Abstract
Background
Information and behaviour can spread through interpersonal ties. By targeting influential individuals, health interventions that harness the distributive properties of social networks may be made more effective and efficient than those that do not.
Methods
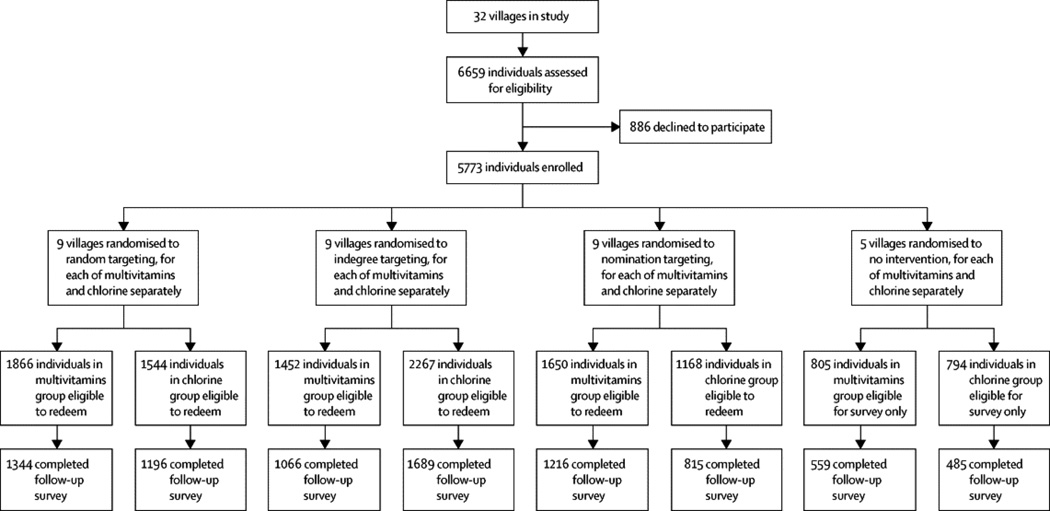
In this block-randomised trial of network targeting methods, we delivered two dissimilar public health interventions to 32 villages in rural Honduras (22–541 participants each; total study population of 5,773): chlorine for water purification, and multivitamins for micronutrient deficiencies. We blocked villages on the basis of network size, socioeconomic status, and baseline rates of water purification. We then randomised villages, separately for each intervention, to one of three targeting methods, introducing the interventions to 5% samples composed either of: (1) randomly selected villagers (n=9 villages for each intervention), (2) villagers with the most social ties (n=9), or (3) nominated friends of random villagers (n=9; the last strategy exploiting the “friendship paradox” of social networks). Primary endpoints were the proportion of available products redeemed by the entire population under each targeting method. Participants and data collectors were not aware of the targeting methods. The trial is registered with ClinicalTrials.gov (NCT01672580).
Findings
For each intervention, 9 villages (each with 1–20 initial target individuals) were randomised to each of the three targeting methods. Targeting the most highly connected individuals produced no greater adoption of the interventions than random targeting. Targeting nominated friends, however, increased adoption of the nutritional intervention by 12·2% compared to random targeting (95% CI, 6·9 to 17·9).
Interpretation
Introducing a health intervention to the nominated friends of random individuals can enhance that intervention’s diffusion by exploiting intrinsic properties of human social networks. This method has the additional advantage of scalability because it can be implemented without mapping the network. Deploying certain types of health interventions via network targeting, without increasing the number of individuals targeted or the resources used, may enhance the adoption and efficiency of those interventions, thereby improving population health.
Funding
NIH, Bill and Melinda Gates Foundation, Star Family Foundation, and the Canadian Institutes of Health Research. We thank The Clorox Company and Tishcon Corporation for their donations of supplies used in the study in Honduras.
INTRODUCTION
Advances in understanding of the structure1–3 and function4,5 of social networks have opened new frontiers for interventions to improve the health of individuals and populations.6–9 Since knowledge and behaviour can spread across interpersonal ties,10,11 and since the networks formed by such ties tend to amplify this spread,12–14 changes in one person’s behaviour can cascade out across a social network, producing behavioural changes in other people in the population-at-large. Such cascades offer the prospect of increasing the effectiveness of public health campaigns that seek to disseminate salubrious practices, and could prove especially beneficial in low-resource settings.15 Here, we report a randomised controlled trial of strategies designed to maximize the likelihood of such behavioural cascades.
Deliberately fostering cascade effects requires the identification of potentially influential individuals among whom to launch an intervention. However, whom in a social network to target with the relevant knowledge or behaviour so as to maximize such diffusion is not clear. Simulation results suggest, for instance, that targeting highly connected (or high “degree”) individuals in networks could enhance the population-level efficacy of prophylactic interventions.16,17 Other research, meanwhile, suggests more complex methods for the optimal targeting of interventions.14,18,19
Most such methods require mapping whole social networks to identify targets. Such mapping is costly, time-consuming, and often infeasible in real-world, face-to-face situations. If network analysis is to meaningfully inform the design of policy and interventions, then simple, cost-effective procedures must be developed to identify structurally influential individuals without mapping their entire networks. We therefore explore both a conventional measure of network centrality (“indegree:” the number of times a person is named as a social contact by other people), and an alternative strategy that does not require ascertainment of global network structure (namely, seeding a network via the friends of randomly selected individuals). The latter strategy exploits the so-called “friendship paradox” of human social networks: on average, the friends of randomly selected individuals are more central in the network than the individuals who named them; colloquially, “your friends have more friends than you do.”7,20 But despite its theoretical promise and its demonstrated efficacy in the early detection of outbreaks,7 friendship nomination, to our knowledge, has never been tested experimentally as a targeting strategy for a real-world network intervention. If effective, this strategy would identify targets likely to foster cascades without mapping whole networks, a crucial condition for scalable network strategies in resource-limited settings.
