Abstract
Background
Infection with Human Immunodeficiency virus (HIV) is an important risk factor for Tuberculosis (TB). Anti-Retroviral Therapy (ART) has improved the prognosis of HIV and reduced the risk of TB infected patients. Isoniazid Preventive Therapy (IPT) aims to reduce the development of active TB in patients with latent TB.
Objective
Systematically review and synthesize effect estimates of IPT for TB prevention in adult HIV patients. Secondary objectives were to assess the effect of IPT on HIV disease progression, all-cause mortality and adverse drug reaction (ADR).
Search Strategy
Electronic databases were searched to identify relevant articles in English available by September 11th 2015.
Selection Criteria
Research articles comparing IPT to placebo or no treatment in HIV infected adults using randomized clinical trials.
Data Analysis
A qualitative review included study-level information on randomization and treatment allocation. Effect estimates were pooled using random-effects models to account for between-study heterogeneity.
Main Results
This review assessed ten randomized clinical trials that assigned 7619 HIV patients to IPT or placebo. An overall 35% of TB risk reduction (RR = 0.65, 95% CI (0.51, 0.84)) was found in all participants, however, larger benefit of IPT was observed in Tuberculin Skin Test (TST) positive participants, with pooled relative risk reduction of 52% [RR = 0.48; 95% CI (0.29, 0.82)] and with a prediction interval ranging from 0.13 to 1.81. There was no statistically significant effect of IPT on TB occurrence in TST negative or unknown participants. IPT also reduced the risk of HIV disease progression in all participants (RR = 0.69; 95% CI (0.48, 0.99)) despite no benefits observed in TST strata. All-cause mortality was not affected by IPT although participants who had 12 months of IPT tend to have a reduced risk (RR = 0.65; 95% CI(0.47, 0.90)). IPT had an elevated, yet statistically non-significant, risk of adverse drug reaction [RR = 1.20; 95% CI (1.20, 1.71)]. Only a single study assessed the effect of IPT in combination with ART in preventing TB and occurrence of multi-drug resistant tuberculosis.
Conclusions
IPT use substantially contributes in preventing TB in persons with HIV in general and in TST positive individuals in particular. More evidence is needed to explain discrepancies in the protective effect of IPT in these individuals.
Introduction
Mycobacterium tuberculosis (TB) is the most common cause of bacterial infection in humans[1–3] and is globally a leading cause of morbidity and mortality, especially in developing countries.[4] Human Immunodeficiency Virus (HIV) infection is the strongest risk factor for TB and over 4 million people are co-infected with both organisms, the majority of whom reside in Africa.[5] Such co-infection worsens the prognosis of HIV infection by increasing HIV replication[6–8] and may result in rapid progression of HIV and subsequent immunosuppression,[9–12] and a higher risk of acquiring other, potentially lethal, opportunistic infections.[13], [14]
Randomized controlled trials have demonstrated that a course of Isoniazid Preventive Therapy (IPT) reduces the incidence of TB disease in HIV-negative populations at risk of developing active disease.[15] In HIV infected patients, IPT reduced reactivation of latent TB infection, both in industrialized countries[16–18] as well as in developing countries.[19–21] Also, observational studies in HIV-positive injecting drug users (IDU) suggested a potential benefit of IPT.[16], [17], [22–24]. The benefit appears to be higher in Tuberculin Skin Test (TST) positive patients than in TST negatives.[25] In addition, recent observational studies reported that the benefit of IPT increased if it is delivered in combination to ART.[26–28]
A previous meta-analysis found that IPT was efficacious in TST positive participants in reducing risk of TB.[29–31] Based on these data, the World Health Organization (WHO) in 2004 recommended IPT for HIV-infected persons[32–34] and most high TB/HIV burden countries have adopted antiretroviral therapy (ART) and the WHO recommended TB/HIV collaborative activities including IPT since 2005. However, previous meta-analyses did not include studies with participants on ART nor assessed the effect of IPT in different dose and duration strata.[35] In addition, the recently updated WHO guidelines[36] does not precisely outline the optimal dosing and duration of IPT treatment in patients receiving ART.[30]
This study aims to synthesize evidence from randomized controlled trials regarding the protective effect of IPT on all-type or confirmed TB in HIV infected patients, and assess the combined benefit of ART an IPT on prevention of active TB. Secondary objectives were to assess the effect of IPT on HIV disease progression, all-cause mortality and adverse drug reaction (ADR) as well as to determine the effect of IPT in strata of TST positive and negative patients, and by dosing and duration of the IPT regimen.
Methods
Eligibility and selection
This systematic review included clinical trials assessing the effect of IPT on prevention of active TB or reactivation of latent TB disease in adults living with HIV with or without ART. Studies were eligible when the intervention (IPT) was applied to adult HIV patients (excluding pregnant women), had at least one year follow-up after finishing IPT and used a randomized study design. Furthermore, reported data included the occurrence of active TB disease.
Search methods for the identification of studies
A comprehensive search of PubMed, Excerpta Medica dataBASE (EMBASE), the Cochrane Central Register of Controlled Trials (CENTRAL) and Cumulative Index of Nursing and Allied Health (CINAHL) was performed to identify all relevant studies in the English language available from the start of MEDLINE to September 11th 2015. Both text words in title, abstract and medical subject heading (MeSH) terms were used in varying combinations. The literature search strategy was adapted to suit each database (Table 1). Moreover, the website of Health Internet Internetwork and Research Initiative (HINARI) and Google Scholar databases were checked for potential eligible studies.
Table 1. Search strategy.
| Domain, intervention, outcome and design | ||||
|---|---|---|---|---|
| HIV/AIDS (1) | Isoniazid (2) | Tuberculosis (3) | Randomization (4) | |
| Search terms | Human immunodeficiency OR virus, OR HIV, OR Acquired immunodeficiency syndrome, OR AIDS | Isoniazid, INH, OR Prevention, OR Preventive, OR Prophylaxis, OR Prophylactic | Tuberculosis OR TB | Randomized OR Randomization |
| Search fields | MeSH AND Title/Abstract | MeSH AND Title/Abstract | MeSH AND Title/Abstract | MeSH AND Title/Abstract |
| Final search | (((#1) AND #2) AND #3) AND #4 | Date of search closure at September 11th 2015 | ||
HIV: Human Immunodeficiency Virus; AIDS: Acquired Immunodeficiency Syndrome; INH: Isoniazid; TB: Tuberculosis; MeSH: Medical Subject Headings
Data collection
A pre-defined data extraction tool was used to extract information on IPT and outcome related variables. Details on the intervention included the regimen of the preventive therapy, dosing, duration, randomization processes, follow up periods, and combined use of IPT and ART. In addition, study area, publication year, and methods of outcome ascertainment were extracted. (S1 Table) We assessed comparability of the target population, intervention and outcome across the selected studies.
Outcome
The primary outcome of interest was the onset of active TB disease. The ascertainment was mostly based on detection of acid fast bacilli (AFB), pathology, clinical features and X-ray (all-types of TB) or confirmed by culture positivity of sputum or biopsy (confirmed TB). In addition, HIV disease progression, all-cause mortality and ADR which caused withdrawal of patients from the follow-up were considered as secondary end points.
