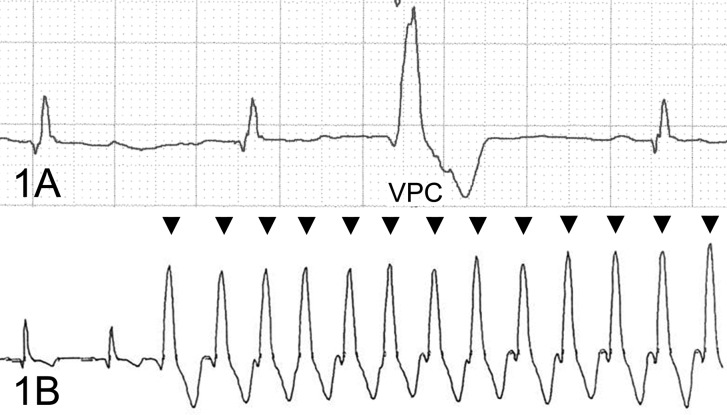
A 7-year-old female boxer dog died suddenly without any clinical signs. In the screening examination at 3 years of age, the electrocardiogram showed ventricular premature complexes, which were of a left bundle branch block morphology (Fig. 1A). Additionally, the Holter monitoring showed ventricular premature complexes of more than 1,000 times a day and occasional ventricular tachycardia (Fig. 1B). These findings suggested that the dog was afflicated with ARVC. However, no clinical signs (syncope, exercise intolerance, etc.) were detected until sudden death at 7 years of age. The heart was removed and fixed in 10% formalin for postmortem diagnosis. On the transversal cross section after fixation, slight thinning of the right ventricular wall and yellowish change around the outer layer of the ventricular wall were found (Fig. 2). For the histopathological examination, the heart was trimmed, embedded in paraffin, sectioned and stained with hematoxylin and eosin (HE), Masson’s trichrome and modified elastica van Gieson (using Sirius red substitute for fuchsin acid). Oil red O stain was also performed with formalin-fixed frozen sections. For transmission electron microscopy, the specimens were cut from four different parts: the outer and middle muscle layers of the right ventricular wall and the inner muscle layer and papillary muscles of the left ventricular wall. They were cut into small blocks and washed in distilled water for 30 min. They were post-fixed in 1% osmium tetra-oxide and embedded in epoxy resin. Ultra-thin sections were mounted on copper grids, stained with uranyl acetate and lead citrate, and examined with an H-7600 transmission electron microscope (Hitachi High-Tech Fielding Co., Tokyo, Japan).
Fig. 1.
Electrocardiogram; a boxer dog with ARVC. (A) The electrocardiogram showed ventricular premature complexes (VPC), which were left bundle branch block morphology, at 3 years old. Lead II, 50 mm/s, 0.5 cm/mV. (B) Ventricular tachycardia is observed by the Holter monitoring (arrowheads).
Fig. 2.
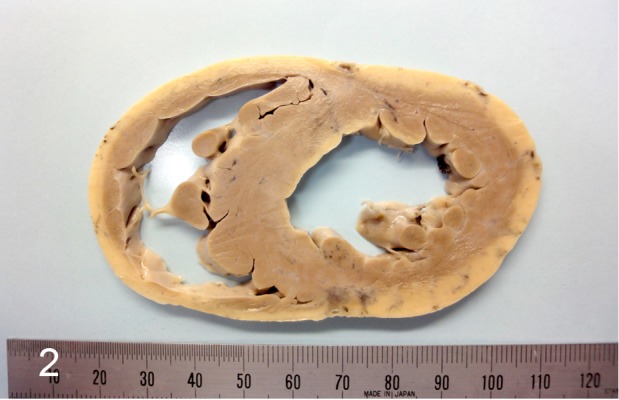
Gross features on the transversal cross section of the heart after fixation in 10% formalin. Thinning of right ventricular wall and yellowish change at outer layer of the ventricular wall are noted.