We therefore conducted a randomised controlled trial of network targeting algorithms using two common but dissimilar public health interventions: chlorine for water purification, and multivitamins for micronutrient deficiencies. Thirty-two villages in rural Honduras were randomly assigned to one of three targeting methods. In some villages, interventions were introduced to individuals occupying the highest indegree positions in the network. In other villages, interventions were introduced to the nominated friends of randomly chosen individuals. In still other villages, targets were selected randomly. In the weeks following the introduction of the interventions, we tracked the diffusion of products and knowledge among all villagers, targeted and untargeted.
METHODS
Overview
Our objective is to assess which targeting methods produce the greatest cascades or spillover effects and hence maximise population-level behaviour change. In comparable villages, we delivered the same public health interventions to the same fraction of the population (5%), varying only the method of selecting targets. Since we are interested in the production of spillovers that change the knowledge and behaviour of untargeted individuals, we prospectively followed all members of all villages to measure their knowledge and behaviour with respect to the interventions.
Study Setting
We conducted our study in 32 villages of the Department of Lempira, Honduras. Lempira is a rural, mountainous, coffee-growing region in which geographic barriers and limited transportation tend to isolate villages from one another. The villages ranged in size from 22–541 residents. Between 79·5% and 96·8% of all adults in each village participated in the study, with an average participation rate of 86·7%. Average age was 35 years, 20·1% of respondents were unmarried, and respondents had an average of 4·2 years of formal schooling. A total of 5,773 individuals participated in the study.
We measured the entire social network of each village by asking all residents to identify spouses, siblings, and friends from a photographic census (see Supplementary Information, SI). We subsequently conducted a public health needs assessment with community leaders, who identified diarrheal illness and nutritional deficiencies as prevalent local health concerns; we therefore selected chlorine for water purification and multivitamins for micronutrient deficiencies as the public health interventions to deploy.
We conducted a baseline survey to evaluate knowledge, attitudes, and behaviours surrounding water purification and nutrition, and followed villagers (whether targeted or not) for their knowledge and behaviours (see SI).
The study was approved by our institutional review board; all participants provided informed consent. Our protocol was registered on clinicaltrials.gov (NCT01672580) and we did not deviate from our original analytic plan.
Blocking, Randomisation, and Intervention
In the design of this study, we used design-of-experiments principles, including randomisation, blocking, and the ideas of orthogonalisation and balanced treatment assignments (applied with respect to the handling of the two interventions – see SI) to obtain precise and unbiased estimates of the effects of three network targeting strategies (random, highest indegree, and friendship nomination) on the diffusion and uptake of two health interventions (multivitamins for micronutrient deficiencies, and chlorine for water purification). We randomised villages to targeting mechanisms in order to ensure that the distribution of potential (observed and unobserved) confounding variables would be the same (in expectation) across the villages.
To better isolate the effect of targeting method from potential village-level influences on product adoption, which in 32 villages could be unevenly distributed across targeting methods by chance alone, we divided the 32 villages into eight blocks on the basis of network size, mean socioeconomic status, and baseline rates of water purification (see SI). We used the results of a factor analysis to form an aggregate score that explained most of the variance in the three blocking variables. We then found the assignment of villages to blocks that minimised the ratio of within-block to between-block variance in the distribution of the composite score (see SI).
After blocking, we randomised each village in each block to targeting methods for multivitamins and chlorine (indegree targeting, nominated friends targeting, random targeting, or no intervention), using a fractional-factorial design (see SI for details). That is, for each of the two interventions separately, nine of the 32 villages were targeted randomly, nine by highest indegree, and nine by the nominated friends technique. Six villages received only one intervention and two villages received neither intervention. For each village receiving both multivitamin and chlorine interventions, we used a different targeting method for each intervention. Participants and data collectors were not aware of the targeting methods.
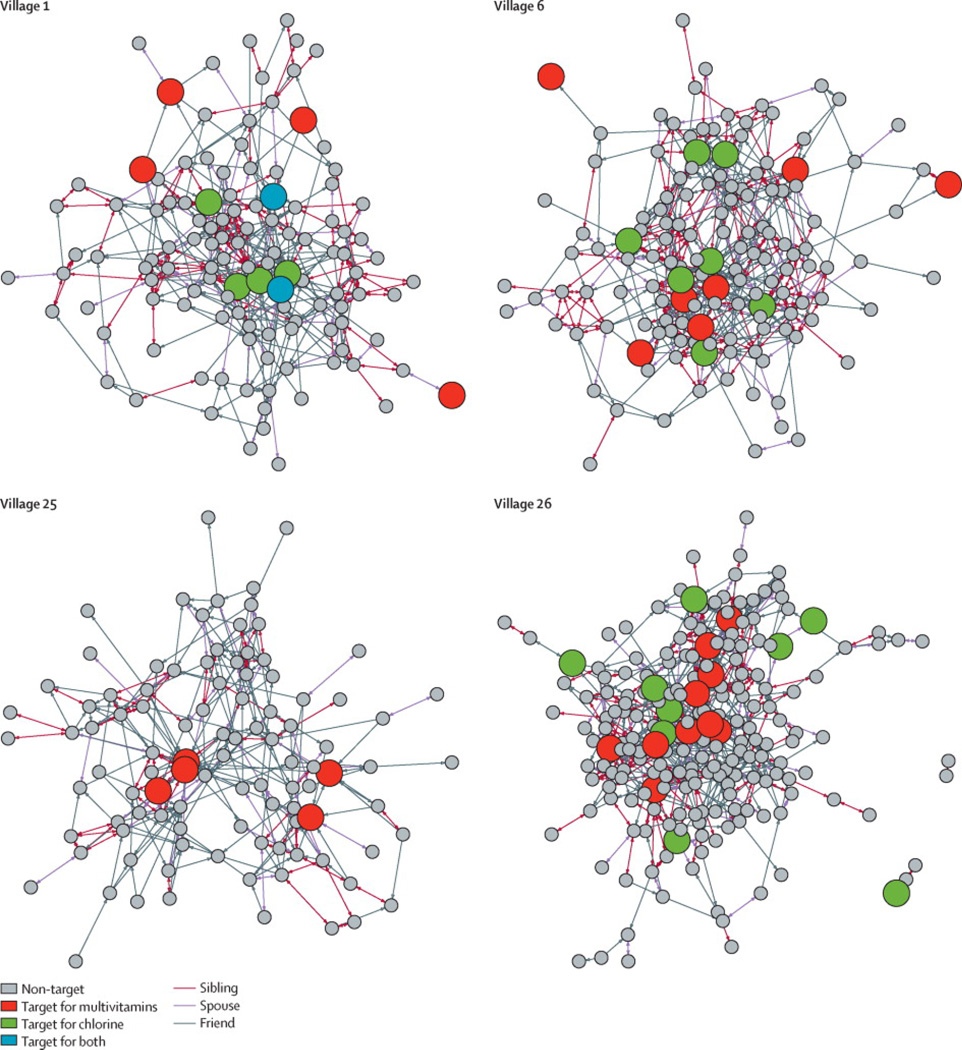
In indegree-targeted villages, we targeted the 5% of villagers named as a contact most often by others in their village. In the nomination-targeted villages, we targeted a 5% sample of villagers composed of one randomly chosen friend nominated by each member of a 5% random sample of villagers. In the randomly targeted villages, we targeted a random 5% sample of villagers (see SI for details of targeting algorithms). Figure 1 shows the targeting of each intervention for a representative block of villages.
