Abstract
Fifty-five samples (15.62%) collected from dogs and cats were identified as canine parvovirus (CPV) infection in Beijing during 2010–2013. The nucleotide identities and aa similarities were 98.2–100% and 97.7–100%, respectively, when compared with the reference isolates. Also, several synonymous and non-synonymous mutations were also recorded for the first time. New CPV-2a was dominant, accounting for 90.90% of the samples. Two of the 16 samples collected from cats were identified as new CPV-2a (12.5%), showing nucleotide identities of 100% with those from dogs. Twelve samples (15.78%) collected from completely immunized dogs were found to be new CPV-2a, which means CPV-2 vaccines may not provide sufficient protection for the epidemic strains.
Keywords: Beijing, canine parvovirus, molecular epidemiology
Canine parvovirus type 2 (CPV-2) is the etiological agent of an epizootic severe gastroenteritis of dogs [2, 10, 38]. It has genomic substitution rates similar to those of RNA viruses, with values of about 10−4 substitutions per site per year, and host-immunity pressure may contribute to the progressive emergence of CPV-2 antigenic variants [34]. During 1979–1981, an antigenic variant, CPV-2a, was found. In 1984, a second variant, CPV-2b, was identified [23, 27, 28]. These antigenic types differ from the original CPV-2 in the VP2 gene, with five distinct amino acid differences that are mostly in the VP2 domain interacting with the host-cell transferrin receptor (TfR) [4, 8, 29]. During the last few decades, CPV-2a/2b with the Ser297Ala mutation was designated as new CPV-2a/2b [11, 23, 26]. Currently, new CPV-2a and new CPV-2b appear to have replaced the prototype CPV-2a and CPV-2b and to have become the predominant types, and they appear to be co-circulating in many countries [13, 16, 24, 26, 40, 41]. Another antigenic variant having an amino acid substitution (Asp426Glu), named as CPV-2c, was first reported in Italy in 2000 [6], and it is the most predominant variant in Italy, Germany, Uruguay and Argentina currently [10,11,12, 25, 39]. CPV-2 is considered the main pathogen responsible for acute gastroenteritis in dogs. However, CPV variants can also infect feline hosts [5, 35]. In Germany, CPV was previously detected in only approximately 10% of feline samples [37], but in Vietnam and Taiwan, reports estimated that up to approximately 80% of diseased cats were infected with CPV [19]. Some reports previously found that new-type CPV-2a and CPV-2b have been the predominant types in some provinces of China since the 1990s [42], while the epidemic situation of CPV variants in the domestic cat in mainland of China is still unknown.
In the present study, to clarify the evolution of CPV-2, which has recently been considered to be epidemic in China, and compare the epidemic isolates with the vaccine strain, the VP2 gene sequences of CPV-2 detected in Beijing from 2010 to 2013 were analyzed and compared with strains from China, Korea and other areas throughout the world. A total of 352 samples (blood, feces and secretions) from domestic dogs and domestic cats with diarrhea or bloody diarrhea were collected during 2010–2013, and subjected PCR. The primers used for PCR are shown in Table 1. A 1752 bp fragment covering the full-length sequence of the VP2 gene was successfully amplified from 55 samples (15.63%); among them, BJ-E13-2012 and BJ-E53-2012 collected from cats were identified as new CPV-2a (12.5%) and showed nucleotide identities of 100% with CPV strains collected from domestic dogs, but their infectiousness and pathogenicity in cats and dogs still need a further study. The sequences were submitted to GenBank. The GenBank accession numbers are KF803589 to KF803643. Detailed information on the origin and accession numbers of the CPV-positive samples is shown in Table 2. The resulting sequences were aligned with CPV reference isolates retrieved from the GenBank database. Sequences comparisons showed no deletion or shift in any sequences of the detected samples, and the nucleotide identities and deduced aa sequences identities were 98.2–100% and 97.7–100%, respectively, when compared with the CPV reference isolates.
Table 1. The primers used for PCR detection of CPV.
| Primer | Sequence (5′→3′) | Positions (in VP2 gene) |
Fragment length |
|---|---|---|---|
| CPV-1F CPV-1R | TTAAAGACTGTTTCAGAATCTGC AATCTCTCAGGTGTTTCTCCTGTT | 448–470 1170–1193 | 746 bp |
| CPV-2F CPV-2R | GGCGAATTCATGAGTGATGGAGCAGTTC CGCCTCGAGATATAATTTTCTAGGTGCT | 1–19 1733–1752 | 1,752 bp |
Table 2. Detailed informations of the CPV-positive samples.