Determinant
The primary determinant was IPT stratified by TST status. TST reactivity demonstrates a functional anti-mycobacterial response; a TST negative status can therefore indicate a lack of TB exposure, but also result from severe immune deficiency (anergy) with or without underlying latent TB. A TST positive status, therefore, demonstrates TB exposure as well as a preserved immune response. The latter could affect the efficacy of IPT. Analyses were further stratified by dosage and duration of the IPT regimen.
Statistical Methods
The reviewed articles’ unit of analysis was the individual patient. All the randomization and intervention allocation was patient-based. Due to the underlying clinical heterogeneity of selected studies, a random-effects model was used to estimate the pooled effect of IPT, using the method of Hartung and Knapp.[37], [38] The between-study heterogeneity in treatment effect was assessed by the I squared (I2) statistic and 95% prediction intervals (95% PI). The I2 statistic indicates the extent of variability that cannot be explained by sampling error and ranges between 0 and 100%. Conversely, the prediction interval gives an indication of true treatment effect of IPT that can be expected when the intervention is applied to new studies; it thereby provides a sense of the heterogeneity in expected benefits.[39] Furthermore, subgroup analyses were performed and stratified by baseline TST status (positive, negative, and unknown) and dosing and duration of the regimen. MetaAnalyst[40] and R version 3.2.1[41] were used for the data analysis.
Results
Study selection
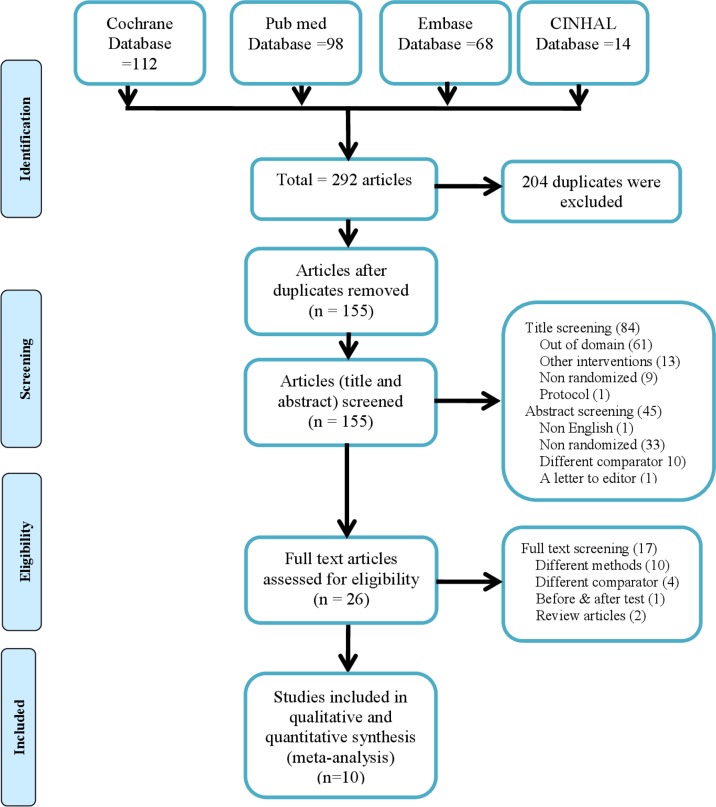
Based on the preset inclusion and exclusion criteria and cross referencing, ten articles were eligible for inclusion in this review from 246 initial hits from four databases. (S1 Text and S2 Text) After de-duplication, 117 studies underwent application of in- and exclusion criteria, leaving ten articles for this meta-analysis. (Fig 1)
Fig 1. Schematic presentation of studies inclusion to the review.
Description of included studies
Of the included studies, eight were conducted in six high TB/HIV burden countries (Uganda, Cote d’Ivoire, Kenya, South Africa (2), Zambia, and Haiti (2))[42] and two were in countries with low TB endemicity (Spain and United States of America (USA)). Table 2 provides details on study characteristics.
Table 2. Description of selected studies for the systematic review and meta-analysis.
| Study population | Exposure | Outcome | Analysis | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Studies | n | Age | TST status | ART use | IPT regimen | Placebo regimen | Duration of follow-up | Method of randomi-zation | Blinding | Ascertainment of TB | Lost to follow-up | Adherence | Other endpoints | Analysis |
| Fitzgerald 2001 (Haiti) | 237 | ≥ 18 | Negative | No | IPT 300 mg + VB6 50 mg daily (12 mo) | VB6 50 mg for (12 mo) | 2.5 years in IPT & 2.4 years in placebo | Unclear | Not clear | Clinical, AFB, Radiology | 23% lost to follow up | No data | all-cause mortality & AIDS | ITT |
| Gordin 1997 (USA) | 517 | ≥ 13 | Negative | No | IPT 300 mg + VB6 50 mg daily (6 mo) | Placebo + VB6 50 mg daily for (6 mo) | 30 months | Unclear | Not clear | Culture, AFB, Radiology, Clinical | 6.2% (IPT), 7% (placebo) | 37% non-adherence | Probable TB, all-cause mortality, AIDS | Not mentioned (PP assumed) |
| Hawken 1997 (Kenya) | 684 | 14–65 | Positive & negative | No | IPT 300 mg daily (6 mo) | Placebo daily (6 mo) | Median 22 months (0–41 months) | Blocked (per 10) | Double blinded | Culture & Histology | 29.8% (at 6 months) | 42% missed 1 week, 27% missed 1 – 4wk and 31% missed > = 5 | AIDS, all-cause mortality, ADR | Not mention-ed (PP assumed) |
| Moham-med 2007 (South Africa) | 98 | ≥ 18 | Negative No | No | IPT 15 mg/kg + VB6 25mg twice weekly (12 mo) | Placebo + VB6 25mg twice weekly (12 mo) | 12 months | Blocked (per 20) | Double blinded | Culture-positive, Radiographic, & AFB | Complete | 15% non-adherence | All-cause mortality, Adherence, ADR | ITT |
| Mwinga 1998 (Zambia) | 1053 | ≥ 15 | Positive & negative | No | IPT 900 mg twice weekly (6 mo) | Placebo twice weekly (6 mo) | 36 months | Blocked (per 30) | Double blinded | AFB, Radiology & Culture | 32.4% (IPT), 30.3% (placebo) | Non-adherence 28% for IPT & 18% for placebo | All-cause mortality & ADR | ITT |
| Pape 1993 (Haiti) | 118 | 18–65 | positive & negative | No | IPT 300 mg + VB6 50 mg daily (12 mo) | VB6 50 mg daily (12 mo) | Placebo 2.45 years & IPT 3.13 years | Computer generated | Double blinded assumed | AFB, Radiology, Histopatho-logy, Culture, Clinical | Unclear | Unclear | AIDS & all-cause mortality | ITT |
| Rangaka (2014) (South Africa) | 1329 | ≥ 18 | Both TST & IGRA positive & negative | Both at ART at base-line | IPT (<50kg 200 mg or ≥50kg 300mg) daily and VB6 (12 mo) | Placebo daily for (6 mo) | >2.5 years (maximum 3.7 years) | Random number generator | Double blinded | AFB, Culture and Histo-pathology | 11% | No data | All-cause mortality & ADR | Modified ITT |
| Rivero 2003 (Spain) | 319 | 18–65 | Negative | No | IPT 5mg/kg (300mg max) daily for 6 months | No IPT | 2 years | Unclear | Open label | AFB, Culture and clinical | 6.1% (IPT) | 79.3% (IPT) | All-cause mortality and ADR | Not mention-ed (PP assumed) |