Histopathologically, loss of cardiocytes and fibrofatty replacement occurred in the outer and middle muscle layers of the right ventricular wall, extending from the epicardium toward the endocardium (Fig. 3A). The lesion was composed of fatty infiltration, interstitial fibrosis and coexistence of the various-sized surviving cardiocytes, from hypertrophied large to atrophied small ones (Fig. 3B). These cardiocytes occasionally had vacuoles, which were negative for oil red O staining (Fig. 3C). Some cardiocytes had lipofuscins, which stained greenish blue by the Schmorl method. Mononuclear cells infiltrated multifocally, but necrotic cardiocyte features, such as karyopyknosis and hypereosinophilic sarcoplasm with loss of cross-striations, were not visible. In the outer muscle layer of the left ventricular wall, loss of cardiocytes and fibrofatty replacement occurred mainly in the confined area near the epicardium. As compared to the right ventricular wall, the area of fibrofatty replacement was limited (Fig. 4A). The cardiocytes with vacuoles were distributed in the fibrofatty replacement area. The cardiocytes of all layers in the left ventricular wall were hypertrophied and had lipofuscins. These findings were similar to the ones previously reported in boxer dogs with ARVC [1, 2, 5].
Fig. 3.
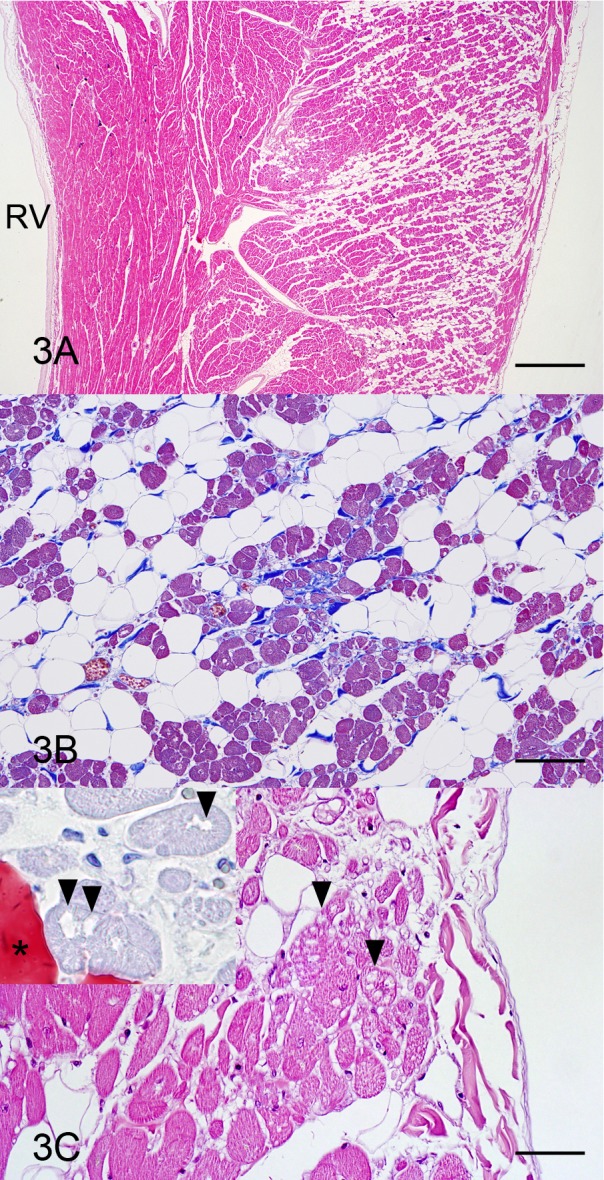
Heart, right ventricular wall; a boxer dog with ARVC. (A) Loss of cardiocytes and fibrofatty replacement are observed in the outer and middle muscle layers. RV, right ventricle. HE stain. Bar=1 mm. (B) Prominent fatty infiltration, discontinuous interstitial fibrosis and various-sized cardiocytes are present. Masson’s trichrome stain. Bar=100 µm. (C) The cardiocytes having vacuoles (arrowheads) are found in fibrofatty replacement area. HE stain. Bar=50 µm. Inset: The vacuoles (arrowheads) are negative for oil red O, and conversely, infiltrated fat cell (asterisk) is positive. Oil red O stain.
