Abstract
Objective
To explore the relationship between TβRII [type II TGFβ (transforming growth factor β) receptor] expression and clinicopathological characteristics, and to evaluate the prognostic significance of TβRII expression in breast cancer.
Methods
Clinicopathological data and prognostic information of 108 patients with histologically confirmed breast cancer who were surgically treated at China Medical University between January 2007 and September 2008 were reviewed and the association between the clinicopathological characteristics and TβRII expression was analyzed by chi-square test and multivariate analysis. The expression of TβRII was assessed by immunohistochemistry.
Results
Of the 108 patients, 60 cases were TβRII positive and 48 cases were negative. There was no significant association between TβRII expression of the patients older than 40 years and that of the younger than 40 years (56.0% vs 50.0%; P = 0.742). The TβRII expression rate was significantly increased in patients with lymph node metastasis compared to those without lymph node metastasis (67.40% vs 46.8%; P = 0.033). Statistically significant relationships were found between increasing tumor clinical stage and high TβRII expression (P = 0.011). TβRII expression was not associated with the expression of ER(estrogen receptor)、PR, (progesterone receptor)、Her-2 (human epidermal growth factor receptor 2) (P = 0.925,P = 0.861, and P = 0.840, respectively). Patients with high TβRII expression showed poorer 5-year disease-free survival (DFS) compared to those with low expression (66.7% vs 45.6%; P = 0.028) by univariate analysis. Survival analysis demonstrated that TβRII was associated with poor DFS (P = 0.011). Subgroup analysis revealed that TβRII expression was associated with shorter DFS in patients with lymph node metastasis, ER-positive, PR-positive or Her-2-negative tumors (P = 0.006, P = 0.016, P = 0.022, and P = 0.033, respectively). Cox regression analysis revealed that high TβRII expression was related to poor 5-year DFS, and it was an independent factor for predicting the poor outcome for breast cancer patients (P = 0.016).
Conclusions
High levels of TβRII expression were associated with lymph node metastasis, increasing tumor clinical stage, and poorer 5-year DFS in patients with breast cancer. TβRII may be a potential prognostic marker for breast cancer.
Introduction
Breast cancer accounts for 23% (1.38 million) of all new cancer cases and 14% (458,400) of all deaths by cancer [1]. At present, breast cancer is the most common malignancy in women and the leading cause of death for cancer among females globally [2]. Although various breast cancer treatments including surgery, radiotherapy, endocrine therapy, trastuzumab, cytotoxic therapy, and other biological agent therapies have improved patient survival in recent years, the overwhelming majority of deaths due to cancer are because of recurrence and metastasis. Many studies have revealed that TGFβ (transforming growth factor β) signaling plays a major role in cancer metastasis, and regulation of TGFβ signaling is important for breast cancer therapy [3,4].
The TGFβ superfamily is a large, evolutionarily conserved family of secreted multifunctional peptides [5], composed of 33 structurally similar cytokines (bone morphogenic proteins, activins, and TGF-β ligands), which are important factors in developmental biology, including mammary gland development [6]. TGFβ signaling has tumor-suppressive and tumorigenic effects in accordance with tumor stage [7,8]. The effects of TGFβ are mediated by three TGFβ ligands, TGFβ1, TGFβ2 and TGFβ3 through binding to a heteromeric complex of transmembrane TGFβ serine/threonine kinase type I and type II receptors (TβRI and TβRII). TβRII [type II TGFβ receptor] is crucial for the regulation of TGFβ signaling in tumor initiation, progression, and metastasis [9].