| TEMPRANO 2015 | 2056 | ≥ 18 | Both IGRA positive & negativeNo TST | Early versus deferred ART | IPT 300 mg for six months | No IPT | 30 months | Stratified, computer generated, sequentially numbered, & bock randomization | Open label | AFB, Culture and clinical | 2.2% (IPT) 3.4% (No IPT) | Deferred IPT 93% Early IPT 94% | Grade III or IV illness, Virologic suppression, & Adherence | ITT |
| Whalen 1997 (Uganda) | 2736 | ≥ 18 | positive & negative (Anergy) | ART excluded | IPT 300 mg daily (6 mo) | Vit C 250 mg daily (6 mo) | 15 months | Blocked (per 6) | Blinded | AFB & Culture | 12% TST positive & 14% TST negatives | No data | All-cause mortality & ADR | ITT |
ADR: Adverse Drug Reaction; AFB: Acid fast bacilli; AIDS: Acquired immune deficiency Syndrome; ART: Antiretroviral Therapy; IGRA: Interferon Gamma Release Assay; IPT: Isoniazid Preventive Therapy; ITT: Intention To Treat; LTF: Lost To Follow-up; PP: Per Protocol; TB: Tuberculosis; TST: Tuberculin Skin Test; VB6: Vitamin B 6 (Pyridoxine)
All studies randomized participants to IPT or placebo or no IPT intervention; however, the dosage and duration of IPT varied. The total duration of the IPT treatment course ranged from 6 to 12 months with subsequent follow up of 12–44 months. Most of the included studies (n = 7) assessed 300mg of IPT daily for a duration of six months (n = 4) or twelve months (n = 3). A Zambian study assessed 900 mg of IPT twice weekly for 12 months and another South African study assessed 15mg/kg of IPT twice weekly. Six studies assessed the effect of Isoniazid alone in comparison to placebo or no intervention whereas two studies assessed the effect of Isoniazid alone compared to combinations of Rifampicin and Pyrazinamide with placebo or no intervention. The recent TEMPRANO study compared IPT treatment effect to no IPT in the setting of deferred ART or early ART.
With the exception of two studies, the included studies assessed the TST status at baseline. Four studies included both TST positive and negative individuals while three studies solely considered TST negatives. The Ugandan study separately investigated the effect of IPT in TST positive and anergic patients. In most African countries ART was widely introduced in 2004 and five studies conducted before this period lack information regarding ART use. Five studies used a block randomization, two studies used computer generated randomization and the remaining two studies used unclear randomization methods.
Methodological quality assessment
The qualitative assessment on data completion, randomization, treatment concealment and outcome blinding did not reveal major methodological flaws across studies. In some studies, exclusion of possible pre-existing TB disease by ascertainment of clinical symptoms and signs at baseline may have missed few cases of pre-existing TB. Ascertainment of the outcome of TB was active and mostly based on AFB test confirmed by culture positivity of sputum or biopsy. Most of the included studies used family-reported all-cause mortality. There was no indication of selective reporting[43] (Table 3).
Table 3. Risk of bias summary of included studies.
| Studies | Fitzgerald 2001 | Gordin 1997 | Hawken 1997 | Mohammed 2007 | Mwinga 1998 | Pape 1993 | Rangaka 2014 | Rivero 2003 | TEMPRANO 2015 | Whalen 1997 |
|---|---|---|---|---|---|---|---|---|---|---|
| Optimal exclusion of TB patients at enrolment | ? | + | + | + | + | + | ? | + | + | + |
| Random sequence generation | + | + | + | + | + | + | + | + | + | + |
| Allocation concealment | - | + | + | + | + | - | + | ? | - | + |
| Placebo control | + | + | + | + | + | + | + | - | - | + |
| Blinding of participants, personnel, and outcome assessors (TB, ADR, and AIDS) | ? | + | ? | + | + | - | + | ? | - | + |
| Completion of IPT course with adequate adherence | ? | - | + | + | - | + | + | - | + | + |
| Lost to follow up described | + | + | + | + | + | + | + | + | + | + |
| Both primary and secondary outcomes were reported | + | + | + | + | + | + | + | + | + | + |
TB: Tuberculosis; ADR: Adverse Drug Reaction; AIDS: Acquired Immuno Deficiency Syndrome; IPT: Isoniazid Preventive Therapy
+ Low risk of bias
? Unclear (probable) risk of bias
- High risk of bias
Assessment of heterogeneity
Among the included studies, there was substantial clinical and methodological variability, complicating interpretation of study findings. The clinical heterogeneity emanates in part from baseline variations of subjects such as TST status variation. In addition, the IPT intervention differed in terms of dose, duration, provision of IPT with other preventive therapies, adherence levels achieved and subsequent follow-up. Other methodological variation across studies was mostly related to differences in randomization techniques, outcome ascertainment and analysis methods. Finally, eight studies were conducted in low income and high TB burden countries; however, the other two studies were conducted in a high income country with lower levels of endemicity. Given these differences, it is recommended to allow for the between-study heterogeneity in treatment effect and to adopt random effects models.[37], [38], [44] Despite the presence of clinical heterogeneity between the included studies, an acceptable degree of statistical heterogeneity was found for the analysis including all relevant studies. A somewhat larger statistical heterogeneity was observed in the subset of studies where IPT effect on all-cause mortality was assessed in TST positive participants (I2 = 54.7%) and IPT effect on HIV disease progression (I2 = 59.4%). Prediction intervals were determined to indicate an anticipated variation in effect estimates.
