Abstract
Essential life processes are heavily controlled by a variety of positive and negative feedback systems. Cytokinesis failure, ultimately leading to aneuploidy, is appreciated as an early step in tumor formation in mammals and is deleterious for all cells. Further, the growing list of cancer predisposition mutations includes a number of genes whose proteins control mitosis and/or cytokinesis. Cytokinesis shape control is also an important part of pattern formation and cell-type specialization during multi-cellular development. Inherently mechanical, we hypothesized that mechanosensing and mechanical feedback are fundamental for cytokinesis shape regulation. Using mechanical perturbation, we identified a mechanosensory control system that monitors shape progression during cytokinesis. In this review, we summarize these findings and their implications for cytokinesis regulation and for understanding the cytoskeletal system architecture that governs shape control.
Keywords: actin, cell mechanics, checkpoint, control system, cytokinesis, mechanical feedback, myosin-II
INTRODUCTION
The human body relies on controlled cell division to replenish numerous cell types continually, with ~108–109 cell division events occurring at every moment in time. Successful mitosis requires that the cell's genetic material be accurately replicated, separated, and properly positioned so that cytokinesis produces two genetically equivalent daughter cells. Since Boveri's work in the early 20th century, successful cell division has been appreciated as critical to human health. Failure of cytokinesis is tumorigenic in mouse models and many cancer-associated genes lead to mitotic and/or cytokinetic failure.1-4 For example, BRCA2, one of the first genes associated with familial breast cancers and later implicated in other types of cancers, is required for myosin-II localization at the cleavage furrow cortex and for successful completion of cytokinesis.5-7 Given the high rate of cell proliferation and the direct connection between cytokinesis failure and tumorigenesis, it is perhaps not surprising that nearly one in two U.S. citizens will develop some form of invasive tumor during their life time (American Cancer Society 2006 Annual Report). Thus, a complex, dynamic process such as cytokinesis likely utilizes many types of corrective mechanisms to ensure that cytokinesis completes with a nearly 100% success rate. Uncovering how cell division is regulated will have broad implications in many areas of research, providing insight into numerous genetic disorders and tumor cell biology as well as lead to the identification of new therapeutic targets. In this article, we summarize the molecular and mechanical basis for a mechanosensory and mechanical feedback system that we recently discovered that provides a control system to regulate cell shape progression during cytokinesis.8
CONVENTIONAL VIEW OF CYTOKINESIS
Dividing cells undergo a series of shape changes that begin in mitosis and continue throughout cytokinesis (Fig. 1). Stereotypically, the mother cell rounds up by metaphase and then elongates during anaphase with the long axis of the cell parallel to the spindle axis. By telophase, the cleavage furrow begins to ingress until a thin bridge is formed. The bridge dwells until it is ultimately severed yielding two daughter cells.
Figure 1.
Stereotypical, open-loop cytokinesis. (A) Cartoon depicts the series of shapes that cells undergo during cytokinesis. Region of myosin-II enrichment is shown in green. Microtubules are shown in red and depict the microtubule rearrangements typical of Dictyostelium. Nuclei are shown as blue circles. Dictyostelium cells have a closed mitosis (similar to yeast) in which the nuclear envelope does not completely disassemble as in higher metazoans. (B) Diagram depicts an open loop system in which the mitotic spindle delivers signals that direct the accumulation of contractile proteins to the equatorial cortex. The contractile proteins generate force, which changes the cell shape until cytokinesis is completed, producing two daughter cells. However, mechanical perturbations can also affect the forces acting on the cell, and if these disturbances go undetected, cytokinesis can be defective.