FIGURE 1. Targeting of interventions for one block of villages.
Within each block of four villages, we randomly assigned each village to a targeting method (indegree, nominated, random, or none). We did this for each of the two interventions in a fractional factorial design (see SI). In the indegree-targeted villages, we targeted the 5% of villagers with highest indegree. In the nomination-targeted villages, we targeted one randomly chosen friend nominated by each member of a 5% random sample of villagers. In the randomly targeted villages, we targeted a 5% random sample of villagers. In the villages receiving both interventions (chlorine and vitamins), a small proportion of villagers were drawn, by chance, as targets for both interventions (e.g., two blue nodes in Village 1). In the block shown, Village 1 received multivitamins (red nodes) by random targeting and chlorine (green nodes) by nomination targeting. Village 6 received multivitamins by nomination targeting and chlorine by indegree targeting. Village 25 received multivitamins only, by indegree targeting. Village 26, finally, received multivitamins by indegree targeting and chlorine by random targeting. Some visible groups of siblings are not fully inter-connected given a deliberate feature of the “name generator” used to map the network (see SI).
Over the course of a single day for each village, we delivered to each targeted individual an intervention consisting of a health product, instructions for use, and an educational component (see SI). We also gave targeted individuals supplementary information about the interventions that was not generally known at baseline or circulated by other means, and asked them to relay this information to others, allowing us to track the diffusion of knowledge as well as of product adoption by the study’s completion. Multivitamin targets received 60 adult multivitamins (see SI for formulation). Chlorine targets received a 250 ml plastic bottle of sodium hypochlorite with a medicine dropper. A small proportion of villagers were drawn, by chance, as targets for both products, and so received both (see SI).
Each targeted villager also received four tickets to distribute to friends or family outside the household but within the village, who could in turn redeem the ticket for the same product at a local store for a nominal fee to the shopkeeper. Targets were asked to instruct the villagers to whom they gave tickets about the correct usage and benefits of the product. Each of these initial (“first wave”) ticket redeemers received, with their product, a packet of 4 additional (“second wave”) tickets for distribution to additional villagers. Each ticket was uniquely identified and was signed, dated, and checked by a participating shopkeeper against a list of eligible study participants upon redemption, which enabled us to track the diffusion of products through the village networks over time.
Follow-up Survey
We used ticket redemption for multivitamins and chlorine as our primary measure of behaviour because ticket redemption was the most accurately and comprehensively recorded measure of product uptake (i.e., we know the identity without exception of every individual who redeemed a ticket, and the exact date on which she did so), and because it allowed us to trace with the greatest social and temporal resolution the rate and extent of product diffusion through the village networks, without relying on participants’ recollection or self-report. We supplemented this “hard” behavioural measure (ticket redemption) with self-reports of knowledge and practice as well, conducting an extensive follow-up survey in all villages, six weeks after the interventions, in which we asked villagers (whether or not they had redeemed a ticket) about their use of the products, their attitudes concerning the products’ utility and effectiveness, and a series of factual questions about their correct usage and benefits (from which the knowledge scores, our secondary outcomes, were derived, as detailed in the SI).
After completion of the entire trial, we donated additional multivitamins and other supplies to all villages in the study.
Data and Analysis
We evaluated the effect of targeting method at both the population and the individual level. To assess the effect of targeting method on the diffusion of interventions at the population level, we calculated, for each day after the initial delivery of interventions to the targeted villagers, the proportion of available tickets redeemed for each product in each group of villages (i.e., indegree, nomination, and random targeting). This was our primary measure of behaviour change. To assess knowledge transmission, we formed composite 0–10 scores for each intervention, using the first component from a principal components analysis of the knowledge and usage questions from the follow-up survey (see SI). We then calculated, for each intervention, the proportion of respondents in each treatment arm (indegree, nomination, and random) achieving scores in the top quartile; other measures of knowledge produced similar results (see SI). We used chi-square tests to assess whether there was any difference in the proportions of tickets redeemed or high scores attained across the three treatment arms for each intervention.