| Strains | GenBank ID | Sampling year | Host | Age | Clinical signs | Sample source | Vaccinated | Genetype |
|---|---|---|---|---|---|---|---|---|
| BJ-A1 | KF803589 | 2010 | Domestic dog | 2 months | Vomit and diarrhea | Feces | Not completely | New CPV-2a |
| BJ-A2 | KF803590 | 2010 | Domestic dog | 2 months | Vomit and diarrhea | Blood | Not completely | New CPV-2a like |
| BJ-A45 | KF803591 | 2010 | Domestic dog | 3 months | Cough and diarrhea | Feces | Not completely | CPV-2a |
| BJ-A48 | KF803592 | 2010 | Domestic dog | 10 months | Cough and diarrhea | Secretions | Not completely | New CPV-2a |
| BJ-A49 | KF803593 | 2010 | Domestic dog | 18 months | Recovery phase | Feces | Yes | New CPV-2a |
| BJ-A50 | KF803594 | 2010 | Domestic dog | 2 months | Recovery phase | Feces | Not completely | New CPV-2a like |
| BJ-A53 | KF803595 | 2010 | Domestic dog | 2 months | Recovery phase | Feces | Yes | New CPV-2a |
| BJ-A57 | KF803596 | 2010 | Domestic dog | 2 months | Restored from diarrhea | Feces | Yes | New CPV-2a |
| BJ-A61 | KF803597 | 2010 | Domestic dog | 4 months | Restored from diarrhea | Feces | Not completely | New CPV-2a |
| BJ-A63 | KF803598 | 2010 | Domestic dog | 4 months | Restored from diarrhea | Feces | Yes | New CPV-2b |
| BJ-A64 | KF803599 | 2010 | Domestic dog | 6 months | Injury | Feces | Not completely | New CPV-2a |
| BJ-A68 | KF803600 | 2010 | Domestic dog | 6 months | Injury | Blood | Yes | New CPV-2a like |
| BJ-A69 | KF803601 | 2010 | Domestic dog | 6 months | Diarrhea | Feces | Yes | New CPV-2a like |
| BJ-A72 | KF803602 | 2010 | Domestic dog | 7 months | Restored from diarrhea | Feces | Not completely | New CPV-2a |
| BJ-A108 | KF803603 | 2010 | Domestic dog | 2 months | Diarrhea and anorexia | Feces | Not completely | New CPV-2b |
| BJ-B4 | KF803604 | 2011 | Domestic dog | 3 months | Diarrhea | Feces | Not completely | New CPV-2a |
| BJ-B5 | KF803605 | 2011 | Domestic dog | 2 months | Vomit and diarrhea | Feces | Not completely | New CPV-2a |
| BJ-B6 | KF803606 | 2011 | Domestic dog | 2 months | Vomit and diarrhea | Feces | Not completely | New CPV-2b |
| BJ-B8 | KF803607 | 2011 | Domestic dog | 2 months | Vomit | Feces | Not completely | New CPV-2a |
| BJ-B10 | KF803608 | 2011 | Domestic dog | 2 months | Vomit and diarrhea | Feces | Not completely | New CPV-2a |
| BJ-B11 | KF803609 | 2011 | Domestic dog | 2 months | Vomit and diarrhea | Feces | Yes | New CPV-2a |
| BJ-B13 | KF803610 | 2011 | Domestic dog | 2 months | Vomit and diarrhea | Feces | Not completely | New CPV-2a |
| BJ-B16 | KF803611 | 2011 | Domestic dog | 2 months | Vomit and diarrhea | Feces | Not completely | New CPV-2b |
| BJ-B19 | KF803612 | 2011 | Domestic dog | 12 months | Vomit and diarrhea | Feces | Not completely | New CPV-2a |
| BJ-B21 | KF803613 | 2011 | Domestic dog | 3 months | Vomit and diarrhea | Feces | Not completely | New CPV-2a |
| BJ-B22 | KF803614 | 2011 | Domestic dog | 2 months | Vomit and diarrhea | Feces | Not completely | New CPV-2a |
| BJ-B25 | KF803615 | 2011 | Domestic dog | 2 months | Vomit and diarrhea | Feces | Not completely | New CPV-2a |
| BJ-B26 | KF803616 | 2011 | Domestic dog | 2 months | Cough and diarrhea | Secretions | Not completely | New CPV-2a |
| BJ-B28 | KF803617 | 2011 | Domestic dog | 3 months | Diarrhea | Feces | Not completely | New CPV-2a |
| BJ-B31 | KF803618 | 2011 | Domestic dog | 4 months | Vomit and bloody | Feces | Not completely | New CPV-2a |
| BJ-B32 | KF803619 | 2011 | Domestic dog | 12 months | Vomit and diarrhea | Feces | Not completely | New CPV-2a |
| BJ-B33 | KF803620 | 2011 | Domestic dog | 2 months | Diarrhea and bloody | Feces | Yes | New CPV-2a |
| BJ-B34 | KF803621 | 2011 | Domestic dog | 3 months | Vomit and bloody | Feces | Not completely | New CPV-2a |