In the literature, cytokinesis is often subdivided into phases: cleavage plane specification, contractile ring assembly, contraction, and resolution into two daughter cells. In this framework, the mitotic spindle delivers the signals that spatiotemporally control contractile ring assembly,9-17 while the constriction of the contractile ring involves force production,9 at least in part, through myosin-II mechanochemistry.18 Indeed, a nearly ubiquitous requirement for myosin-II has been revealed through genetic experiments in a variety of organisms.19-21 However, for Dictyostelium and higher metazoans, the contractile ring assembly and contraction phases are overlapping processes, and contraction is itself a multi-phasic process.22,23 In Dictyostelium, myosin-II accumulation at the cleavage furrow is biphasic with the amount and concentration of this mechanoenzyme increasing during cell elongation, peaking during cleavage furrow ingression, and then decreasing during late bridge thinning.24 Concurrent with myosin-II biphasic dynamics, actin binding proteins differentially localize to the polar (RacE, dynacortin, coronin) or cleavage furrow (cortexillin-I) cortex. In light of such sophisticated cytoskeletal machinery, it is not surprising that morphological analysis reveals that the cleavage furrow thins with a complex dynamic made of fast and slow phases, which contribute to furrow ingression in different ways in different genetic backgrounds.22 The coordinated action of actin binding and actin-based myosin-II motor proteins differentially localizing to the polar or cleavage furrow cortex also control the cell's mechanical properties during cytokinesis.10,11 To summarize, cytokinesis is portrayed as a precisely timed sequence of biochemical events in which the contractile ring assembles, constricts to drive the shape changes of cytokinesis, and disassembles in conjunction with bridge-thinning and separation (Fig. 1A).25,26 However, this view describes a system that does not include an inherent ability to correct for errors in the cell division process because the cell is unable to determine if it is going through the proper shape changes (Fig. 1B).
CELL SHAPE IS MONITORED AND CONTROLLED
The presence of feedback loops in a system allows it to monitor its output and to counteract the effects of deleterious disturbances. Feedback loops are a critical part of all aspects of cell biology where the cell ensures successful completion of any process in the presence of environmental perturbations, which can be chemical, thermal, or mechanical in nature. Cells utilize numerous mechanisms to control their progression through the cell cycle. As examples, the metaphase checkpoint relies on feedback to delay spindle elongation until proper kinetochore attachment by microtubules has been established,27,28 while the p53 checkpoint monitors the cell for DNA damage in various phases of the cell cycle.29 A cytokinesis checkpoint that monitors spindle placement has been identified in S. cerevisiae, ensuring that both the mother and daughter cell receive a nucleus during budding.30,31 Surprisingly, if the cell completely fails at cytokinesis, there does not appear to be a checkpoint control preventing further cell cycles.32,33 Without such a checkpoint, monitoring and regulating shape progression must be critical to ensure cytokinesis fidelity and avoid the formation of tetraploid cells.
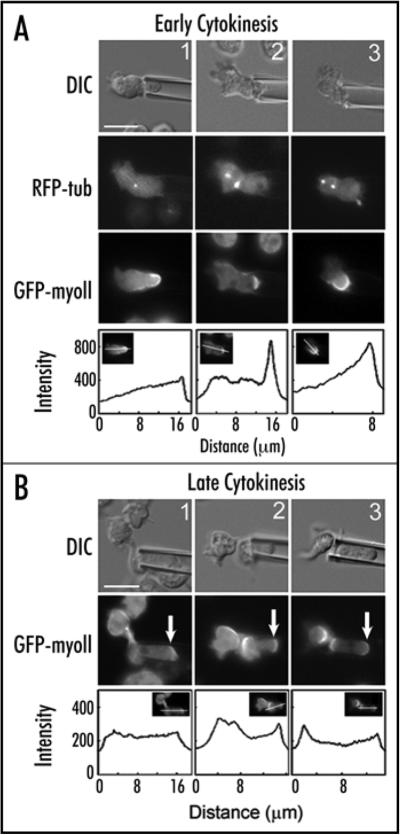
We hypothesized that there must be a control system that monitors and corrects cell shape progression during cytokinesis.8 We found that about 40% of normal dividing (anaphase-telophase) Dictyostelium cells have asymmetries in cell shape that they correct by distributing myosin-II to the distended region of the cell, contracting it to round the cell and then completing division that produces symmetrically shaped daughter cells. This observation suggested that an active mechanism for correcting and controlling shape might exist. We then applied mechanical forces to cells using micropipette aspiration to deform them in specific ways and observe their responses. Mitotic cells in anaphase through the completion of cytokinesis accumulated myosin-II and cortexillin-I at the micropipette (Fig. 2). In early cytokinesis, cells frequently contracted from the pipette, escaping it, and then rerounding so that cell division could be completed, producing two symmetrically sized daughter cells (Fig. 2A). The cells slowed cytokinesis progression if the deformation was induced before significant furrow ingression occurred and required roughly 3 minutes to correct the shape deformation before completing cell division. Cells deformed during late cytokinesis continued with cytokinesis until completion rather than escaping the pipette but still sent myosin to the site of applied load to resist further deformation (Fig. 2B). Significantly, the mechanosensory response only occurred during anaphase through the end of cytokinesis and did not depend on microtubules or spindle orientation. Thus, the mechanosensation appears to be independent of the usual spindle checkpoint controls. The globally distributed actin-crosslinker dynacortin did not move to the micropipette demonstrating that the response is not a general cytoskeletal response. Finally, myosin-II is central to the control system as removal of myosin-II resulted in a three-fold higher failure rate and grossly asymmetric cell division when challenged by mechanical load.