In order to ensure that our aggregate results were not driven by variation in individual-level or village-level characteristics other than targeting method, we also estimated the effect of targeting method on the hazard of individual tickets being redeemed, using mixed-effects Cox models to control for both individual- and village-level characteristics, and controlling for possible interference between the two interventions in villages receiving both (see SI). We also estimated the effect of targeting method on knowledge transmission with multilevel logit models of high knowledge score attainment, again controlling for both individual- and village-level characteristics (see SI).
We account for the presence of simultaneous interventions in certain villages in both the design and the analysis of the study. At the design stage, we use multiple instances of all targeting methods for both interventions, and permute the combinations across villages so as to best mitigate potential interference between interventions, and to allow for simple interference tests to be performed (see SI). In our mixed-effect models of ticket redemption (Tables S4 and S5), we control for interference between the two interventions by including a ticket redeemer's redemption of a ticket for the opposite intervention as a time-varying covariate (see SI). For models of high knowledge score attainment (Table S6), we estimate the effects of the targeting methods of both products on the attainment of a high knowledge score for either product.
We calculated confidence intervals for the ratios of binomial parameters (such as ticket redemption rates) using a skewness-corrected likelihood score-based method,21 and for the ratios of mean target group properties using a method adjusted for heteroscedasticity.22 All analyses were performed in R (3.1).
Role of the Funding Source
The trial was an investigator-initiated study supported by grants from the NIH, the Bill and Melinda Gates Foundation, the Star Family Foundation, and the Canadian Institutes of Health Research. None of the funding sources had any role in the design, conduct, or analysis of the study, the writing of the manuscript, or the decision to submit it for publication. All authors had full access to all the data in the study. Nicholas A. Christakis had final responsibility for the decision to submit for publication.
RESULTS
Consistent with network theory, the targeting methods succeeded in identifying different segments of the village networks in the three treatment arms of the study. Targets in indegree-targeted villages had 2·2 times (95% confidence interval [CI], 2·0 to 2·5) the mean indegree of randomly chosen targets, and 1·6 times (95% CI,1·4 to 1·9) the mean indegree of targets chosen by the nomination method. These nominated targets, meanwhile, had 1·4 times (95% CI, 1·2 to 1·6) the mean indegree of random targets.
We evaluated the basic diffusion of the products by calculating, for each day after initial targeting of the networks, the proportion of available tickets redeemed by eligible villagers. Each of these tickets was redeemed by a study participant who had received a ticket from a targeted villager or from a first-wave ticket redeemer. Both waves of ticket redemption are pooled in our population-level analyses.
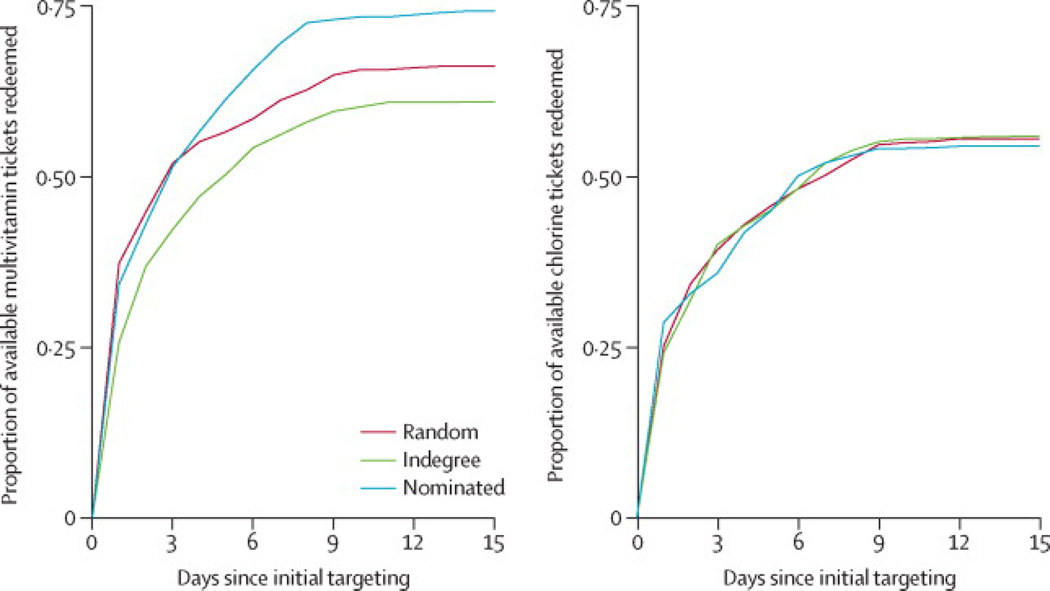
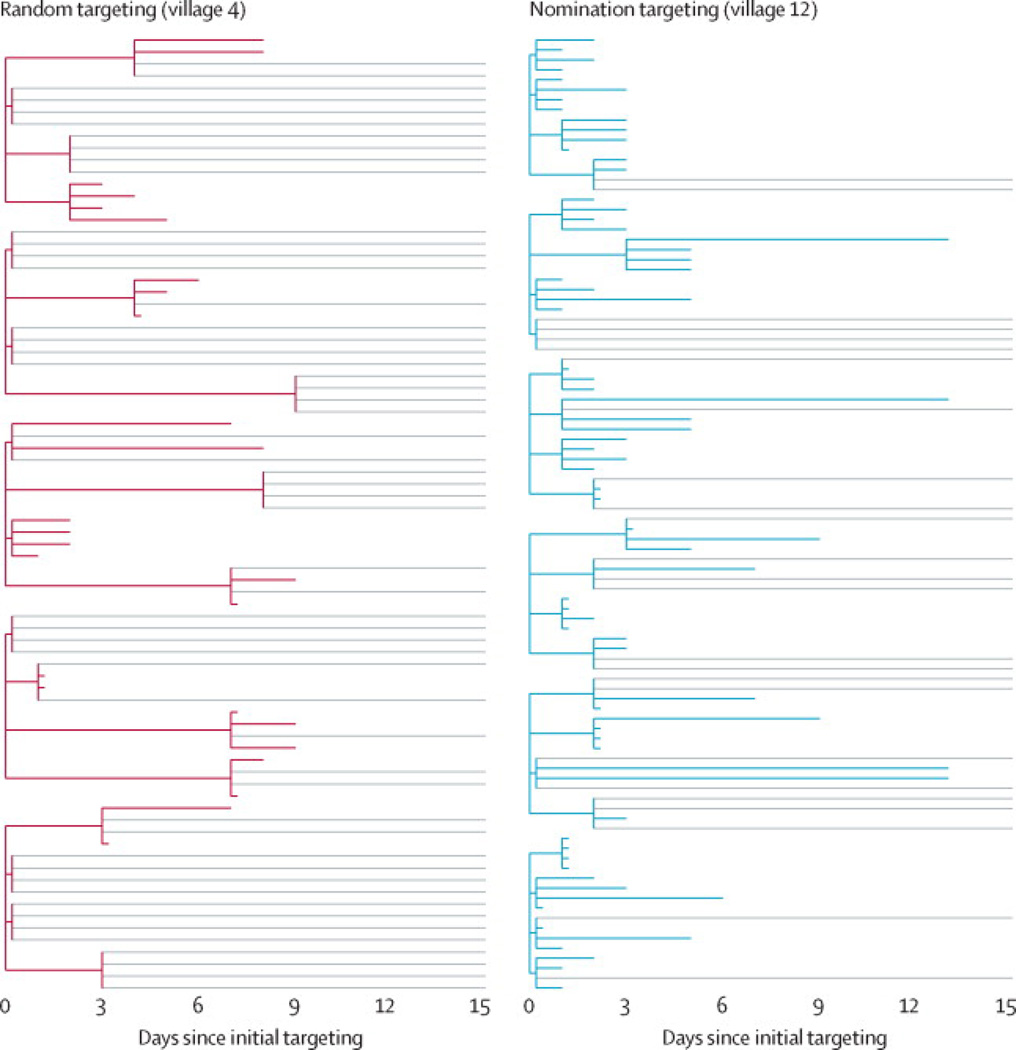