| BJ-B38 | KF803622 | 2011 | Domestic dog | 2 months | Vomit and diarrhea | Feces | Not completely | New CPV-2a |
| BJ-B39 | KF803623 | 2011 | Domestic dog | 3 months | Anorexia | Feces | Not completely | New CPV-2a |
| BJ-B40 | KF803624 | 2011 | Domestic dog | 10 months | Vomit and diarrhea | Feces | Not completely | New CPV-2a |
| BJ-B41 | KF803625 | 2011 | Domestic dog | 3 months | Vomit and diarrhea | Feces | Yes | New CPV-2a |
| BJ-B42 | KF803626 | 2011 | Domestic dog | 4 months | Vomit and diarrhea | Feces | Yes | New CPV-2a |
| BJ-B43 | KF803627 | 2011 | Domestic dog | 3 months | Vomit | Feces | Not completely | New CPV-2b |
| BJ-B44 | KF803628 | 2011 | Domestic dog | 2 months | Vomit and diarrhea | Feces | Not completely | New CPV-2a |
| BJ-D4 | KF803629 | 2012 | Domestic dog | 2 months | Diarrhea | Feces | Not completely | New CPV-2a |
| BJ-D6 | KF803630 | 2012 | Domestic dog | 2 months | Vomit and diarrhea | Feces | Yes | New CPV-2a |
| BJ-D14 | KF803631 | 2012 | Domestic dog | 1 month | Cough | Feces | Not completely | New CPV-2a |
| BJ-D15 | KF803632 | 2012 | Domestic dog | 2 months | Anorexia and Vomit | Feces | Not completely | New CPV-2a |
| BJ-E4 | KF803633 | 2012 | Domestic dog | 2 years | Anorexia and diarrhea | Feces | Not completely | New CPV-2a |
| BJ-E13 | KF803634 | 2012 | Domestic cat | 6 months | Health | Blood | Not completely | New CPV-2a |
| BJ-E14 | KF803635 | 2012 | Domestic dog | 5 years | Health | Blood | Yes | New CPV-2a |
| BJ-E34 | KF803636 | 2012 | Domestic dog | 2 months | Anorexia and diarrhea | Feces | Not completely | New CPV-2a |
| BJ-E53 | KF803637 | 2012 | Domestic cat | 7 months | Diarrhea | Blood | Not completely | New CPV-2a |
| BJ-E64 | KF803638 | 2012 | Domestic dog | 3 months | Vomit | Feces | Not completely | New CPV-2a |
| BJ-E81 | KF803639 | 2012 | Domestic dog | 7 months | Diarrhea | Feces | Not completely | New CPV-2a |
| BJ-P21 | KF803640 | 2013 | Domestic dog | 2 months | Vomit and diarrhea | Feces | Not completely | New CPV-2a |
| BJ-P27 | KF803641 | 2013 | Domestic dog | 7 months | Vomit and diarrhea | Feces | Not completely | New CPV-2a |
| BJ-P33 | KF803642 | 2013 | Domestic dog | 3 months | Vomit and diarrhea | Feces | Not completely | New CPV-2a |
| BJ-P34 | KF803643 | 2013 | Domestic dog | 4 months | Vomit and diarrhea | Feces | Not completely | New CPV-2a |
Mutations in the VP2 protein are associated with the CPV genotypes, so the nucleotide sequences were translated into aa sequences. All samples, except one original CPV2a (BJ-A45-2010), were identified as “new CPV-2a/2b” reported worldwide and were identified as “new CPV-2a/2b” [9, 20, 22]. Previous research has shown residue 297 is under strong positive selection [30, 35]. Also, the 297Ala variant can not be distinguished serologically and does not change the viral antigenic type, but it potentially has a marked influence on host adaptation. Only four samples (BJ-A63-2010, BJ-B6-2011, BJ-B16-2011 and BJ-B43-2011) with Asn426Asp and ser297Ala mutations were characterized as new CPV-2b (7.27%). New CPV-2a accounted for 90.9%, while no CPV-2c was detected. Five samples (BJ-A2-2010, BJ-A49-2010, BJ-A68-2010, BJ-A69-2010 and BJ-A108-2010) also present Met at 87 position (9.09%), and six samples (BJ-A2-2010, BJ-A45-2010, BJ-A50-2010, BJ-A68-2010, BJ-A69-2010 and BJ-A108-2010) present Ile at 101 position (10.91%), which characterized as new CPV-2a like. Four samples presented Val139Ile mutation (7.27%). There are 24 samples presented Phe267Tyr mutation (43.64%). The Phe267Tyr change also has been described in Asiatic CPV-2b from Vietnam [25] and in CPV-2a from Thailand [32] and China [31, 35]. Residue 267 is not exposed on the capsid surface [1], so substitutions in