Figure 2.
Myosin-II redistributes to the site of cell deformation during anaphase through the end of cytokinesis. (A) Three examples of mitotic cells aspirated during anaphase. DIC images show the cell and the micropipette. RFP-tubulin images reveal anaphase spindles that are often bowed during the cell deformation. GFP-myosin-II becomes enriched under the micropipettes. Line scans show the increased GFP-myosin-II accumulation at the micropipette. (B) Three examples of late stage dividing cells aspirated by the micropipette (DIC). GFP-myosin-II has accumulated at the micropipette (arrows) and at the cleavage furrow cortex. Line scans show the increased GFP-myosin-II accumulation at the micropipette. Scale bars in (A and B) are 10 mm and apply to all panels.
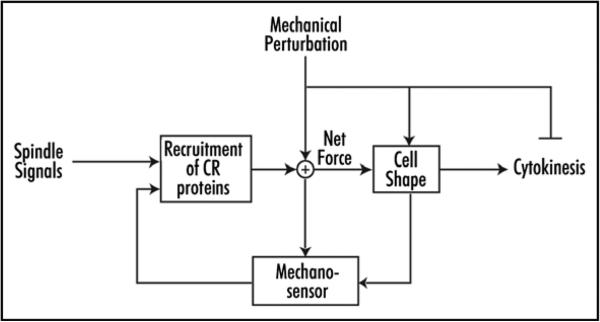
This mechanosensory response has some of the hallmarks of a checkpoint that monitors cell shape (Fig. 3). Typically, the cell cycle is viewed as a series of well-ordered discrete states. At any stage, subsequent progression requires that checkpoints be satisfied, leading to irreversible transitions to the next state. These distinct states can be viewed as multiple stable steady-states of a dynamical system and the corresponding transitions as a switch from one state to the other, leading to bi-stability.34 Feedback loops are required to create this bi-stability.35,36 While also ensuring that mitosis completes successfully, the feedback loop uncovered in our experiments acts in different but complementary ways. First, it halts the progress through mitosis, delaying cytokinesis until the cell is able to escape the micropipette. However, this delay serves as a “soft” rather than “hard” checkpoint in that cells aspirated late in cytokinesis can still divide. At the same time, the feedback loop acts to reject the effect of the external mechanical disturbances thus allowing the cell cycle to continue but ensuring that the transition between states occurs in a well-ordered manner.
Figure 3.
Diagram depicts a mechanosensory system that monitors cell shape during cytokinesis. This system is closed loop in which cell shape is monitored and fed back to redirect contractile protein accumulation to regions of shape deformation. Upon correcting cell shape, the contractile proteins reaccumulate at the equatorial cortex, allowing symmetrical cell division to complete.
Direct monitoring of cell shape is likely to be as important to cytokinesis fidelity as monitoring chromosome spindle attachment. Symmetrical cell division would help ensure that the chromosomes are segregated properly. While the spindle pulls the chromosomes evenly into the two hemispheres of the mother cell, the cleavage furrow must then invaginate through the cell's equator, ensuring that the chromosomes are partitioned correctly into two daughter cells. In Dictyostelium, mutants deficient in cortical cytoskeletal proteins, the actin crosslinker cortexillin-I or the actin modulating protein Aip1, have trouble elongating the spindle and properly segregating chromosomes into the daughter cells.37 Similarly, in yeast, the boi1 and boi2 proteins, which are related to actin-membrane-associated anillin proteins, have a defective checkpoint control for chromosomal material trapped in the bud neck.31 In our work, we have discovered that global proteins as well as equatorial proteins control the furrow ingression dynamics. While inhibition of equatorial proteins myosin-II and cortexillin-I slow the initial phase of furrow ingression, removal of global proteins, actin crosslinker dynacortin and RacE small GTPase, leads to a dramatic increase in furrow-thinning rates late in cytokinesis.22,38 As the cleavage furrow cortex generates nN-scale forces,8,22,24,39 which are sufficient to break chromosomes,40-42 the slow evolution of shape during wild type furrow ingression probably helps protect chromosomal integrity.22 Thus, evidence is mounting for a role in the actin network in providing quality control over karyokinesis as well as cytokinesis progression.