Effects of IPT
Overall population
IPT showed significantly reduced relative risk (RR) of TB (RR = 0.65 with 95% CI: 0.51, 0.84), substantial heterogeneity was however present in this effect (95% PI: 0.37, 1.17) (Table 4) Similar results were found for the effect of IPT toward confirmed TB (RR = 0.69, 95% CI: 0.48, 0.99) with a prediction interval (95%) of 0.38 to 1.24) (Table 5). IPT tended to reduce the risk of all-cause mortality yet statistically non-significant RR = 0.90(95% CI (0.79, 1.02) and 95% PI (0.71, 1.13)) (Table 6). The effect of IPT was almost null on risk of HIV disease progression RR = 0.99; 95% CI (0.73, 1.34) and wide 95% PI (0.31, 3.18). (Table 7)
Table 4. The pooled estimates of isoniazid preventive therapy effect on all-types of Tuberculosis.
| Exposure category | Number of studies | Sample size | Pooled RR* (95% CI) | 95% PI | |
|---|---|---|---|---|---|
| Overall estimate | 10 | 7619 | 0.65 (0.51, 0.84) | (0.37, 1.17) | |
| TST | Positive | 5 | 1703 | 0.48 (0.29, 0.82) | (0.13, 1.81) |
| Negative | 9 | 3140 | 0.79 (0.58, 1.08) | (0.54, 1.16) | |
| Unknown | 4 | 2776 | 0.68 (0.42, 1.10) | (0.11, 4.23) | |
| ART | Treated | 2 | 2226 | 0.67(0.47, 0.96) | Not estimable** |
| Not treated | 8 | 4234 | 0.73 (0.53, 1.02) | (0.33, 1.60) | |
| IPT dose | 300mg | 8 | 6819 | 0.62 (0.47, 0.82) | (0.34, 1.12) |
| 900mg | 2 | 800 | 0.89 (0.36, 2.18) | Not estimable** | |
| IPT duration | 6 months | 6 | 5837 | 0.61 (0.45, 0.82) | (0.30, 1.22) |
| 12 months | 4 | 1782 | 0.79 (0.45, 1.37) | (0.11, 5.73) | |
* Random effect model
**Prediction interval can only be estimated for more than two studies
ART: Antiretro viral therapy; CI: Confidence interval; IPT: Isoniazid preventive therapy; PI: Prediction interval; RR: Relative Risk; TST: Tuberculin Skin Test
Table 5. The pooled estimates of isoniazid preventive therapy effect on confirmed Tuberculosis.
| Exposure category | Number of studies | Sample size | Pooled RR* (95% CI) | 95% PI | |
|---|---|---|---|---|---|
| Overall estimates | 5 | 3392 | 0.69 (0.48, 0.99) | (0.38, 1.24) | |
| TST | Positive | 1 | 112 | 0.13 (0.01, 2.32) | Not estimable** |
| Negative | 3 | 1021 | 0.77 (0.36, 1.64) | (0.01, 106.22) | |
| Unknown | 2 | 930 | 0.82 (0.47, 1.43) | Not estimable** | |
* Random effect model
**Prediction interval can only be estimated for more than two studies
CI: Confidence interval; IPT: Isoniazid preventive therapy; PI: Prediction interval; RR: Relative Risk; TST: Tuberculin Skin Test
Table 6. The pooled estimates of isoniazid preventive therapy effect on all-cause mortality.
| Exposure category | Number of studies | Sample size | Pooled RR* (95% CI) | 95% PI | |
|---|---|---|---|---|---|
| Overall estimates | 10 | 7657 | 0.90 (0.79, 1.02) | (0.71, 1.13) | |
| TST | Positive | 4 | 1311 | 0.67 (0.34, 1.35) | (0.04, 10.10) |
| Negative | 7 | 2428 | 1.02 (0.90, 1.15) | (0.86, 1.20) | |
| Unknown | 3 | 2400 | 0.74 (0.52, 1.05) | (0.08, 7.19) | |
| ART | Treated | 2 | 2226 | 0.77 (0.46, 1.28) | Not estimable** |
| Not treated | 8 | 4272 | 0.92 (0.81, 1.05) | (0.72, 1.18) | |
| IPT dose | 300mg | 8 | 6819 | 0.91 (0.80, 1.03) | (0.73, 1.13) |
| 900mg | 2 | 838 | 0.81 (0.46, 1.40) | Not estimable** | |
| IPT duration | 6 months | 6 | 5855 | 0.95 (0.85, 1.07) | (0.81, 1.12) |
| 12 months | 4 | 1802 | 0.65 (0.47, 0.90) | (0.32, 1.32) | |
* Random effect model
**Prediction interval can only be estimated for more than two studies
ART: Antiretro viral therapy; CI: Confidence interval; IPT: Isoniazid preventive therapy; PI: Prediction interval; RR: Relative Risk; TST: Tuberculin Skin Test
Table 7. The pooled estimates of isoniazid preventive therapy effect on HIV disease progression.
| Exposure category | Number of studies | Sample size | Pooled RR* (95% CI) | 95% PI | |
|---|---|---|---|---|---|
| Overall estimates | 4 | 4929 | 0.99 (0.73, 1.34) | (0.31, 3.18) | |
| TST | Positive | 1 | 63 | 0.36 (0.15, 0.85) | Not estimable** |
| Negative | 3 | 809 | 1.00 (0.88, 1.15) | (0.42, 2.40) | |
| Unknown | 1 | 2056 | 1.38 (0.79, 2.40) | Not estimable** | |
* Random effect model
**Prediction interval can only be estimated for more than two studies
CI: Confidence interval; PI: Prediction interval; RR: Relative Risk; TST: Tuberculin Skin Test
TST positive
In TST positive participants, five studies including a total of 1703 participants demonstrated an effect of IPT on all types of TB (probable to confirmed) with a pooled RR of 0.48; (95%CI 0.29–0.82) and 95% PI ranging from 0.13 to 1.81. Only one study reported the results for confirmed TB [RR = 0.13; 95% CI (0.01, 2.32)]. Four studies, including 1311 participants, reported the effect of IPT on all-cause mortality with pooled RR of 0.67; 95% CI (0.34, 1.35) with a wide prediction interval of (0.04, 10.10). Data about the effect of IPT on HIV disease progression was could be derived from one study only (RR = 0.36; 95% CI (0.15, 0.85)). (Tables 4–7)
TST negative
Nine studies reported data for TST negative (n = 3140) participants. The pooled relative risk for IPT on all types of TB was 0.79 (95% CI: 0.58, 1.08) with a prediction interval of (0.54, 1.16). For confirmed TB only, data was obtained from three studies including 1021 participants. The pooled RR was 0.77 (95% CI: 0.36, 1.64). All-cause mortality was determined from seven studies with 2428 participants. The pooled RR of IPT on all cause-mortality was 1.02 (95% CI: 0.90, 1.15) with a prediction interval of (95% PI: 0.86, 1.1.20). Three studies with 809 participants could deliver data about HIV disease progression, RR = 1.00; 95% CI (0.88, 1.15) with a prediction interval of from 0.42 to 2.40. (Tables 4–7)
TST unknown
In participants with an unknown TST status, the pooled relative risk of IPT on all types of TB was 0.68; 95% CI (0.42, 1.10) with a very wide prediction interval of (95% PI: 0.11, 4.23). Based on two studies with 930 participants, the pooled RR of IPT on confirmed TB was 0.82; 95% CI (0.47, 1.43). Similarly, the other three studies had data on all-cause mortality and the pooled RR was 0.74; 95% CI (0.52, 1.05). One study with 2056 participants found an elevated risk of HIV disease progression yet statistically non-significant RR = 1.38; 95% CI (0.79, 2.40).