Fig. 4.
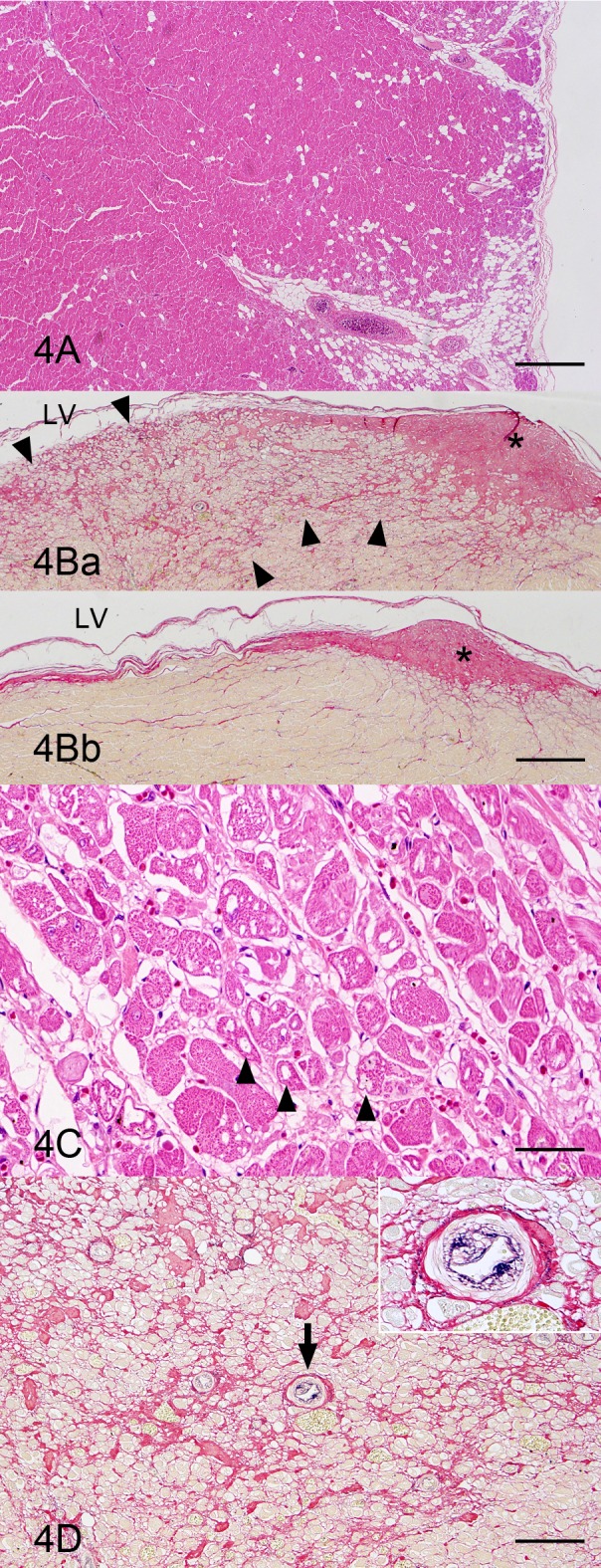
Heart; left ventricular wall; a boxer dog with ARVC. (A) Loss and fibrofatty replacement of cardiocytes are observed in the outer muscle layer. As compared to the right ventricular wall (Fig. 3A), the lesion is limited. HE stain. Bar=1 mm. (B) Fibrosis is remarkable in the papillary muscle region (Fig. 4Ba) compared to the intact region of this animal (Fig. 4Bb). The fibrosis (arrowheads) occurs apart from a tendinous cord (asterisk). LV, left ventricle. Modified elastica van Gieson stain. Bar=500 µm. (C) The cardiocytes having vacuoles are distributed in the papillary muscle region where fibrosis is observed. HE stain. Bar=50 µm. (D) The intimal thickening of small arteries (arrow and inset) at the fibrosis region in the papillary muscle is evident. Modified elastica van Gieson stain. Bar=100 µm.