TβRII is the specific receptor for TGFβ ligands. TβRII, also known as TGFβR2 (TGFβ receptor type-2), is a 567 amino acid single-pass type I membrane protein that contains one protein kinase domain. Gobbi et al. showed that downregulated TβRII was associated with an increased risk of developing invasive breast cancer, and the absence of TβRII correlated with high-grade human carcinoma in situ and invasive breast cancer [7,10]. Previous studies revealed that the complete loss of TβRII tissue expression in breast cancers was associated with the development of distant metastases and poor overall survival, and absence of TβRII in carcinoma cells promotes mammary tumor growth [9,11]. TβRII can act as a tumorsuppressor gene [12,13], and decreased expression in other cancers such as head and neck squamous cell carcinoma is related to aggressive cellular behavior [11,14–17]. Moreover, Chen et al. reported that decreased TβRII more common in non-small cell lung cancer patients with lymph node metastasis and increasing pathological stage [18]. However, analysis of clinical tumor samples has demonstrated that breast cancer patients with high TβRII expression have poor progression-free survival [19,20]. Takanami et al. found that the presence of immunoreactivity for TβRI and/or TβRII is correlated with poor prognosis in lung adenocarcinoma [21].
These paradoxical findings support the notion that TGFβ functions as a tumor suppressor or a tumor promoter in cancer development. In the present study, TβRII expression was evaluated to analyze its relationship with clinicopathological features and prognosis in a cohort of 108 breast cancer patients.
Materials and Methods
Patients
A total of 108 patients with histologically confirmed breast cancer who were surgically treated at China Medical University between January 2006 and September 2007 were included in this study. The mean age was 51.26 years (range, 33–75years). Lymph node metastasis was present in 46 patients. The patients were classified into clinical stages I (n = 30), II (n = 64) and III (n = 14), according to the TNM staging system. The criteria to include a patient in this study were as follows: (a) curative operations were performed; (b) resected specimens were pathologically examined; (c) more than 10 lymph nodes were pathologically examined after the operation; and (d) a complete medical record was available including pathologic tumor size, lymph node status, tumor clinical stage and biomarkers status (ER, PR and HER-2). In addition, follow-up data was available for 108 patients, who were followed-up from 5 to 75 months (median, 54.96 months; mean 61.00 months). The study protocol was approved by the Ethics Committee of China Medical University. Written informed consent was obtained from all the participants involved in the study.
Tissue specimens
Thin slices of tumor tissue of all cases received in our histopathology unit were fixed in 4% formaldehyde solution (pH 7.0) for periods not exceeding 24h. The tissues were processed routinely for paraffin embedding and 4μm-thick sections were cut and placed on glass slides coated with 3-aminopropyl triethoxysilane for immunohistochemistry.
Immunohistochemistry
Rabbit-anti-human monoclonal antibody against TβRII (sc-400; Santa Cruz) and Ready-to-SP (streptavidin-peroxidase) immunohistochemical detection kit (SP-9001; Santa Cruz) were used in this study. The sections were deparaffinized in xylene (I, II, and III) for 15 min each, and rehydrated with graded ethanol solutions for 35 min. Endogenous peroxidase was blocked by incubating the sections in 3% hydrogen peroxide methanol for 10 min. Heatinduced antigen retrieval at 100°C for 2.5 min was performed in 10 mmol/l citrate buffer solution (pH 6.0) in a pressure cooker. After blocking nonspecific reactivity with 10% normal goat serum for 15 min at room temperature, sections were incubated overnight at 4°C with a primary rabbit-anti-human antibody against TβRII (1:500) followed by 15 min incubation at 37°C with a secondary goat-anti-rabbit antibody. Negative control was prepared by substituting the primary antibody with PBS. The samples were subsequently treated with the streptavidin biotin complex for 15 min. Staining was visualized using a diaminobezidine solution, and sections were counterstained with hematoxylin, dehydrated, and cover-slipped with mounting medium.
Evaluation of immunostaining
The presence of brown-yellow particles on the cell nucleus following the immunohistochemical assay indicated positively-stained cells, and the degree of the staining was semi-quantitatively classified according to the percentage of positive cells. The specimens were labeled with (-) if they contained <5% positive cells, (+) for 5-20% positive cells, and (++) for >20% positive cells. We considered specimens graded as ++ to be TβRII positive.
Statistical analysis
SPSS statistics software (Version 17.0, SPSS Inc., Chicago, IL, USA) was used for all statistical analyses. The association between TβRII expression and the clinicopathological characteristics were analyzed using the Chi-squared test. Five-year disease-free survival (DFS) was showed as the number of months from surgery to the occurrence of an event (distant metastasis or disease-related death). Survival curves were constructed using the Kaplan-Meier method, and the survival comparison was examined using the log-rank test. Multivariate analysis for DFS was carried out using Cox proportional hazards model,where some potential prognostic factors were included. All tests were two-tailed, with a confidence interval of 95%. A P-value of less than 0.05 was considered to indicate a statistically significant difference.