ART treatment
IPT-ART combination treatment could be estimated from two studies with 2226 participants. IPT reduced risk of all-types of TB in participants treated with ART RR = 0.67 (95% CI 0.47, 0.96). The pooled effect from studies (n = 8) which did not include participants on ART was not statistically significant RR = 0.73; 95% CI (0.53, 1.02) and 95% PI (0.33, 1.60). In the same studies, the pooled effect of IPT on all-cause mortality for participants on ART was 0.77; 95% CI (0.46, 1.28) and 0.92; 95% CI (0.81, 1.05) for those not on ART. (Tables 4, 6 & 8)
Table 8. The pooled estimates of isoniazid preventive therapy effect on adverse drug reaction.
| Exposure category | Number of studies | Sample size | Pooled RR* 95% CI | 95% PI | |
|---|---|---|---|---|---|
| Overall estimates | 9 | 7284 | 1.20 (0.85, 1.71) | (0.50, 2.88) | |
| ART | Treated | 2 | 2226 | 1.41 (0.81, 1.71) | Not estimable** |
| Not treated | 7 | 3899 | 2.06 (0.96, 4.40) | (0.24, 17.88) | |
| IPT dose | 300mg | 8 | 6582 | 1.06 (0.79, 1.41) | (0.54, 2.10) |
| 900mg | 1 | 702 | 3.98 (1.13, 13.97) | Not estimable** | |
| IPT duration | 6 months | 7 | 5837 | 1.47 (0.83, 2.60) | (0.31, 6.94) |
| 12 months | 2 | 1447 | 1.09 (0.84, 1.42) | Not estimable** | |
* Random effect model
**Prediction interval can only be estimated for more than two studies
ART: Antiretro viral therapy; CI: Confidence interval; IPT: Isoniazid preventive therapy; PI: Prediction interval; RR: Relative Risk; TST: Tuberculin Skin Test
IPT dose and duration
The 300 mg IPT daily dose had higher benefit than 900mg twice weekly dose in reducing all-types of TB risk, pooled RR = 0.62; 95% CI(0.47, 0.82) and 0.89; 95% (0.36, 2.18) respectively. There were no differences in the pooled effect of different IPT doses on risk of all-cause mortality. Concerning the duration of IPT, 6 months therapy reduced all-types of TB [RR = 0.61; 95% CI (0.45, 0.82)]. The same protective effect of 12 months IPT course observed toward all-types of TB risk reduction yet the pooled estimate was not statistically significant [RR = 0.79; 95% CI (0.45, 1.37)] with a wide 95% PI (0.11, 5.73). (Tables 4, 6 & 8)
For adverse drug reactions participants were not stratified by TST status. All studies included data about the overall number of ADR (5068 participants). Nevertheless, two studies did not have withdrawal from the follow up due to ADR. The pooled RR of IPT compared to placebo for the development of severed ADR was 1.32; 95% CI (0.89, 1.96) with prediction interval of (0.46, 3.75). For the included studies, there appeared a positive association between the dosage of IPT and the risk of adverse drug reaction. The 900mg IPT dose showed higher risks of ADR [RR = 3.98; 95% CI (1.13, 13.97)] in comparison to 300mg daily dose [RR = 1.06; 95% CI (0.79, 1.41)] (Table 8).
The details of the forest plots are annexed (S1 Fig) and additional effect estimates extracted from included studies are also annexed (S2 Table).
Adherence to the IPT (or placebo) regimen could be assessed from seven studies. The studies reported similarity of TB incidence in adherent and non-adherent participants although the proportion of non-adherence varied from study to study. None of these studies described reasons for non-adherence or predictors of non-adherence. (Table 2)
Discussion
IPT has been one of the four main strategies of TB prevention in both HIV positive and negative patients since the introduction of Isoniazid.41,42,43 The clinical trials included in this review demonstrated that IPT was generally efficacious to prevent TB disease. Nevertheless, the width of calculated prediction intervals indicates that IPT may not be a beneficial strategy in all populations. Further research is needed to explore sources of this heterogeneity. Despite an effect on development of TB disease, a limited reduction in all-cause mortality was found; Some of the studies may have been under-powered to detect an effect on mortality or limited follow-up time precluded assessment of a long term effect. From the subgroup analysis, it appears that the six-month 300 mg daily IPT regimen reduced the risk of TB with the lowest number of adverse events.
The results of this meta-analysis confirm that a positive TST is a very strong indicator for the potential benefit of IPT also in countries with high burden of TB.41,44 Patients with a positive TST experienced a relative risk of TB disease of RR = 0.48; 95% CI (0.29, 0.82) compared to untreated patients or treated with placebo. For subjects with negative TST reactions, there was a moderate, but not statistically significant, protective effect (RR = 0.79; 95% CI (0.58, 1.08) compared to untreated patients. This is in agreement with previous reports.31,44 As TST reactivity also demonstrates a functional anti-mycobacterial response, a TST negative status can indicate either a lack of TB exposure, or result from severe immune deficiency with or without underlying latent TB. A TST positive status hence indicates a preserved immune response and this could affect the efficacy of IPT.45
ART contributes to the prevention of TB in HIV positive patients by maintaining the patient’s immune system.[45] Only two of the included studies (Rangaka et.al. and TEMPRANO) assessed the combined effect of ART and IPT on TB, all-cause mortality, or HIV disease progression and found that ART decreased the risk of all-types of TB and increased the risk of ADR. Further evidence from observational cohort studies indicate that antiretroviral therapy may significantly reduce the risk of TB.[28], [46–52] Despite the protective effects of ART, long-term incidence rates of TB remain high in people with HIV.[53–55] This is arguably related to suboptimal adherence,[56] the limited CD4-cell count recovery[57], persisting defects in TB-specific immune responses[58], or a high rate of re-exposure to infectious TB.[59] Even in patients who achieve CD4-cell counts above 500×10⁶/L TB risk remains about two times higher than background.[57] Recent clinical trials[60] and observational studies[26] reported that combined IPT and ART reduced the risk of TB and advanced HIV disease events and IPT achieved an additional TB disease risk reduction in patients on ART. Therefore, clinical trials or well-designed observational studies are needed to assess the TB prevention interventions in addition to ART. The benefit of secondary prophylaxis of IPT should ideally be investigated in high TB burden countries.
The potential benefit of IPT on the prevention of progression of immunodeficiency (HIV disease progression) in TST positives was only assessed in a single study with a RR = 0.36; 95% CI (0.15, 0.85). In TST negatives, however, the effect of IPT is nominal on HIV disease progression [RR = 1.00; 95% CI (0.88, 1.15)]. Data from a meta-analysis of randomized controlled trials of 4136 HIV patients previously demonstrated that IPT did not prevent progression of immunodeficiency.[61]
IPT did not appear to affect all-cause mortality in the total population studied, nor in any of the subgroups based on TST status, which is consistent with findings of other studies.[60] The pooled estimates did not reveal an association between IPT and adverse drug reactions.[61] However, higher dosage and duration was associated with a higher risk of the adverse drug reaction.