this position may not affect the antigenicity of the virus. Fifty four samples presented Ala300Gly mutation (98.18%). Tyr324Ile mutation was first detected in China and Korea in 2004 [20, 35], and reported among the CPV 2a/2b isolates in China [17, 35]. Previous studies have shown that residue 324 is subject to strong positive selection in all parvoviruses of carnivores [17]. Residue 324 is likely to have had an effect on the parvovirus host range [35]. It is adjacent to residue 323, and together with residue 93, affects TfR binding [18]. In this study, fifty one samples presented Tyr324Ile mutation (92.73%) indicated that strain with mutation Tyr324Ile is the major epidemic strain. Thr440Ala has been described in CPV-2a and CPV-2b strains from China, Korea, India, Italy, Brazil and Uruguayan [3, 22, 30, 31] and in CPV-2c strains from the United States [21] and Argentina [7]. The 440 residue is important, because it is located at the top of the three-fold spike, the main antigenic site of the virus [36]. This residue is undergoing positive selection and has evolved in different populations independently, which explains its world-wide presence in unrelated CPV-2 populations [12]. In this study, seventeen samples presented Thr440Ala mutation, which accounted for 30.91%. And, all samples presented Tyr at 305 position and Val at 555 position. Also, Ser192Phe mutation in BJ-A2-2010 was just previously reported in SC02-2011. Asp375Asn mutation in BJ-A45-2010 was just previously reported in CPV-b, GZ0201 and JL0201. Previous report has indicated that residue 375 is associated with the ability of CPV to hemagglutinate or alter pH dependence of hemagglutination [15, 36], so BJ-A45-2010 may have different coagulation feature with others. While the mutations, Pro202Thr, Ile219Vla, Ala347Thr, Gln386Lys, Pro187Gln, Ser188 Gln and Val308Ile, present in this study were interesting, because it has not been detected previously in any other strains. Pro202Thr mutation was firstly reported in BJ-A2-2010, and Ile219Vla, Ala347Thr and Gln386Lys mutations were firstly reported in BJ-A45-2010. Pro187Gln and Ser188 Gln mutations were firstly reported in BJ-A69-2010. Also, Val308Ile mutation was firstly reported in BJ-D6-2012 and BJ-D14-2012. While, Asp427His, The445Asn and Pro512His which appeared in Nanjing strains of 2009–2012 [41] and Thr442Ala and Gln370Arg reported in the giant panda strain B11 [15] were not present in this study. The detail mutation sites, types and rates in VP2 genes were shown in Table 3. The affects of these mutations to virus itself, which appeared in this study, need our further research.
Table 3. Table of statistics of mutation sites, types and rates in VP2 genes.
| aa sites | 87 | 101 | 139 | 187 | 188 | 192 | 202 | 219 | 267 | 297 | 300 | 305 | 308 | 324 | 347 | 375 | 386 | 426 | 440 | 555 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| aa mutation | L→M | T→I | V→I | P→I P→Q |
A→Q | S→F | P→T | I→K | F→Y | S→A | A→G | D→Y | V→I | Y→I | A→T | D→N | Q→K | N→D | T→A | I→V |
| Sample numbers | 5 | 6 | 4 | 2 | 1 | 1 | 1 | 1 | 24 | 54 | 54 | 55 | 2 | 51 | 1 | 1 | 1 | 4 | 17 | 55 |
| Mutation rates (%) | 9.09 | 10.91 | 7.27 | 3.64 | 1.82 | 1.82 | 1.82 | 1.82 | 43.64 | 98.18 | 98.18 | 100 | 3.64 | 82.73 | 1.84 | 1.84 | 1.84 | 7.27 | 30.91 | 100 |
Twelve strains were detected in dogs completely immunized with CPV-2 vaccine (Table 2). Although the effectiveness of CPV-2 vaccine against CPV-2a type has not been evaluated in China, our results showed that 12 out of 55 samples were detected as “new CPV-2a/2b” in dogs completely immunized with CPV-2 vaccine, which suggested that complete immunity may not be provided to dogs even if CPV-2 vaccines are used as previously reported [14, 15, 33, 35].