Results
TβRII expression
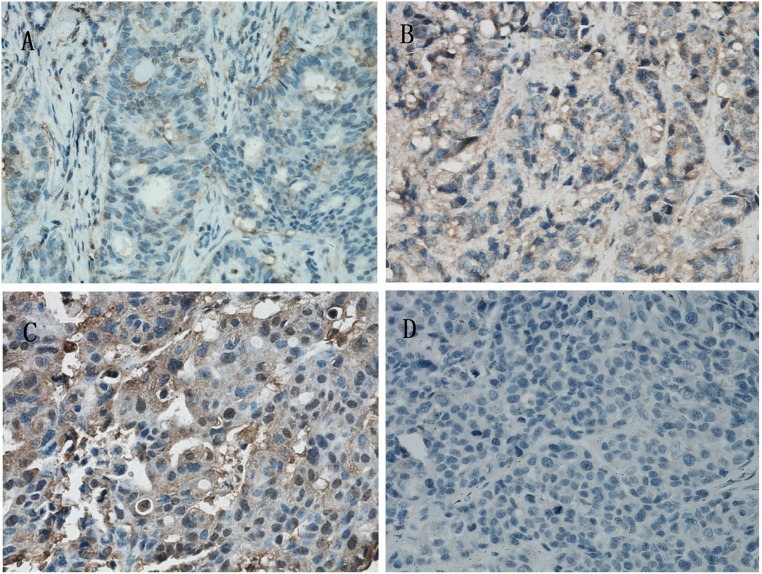
Immunohistochemical detection showed that TβRII was negatively expressed and was labled as (-) (Fig 1A). The TβRII was positively expressed and was labeled as (+) and (++) respectively (Fig 1B and 1C). There was no TβRII expression in negative controls (Fig 1D). As shown in Table 1, 60 tumors (55.5%) displayed positive expression of TβRII and 48 displayed negative expression (44.5%). In patients with tumor size lager than 2.0cm, the percentage of TβRII expression was 50.87% and in the patients with tumor size between 2 cm and 5 cm, the percentage was 60.78%. In the 14 cases classified as tumor stage III, there were 13 cases with positive TβRII expression (92.86%), and in the 94 cases with tumor stage lower than II, there were 47 cases with TβRII expression (50.0%). The percentage of lymph node-positive cases was 67.39% in patients with positive TβRII expression. In this study, in patients with ER-negative, PR-negative, and HER2-negative disease, the percentage of TβRII expression was 56.3%, 54.55%, and 56.10%, respectively.
Fig 1. Immunohistochemical staining of TβRII (transforming growth factor-βreceptor type II) protein expression in breast cancer cells. SP staining; Magnification, ×400.
(A) TβRII expression was labeled as (-). (B) and (C) TβRII protein expression in the two images were labeled as (+) and (++) respectively. (D) The negative controls showed no TβRII expression.
Table 1. Relationships between TβRII and clinicapathological feathers.
| Varible | n | TβRII a - | TβRII + | P-value |
|---|---|---|---|---|
| Age | ||||
| >40 | 98 | 43 | 55 | 0.742 |
| ≤40 | 10 | 5 | 5 | |
| Tumor size | ||||
| ≤2cm | 57 | 28 | 29 | 0.301 |
| 2-5cm | 51 | 20 | 31 | |
| Nodes | ||||
| Nagative | 62 | 33 | 29 | 0.033 |
| Positive | 46 | 15 | 31 | |
| Clinial stage | ||||
| I | 30 | 15 | 15 | |
| II | 64 | 32 | 32 | 0.011 |
| III | 14 | 1 | 13 |
aTβRII,typeIITGFβ(transforming growth factorβ)receptor
Relationships between TβRII expression and clinicopathological features
As shown in Table 1, TβRII expression was not related to age or tumor size (P = 0.742, and P = 0.301, respectively). The expression levels of TβRII in patients with lymph node metastasis were significantly higher compared with patients without lymph node metastasis, and a statistically significant difference was observed between the two groups (P = 0.033). Statistically significant relationships were found between increasing tumor stage and high TβRII expression (P = 0.011).