First, we evaluated ticket redemption rates (representing product adoption) and knowledge scores at follow-up (representing knowledge diffusion) for the multivitamin intervention (Figure 2, left panel). In nomination-targeted villages, 74·3% of available multivitamin tickets were redeemed, compared to 66·2%in randomly targeted villages and 61·0% in indegree-targeted villages. All pairwise differences in redemption rates are statistically significant (p< 0·01) after correction for multiple comparisons (see SI). Hence, targeting nominated friends increased population-level adoption of a nutritional intervention by 12·2% (95% CI,6·9 to 17·9), an 8·1 percentage point increase in product adoption. Figure 3 illustrates the effect of targeting method on product diffusion cascades for two representative villages: compared to random targeting, nomination targeting produced both more rapid and more thorough adoption of the multivitamin intervention.
FIGURE 2. Diffusion of interventions.
The left panel shows the pooled proportion of available multivitamin tickets redeemed by day after initial targeting, by treatment arm. The right panel shows the equivalent measure for the chlorine intervention.
FIGURE 3. Multivitamin diffusion through two representative villages.
In all villages receiving a given intervention, 5% of the adult population was initially targeted with the intervention. The left panel shows the diffusion of multivitamins in a village whose five initial targets were selected at random. The right panel represents a village of comparable size whose six initial targets were the nominated friends of random villagers. Every targeted villager received four tickets to distribute to contacts outside the household, who could in turn redeem the ticket for the same product and for four additional tickets to give to others. The colored lines represent completed ticket-redemption paths: shorter lines represent tickets redeemed more quickly. Light gray lines represent available tickets that were not redeemed. Compared to the randomly targeted village, multivitamins in the nomination-targeted village diffused more rapidly (the average time-to-redemption of first-wave tickets is substantially shorter) and more completely (a greater proportion of available tickets was ultimately redeemed).
These results concord with our multilevel Cox models of ticket redemption, in which we estimate a hazard ratio of 1·65 (95% CI, 1·10 to 2·47) for redemption of first-wave tickets under nomination targeting, compared to random targeting, and controlling for individual-target-level and village-level characteristics (see SI). For the redemption of second-wave tickets, our estimated hazard ratio of 1·15 under nomination targeting compared to random targeting does not reach statistical significance (p=0·54); a larger sample may have allowed us to detect effects out to two degrees of separation from the initial targets.
With respect to knowledge, 30·8% of untargeted ticket recipients attained high knowledge scores in nomination-targeted villages, compared to 27·6% in both indegree and randomly targeted villages. Though the differences in aggregate proportions are not statistically significant (p>0·05), a multilevel logit model controlling for both individual-level and village-level characteristics reveals a positive and statistically significant effect of nomination targeting (compared to random targeting) on the attainment of high multivitamin knowledge scores (OR=1·66, 95% CI,1·02 to 2·70).
Conditional on ticket redemption, there was no significant variation in self-reported product use or belief in the products’ effectiveness by targeting method (random, indegree, or friend nomination). Self-reported continued product use among confirmed ticket redeemers was uniformly high (>90%). These results suggest that ticket redemption (in which we did observe significant variation by targeting method, both at the population level, as well as in mixed-effects models accounting for individual-level and village-level covariates and for possible interference between interventions) is indeed a valid omnibus measure of product adoption and continued use, while the knowledge scores we report provide additional data about the differential spread of health information not predicted by product use alone.