Cell size and shape are also beginning to be appreciated as potential regulators of signal transduction.43 In this case, if cell division led to daughter cells of unequal size, each daughter could respond differently to external cues, leading to differences in cell fate of the two daughter cells. In situations where asymmetric daughter cells are needed, such as during early embryogenesis in C. elegans,44 the correct daughter shapes could be attained by modifying the feedback system.
HOW MIGHT A MECHANOSENSORY SYSTEM WORK?
A mechanical feedback control system requires two components: a mechanosensor and a mechanotransducer. Paradigms for mechanosensors fall largely into three classes (Fig. 4).45 In one class, the entire actin network serves as a mechanosensor. This scheme involves strains in the actin network that lead to unfolding of cytoskeletal associated proteins and creation of binding sites for new proteins. This class of sensor is best exemplified by the focal adhesion complex.46,47
Figure 4.
Classes of potential mechanosensors that could sense cellular deformation. (A) The actin network itself can function as a mechanosensor. Strain induced by applied force (F) on the network can lead to local unfolding of actin-associated proteins, which can create new binding sites, allowing other proteins (green ball) to associate. (B) Strain in the membrane can lead to channel opening, allowing ions to move. Many types of channels are known to be strain-sensitive. (C) Myosins are strain sensitive. Load against the motor increases the strongly bound state time (ts) typically by inhibiting ADP-release for most myosin classes. If myosin binding changes the actin network, stabilizing actin-myosin interactions by increasing ts could lead to accumulation of other actin-associated proteins.
In a second class, stretch-activated membrane channels function as mechanosensors. Many membrane channels, including the PKD2 Ca2+ channel and inner rectifier K+ channel in eukaryotes as well as the prokaryotic MscS and MscL channels, display some form of activation upon stretching.48 Thus, it is possible that the first phase of mechanosensing requires stretch of the membrane, leading to channel opening that triggers the recruitment of myosin-II.
In a third class, myosins themselves have mechanosensing ability due to strain that alters their binding properties. This class of behaviors is best demonstrated by myoIC during adaptation in hearing.49 Myosin-II is an attractive candidate for providing mechanosensory functions during cytokinesis. Micropipette aspiration induces mechanical strain (deformation) on the cortical actin network that could be sensed directly by myosin-II. Myosin naturally couples biochemical (nucleotide) states and protein conformations to force generation. Several studies have shown that a variety of myosin family members respond to mechanical loads by altering biochemical rates, which is particularly important in processive myosins, allowing gating of the two heads.50-52 Thus, myosin-II is already poised to sense mechanical strains on the actin network and could serve as a mechanotransducer by altering the actin conformation, promoting the binding of other proteins (such as cortexillin-I).
Once the shape deformation has been sensed, the cell accumulates myosin-II and cortexillin-I at the cortex underlying the micropipette. From mechanical measurements, these proteins would increase the cortical resistance (cortical viscoelasticity) of the underlying actin network,53-55 which would counteract further shape deformation. Then, myosin-II mediated dynamic rearrangements of the cortical actin network would lead to contractility of the cortex away from the pipette, allowing the cell to pull itself from the micropipette.55 Once the shape has been corrected, allowing the strain in the network to relax, the contractile proteins disassemble, and cytokinesis proceeds.
MANY ROLES OF MYOSIN-II
The mechanoenzyme myosin-II, which localizes to the cleavage furrow, is vital to many aspects of cell division and has long been recognized as being important to cytokinesis. Myosin-II promotes actin turnover in the contractile ring during cytokinesis56-58 and remodeling of furrow microtubules,58 and drives cell elongation and cleavage furrow ingression.59 Myosin-II has also been shown to affect processes upstream of cytokinesis such as spindle assembly, enabling proper centrosome separation and positioning.60
Dictyostelium cells lacking myosin-II have altered cleavage furrow morphology and bridge-thinning dynamics.22 During early cytokinesis, myoII cells exhibit slower bridge-thinning dynamics than wild type cells, but then late in cytokinesis the bridge-thinning dynamics are faster than wild type cells. This multi-phasic dynamics has been reconciled by considering the viscoelastic nature of cells and utilizing fluid dynamic principles to understand furrow-thinning further. The myoII cells also frequently exhibit asymmetric bridge thinning, which produces unequally-sized daughter cells.8 Overall, myosin-II has a fundamental role in many important cell division processes as well as maintaining proper cortical mechanics during division, and revealing these diverse roles has required innovative genetic, mechanical, and analytical approaches.