This review bears the following strengths. First, participants were stratified based on their TST status, which allowed identification of the subgroups that may benefit most from IPT. In contrast to previous systematic reviews, we assessed HIV disease progression and all-cause mortality across the different TST, ART exposure, IPT dose, and duration strata. Second, this review includes a recent clinical trial assessing the effect of IPT in ART patients which has substantial implications to the contemporary TB/HIV clinical practice of high ART adoption rate. Finally, given the high degree of clinical heterogeneity, the current review did not only present pooled effect estimates that were adjusted for between-study heterogeneity, but also calculated prediction intervals reflecting the expected range of the IPT efficacy in future studies with similar characteristics as those included in this meta-analysis. These intervals suggest that, despite the overall protective effect of IPT in HIV patients, non-beneficial effects may still be expected in some specific TST negative and TST unknown populations for yet unidentified reasons.
This review has also some limitations. A major limitation emanates from the small sample size of participants in TST positives and TST unknowns group rendering the pooled effect size of IPT across different outcomes rather imprecise. Furthermore, only one study assessed the combined effect of IPT with ART on prevention of TB, HIV disease progression and all-cause mortality, so no synthesis could be applied for this subgroup of patients. Despite an anticipated risk of isoniazid-resistant TB after inadvertent isoniazid monotherapy in high TB burden countries,[62] only one study assessed this effect of IPT and found an insignificant risk of drug-resistant TB. Hence, evaluating the risk of drug-resistant TB is a compelling area for future research. This review included manuscript available in English language only. Finally, collecting subject-level data from the included studies and performing an individual participant data meta-analysis could help to reduce the extent of the observed heterogeneity (e.g. by harmonizing the analysis of primary studies and accounting for differences in follow-up), and to analyze the presence of effect modification more thoroughly.[63], [64]
Conclusions
This meta-analysis suggests a protective effect of IPT on development of TB in HIV infected patients, where TST positive patients benefit to a greater extent than patients with a negative TST. The effects on mortality and HIV disease progression were modest but there was some evidence indicating an additional benefit of IPT over ART alone in the prevention of TB disease. Future studies are necessary to determine the risk of drug-resistant TB after IPT use.
Supporting Information
(PDF)
(DOCX)
(DOCX)
(DOC)
(DOCX)
(DOCX)
Acknowledgments
The study was funded by Netherlands Fellowship Program (NUFFIC grant number 11169). The funders had no role in study design, data collection and analysis, preparation of the manuscript, or decision to publish.
Data Availability
Data extracted from included studies in the references.
Funding Statement
The study was funded by the Netherlands Fellowship Program (NUFFIC grant number 11169). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Grigg E. R., “The arcana of Tuberculosis with a brief epidemiologic history of the disease in the USA. Part III,” Am Rev Tuberc Pulm Dis, vol. 78, pp. 426–453, 1958. [DOI] [PubMed] [Google Scholar]
- 2. Galdston I., “The White Plague, Tuberculosis; Man and Society,” Bull. Med. Libr. Assoc., vol. 42, no. 1, pp. 142–143, 1954. [Google Scholar]
- 3. Kristian F. Andvord, “Andvord KF 2002 what can we learn by following the development.pdf,” Int J Tuberc Lung Dis, vol. 6, pp. 562–568, 2002. [PubMed] [Google Scholar]
- 4. Zumla A., Raviglione M., Hafner R., and Fordham von Reyn C., “Tuberculosis,” N. Engl. J. Med., vol. 368, pp. 745–755, 2013. [DOI] [PubMed] [Google Scholar]
- 5. Narain J. P., Raviglione M. C., and Kochi A., “HIV-associated tuberculosis in developing countries: Epidemiology and strategies for prevention,” Tubercle and Lung Disease, vol. 73, no. 6 pp. 311–321, 1992. [DOI] [PubMed] [Google Scholar]
- 6. Goletti D., Weissman D., Jackson R. W., Collins F., a Kinter, and a S. Fauci, “The in vitro induction of human immunodeficiency virus (HIV) replication in purified protein derivative-positive HIV-infected persons by recall antigen response to Mycobacterium tuberculosis is the result of a balance of the effects of endogenous interleu,” J. Infect. Dis., vol. 177, no. 5, pp. 1332–8, May 1998. [DOI] [PubMed] [Google Scholar]
- 7. Goletti D., Carrara S., Vincenti D., Giacomini E., Fattorini L., Garbuglia A. R., et al. , “Inhibition of HIV-1 replication in monocyte-derived macrophages by Mycobacterium tuberculosis,” J.Infect.Dis., vol. 189, no. 4, pp. 624–633, 2004. [DOI] [PubMed] [Google Scholar]
- 8. Hoshino Y., Hoshino S., Gold J. A., Raju B., Prabhakar S., Pine R., et al. , “Mechanisms of polymorphonuclear neutrophil-mediated induction of HIV-1 replication in macrophages during pulmonary tuberculosis.,” J. Infect. Dis., vol. 195, no. 9, pp. 1303–1310, 2007. [DOI] [PubMed] [Google Scholar]
- 9. Hoshino Y., Nakata K., Hoshino S., Honda Y., Tse D. B., Shioda T., et al. , “Maximal HIV-1 replication in alveolar macrophages during tuberculosis requires both lymphocyte contact and cytokines.,” J. Exp. Med., vol. 195, no. 4, pp. 495–505, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mancino G., Placido R., Bach S., Mariani F., Montesano C., Ercoli L., et al. , “Infection of human monocytes with Mycobacterium tuberculosis enhances human immunodeficiency virus type 1 replication and transmission to T cells,” J Infect Dis, vol. 175, no. 6, pp. 1531–1535, 1997. [DOI] [PubMed] [Google Scholar]
- 11. Ranjbar S., Boshoff H. I., Mulder A., Siddiqi N., Rubin E. J., and Goldfeld A. E., “HIV-1 replication is differentially regulated by distinct clinical strains of Mycobacterium tuberculosis,” PLoS One, vol. 4, no. 7, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Reed M. B., Domenech P., Manca C., Su H., Barczak A. K., Kreiswirth B. N., et al. , “A glycolipid of hypervirulent tuberculosis strains that inhibits the innate immune response.,” Nature, vol. 431, no. 7004, pp. 84–87, 2004. [DOI] [PubMed] [Google Scholar]
- 13. Bucy R. P. and Kilby J. M., “Perspectives on inducing efficient immune control of HIV-1 replication—a new goal for HIV therapeutics?,” AIDS, vol. 15 Suppl 2, pp. S36–S42, 2001. [DOI] [PubMed] [Google Scholar]