In the inner muscle layer including the papillary muscles of the left ventricular wall, fibrofatty replacement was not observed, but multifocal fibrosis was remarkable. The fibrosis occurred apart from tendinous cords (Fig. 4B). The cardiocytes with vacuoles were also distributed near the fibrotic area in the inner muscle layer including the papillary muscles of the left ventricular wall (Fig. 4C). The intimal thickening of the small arteries was clearly shown by the modified elastica van Gieson stain near the fibrotic area of the inner muscle layer including the papillary muscles of the left ventricular wall (Fig. 4D). In the left ventricular side of the interventricular septum, hypertrophic cardiocytes, slight fibrosis and intimal thickening of small arteries were observed as in the left ventricular free wall. The vascular lesions were present only in these mural arteries in the inner muscle layer of the left ventricular wall and in the interventricular septum. Other mural arteries and coronary arteries remained intact.
Cardiac conduction systems, such as the sinoatrial node, atrioventricular node and the bundle of His, showed no particular lesions in the HE and Masson’s trichrome stainings.
Ultrastructurally, the cardiocytes of the outer or middle muscle layers of the right ventricular wall had some vacuoles, which were surrounded by a single layer membrane (Fig. 5A). Many highly electron dense materials corresponding to lipofuscins upon light microscopy were found in the cardiocytes (Fig. 5B). The intercalated disks were of a high electron density, and the myofibrils were loosely associated around the intercalated disks (Fig. 5C). In the inner muscle layer and papillary muscles of the left ventricular wall, the cardiocytes also had many vacuoles and high electron dense materials corresponding to lipofuscins. The intercalated disks also had a high electron density, and the myofibrils were loosely associated around the intercalated disks. There were no morphological differences in the abnormalities of the cardiocytes between the outer or middle muscle layers of the right ventricular wall and the inner muscle layer including the papillary muscles of the left ventricular wall. There were no lipid-like structures in the cardiocytes.
Fig. 5.

Heart; right ventricular wall; a boxer dog with ARVC. (A) The cardiocyte of outer muscle layer has some vacuoles which are surrounded by a single layer membrane. Electron micrograph. Bar=1 µm. (B) Many high electron dense materials (arrows) corresponding to lipofuscins on light microscopy are in the cardiocytes. Electron micrograph. Bar=2 µm. (C) The intercalated disk in the cardiocytes is of a high electron density (black arrowheads), and the myofibrils come loose around the intercalated disks (white arrowheads). Electron micrograph. Bar=0.5 µm.
We diagnosed this case as ARVC according to information gathered from this study including an abnormal electrocardiogram, findings from the Holter monitoring, sudden death without clinical signs, distinctive histopathological findings and abnormal ultrastructural features [1, 2, 5, 13,14,15]. The loss of cardiocytes and fibrofatty replacement in the right ventricular wall, were particularly characteristic of ARVC and have been occasionally observed in both the atria and left ventricular walls in boxer dogs [1, 5]. In our case, these histopathological findings were also detected in both the left and right ventricular walls. In human ARVC, d’Amati et al. reported a unique morphological pattern suggesting a progressive transdifferentiation from myocytes to adipocytes to help explain the pathogenetic mechanism of adipose replacement [3]. As an evidence of transdifferentiation, they found myocytes adjacent to the adipose tissue showing multiple sarcoplasmic vacuoles and found these cells to have both myocytic and adipocytic characteristics according to the immunohistochemical and ultrastructural examinations [3]. In boxer dogs with ARVC, vacuoles in cardiocytes were reported, but not proved to be lipid vacuoles [2]. In our case, no lipid droplets stained with oil red O in the cardiocytes showing vacuolation, and no lipid-like structures were observed upon ultrastructural examination. We presume the non-lipid vacuoles in the cardiocytes were dilated endoplasmic reticula, because they were encircled with a single layer membrane. Therefore, we surmise that the fat cells in the fibrofatty replacement area were derived from the coronary or intramural connective tissue to fill up the vacancy, created by the gentle cardiocyte loss in this case.