MECHANOSENSING IN OTHER CONTEXTS
Mechanosensing and mechanotransduction are fundamental to a wide variety of cellular processes critical to healthy states.45,61 Tumor cells can grow in the absence of surface attachment, a feature that classically defines cellular transformation, indicating that changes in mechanotransduction are an important part of cancer progression. Bone remodeling, blood pressure regulation, hearing, and exercise-induced skeletal muscle growth are all examples of normal processes that depend on mechanosensing. Furthermore, nonmuscle myosin-IIs have been implicated in mechanosensing of substrate stiffness and contribute to how stem cells sense and differentiate in response to various mechanical environments that mimic bone, muscle and brain tissue.62 Though the molecular mechanisms for mechanosensing and mechanotransduction are not fully elucidated in any system, the actin cytoskeleton and myosin-II motors clearly play an integral role in mechanotransduction.
CONCLUSION
Overall, mechanosensation is a universally important cellular response. Understanding how this mitosis-specific system works will have important implications for general cellular mechanosensation. Moreover, because mechanosensation on the whole organ or organism level ultimately reduces to cellular responses, dissecting this system in Dictyostelium will have implications for these systems as well.
A fundamental question that needs to be addressed for the mitotic mechanosensory response is how the cell detects its current shape. How does a cell sense deformation/shape irregularities and how does a cell know when its shape has been corrected? Do cells sense force locally through a molecular scale strain or sense shape deformations globally suggesting that the mechanosensing is a network or system response?
Also of importance is to understand the role of myosin in the response. Cell shape sensing requires a molecular strain trigger. What is the molecular scale strain that is sensed and is myosin-II itself the strain sensor? Myosin-II already has all of the critical features for a sensor: force-dependent biochemical steps (strain-sensing) and the ability to generate force on the actin network (force transduction). Other questions include what other factors are recruited; how does the system lead to shape correction; and why is the response cell-cycle stage specific. Extreme cell deformations that lead to membrane-cortex rupture trigger myosin-II recruitment during interphase;63,64 yet, the mitosis-specific response occurs in cells that do not apparently have a membrane cortex rupture. We suspect that the cell-cycle stage-specificity reflects differences in the cortex composition and/or mechanics that make the mitotic cell exquisitely suited to undergo its dramatic shape change (producing two daughters in under 5 min.!). Indeed, understanding the mechanosensory response will provide insight into cytokinesis fidelity, cell shape change, cellular contractile systems, and cell mechanics.
Traditional genetic, pharmacological, and cell biological approaches have uncovered numerous molecules involved in cytokinesis and helped establish the order of events. Because cytokinesis is an inherently mechanical process, these approaches must be combined with biophysical techniques that can probe the mechanical properties of cells. By using mechanical perturbation, our data have revealed novel roles for contractile proteins during mitosis and discovered a novel mechanosensory system that controls the global cell shape changes ensuring robust cytokinesis.
ACKNOWLEDGEMENTS
This work was supported by a Burroughs-Wellcome Career Development Award (D.N.R.), a Beckman Young Investigator Award (D.N.R.), the NIH (R01#GM066817 to D.N.R., GM071920 to P.A.I.) and the NSF (DMS0083500 to P.A.I.).