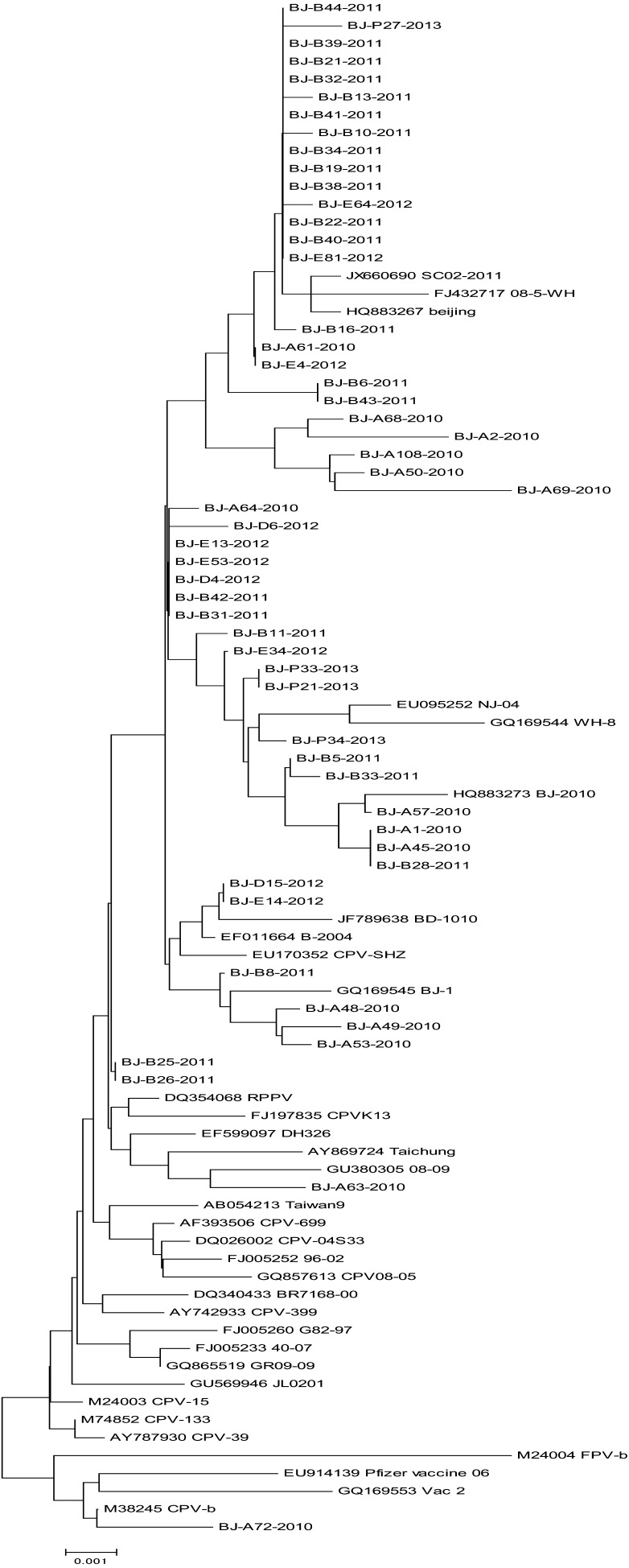
To examine the phylogenetic relationships of the 55 samples with the reference isolates, a phylogenetic tree based on the nucleotide sequence from 1 to 1752 nt of the VP2 gene was built. As shown in Fig. 1, all the samples clustered in CPV group, while separated from FPV-b. Most of them are with the closest relationship with the Chinese isolates, such as Guanzhou (JX66-690, SC02-2011), Beijing (HQ883267, Beijing; HQ883273, BJ-2010), Wuhan (FJ432717, 08-5-WH), Nanjing (EU095252, NJ-04) and so on. All sequences are with the distant relationship with Brazil, France, Japan, Vietnam, the United States and Italy isolates. However, BJ-B25-2011, BJ-B26-2011, BJ-A72-2010 and BJ-A63-2010 are with the distant relationship with others. Interestingly, BJ-A72-2010 is with the closest relationship with vaccine strains (EU914139, Pfizer vaccine 06 GQ169553, Vac 2), CPV-2 representative strain (M38245, CPV-b) and FPV representative strain (M24004, FPV-b). BJ-A63-2010 is with the closest relationship with South Korea strain (EF599097, DH326), Taiwan strain (AY869724, Taichung) and Chinese CPV 2c strain (GU380305 08-09). As the phylogenetic tree shows, most of the viruses isolated in China formed a large cluster and certain mutations detected in Chinese CPVs probably arose during the process of local adaptation, as indicated by previous surveys [15, 30].
Fig. 1.
Phylogenies of the VP2 gene sequences. The phylogenetic analyses were performed using the MEGA software, version 4.0 (http://www.megasoftware.net/). The neighbor-joining method was chosen to draw the nucleotide phylogenetic tree. The reliability of the phylogenetic tree obtained for the VP2 region was evaluated by running 1,000 replicates in the bootstrap test.
In summary, new CPV-2a is the prominent type of CPV in Beijing from 2010 to 2013. Few samples still present Met at 87 position, Ile at 101 position and Ala at 300 position. Most samples contained the mutation Phe267Tyr, Ala300Gly, Tyr324Ile and Thr440Ala. All samples presented Tyr at 305 position and Val at 555 position. While, Pro187Gln, Ser188 Gln, Ile219Vla, Ala347Thr, Gln386Lys and Val308Ile mutations were firstly reported in present study. Also, new CPV-2a was detected in cats with diarrhea, and CPV-2 vaccines may not provide complete immunity to dogs in China. Due to the continuing evolution of CPV, monitoring the prominent type of CPV, and detecting genetic mutation and antigenic changes, will be necessary to find new vaccines and control CPV infection in China.