Relationships between TβRII expression and ER,PR,and Her-2
As shown in Table 2, the associations between the expression of TβRII and ER, PR, and Her-2 were not found to be significantly different (P = 0.925, P = 0.84, and P = 0.861, respectively).
Table 2. Relationships between TβRII and ER, PR, Her-2.
| Variable | n | TβRII - | TβRII + | P-value |
|---|---|---|---|---|
| ER a | ||||
| Nagative | 32 | 14 | 18 | 0.925 |
| Positive | 76 | 34 | 42 | |
| PR b | ||||
| Nagative | 44 | 20 | 24 | 0.84 |
| Positive | 64 | 28 | 36 | |
| Her-2 c | ||||
| Nagative | 82 | 36 | 46 | 0.861 |
| Positive | 26 | 12 | 14 |
aER,estrogen receptor.
bPR, progesterone receptor.
cHer-2,human epidermal growth factor receptor 2.
Survival outcome
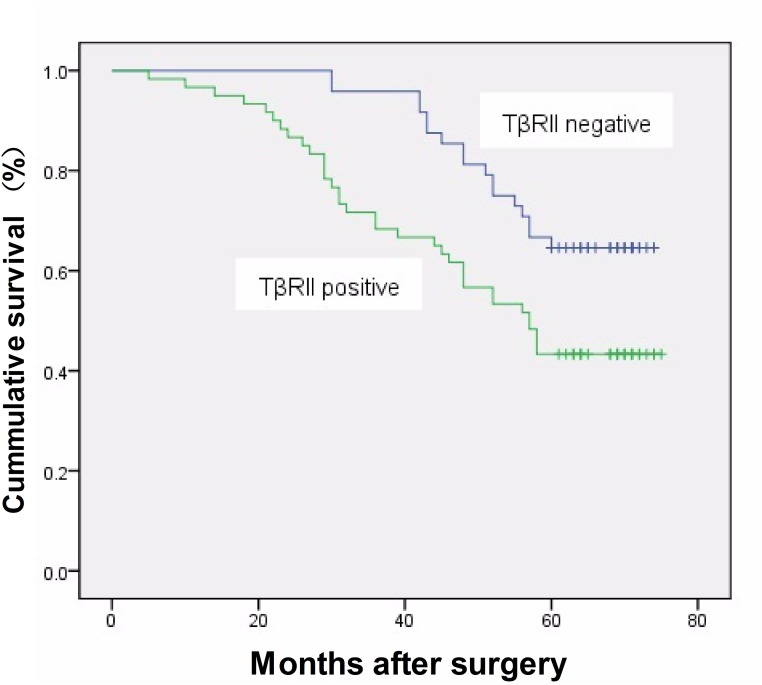
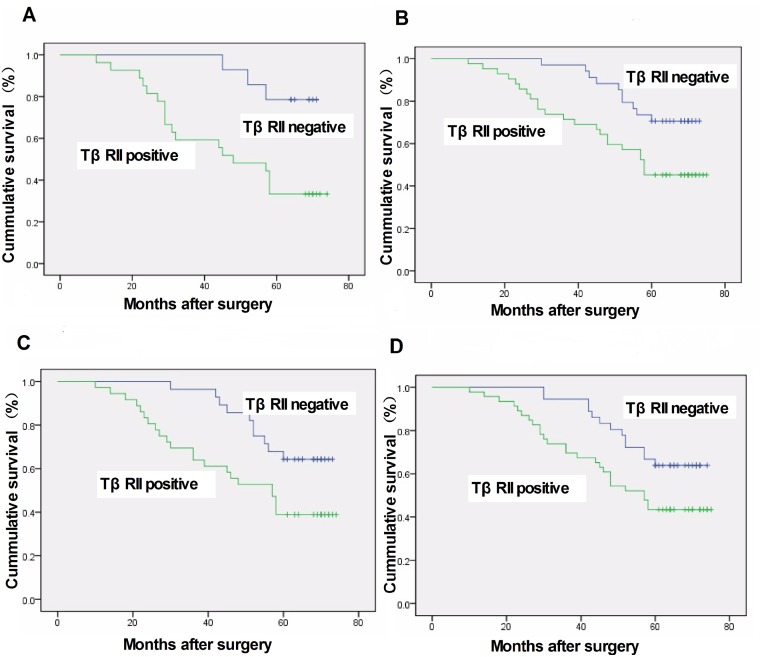
After a median follow-up time of 54.96 months (range, 5–75 months), 51 women (54.4%) had relapsed and died, and 5-year DFS rate was 45.6%. Patients with stage I or II disease had a significantly longer DFS rate than those with stage III disease (50.0% vs. 60.94% vs. 21.43%; P = 0.026). Univariate analysis showed that elevated TβRII expression was associated with poor DFS of breast cancer (P = 0.028). However, there was no association of survival rates and tumor size, lymph node status, and the expression of ER, PR, and HER-2. Survival analysis demonstrated that TβRII was associated with poor DFS (P = 0.011, log rank test; Fig 2). Subgroup analysis revealed that TβRII expression was associated with a shorter DFS rate in patients with lymph node metastasis, ER-positive, PR-positive, and Her-2-negative tumors (P = 0.006, P = 0.016, P = 0.022, and P = 0.033, respectively; log rank test; Fig 3A–3D). Furthermore, multivariate analysis using Cox proportional hazards model revealed that TβRII was an independent prognostic factor for breast cancer.
Fig 2. TβRII expression was significantly associated with 5-year disease-free survival (P = 0.011).
Fig 3. Five-year disease-free survival rate of breast cancer patients stratified according to TβRII expression, other clinicopathological characteristics and immunohistochemical markers.
(A) TβRII expression was associated with poorer prognosis in lymph node-positive patients (P = 0.006).(B) TβRII expression was associated with shorter 5-year disease-free survival in patients with ER-positive breast cancer (P = 0.016).(C) TβRII expression was associated with shorter 5-year disease-free survival in the patients with PR-positive breast cancer (P = 0.022).(D) In patients lacking HER2 expression, there was a significant association between TβRII expression and 5-year disease-free survival (P = 0.033).