The left ventricular wall in our case showed not only fibrofatty replacement of cardiocytes in the outer muscle layer but also cardiac fibrosis without fatty infiltration in the inner muscle layer including the papillary muscle. Basso et al. reported that 48% of boxer dogs with ARVC had fatty or fibrofatty replacement in the left ventricular wall [1]. However, to the best of our knowledge, left ventricular cardiac fibrosis without fatty replacement at the inner muscle layer has not been previously reported in boxer dogs with ARVC. In our case, there was no histopathological relationship between fibrofatty replacement and cardiac fibrosis, because the distribution was different. The relationship between ARVC and cardiac fibrosis remains unclear, and the pathogenetical relationship between cardiac fibrosis and intimal thickening of the small arteries of the left ventricular wall and septa is not sufficiently explained. However, with regard to these relationships, we hypothesize the following: it is known that one of the causes of cardiac fibrosis is long-standing myocardial damage [10]. In our case, intimal thickening of the small arteries was detected near the cardiac fibrotic region. Therefore, the small arteries with intimal thickening may have induced the regional ischemia [9]. The regional ischemia results in oxygen deficiency damaging the cardiocytes, which require high oxygen. The cause of the intimal thickening may be a reaction to vascular endothelial injury. One of the possible causes of endothelial injury is an abnormality of the local arterial blood flow [8]. Arrhythmia due to ARVC may cause the abnormality of the local arterial blood flow. Therefore, we have speculated that the cardiac fibrosis in this case was secondary to ARVC over a long-term progression.
Acknowledgments
We would like to thank Mr. Stephen Filiatrault and Ms. Kanae Tamatsukuri for language editing.
REFERENCES
- 1.Basso C., Fox P. R., Meurs K. M., Towbin J. A., Spier A. W., Calabrese F., Maron B. J., Thiene G.2004. Arrhythmogenic right ventricular cardiomyopathy causing sudden cardiac death in boxer dogs: a new animal model of human disease. Circulation 109: 1180–1185. doi: 10.1161/01.CIR.0000118494.07530.65 [DOI] [PubMed] [Google Scholar]
- 2.Boujon C. E., Amberger C. N.2003. Arrhythmogenic right ventricular cardiomyopathy (ARVC) in a boxer. J. Vet. Cardiol. 5: 35–41. doi: 10.1016/S1760-2734(06)70043-1 [DOI] [PubMed] [Google Scholar]
- 3.d’Amati G., di Gioia C. R., Giordano C., Gallo P.2000. Myocyte transdifferentiation: a possible pathogenetic mechanism for arrhythmogenic right ventricular cardiomyopathy. Arch. Pathol. Lab. Med. 124: 287–290. [DOI] [PubMed] [Google Scholar]
- 4.Gomes J., Finlay M., Ahmed A. K., Ciaccio E. J., Asimaki A., Saffitz J. E., Quarta G., Nobles M., Syrris P., Chaubey S., McKenna W. J., Tinker A., Lambiase P. D.2012. Electrophysiological abnormalities precede overt structural changes in arrhythmogenic right ventricular cardiomyopathy due to mutations in desmoplakin-A combined murine and human study. Eur. Heart J. 33: 1942–1953. doi: 10.1093/eurheartj/ehr472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Meurs K. M.2004. Boxer dog cardiomyopathy: an update. Vet. Clin. North Am. Small Anim. Pract. 34: 1235–1244, viii. doi: 10.1016/j.cvsm.2004.05.003 [DOI] [PubMed] [Google Scholar]