References
- 1.Fujiwara T, Bandi M, Nitta M, Ivanova EV, Bronson RT, Pellman D. Cytokinesis failure generating tetraploids promotes tumorigenesis in p53-null cells. Nature. 2005;437:1043–7. doi: 10.1038/nature04217. [DOI] [PubMed] [Google Scholar]
- 2.Giet R, Petretti C, Prigent C. Aurora kinases, aneuploidy and cancer, a coincidence or a real link? Trends Cell Biol. 2005;15:241–50. doi: 10.1016/j.tcb.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 3.Meraldi P, Honda R, Nigg EA. Aurora-A overexpression reveals tetraploidization as a major route to centrosome amplification in p53−/− cells. EMBO J. 2002;21:483–92. doi: 10.1093/emboj/21.4.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gisselsson D. Mitotic instability in cancer: Is there a method in the madness? Cell Cycle. 2005;4:1007–10. doi: 10.4161/cc.4.8.1884. [DOI] [PubMed] [Google Scholar]
- 5.Wooster R, Neuhausen SL, Mangion J, Quirk Y, Ford D, Collins N, Nguyen K, Seal S, Tran T, Averill D. Localization of a breast cancer susceptibility gene, BRCA2, to chromosome 13q12-13. Science. 1994;265:2088–90. doi: 10.1126/science.8091231. [DOI] [PubMed] [Google Scholar]
- 6.Nathanson KL, Wooster R, Weber BL. Breast cancer genetics: What we know and what we need. Nat Med. 2001;7:552–6. doi: 10.1038/87876. [DOI] [PubMed] [Google Scholar]
- 7.Daniels MJ, Wang Y, Lee M, Venkitaraman AR. Abnormal cytokinesis in cells deficient in the breast cancer susceptibility protein BRCA2. Science. 2004;306:876–9. doi: 10.1126/science.1102574. [DOI] [PubMed] [Google Scholar]
- 8.Effler JC, Kee Y-S, Berk JM, Tran MN, Iglesias PA, Robinson DN. Mitosis-specific mechanosensing and contractile protein redistribution control cell shape. Curr Biol. 2006;16:1962–7. doi: 10.1016/j.cub.2006.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rappaport R. Cytokinesis in Animal Cells. Cambridge University Press; Cambridge: 1996. [Google Scholar]
- 10.Robinson DN, Spudich JA. Towards a molecular understanding of cytokinesis. Trends Cell Biol. 2000;10:228–37. doi: 10.1016/s0962-8924(00)01747-5. [DOI] [PubMed] [Google Scholar]
- 11.Robinson DN, Spudich JA. Mechanics and regulation of cytokinesis. Curr Opin Cell Biol. 2004;16:182–8. doi: 10.1016/j.ceb.2004.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shuster CB, Burgess DR. Parameters that specify the timing of cytokinesis. J Cell Biol. 1999;146:981–92. doi: 10.1083/jcb.146.5.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bringmann H, Hyman A. A cytokinesis furrow is positioned by two consecutive signals. Nature. 2005;436:731–4. doi: 10.1038/nature03823. [DOI] [PubMed] [Google Scholar]
- 14.Rappaport R. Geometrical relations of the cleavage stimulus in constricted sand dollar eggs. J Exp Zool. 1964;155:225–30. doi: 10.1002/jez.1401550209. [DOI] [PubMed] [Google Scholar]
- 15.Wu D, Asiedu M, Adelstein RS, Wei Q. A novel guanine nucleotide exchange factor MyoGEF is required for cytokinesis. Cell Cycle. 2006;5:1234–9. doi: 10.4161/cc.5.11.2815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bringmann H. Cytokinesis and the spindle midzone. Cell Cycle. 2005;4:1709–12. doi: 10.4161/cc.4.12.2215. [DOI] [PubMed] [Google Scholar]
- 17.Geddis AE, Kaushansky K. Endomitotic megakaryocytes form a midzone in anaphase but have a deficiency in cleavage furrow formation. Cell Cycle. 2006;5:538–45. doi: 10.4161/cc.5.5.2537. [DOI] [PubMed] [Google Scholar]
- 18.Yumura S, Uyeda TQP. Transport of myosin II to the equatorial region without its own motor activity in mitotic Dictyostelium cells. Mol Biol Cell. 1997;8:2089–99. doi: 10.1091/mbc.8.10.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.DeLozanne A, Spudich JA. Disruption of the Dictyostelium myosin heavy chain gene by homologous recombination. Science. 1987;236:1086–91. doi: 10.1126/science.3576222. [DOI] [PubMed] [Google Scholar]