Acknowledgments
We are grateful to the animal health workers who were involved in this study and collected animal blood samples. This work was supported by the Special Fund for Agro-Scientific Research in the Public Interest (no. 201303042), the National High Technology Research and Development Program of China (no. 2012AA101302), and a project supported by the National Science Foundation for Young Scientists of China (no. 31302130).
References
- 1.Agbandje M., Parrish C.R., Rossmann M.G.1995. The Structure of Parvoviruses. Semin. Virol. 6: 299–309. doi: 10.1006/smvy.1995.0036 [DOI] [Google Scholar]
- 2.Appel M. J., Scott F. W., Carmichael L. E.1979. Isolation and immunisation studies of a canine parco-like virus from dogs with haemorrhagic enteritis. Vet. Rec. 105: 156–159. doi: 10.1136/vr.105.8.156 [DOI] [PubMed] [Google Scholar]
- 3.Battilani M., Ciulli S., Tisato E., Prosperi S.2002. Genetic analysis of canine parvovirus isolates (CPV-2) from dogs in Italy. Virus Res. 83: 149–157. doi: 10.1016/S0168-1702(01)00431-2 [DOI] [PubMed] [Google Scholar]
- 4.Battilani M., Scagliarini A., Ciulli S., Morganti L., Prosperi S.2006. High genetic diversity of the VP2 gene of a canine parvovirus strain detected in a domestic cat. Virology 352: 22–26. doi: 10.1016/j.virol.2006.06.002 [DOI] [PubMed] [Google Scholar]
- 5.Battilani M., Balboni A., Giunti M., Prosperi S.2013. Co-infection with feline and canine parvovirus in a cat. Vet. Ital. 49: 127–129. [PubMed] [Google Scholar]
- 6.Buonavoglia C., Martella V., Pratelli A., Tempesta M., Cavalli A., Buonavoglia D., Bozzo G., Elia G., Decaro N., Carmichael L.2001. Evidence for evolution of canine parvovirus type 2 in Italy. J. Gen. Virol. 82: 3021–3025. [DOI] [PubMed] [Google Scholar]
- 7.Calderón M. G., Romanutti C., D’ Antuono A., Keller L., Mattion N., La Torre J.2011. Evolution of canine parvovirus in Argentina between years 2003 and 2010: CPV2c has become the predominant variant affecting the domestic dog population. Virus Res. 157: 106–110. doi: 10.1016/j.virusres.2011.02.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cavalli A., Martella V., Desario C., Camero M., Bellacicco A. L., De Palo P., Decaro N., Elia G., Buonavoglia C.2008. Evaluation of the antigenic relationships among canine parvovirus type 2 variants. Clin. Vaccine Immunol. 15: 534–539. doi: 10.1128/CVI.00444-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chinchkar S. R., Mohana Subramanian B., Hanumantha Rao N., Rangarajan P. N., Thiagarajan D., Srinivasan V. A.2006. Analysis of VP2 gene sequences of canine parvovirus isolates in India. Arch. Virol. 151: 1881–1887. doi: 10.1007/s00705-006-0753-8 [DOI] [PubMed] [Google Scholar]
- 10.Decaro N., Desario C., Campolo M., Elia G., Martella V., Ricci D., Lorusso E., Buonavoglia C.2005. Clinical and virological findings in pups naturally infected by canine parvovirus type 2 Glu-426 mutant. J. Vet. Diagn. Invest. 17: 133–138. doi: 10.1177/104063870501700206 [DOI] [PubMed] [Google Scholar]
- 11.Decaro N., Martella V., Desario C., Bellacicco A. L., Camero M., Manna L., d’Aloja D., Buonavoglia C.2006. First detection of canine parvovirus type 2c in pups with haemorrhagic enteritis in Spain. J. Vet. Med. B Infect. Dis. Vet. Public Health 53: 468–472. doi: 10.1111/j.1439-0450.2006.00974.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Decaro N., Buonavoglia C.2012. Canine parvovirus—a review of epidemiological and diagnostic aspects, with emphasis on type 2c. Vet. Microbiol. 155: 1–12. doi: 10.1016/j.vetmic.2011.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dogonyaro B. B., Bosman A. M., Sibeko K. P., Venter E. H., van Vuuren M.2013. Genetic analysis of the VP2-encoding gene of canine parvovirus strains from Africa. Vet. Microbiol. 165: 460–465. doi: 10.1016/j.vetmic.2013.04.022 [DOI] [PubMed] [Google Scholar]