Discussion
TGF-β signaling regulates multiple aspects of tumor progression, including the proliferation, apoptosis, and metastasis of tumor cells, as well as the maintenance of tumor-initiating cells; TGF-β has either a tumor suppressing or tumor promoting function depending on cellular context [15].
In the present study, our data demonstrated that positive TβRII expression was associated with lymph node metastasis and increasing clinical stage. This observation is consistent with the concept that in late-stage human cancers, the TGF-β signaling pathway functions as a tumor-promoter, which is associated with an aggressive tumor phenotype and metastasis [22,23]. Previous works suggests that decreased TβRII is associated with an increased risk of developing invasive breast cancer, and that TβRII is a marker of poor prognosis [7,11]. In the TGF-β signaling pathways, TβRII is critical for transcription. Tumor cells are less sensitivive to TGF-β-mediated growth inhibitory responses upon TβRII down-regulation [24]. In contrast, our study showed that higher TβRII expression was correlated with poorer survival outcome. There may be some possible reasons behind these discrepant results, such as the ethnicity of the patient population, the sample size, and the varying clinicopathological features including clinical stage, tumor histological type, grade, and others. However, perhaps more importantly,the methods of evaluating TβRII expression by immunohistochemistry are different in various studies. Perhaps, the additional methods to evaluate the TβRII expression can provide more information for the discrepancy from different studies. So, the improved experimental design must be needed for the further research.
Several studies have concentrated more on the relationship between ER and TGF-β signaling. It is reported that ER and ER-α suppressed breast cancer metastasis by inhibiting TGF-β signaling [25]. ER-negative tumors that express TβRII or demonstrate a TGF-β response transcript signature have been associated with reduced overall survival [26]. High expression of TβRII is associated with ER positivity [19]. In our study, after the subgroup analysis, ER positive tumors that expressed TβRII was associated with shorter DFS.