- 20.Bezanilla M, Forsburg SL, Pollard TD. Identification of a second myosin-II in Schizosaccharomyces pombe: Myp2p is conditionally required for cytokinesis. Mol Biol Cell. 1997;8:2693–705. doi: 10.1091/mbc.8.12.2693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Straight AF, Cheung A, Limouze J, Chen I, Westwood NJ, Sellers JR, Mitchison TJ. Dissecting temporal and spatial control of cytokinesis with a myosin II inhibitor. Science. 2003;299:1743–7. doi: 10.1126/science.1081412. [DOI] [PubMed] [Google Scholar]
- 22.Zhang W, Robinson DN. Balance of actively generated contractile and resistive forces controls cytokinesis dynamics. Proc Natl Acad Sci USA. 2005;102:7186–91. doi: 10.1073/pnas.0502545102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reichl EM, Effler JC, Robinson DN. The stress and strain of cytokinesis. Trends Cell Biol. 2005;15:200–6. doi: 10.1016/j.tcb.2005.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Robinson DN, Cavet G, Warrick HM, Spudich JA. Quantitation of the distribution and flux of myosin-II during cytokinesis. BMC Cell Biology. 2002;3:4. doi: 10.1186/1471-2121-3-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wu J-Q, Kuhn JR, Kovar DR, Pollard TD. Spatial and temporal pathway for assembly and constriction of the contractile ring in fission yeast cytokinesis. Dev Cell. 2003;5:723–34. doi: 10.1016/s1534-5807(03)00324-1. [DOI] [PubMed] [Google Scholar]
- 26.Guertin DA, Trautmann S, McCollum D. Cytokinesis in eukaryotes. Microbiol Mol Biol Rev. 2002;66:155–78. doi: 10.1128/MMBR.66.2.155-178.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pinsky BA, Biggins S. The spindle checkpoint: tension versus attachment. Trends Cell Biol. 2005;15:486–93. doi: 10.1016/j.tcb.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 28.Pot I, Knockleby J, Aneliunas V, Nguyen T, Ah-Kye S, Liszt G, Snyder M, Hieter P, Vogel J. Spindle checkpoint maintenance requires Ame1 and Okp1. Cell Cycle. 2005;4:1448–56. doi: 10.4161/cc.4.10.2106. [DOI] [PubMed] [Google Scholar]
- 29.Stukenberg PT. Triggering p53 after cytokinesis failure. J Cell Biol. 2004;165:607–8. doi: 10.1083/jcb.200405089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Muhua L, Adames NR, Murphy MD, Shields CR, Cooper JA. A cytokinesis checkpoint requiring the yeast homologue of an APC-binding protein. Nature. 1998;393:487–91. doi: 10.1038/31014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Norden C, Mendoza M, Dobbelaere J, Kotwaliwale CV, Biggins S, Barral Y. The nocut pathway links completion of cytokinesis to spindle midzone function to prevent chromosome breakage. Cell. 2006;125:85–98. doi: 10.1016/j.cell.2006.01.045. [DOI] [PubMed] [Google Scholar]
- 32.Uetake Y, Sluder G. Cell cycle progression after cleavage failure: mammalian somatic cells do not possess a “tetraploidy checkpoint”. J Cell Biol. 2004;165:609–15. doi: 10.1083/jcb.200403014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wong C, Stearns T. Mammalian cells lack checkpoints for tetraploidy, aberrant centrosome number, and cytokinesis failure. BMC Cell Biology. 2005;6:6. doi: 10.1186/1471-2121-6-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ingolia NT, Murray AW. The ups and downs of modeling the cell cycle. Curr Biol. 2004;14:R771–R7. doi: 10.1016/j.cub.2004.09.018. [DOI] [PubMed] [Google Scholar]
- 35.Ferrell JE. Self-perpetuating states in signal transduction: positive feedback, double-negative feedback and bistability. Curr Opin Cell Biol. 2002;14:140–8. doi: 10.1016/s0955-0674(02)00314-9. [DOI] [PubMed] [Google Scholar]
- 36.Brandman O, Ferrell JE, Li R, Meyer T. Interlinked fast and slow positive feedback loops drive reliable cell decisions. Science. 2005;310:496–8. doi: 10.1126/science.1113834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gerisch G, Faix J, Köhler J, Müller-Tautenberger A. Actin-binding proteins required for reliable chromosome segregation in mitosis. Cell Motil Cytoskeleton. 2004;57:18–25. doi: 10.1002/cm.10150. [DOI] [PubMed] [Google Scholar]