- 14.Elia G., Cavalli A., Cirone F., Lorusso E., Camero M., Buonavoglia D., Tempesta M.2005. Antibody levels and protection to canine parvovirus type 2. J. Vet. Med. B Infect. Dis. Vet. Public Health 52: 320–322. doi: 10.1111/j.1439-0450.2005.00870.x [DOI] [PubMed] [Google Scholar]
- 15.Guo L., Yang S. L., Chen S. J., Zhang Z., Wang C., Hou R., Ren Y., Wen X., Cao S., Guo W., Hao Z., Quan Z., Zhang M., Yan Q. G.2013. Identification of canine parvovirus with the Q370R point mutation in the VP2 gene from a giant panda (Ailuropoda melanoleuca). Virol. J. 10: 163. doi: 10.1186/1743-422X-10-163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hirayama K., Kano R., Hosokawa-Kanai T., Tuchiya K., Tsuyama S., Nakamura Y., Sasaki Y., Hasegawa A.2005. VP2 gene of a canine parvovirus isolate from stool of a puppy. J. Vet. Med. Sci. 67: 139–143. doi: 10.1292/jvms.67.139 [DOI] [PubMed] [Google Scholar]
- 17.Hoelzer K., Shackelton L. A., Parrish C. R., Holmes E. C.2008. Phylogenetic analysis reveals the emergence, evolution and dispersal of carnivore parvoviruses. J. Gen. Virol. 89: 2280–2289. doi: 10.1099/vir.0.2008/002055-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hueffer K., Parker J. S., Weichert W. S., Geisel R. E., Sgro J. Y., Parrish C. R.2003. The natural host range shift and subsequent evolution of canine parvovirus resulted from virus-specific binding to the canine transferrin receptor. J. Virol. 77: 1718–1726. doi: 10.1128/JVI.77.3.1718-1726.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ikeda Y., Mochizuki M., Naito R., Nakamura K., Miyazawa T., Mikami T., Takahashi E.2000. Predominance of canine parvovirus (CPV) in unvaccinated cat populations and emergence of new antigenic types of CPVs in cats. Virology 278: 13–19. doi: 10.1006/viro.2000.0653 [DOI] [PubMed] [Google Scholar]
- 20.Jeoung S. Y., Ahn S. J., Kim D.2008. Genetic analysis of VP2 gene of canine parvovirus isolates in Korea. J. Vet. Med. Sci. 70: 719–722. doi: 10.1292/jvms.70.719 [DOI] [PubMed] [Google Scholar]
- 21.Kapil S., Cooper E., Lamm C., Murray B., Rezabek G., Johnston L., 3rd, Campbell G., Johnson B.2007. Canine parvovirus types 2c and 2b circulating in North American dogs in 2006 and 2007. J. Clin. Microbiol. 45: 4044–4047. doi: 10.1128/JCM.01300-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kang B. K., Song D. S., Lee C. S., Jung K. I., Park S. J., Kim E. M., Park B. K.2008. Prevalence and genetic characterization of canine parvoviruses in Korea. Virus Genes 36: 127–133. doi: 10.1007/s11262-007-0189-6 [DOI] [PubMed] [Google Scholar]
- 23.Martella V., Decaro N., Elia G., Buonavoglia C.2005. Surveillance activity for canine parvovirus in Italy. J. Vet. Med. B Infect. Dis. Vet. Public Health 52: 312–315. doi: 10.1111/j.1439-0450.2005.00875.x [DOI] [PubMed] [Google Scholar]
- 24.Mukhopadhyay H. K., Matta S. L., Amsaveni S., Antony P. X., Thanislass J., Pillai R. M.2014. Phylogenetic analysis of canine parvovirus partial VP2 gene in India. Virus Genes 48: 89–95. doi: 10.1007/s11262-013-1000-5 [DOI] [PubMed] [Google Scholar]
- 25.Nakamura M., Tohya Y., Miyazawa T., Mochizuki M., Phung H. T., Nguyen N. H., Huynh L. M., Nguyen L. T., Nguyen P. N., Nguyen P. V., Nguyen N. P., Akashi H.2004. A novel antigenic variant of Canine parvovirus from a Vietnamese dog. Arch. Virol. 149: 2261–2269. doi: 10.1007/s00705-004-0367-y [DOI] [PubMed] [Google Scholar]
- 26.Ohshima T., Hisaka M., Kawakami K., Kishi M., Tohya Y., Mochizuki M.2008. Chronological analysis of canine parvovirus type 2 isolates in Japan. J. Vet. Med. Sci. 70: 769–775. doi: 10.1292/jvms.70.769 [DOI] [PubMed] [Google Scholar]
- 27.Parrish C. R., O’Connell P. H., Evermann J. F., Carmichael L. E.1985. Natural variation of canine parvovirus. Science 230: 1046–1048. doi: 10.1126/science.4059921 [DOI] [PubMed] [Google Scholar]