- 6.Meurs K. M., Lacombe V. A., Dryburgh K., Fox P. R., Reiser P. R., Kittleson M. D.2006. Differential expression of the cardiac ryanodine receptor in normal and arrhythmogenic right ventricular cardiomyopathy canine hearts. Hum. Genet. 120: 111–118. doi: 10.1007/s00439-006-0193-2 [DOI] [PubMed] [Google Scholar]
- 7.Meurs K. M., Mauceli E., Lahmers S., Acland G. M., White S. N., Lindblad-Toh K.2010. Genome-wide association identifies a deletion in the 3′ untranslated region of striatin in a canine model of arrhythmogenic right ventricular cardiomyopathy. Hum. Genet. 128: 315–324. doi: 10.1007/s00439-010-0855-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mosier D. A.2012. Thrombosis. pp. 78–82. In: Pathology Basis of Veterinary Disease (Zachary, J. F. and McGavin, M. D. eds.), Elsevier, St. Louis. [Google Scholar]
- 9.Mosier D. A.2012. Decreased tissue perfusion. pp. 84–86. In: Pathology Basis of Veterinary Disease (Zachary, J. F. and McGavin, M. D. eds.), Elsevier, St. Louis. [Google Scholar]
- 10.Mosier D. A.2012. Cell degeneration and death. pp. 553–556. In: Pathology Basis of Veterinary Disease (Zachary, J. F. and McGavin, M. D. eds.), Elsevier, St. Louis. [Google Scholar]
- 11.Mosier D. A.2012. Arrhythmogenic right ventricular cardiomyopathy. p. 583. In: Pathology Basis of Veterinary Disease (Zachary, J. F. and McGavin, M. D. eds.), Elsevier, St. Louis. [Google Scholar]
- 12.Nakao S., Hirakawa A., Yamamoto S., Kobayashi M., Machida N.2011. Pathological features of arrhythmogenic right ventricular cardiomyopathy in middle-aged dogs. J. Vet. Med. Sci. 73: 1031–1036. doi: 10.1292/jvms.11-0080 [DOI] [PubMed] [Google Scholar]
- 13.Oxford E. M., Danko C. G., Kornreich B. G., Maass K., Hemsley S. A., Raskolnikov D., Fox P. R., Delmar M., Moïse N. S.2011. Ultrastructural changes in cardiac myocytes from Boxer dogs with arrhythmogenic right ventricular cardiomyopathy. J. Vet. Cardiol. 13: 101–113. doi: 10.1016/j.jvc.2011.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oxford E. M., Everitt M., Coombs W., Fox P. R., Kraus M., Gelzer A. R., Saffitz J., Taffet S. M., Moïse N. S., Delmar M.2007. Molecular composition of the intercalated disc in a spontaneous canine animal model of arrhythmogenic right ventricular dysplasia/cardiomyopathy. Heart Rhythm 4: 1196–1205. doi: 10.1016/j.hrthm.2007.05.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oyama M. A., Reiken S., Lehnart S. E., Chittur S. V., Meurs K. M., Stern J., Marks A. R.2008. Arrhythmogenic right ventricular cardiomyopathy in Boxer dogs is associated with calstabin2 deficiency. J. Vet. Cardiol. 10: 1–10. doi: 10.1016/j.jvc.2008.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rickelt S., Pieperhoff S.2012. Mutations with pathogenic potential in proteins located in or at the composite junctions of the intercalated disk connecting mammalian cardiomyocytes: a reference thesaurus for arrhythmogenic cardiomyopathies and for Naxos and Carvajal diseases. Cell Tissue Res. 348: 325–333. doi: 10.1007/s00441-012-1365-0 [DOI] [PMC free article] [PubMed] [Google Scholar]