However there are still some other components involved in the TGF-β signaling pathway, including TGF-β receptors I, nuclear phosphorylated-Smad2 and Smad4. Previous study shown that the presence of phosphorylated-Smad2 (p-Smad2, indicative of active canonical TGF-β signaling) was associated with positive nodal status [20] and it was reported that high expression of Smad4 is associated with a favorable prognosis [19]. These TβRII downstream targets were also associated with the prognosis of breast cancer. In further studies, these biomarkers should be combined to explore the interactions and provide more information about the TGF-β signaling pathway.
In summary, our results demonstrate that high expression of TβRII in breast cancer cells may be a prognostic marker for breast cancer patients and the deep research is needed to provide useful information for evaluating the effect of TGF-β in the development of breast cancer.
Supporting Information
(PDF)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
These authors have no support or funding to report.
References
- 1. Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D: Global cancer statistics, 2011, 61, pp 69–90. [DOI] [PubMed] [Google Scholar]
- 2. Goto N, Hiyoshi H, Ito I, Iida K, Nakajima Y, Nagasawa K, et al. : Identification of a novel compound that suppresses breast cancer invasiveness by inhibiting transforming growth factor-β signaling via estrogen receptor α. Journal of Cancer 2014;5:336–343. 10.7150/jca.7202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Deckers M, van Dinther M, Buijs J, Que I, Lowik C, van der Pluijm G, et al. : The tumor suppressor Smad4 is required for transforming growth factor beta-induced epithelial to mesenchymal transition and bone metastasis of breast cancer cells. Cancer Res 2006;66:2202–2209. [DOI] [PubMed] [Google Scholar]
- 4. Kang Y, He W, Tulley S, Gupta GP, Serganova I, Chen CR,et al. : Breast cancer bone metastasis mediated by the Smad tumor suppressor pathway. Proc Natl Acad Sci U S A 2005;102:13909–13914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Makris EA, Hadidi P, Athanasiou KA: The knee meniscus: Structure-function, pathophysiology, current repair techniques, and prospects for regeneration. Biomaterials 2011;32:7411–7431. 10.1016/j.biomaterials.2011.06.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Arteaga CL, Moses HL: TGF-beta in mammary development and neoplasia. J Mammary Gland Biol Neoplasia 1996;1:327–329. [DOI] [PubMed] [Google Scholar]
- 7. Gobbi H, Dupont WD, Simpson JF, Plummer WJ, Schuyler PA, Olson SJ,et al. : Transforming growth factor-beta and breast cancer risk in women with mammary epithelial hyperplasia. J Natl Cancer Inst 1999;91:2096–2101. [DOI] [PubMed] [Google Scholar]
- 8. Wiercinska E, Naber HP, Pardali E, van der Pluijm G, van Dam H, Ten DP: The TGF-beta/Smad pathway induces breast cancer cell invasion through the up-regulation of matrix metalloproteinase 2 and 9 in a spheroid invasion model system. Breast Cancer Res Treat 2011;128:657–666. 10.1007/s10549-010-1147-x [DOI] [PubMed] [Google Scholar]
- 9. Novitskiy SV, Pickup MW, Gorska AE, Owens P, Chytil A, Aakre M,et al. : TGF- receptor II loss promotes mammary carcinoma progression by Th17-Dependent mechanisms. Cancer Discovery 2011;1:430–441. 10.1158/2159-8290.CD-11-0100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gobbi H, Arteaga CL, Jensen RA, Simpson JF, Dupont WD, Olson SJ,et al. : Loss of expression of transforming growth factor beta type II receptor correlates with high tumour grade in human breast in-situ and invasive carcinomas. Histopathology 2000;36:168–177. [DOI] [PubMed] [Google Scholar]