- 28.Parrish C. R.1991. Mapping specific functions in the capsid structure of canine parvovirus and feline panleukopenia virus using infectious plasmid clones. Virology 183: 195–205. doi: 10.1016/0042-6822(91)90132-U [DOI] [PubMed] [Google Scholar]
- 29.Parker J. S., Murphy W. J., Wang D., O’Brien S. J., Parrish C. R.2001. Canine and feline parvoviruses can use human or feline transferrin receptors to bind, enter, and infect cells. J. Virol. 75: 3896–3902. doi: 10.1128/JVI.75.8.3896-3902.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pereira C. A., Leal E. S., Durigon E. L.2007. Selective regimen shift and demographic growth increase associated with the emergence of high-fitness variants of canine parvovirus. Infect. Genet. Evol. 7: 399–409. doi: 10.1016/j.meegid.2006.03.007 [DOI] [PubMed] [Google Scholar]
- 31.Pérez R., Bianchi P., Calleros L., Francia L., Hernández M., Maya L., Panzera Y., Sosa K., Zoller S.2012. Recent spreading of a divergent canine parvovirus type 2a (CPV-2a) strain in a CPV-2c homogenous population. Vet. Microbiol. 155: 214–219. doi: 10.1016/j.vetmic.2011.09.017 [DOI] [PubMed] [Google Scholar]
- 32.Phromnoi S., Sirinarumitr K., Sirinarumitr T.2010. Sequence analysis of VP2 gene of canine parvovirus isolates in Thailand. Virus Genes 41: 23–29. doi: 10.1007/s11262-010-0475-6 [DOI] [PubMed] [Google Scholar]
- 33.Pratelli A., Cavalli A., Martella V., Tempesta M., Decaro N., Carmichael L. E., Buonavoglia C.2001. Canine parvovirus (CPV) vaccination: comparison of neutralizing antibody responses in pups after inoculation with CPV2 or CPV2b modified live virus vaccine. Clin. Diagn. Lab. Immunol. 8: 612–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shackelton L. A., Parrish C. R., Truyen U., Holmes E. C.2005. High rate of viral evolution associated with the emergence of carnivore parvovirus. Proc. Natl. Acad. Sci. U.S.A. 102: 379–384. doi: 10.1073/pnas.0406765102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stuetzer B., Hartmann K.2014. Feline parvovirus infection and associated diseases. Vet. J. 201: 150–155. doi: 10.1016/j.tvjl.2014.05.027 [DOI] [PubMed] [Google Scholar]
- 36.Tsao J., Chapman M. S., Agbandje M., Keller W., Smith K., Wu H., Luo M., Smith T. J., Rossmann M. G., Compans R. W., et al. 1991. The three-dimensional structure of canine parvovirus and its functional implications. Science 251: 1456–1464. doi: 10.1126/science.2006420 [DOI] [PubMed] [Google Scholar]
- 37.Truyen U., Evermann J. F., Vieler E., Parrish C. R.1996. Evolution of canine parvovirus involved loss and gain of feline host range. Virology 215: 186–189. doi: 10.1006/viro.1996.0021 [DOI] [PubMed] [Google Scholar]
- 38.Truyen U.2006. Evolution of canine parvovirus—a need for new vaccines? Vet. Microbiol. 117: 9–13. doi: 10.1016/j.vetmic.2006.04.003 [DOI] [PubMed] [Google Scholar]
- 39.Touihri L., Bouzid I., Daoud R., Desario C., El Goulli A. F., Decaro N., Ghorbel A., Buonavoglia C., Bahloul C.2009. Molecular characterization of canine parvovirus-2 variants circulating in Tunisia. Virus Genes 38: 249–258. doi: 10.1007/s11262-008-0314-1 [DOI] [PubMed] [Google Scholar]
- 40.Yi L., Tong M., Cheng Y., Song W., Cheng S.2014. Phylogenetic Analysis of Canine Parvovirus VP2 Gene in China. Transbound. Emerg. Dis.. doi: 10.1111/tbed.12268 [DOI] [PubMed] [Google Scholar]
- 41.Zhao Y., Lin Y., Zeng X., Lu C., Hou J.2013. Genotyping and pathobiologic characterization of canine parvovirus circulating in Nanjing, China. Virol. J. 10: 272. doi: 10.1186/1743-422X-10-272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang R., Yang S., Zhang W., Zhang T., Xie Z., Feng H., Wang S., Xia X.2010. Phylogenetic analysis of the VP2 gene of canine parvoviruses circulating in China. Virus Genes 40: 397–402. doi: 10.1007/s11262-010-0466-7 [DOI] [PubMed] [Google Scholar]