- 14. Merchant R. H. and a Quadir Z., “Management of opportunistic infections in pediatric HIV.,” Indian J. Pediatr., vol. 69, no. 11, pp. 973–7, November 2002. [DOI] [PubMed] [Google Scholar]
- 15. Ferebee S., “Controlled chemoprophylaxis trials in tuberculosis: a general review,” Bibl Tuberc., vol. 26, pp. 28–106, 1970. [PubMed] [Google Scholar]
- 16. Selwyn P. A., Hartel D., Lewis V. A., Schoenbaum E. E., Vermund S. H., Klein R. S., et al. , “A prospective study of the risk of tuberculosis among intravenous drug users with human immunodeficiency virus infection,” N Engl J Med, vol. 320, no. 9, pp. 545–550, 1989. [DOI] [PubMed] [Google Scholar]
- 17. Guelar A., Gatell J. M., Verdejo J., Podzamczer D., Lozano L., Aznar E., et al. , “A prospective study of the risk of tuberculosis among HIV-infected patients.,” AIDS, vol. 7, no. 10, pp. 1345–9, 1993. [DOI] [PubMed] [Google Scholar]
- 18. Pape J. W., Jean S. S., Ho J. L., Hafner A., and Johnson W. D., “Effect if isoniazid prophylaxis on incidence of active tuberculosis and progression of HIV infection,” Lancet, vol. 342, no. 8866, pp. 268–272, 1993. [DOI] [PubMed] [Google Scholar]
- 19. Churchyard G. J., Fielding K. L., Lewis J. J., Coetzee L., Corbett E. L., Godfrey-Faussett P., et al. , “A trial of mass isoniazid preventive therapy for tuberculosis control.,” N. Engl. J. Med., vol. 370, no. 4, pp. 301–10, 2014. [DOI] [PubMed] [Google Scholar]
- 20. Fielding K. L., Grant A. D., Hayes R. J., Chaisson R. E., Corbett E. L., and Churchyard G. J., “Thibela TB: Design and methods of a cluster randomised trial of the effect of community-wide isoniazid preventive therapy on tuberculosis amongst gold miners in South Africa,” Contemp. Clin. Trials, vol. 32, no. 3, pp. 382–392, 2011. [DOI] [PubMed] [Google Scholar]
- 21. Gordin F., “Rifampin and Pyrazinamide vs Isoniazid for Prevention of Tuberculosis in HIV-Infected Persons: An International Randomized Trial,” JAMA: The Journal of the American Medical Association, vol. 283, no. 11 pp. 1445–1450, 2000. [DOI] [PubMed] [Google Scholar]
- 22. Moreno S., Baraia-Etxaburu J., Bouza E., Parras F., Pérez-Tascón M., Miralles P., et al. , “Risk for developing tuberculosis among anergic patients infected with HIV.,” Ann. Intern. Med., vol. 119, no. 3, pp. 194–198, 1993. [DOI] [PubMed] [Google Scholar]
- 23. Graham N. M., Galai N., Nelson K. E., Astemborski J., Bonds M., Rizzo R. T., et al. , “Effect of isoniazid chemoprophylaxis on HIV-related mycobacterial disease.,” 1996. [PubMed] [Google Scholar]
- 24. de Souza C. T. V., Hökerberg Y. H. M., Pacheco S. J. B., Rolla V. C., and Passos S. R. L., “Effectiveness and safety of isoniazid chemoprophylaxis for HIV-1 infected patients from Rio de Janeiro.,” Mem. Inst. Oswaldo Cruz, vol. 104, no. 3, pp. 462–467, 2009. [DOI] [PubMed] [Google Scholar]
- 25. Kerkhoff A. D., Kranzer K., Samandari T., Nakiyingi-Miiro J., Whalen C. C., Harries A. D., et al. , “Systematic Review of TST Responses in People Living with HIV in Under-Resourced Settings: Implications for Isoniazid Preventive Therapy,” PLoS One, vol. 7, no. 11, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ayele H. T., van Mourik M. S., and Bonten M. J., “Effect of isoniazid preventive therapy on tuberculosis or death in persons with HIV: a retrospective cohort study,” BMC Infect. Dis., vol. 15, no. 1, p. 334, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Golub J. E., Saraceni V., Cavalcante S. C., Pacheco A. G., Moulton L. H., King B. S., et al. , “The impact of antiretroviral therapy and isoniazid preventive therapy on tuberculosis incidence in HIV-infected patients in Rio de Janeiro, Brazil.,” AIDS, vol. 21, no. 11, pp. 1441–1448, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Golub J. E., Pronyk P., Mohapi L., Thsabangu N., Moshabela M., Struthers H., et al. , “Isoniazid preventive therapy, HAART and tuberculosis risk in HIV-infected adults in South Africa: a prospective cohort.,” AIDS, vol. 23, no. 5, pp. 631–636, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bucher H. C., Griffith L. E., Guyatt G. H., Sudre P., Naef M., Sendi P., et al. , “Isoniazid prophylaxis for tuberculosis in HIV infection: a meta-analysis of randomized controlled trials.,” AIDS, vol. 13, no. 4, pp. 501–507, 1999. [DOI] [PubMed] [Google Scholar]
- 30. Akolo C., Adetifa I., Shepperd S., and Volmink J., “Treatment of latent tuberculosis infection in HIV infected persons,” Cochrane Database Syst. Rev., no. 1, p. 171, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wilkinson D., Squire S. B., and Garner P., “Effect of preventive treatment for tuberculosis in adults infected with HIV: systematic review of randomised placebo controlled trials,” Bmj, vol. 317, no. 7159, pp. 625–629, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.WHO Stop TB, “INTERIM POLICY ON COLLABORATIVE TB/HIV ACTIVITIES,” Geneva, Switzerland, 2004.
- 33. Date A. A., Vitoria M., Granich R., Banda M., Fox M. Y., and Gilks C., “Implementation of co-trimoxazole prophylaxis and isoniazid preventive therapy for people living with HIV.,” Bull. World Health Organ., vol. 88, no. 4, pp. 253–9, April 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Gupta S. S., Granich R., Suthar A. B., Smyth C., Baggaley R., Sculier D., et al. , “Three I’s for HIV/TB and early ART to prevent HIV and TB: Policy review of HIV and TB guidelines for high HIV/TBburden African countries,” J. Int. AIDS Soc., vol. 15, p. 213, 2012. [Google Scholar]
- 35.WHO, “Global tuberculosis report 2013,” Geneva, Switzerland, 2013.
- 36.World Health Organization, Guidelines for intensified tuberculosis case-finding and isoniazid preventive therapy for people living with HIV in resourceconstrained settings. 2015.
- 37. Hartung J., “An alternative method for meta-analysis,” Biometrical J., vol. 41, no. 8, pp. 901–916, 1999. [Google Scholar]
- 38. Hartung J. and Knapp G., “A refined method for the meta analysis of controlled CLINICAL TRIALS with binary outcome.,” Stat Med, vol. 20, no. 24, pp. 3875–3889, 2001. [DOI] [PubMed] [Google Scholar]
- 39. Riley R. D., Higgins J. P. T., and Deeks J. J., “Interpretation of random effects meta-analyses.,” BMJ, vol. 342, p. d549, 2011. [DOI] [PubMed] [Google Scholar]
- 40. Wallace B. C., Schmid C. H., Lau J., and Trikalinos T. A., “Meta-Analyst: software for meta-analysis of binary, continuous and diagnostic data.,” BMC Med. Res. Methodol., vol. 9, p. 80, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.R Development Core Team, “R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. ISBN 3-900051-07-0, URL http://www.R-project.org/.,” R Found. Stat. Comput. Vienna, Austria., 2012.