- 11. Paiva CE, Drigo SA, Rosa FE, Moraes NF, Caldeira JR, Soares FA,et al. : Absence of transforming growth factor-beta type II receptor is associated with poorer prognosis in HER2-negative breast tumours. Ann Oncol 2010;21:734–740. 10.1093/annonc/mdp518 [DOI] [PubMed] [Google Scholar]
- 12. Chowdhury S, Ammanamanchi S, Howell GM: Epigenetic targeting of transforming growth factor beta receptor II and implications for cancer therapy. Mol Cell Pharmacol 2009;1:57–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Brattain MG, Markowitz SD, Willson JK: The type II transforming growth factor-beta receptor as a tumor-suppressor gene, 1996, 8, pp 49–53. [DOI] [PubMed] [Google Scholar]
- 14. Mamiya T, Yamazaki K, Masugi Y, Mori T, Effendi K, Du W, et al. : Reduced transforming growth factor-beta receptor II expression in hepatocellular carcinoma correlates with intrahepatic metastasis. Lab Invest 2010;90:1339–1345. 10.1038/labinvest.2010.105 [DOI] [PubMed] [Google Scholar]
- 15. Ikushima H, Miyazono K: TGFβ signalling: A complex web in cancer progression. Nat Rev Cancer 2010;10:415–424. 10.1038/nrc2853 [DOI] [PubMed] [Google Scholar]
- 16. Yu Y, Kanwar SS, Patel BB, Oh PS, Nautiyal J, Sarkar FH, et al. : MicroRNA-21 induces stemness by downregulating transforming growth factor beta receptor 2 (TGFbetaR2) in colon cancer cells. Carcinogenesis 2012;33:68–76. 10.1093/carcin/bgr246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Leemans CR, Braakhuis BJ, Brakenhoff RH: The molecular biology of head and neck cancer. Nat Rev Cancer 2011;11:9–22. 10.1038/nrc2982 [DOI] [PubMed] [Google Scholar]
- 18. Chen H, Wang JW, Liu LX, Yan JD, Ren SH, Li Y, et al. : Expression and significance of transforming growth factor-beta receptor type II and DPC4/Smad4 in non-small cell lung cancer. Exp Ther Med 2015;9:227–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. de Kruijf EM, Dekker TJ, Hawinkels LJ, Putter H, Smit VT, Kroep JR,et al. : The prognostic role of TGF-beta signaling pathway in breast cancer patients. Ann Oncol 2013;24:384–390. 10.1093/annonc/mds333 [DOI] [PubMed] [Google Scholar]
- 20. Figueroa JD, Flanders KC, Garcia-Closas M, Anderson WF, Yang XR, Matsuno RK,et al. : Expression of TGF-β signaling factors in invasive breast cancers: Relationships with age at diagnosis and tumor characteristics. Breast Cancer Res Tr 2010;121:727–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Takanami I, Tanaka F, Hashizume T, Kodaira S: Roles of the transforming growth factor beta 1 and its type I and II receptors in the development of a pulmonary adenocarcinoma: Results of an immunohistochemical study. J Surg Oncol 1997;64:262–267. [DOI] [PubMed] [Google Scholar]
- 22. Derynck R, Akhurst RJ, Balmain A: TGF-beta signaling in tumor suppression and cancer progression. Nat Genet 2001;29:117–129. [DOI] [PubMed] [Google Scholar]
- 23. Wakefield LM, Roberts AB: TGF-beta signaling: Positive and negative effects on tumorigenesis. Curr Opin Genet Dev 2002;12:22–29. [DOI] [PubMed] [Google Scholar]
- 24. Mishra S, Deng JJ, Gowda PS, Rao MK, Lin CL, Chen CL, et al. : Androgen receptor and microRNA-21 axis downregulates transforming growth factor beta receptor II (TGFBR2) expression in prostate cancer. Oncogene 2014;33:4097–4106. 10.1038/onc.2013.374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Goto N, Hiyoshi H, Ito I, Tsuchiya M, Nakajima Y, Yanagisawa J: Estrogen and antiestrogens alter breast cancer invasiveness by modulating the transforming growth factor-beta signaling pathway. Cancer Sci 2011;102:1501–1508. 10.1111/j.1349-7006.2011.01977.x [DOI] [PubMed] [Google Scholar]
- 26. Buck MB, Fritz P, Dippon J, Zugmaier G, Knabbe C: Prognostic significance of transforming growth factor beta receptor II in estrogen receptor-negative breast cancer patients. Clin Cancer Res 2004;10:491–498. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.