We accounted for potential interference between the two interventions at both the village and the individual level. All such robustness tests support a positive effect of nomination targeting on the uptake of the multivitamin (MVI) intervention (see SI). The mean village-level MVI ticket redemption rate is statistically indistinguishable between those villages that received both MVI and chlorine interventions (on average, 73.1% of these villages’ available MVI tickets were redeemed), and those villages that received the MVI intervention alone (on average, 73.8% of these villages’ available MVI tickets were redeemed); note that these are village-level redemption rates, as distinct from the pooled proportions depicted in Figure 2. Likewise, controlling for possible interference in our mixed-effect models does not alter the trend we observe at the village level (see SI).
Finally, we performed corresponding analyses for the chlorine intervention (Figure 2, right panel). For this intervention, final ticket redemption rates were statistically indistinguishable (p>0·05) across targeting methods: 55·6% for random targeting, 55·9% for indegree and 54·5% for nomination. Though we observe the same trend in knowledge scores as for multivitamins (38·4% high scores in nomination-targeted villages, compared to 36·0% and 35·0% in indegree and randomly targeted villages, respectively), the aggregate differences are not statistically significant. Corresponding individual-level models likewise find no significant effect of targeting method on ticket redemption or knowledge scores in the case of the chlorine intervention (p>0·05, see SI).
DISCUSSION
In a randomised controlled trial in 32 villages, we evaluated network-based approaches to maximise population-level behaviour change. Our results yield no evidence that health interventions benefit from targeting only the most highly connected individuals. However, a second technique, in which we selected targets by exploiting the friendship paradox, produced significantly larger cascades of product adoption and health knowledge than either random or indegree targeting. For our nutritional intervention, targeting nominated friends increased population-level behaviour change by 12·2% compared to random targeting; it was also associated with enhanced health knowledge among untargeted individuals.
Of our two network targeting methods, indegree targeting requires the expenditure of substantial resources to map the whole network, since everyone in the population must be asked to whom they are connected in order to identify the individuals receiving the most nominations. It is therefore fortunate that the friendship nomination technique, which exploits the properties of human social networks without requiring that the entire network be mapped, produced the greatest behavioural cascades. Compared to methods requiring whole network (“sociocentric”) data, targeting the nominated friends of a randomly selected group can furnish the benefits of network targeting in a more scalable and less resource-intensive fashion. Indeed, since high-degree individuals in human social networks tend to be friends with one another,3,23 targeting nominated friends may outperform targeting the highest-degree individuals if the latter strategy produces redundant clustering among targets, resulting in an “echo chamber” of influence that fails to reach more dispersed or peripheral parts of the network. Our experimental results concord with recent simulations in which indegree-based targeting produces lower adoption rates than even random targeting.19
Social networks amplify the information and behaviours with which they are seeded,10,11,24 but the nature of this diffusion depends on the innovation being transmitted.6,25,26 The complexity of understanding and implementing a new practice, the visibility of its results, and its perceived advantage over existing methods can all influence adoption patterns.26 Simple information (regarding, for instance, the availability of subsidised multivitamins) can spread by so-called “simple contagion,” requiring only a single contact for transmission between two individuals. Deeper behavioural changes, by contrast, may require reinforcement from multiple social contacts (“complex contagion”),6,25 perhaps because they require significant motivation (as in the case of behaviours like smoking cessation), or because they require changes in longstanding practices and beliefs (as in the case of water chlorination in rural Honduras).
We selected for our study two interventions with very different social and behavioural implications: chlorine is widely available and affordable, even by rural Honduran standards, but its primary use is for washing clothing. Chlorinating water is a relatively complex, multi-step process. Thus, the chlorine intervention demanded the acquisition of new knowledge and a change in the use of a familiar product. By contrast, multivitamins in rural Honduras are easy to use and widely viewed as beneficial, but are relatively more difficult and costly to obtain. The multivitamin intervention, then, demanded substantially less behavioural and ideational change. Consistent with the theory of complex contagion,6,25 these fundamental differences between our two interventions may account in part for the robust success of nomination targeting in increasing the adoption of multivitamins, but not of chlorine for water purification, for which an altogether different targeting method might have been effective.
Diarrheal illness and malnutrition account for a sizeable burden of disease in rural Honduras,27,28 which is why we evaluated water purification and multivitamins in our setting. But countless other health interventions in the developed and developing world stand to benefit from network targeting, including polio vaccination,16,29 anti-malarial bednets, maternal health care, safe sex practices, smoking cessation,30 substance abuse prevention,12 helmet and seatbelt use,8 and many more. The extraordinary variety of health states and behaviours observed to spread through social networks4,5,11 likely means that no single targeting method will prove optimal for all interventions. Simulations16,17,19,29 and observational studies14 suggest strategies to be tested in experiments such as ours, the results of which can in turn inform the design of more accurate models of behavioural cascades. Future research will continue to characterise the targeting methods best suited to different types of interventions, and, though no universally optimal solution is likely to be found, general principles, such as the efficiency of nomination-based targeting for the diffusion of certain types of phenomena, are likely to emerge.