- 42. World Health Organization, Global Tuberculosis Report 2013, vol. 375 2013. [Google Scholar]
- 43. Green S., Higgins P J.., Alderson T., P., Clarke M., Mulrow D C., and Oxman D A., “Cochrane Handbook: Cochrane Reviews: Ch 8: Assessing risk of bias in included studies,” in Cochrane Handbook for: Systematic Reviews of Interventions, vol. 6, 2011, pp. 3–10. [Google Scholar]
- 44.M. Borenstein, L. Hedges, and H. Rothstein, Meta-Analysis Fixed effect vs. random effects. 2007.
- 45. Lawn S. D., Wood R., De Cock K. M., Kranzer K., Lewis J. J., and Churchyard G. J., “Antiretrovirals and isoniazid preventive therapy in the prevention of HIV-associated tuberculosis in settings with limited health-care resources,” Lancet Infect. Dis., vol. 10, pp. 489–498, 2010. [DOI] [PubMed] [Google Scholar]
- 46. Jones J. L., Hanson D. L., Dworkin M. S., Decock K. M., Adult T., and Spectrum A., “HIV-associated tuberculosis in the era of highly active antiretroviral therapy,” Int. J. Tuberc. Lung Dis., vol. 4, no. June, pp. 1026–1031, 2000. [PubMed] [Google Scholar]
- 47. Girardi E., Antonucci G., Vanacore P., Libanore M., Errante I., Matteelli A., et al. , “Impact of combination antiretroviral therapy on the risk of tuberculosis among persons with HIV infection.,” AIDS, vol. 14, no. 13, pp. 1985–91, 2000. [DOI] [PubMed] [Google Scholar]
- 48. Santoro-Lopes G., de Pinho A. M., Harrison L. H., and Schechter M., “Reduced risk of tuberculosis among Brazilian patients with advanced human immunodeficiency virus infection treated with highly active antiretroviral therapy,” Clin Infect Dis, vol. 34, no. 4, pp. 543–546, 2002. [DOI] [PubMed] [Google Scholar]
- 49. Badri M., Wilson D., and Wood R., “Effect of highly active antiretroviral therapy on incidence of tuberculosis in South Africa: a cohort study.,” Lancet, vol. 359, no. 9323, pp. 2059–64, 2002. [DOI] [PubMed] [Google Scholar]
- 50. Miranda A., Morgan M., Jamal L., Laserson K., Barreira D., Silva G., et al. , “Impact of antiretroviral therapy on the incidence of tuberculosis: The Brazilian experience, 1995–2001,” PLoS One, vol. 2, no. 9, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Muga R., Ferreros I., Langohr K., de Olalla P. G., Del Romero J., Quintana M., et al. , “Changes in the incidence of tuberculosis in a cohort of HIV-seroconverters before and after the introduction of HAART.,” AIDS, vol. 21, no. 18, pp. 2521–2527, 2007. [DOI] [PubMed] [Google Scholar]
- 52. Moreno S., Jarrin I., Iribarren J. A., Perez-Elías M. J., Viciana P., Parra-Ruiz J., et al. , “Incidence and risk factors for tuberculosis in HIV-positive subjects by HAART status.,” Int. J. Tuberc. Lung Dis., vol. 12, no. 12, pp. 1393–1400, 2008. [PubMed] [Google Scholar]
- 53. Lawn S. D., Myer L., Bekker L.-G., and Wood R., “Burden of tuberculosis in an antiretroviral treatment programme in sub-Saharan Africa: impact on treatment outcomes and implications for tuberculosis control.,” AIDS, vol. 20, no. 12, pp. 1605–1612, 2006. [DOI] [PubMed] [Google Scholar]
- 54. Lawn S. D., Badri M., and Wood R., “Tuberculosis among HIV-infected patients receiving HAART: long term incidence and risk factors in a South African cohort.,” AIDS, vol. 19, no. 18, pp. 2109–2116, 2005. [DOI] [PubMed] [Google Scholar]
- 55. Girardi E., Sabin C. A., d’Arminio Monforte A., Hogg B., Phillips A. N., Gill M. J., et al. , “Incidence of Tuberculosis among HIV-infected patients receiving highly active antiretroviral therapy in Europe and North America.,” Clin. Infect. Dis., vol. 41, no. 12, pp. 1772–1782, 2005. [DOI] [PubMed] [Google Scholar]
- 56. Mesfin N., Deribew A., Yami A., Solomon T., Van Geertruyden J. P., and Colebunders R., “Predictors of antiretroviral treatment-associated tuberculosis in Ethiopia: a nested case-control study,” International Journal of STD & AIDS, vol. 23, no. 2 pp. 94–98, 2012. [DOI] [PubMed] [Google Scholar]
- 57. Lawn S. D., Myer L., Edwards D., Bekker L.-G., and Wood R., “Short-term and long-term risk of tuberculosis associated with CD4 cell recovery during antiretroviral therapy in South Africa.,” AIDS, vol. 23, no. 13, pp. 1717–1725, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Lawn S. D., Bekker L.-G., and Wood R., “How effectively does HAART restore immune responses to Mycobacterium tuberculosis? Implications for tuberculosis control.,” AIDS (London, England), vol. 19, no. 11 pp. 1113–1124, 2005. [DOI] [PubMed] [Google Scholar]
- 59. Kwan C. K. and Ernst J. D., “HIV and tuberculosis: a deadly human syndemic.,” Clin. Microbiol. Rev., vol. 24, no. 2, pp. 351–76, April 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Samandari T., Agizew T. B., Nyirenda S., Tedla Z., Sibanda T., Shang N., et al. , “6-month versus 36-month isoniazid preventive treatment for tuberculosis in adults with HIV infection in Botswana: a randomised, double-blind, placebo-controlled trial.,” Lancet, vol. 377, no. 9777, pp. 1588–98, May 2011. [DOI] [PubMed] [Google Scholar]
- 61. Akolo C., Adetifa I., Shepperd S., and Volmink J., “Treatment of latent tuberculosis infection in HIV infected persons (Review),” no. 1, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Mills H. L., Cohen T., and Colijn C., “Community-wide isoniazid preventive therapy drives drug-resistant tuberculosis: a model-based analysis.,” Sci. Transl. Med., vol. 5, no. 180, p. 180ra49, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Riley R. D., Lambert P. C., and Abo-Zaid G., “Meta-analysis of individual participant data: rationale, conduct, and reporting.,” BMJ, vol. 340, p. c221, 2010. [DOI] [PubMed] [Google Scholar]
- 64. Debray TPA, Moons KGM, van Valkenhoef G, Efthimiou O, Hummel N, Groenwold RHH, “Methods for Individual Participant Data meta-analysis of relevant treatment effect: a systematic review,” Res Syn Meth., vol. (Accepted, 2015. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
(DOCX)
(DOCX)
(DOC)
(DOCX)
(DOCX)
Data Availability Statement
Data extracted from included studies in the references.