Our study has limitations. First, we used ticket redemption and associated survey responses as our primary measures of behaviour, and did not observe the use of the products in people’s homes. Second, the villages in our study were isolated from one another, which, while ideal for a controlled experiment, could yield different patterns of diffusion than would more intermingled populations. Third, longer-term follow-up in the villages would allow us to determine whether the knowledge and behavioural changes associated with a given targeting method persist over time. While “fadeout” is endemic to certain educational interventions, this effect may vary according to network structure and the targeting method of the intervention.31
Network targeting may prove most useful in settings in which limited resources or infrastructure render broadcast interventions infeasible, but even when networks are more fully characterised (e.g., with “big data” techniques32), methods to efficiently identify hubs of influence, as well as the people around the hubs who are likely to be influenceable, may prove useful in the design of more cost-effective campaigns. In this way, deploying health interventions via network targeting, without increasing the number of people targeted or the expenses incurred, may enhance the spread and adoption of those interventions, and thereby improve population health.
Supplementary Material
RESEARCH IN CONTEXT PANEL.
Systematic Review
We searched Google Scholar for "social network" OR "social networks" OR “network” AND "intervention" OR "trial." From our review of the available literature, we concluded that there have been numerous observational studies of contagion in social networks, but very few efforts to exploit network phenomena to maximise the diffusion of desirable knowledge or behavior with respect to health. The few trials conducted to this end have typically involved either small face-to-face populations or large online networks. We found evidence that a randomised, prospective, large-scale trial of network targeting in a face-to-face population could contribute to a better understanding of social networks and health, with implications for the design of public health interventions in the developed and developing world.
Interpretation
In 32 villages, we evaluated network-based approaches to maximise population-level behaviour change. For a nutritional intervention, targeting the friends of randomly selected individuals produced significantly greater population-level adoption and health knowledge than targeting either random or highly connected individuals. This method has the advantage of scalability, because it can be implemented in the field without mapping the network. Our findings suggest that network targeting can be used to efficiently increase the adoption of certain types of public health interventions. Further trials will be needed to characterise the targeting methods best suited to different classes of interventions.
Acknowledgements
We thank Laurie Meneades, Peter Treut, Jen Zhu, Michael DeWit, Bret Abel, Tom Keegan, and our local community health workers and leaders in Honduras—BanyCarballo, MirnaCastellanos, Orlin Chavez, Martina Hernandez, Marco Antonio Miranda, and Dilcia Ponce—for the expert assistance required to collect and assemble the dataset.
FUNDING SOURCES
Dr. Christakis reports grants from the National Institutes of Health (P-01 AG031093, P-30 AG034420), from the Bill and Melinda Gates Foundation, and support from the Star Family Foundation. Mr. Kim was supported by the Canadian Institutes of Health Research and Ms. Hwong was partially supported by NIH F30 AG046978. We thank The Clorox Company and Tishcon Corporation for their donations of supplies used in the study in Honduras.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Trial Registration: clinicaltrials.gov NCT01672580
AUTHORS’ CONTRIBUTIONS
David A. Kim: Study design, data collection, data analysis, data interpretation, literature search, figures, writing
Alison R. Hwong: Study design, data collection, literature search
Derek Stafford: Study design, data collection
D. Alex Hughes: Study design, data collection
A. James O’Malley: Study design, data analysis, data interpretation
James H. Fowler: Study design, data analysis, data interpretation, figures, writing
Nicholas A. Christakis: Study design, data analysis, data interpretation, literature search, figures, writing
DECLARATION OF INTERESTS
ICMJE Conflict of Interest Statements for each author are submitted separately.
Nicholas Christakis had full access to all the data in the study and had final responsibility for the decision to submit for publication.
ETHICS COMMITTEE APPROVAL
The study was approved by the Institutional Review Board of Harvard Medical School.
Contributor Information
David A. Kim, Interfaculty Initiative in Health Policy, Harvard University, 14 Story St., 4th Floor, Cambridge MA 02138, USA.
Alison R. Hwong, Interfaculty Initiative in Health Policy, Harvard University, 14 Story St., 4th Floor, Cambridge MA 02138, USA.
Derek Stafford, Yale Institute for Network Science, Yale University, PO Box 208263, New Haven CT 06520, USA.
D. Alex Hughes, Department of Political Science, University of California, San Diego, 9500 Gilman Drive #0521, La Jolla CA 92093, USA.
A. James O’Malley, Dartmouth Institute of Health Policy and Clinical Practice, Geisel School of Medicine at Dartmouth, 35 Centerra Parkway, Lebanon NH 03766, USA
James H. Fowler, Departments of Political Science and Medical Genetics, University of California, San Diego, 9500 Gilman Drive #0521, La Jolla CA 92093, USA
Nicholas A. Christakis, Departments of Medicine, Ecology and Evolutionary Biology, Biomedical Engineering, and Sociology, Yale University, PO Box 208263, New Haven CT 06520, USA
REFERENCES
- 1.Newman MEJ, Barabási A-L, Watts DJ. The structure and dynamics of networks. Princeton: Princeton University Press; 2006. [Google Scholar]
- 2.Barabási A-L, Albert R. Emergence of Scaling in Random Networks. Science. 1999;286(5439):509–512. doi: 10.1126/science.286.5439.509. [DOI] [PubMed] [Google Scholar]
- 3.Apicella CL, Marlowe FW, Fowler JH, Christakis NA. Social networks and cooperation in hunter-gatherers. Nature. 2012;481(7382):497–501. doi: 10.1038/nature10736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Christakis NA, Fowler JH. The Spread of Obesity in a Large Social Network over 32 Years. New England Journal of Medicine. 2007;357(4):370–379. doi: 10.1056/NEJMsa066082. [DOI] [PubMed] [Google Scholar]
- 5.Christakis NA, Fowler JH. The Collective Dynamics of Smoking in a Large Social Network. New England Journal of Medicine. 2008;358(21):2249–2258. doi: 10.1056/NEJMsa0706154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Centola D. The Spread of Behavior in an Online Social Network Experiment. Science. 2010;329(5996):1194–1197. doi: 10.1126/science.1185231. [DOI] [PubMed] [Google Scholar]
- 7.Christakis NA, Fowler JH. Social Network Sensors for Early Detection of Contagious Outbreaks. PLoS One. 2010;5(9):e12948. doi: 10.1371/journal.pone.0012948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Valente TW. Network Interventions. Science. 2012;337(6090):49–53. doi: 10.1126/science.1217330. [DOI] [PubMed] [Google Scholar]
- 9.Gardy JL, Johnston JC, Sui SJH, et al. Whole-Genome Sequencing and Social-Network Analysis of a Tuberculosis Outbreak. New England Journal of Medicine. 2011;364(8):730–739. doi: 10.1056/NEJMoa1003176. [DOI] [PubMed] [Google Scholar]
- 10.Christakis NA, Fowler JH. Connected: The Surprising Power of Social Networks and How They Shape Our Lives. New York: Little, Brown & Company; 2009. [Google Scholar]
- 11.Fowler JH, Christakis NA. Cooperative behavior cascades in human social networks. Proc Natl Acad Sci USA. 2010 doi: 10.1073/pnas.0913149107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Valente TW, Ritt-Olson A, Stacy A, Unger JB, Okamoto J, Sussman S. Peer acceleration: effects of a social network tailored substance abuse prevention program among high-risk adolescents. Addiction. 2007;102(11):1804–1815. doi: 10.1111/j.1360-0443.2007.01992.x. [DOI] [PubMed] [Google Scholar]
- 13.Rand DG, Arbesman S, Christakis NA. Dynamic social networks promote cooperation in experiments with humans. Proc Natl Acad Sci USA. 2011;108(48):19193–19198. doi: 10.1073/pnas.1108243108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Banerjee A, Chandrasekhar AG, Duflo E, Jackson MO. The Diffusion of Microfinance. Science. 2013;341 doi: 10.1126/science.1236498. [DOI] [PubMed] [Google Scholar]
- 15.Merzel C, D’Afflitti J. Reconsidering Community-Based Health Promotion: Promise, Performance, and Potential. American Journal of Public Health. 2003;93(4):557–574. doi: 10.2105/ajph.93.4.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pastor-Satorras R, Vespignani A. Immunization of complex networks. Physical Review E. 2002;65(3):036104. doi: 10.1103/PhysRevE.65.036104. [DOI] [PubMed] [Google Scholar]
- 17.Bahr DB, Browning RC, Wyatt HR, Hill JO. Exploiting Social Networks to Mitigate the Obesity Epidemic. Obesity. 2009;17(4):723–728. doi: 10.1038/oby.2008.615. [DOI] [PubMed] [Google Scholar]
- 18.Aral S, Muchnik LEV, Sundararajan A. Engineering social contagions: Optimal network seeding in the presence of homophily. Network Science. 2013:1–29. FirstView. [Google Scholar]
- 19.Cho Y, Hwang J, Lee D. Identification of effective opinion leaders in the diffusion of technological innovation: A social network approach. Technological Forecasting and Social Change. 2012;79(1):97–106. [Google Scholar]
- 20.Feld SL. Why Your Friends Have More Friends Than You Do. The American Journal of Sociology. 1991;96(6):1464–1477. [Google Scholar]
- 21.Gart JJ, Nam J-m. Approximate Interval Estimation of the Ratio of Binomial Parameters: A Review and Corrections for Skewness. Biometrics. 1988;44(2):323–338. [PubMed] [Google Scholar]
- 22.Hasler M, Vonk R, Hothorn LA. Assessing non-inferiority of a new treatment in a three-arm trial in the presence of heteroscedasticity. Statistics in Medicine. 2008;27(4):490–503. doi: 10.1002/sim.3052. [DOI] [PubMed] [Google Scholar]
- 23.Newman MEJ. Assortative Mixing in Networks. Physical Review Letters. 2002;89(20):208701. doi: 10.1103/PhysRevLett.89.208701. [DOI] [PubMed] [Google Scholar]
- 24.Bond RM, Fariss CJ, Jones JJ, et al. A 61-million-person experiment in social influence and political mobilization. Nature. 2012;489(7415):295–298. doi: 10.1038/nature11421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Centola D, Macy Michael W. Complex Contagions and the Weakness of Long Ties. American Journal of Sociology. 2007;113(3):702–734. [Google Scholar]
- 26.Rogers E. Diffusion of innovations. New York: Free Press; 2003. [Google Scholar]
- 27.World Health Organization. Country profile of environmental burden of disease: Honduras. [accessed September 8 2013]; http://www.who.int/quantifying_ehimpacts/national/countryprofile/honduras.pdf.
- 28.Solórzano GJO, Molina IB, Turcios-Ruiz RM, et al. Burden of diarrhea among children in Honduras, 2000–2004: estimates of the role of rotavirus. Rev Panam Salud Publica. 2006;20(6):377–384. doi: 10.1590/s1020-49892006001100003. [DOI] [PubMed] [Google Scholar]
- 29.Cohen R, Havlin S, ben-Avraham D. Efficient Immunization Strategies for Computer Networks and Populations. Physical Review Letters. 2003;91(24):247901. doi: 10.1103/PhysRevLett.91.247901. [DOI] [PubMed] [Google Scholar]
- 30.Cobb NK, Graham AL, Abrams DB. Social network structure of a large online community for smoking cessation. 2010;100(7):1282–1289. doi: 10.2105/AJPH.2009.165449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cascio EU, Staiger DO. Knowledge, Tests, and Fadeout in Educational Interventions. National Bureau of Economic Research Working Paper Series. 2012 No. 18038. [Google Scholar]
- 32.Lazer D, Pentland A, Adamic L, et al. Computational Social Science. Science. 2009;323(5915):721–723. doi: 10.1126/science.1167742. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.