- 38.Octtaviani E, Effler JC, Robinson DN. Enlazin, a natural fusion of two classes of canonical cytoskeletal proteins, contributes to cytokinesis dynamics. Mol Biol Cell. 2006;17:5275–86. doi: 10.1091/mbc.E06-08-0767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rappaport R. Cell division: Direct measurement of maximum tension exerted by furrow of echinoderm eggs. Science. 1967;156:1241–3. doi: 10.1126/science.156.3779.1241. [DOI] [PubMed] [Google Scholar]
- 40.Poirier MG, Marko JF. Mitotic chromosomes are chromatin networks without a mechanically contiguous protein scaffold. Proc Natl Acad Sci USA. 2002;99:15393–7. doi: 10.1073/pnas.232442599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Poirier MG, Eroglu S, Chatenay D, Marko JF. Reversible and irreversible unfolding of mitotic newt chromosomes by applied force. Mol Biol Cell. 2000;11:269–76. doi: 10.1091/mbc.11.1.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jannink G, Duplantier B, Sikorav J-L. Forces on chromosomal DNA during anaphase. Biophys J. 1996;71:451–65. doi: 10.1016/S0006-3495(96)79247-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Meyers J, Craig J, Odde DJ. Potential for control of signaling pathways via cell size and shape. Curr Biol. 2006;16:1685–93. doi: 10.1016/j.cub.2006.07.056. [DOI] [PubMed] [Google Scholar]
- 44.Pecreaux J, Röper J-C, Kruse K, Jülicher F, Hyman AA, Grill SW, Howard J. Spindle oscillations during asymmetric cell division require a threshold number of active cortical force generators. Curr Biol. 2006;16:2111–22. doi: 10.1016/j.cub.2006.09.030. [DOI] [PubMed] [Google Scholar]
- 45.Orr AW, Helmke BP, Blackman BR, Schwartz MA. Mechanisms of mechanotransduction. Dev Cell. 2006;10:11–20. doi: 10.1016/j.devcel.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 46.Choquet D, Felsenfeld DP, Sheetz MP. Extracellular matrix rigidity causes strengthening of integrin-cytoskeleton linkages. Cell. 1997;88:39–48. doi: 10.1016/s0092-8674(00)81856-5. [DOI] [PubMed] [Google Scholar]
- 47.Tamada M, Sheetz MP, Sawada Y. Activation of signaling cascade by cytoskeleton stretch. Dev Cell. 2004;7:709–18. doi: 10.1016/j.devcel.2004.08.021. [DOI] [PubMed] [Google Scholar]
- 48.Martinac B. Mechanosensitive ion channels: molecules of mechanotransduction. J Cell Sci. 2004;117:2449–60. doi: 10.1242/jcs.01232. [DOI] [PubMed] [Google Scholar]
- 49.LeMasurier M, Gillespie PG. Hair-cell mechanotransduction and cochlear amplification. Neuron. 2005;48:403–15. doi: 10.1016/j.neuron.2005.10.017. [DOI] [PubMed] [Google Scholar]
- 50.Altman D, Sweeney HL, Spudich JA. The mechanism of myosin VI translocation and its load-induced anchoring. Cell. 2004;116:737–49. doi: 10.1016/s0092-8674(04)00211-9. [DOI] [PubMed] [Google Scholar]
- 51.Veigel C, Molloy JE, Schmitz S, Kendrick-Jones J. Load-dependent kinetics of force production by smooth muscle myosin measured with optical tweezers. Nat Cell Biol. 2003;5:980–6. doi: 10.1038/ncb1060. [DOI] [PubMed] [Google Scholar]
- 52.Purcell TJ, Sweeney HL, Spudich JA. A force-dependent state controls the coordination of processive myosin V. Proc Natl Acad Sci USA. 2005;102:13873–8. doi: 10.1073/pnas.0506441102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pasternak C, Spudich JA, Elson EL. Capping of surface receptors and concomitant cortical tension are generated by conventional myosin. Nature. 1989;341:549–51. doi: 10.1038/341549a0. [DOI] [PubMed] [Google Scholar]
- 54.Girard KD, Chaney C, Delannoy M, Kuo SC, Robinson DN. Dynacortin contributes to cortical viscoelasticity and helps define the shape changes of cytokinesis. EMBO J. 2004;23:1536–46. doi: 10.1038/sj.emboj.7600167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Girard KD, Kuo SC, Robinson DN. Dictyostelium myosin-II mechanochemistry promotes active behavior of the cortex on long time-scales. Proc Natl Acad Sci USA. 2006;103:2103–8. doi: 10.1073/pnas.0508819103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Guha M, Zhou M, Wang Y-l. Cortical actin turnover during cytokinesis requires myosin II. Curr Biol. 2005;15:732–6. doi: 10.1016/j.cub.2005.03.042. [DOI] [PubMed] [Google Scholar]
- 57.Murthy K, Wadsworth P. Myosin-II-dependent localization and dynamics of F-actin during cytokinesis. Curr Biol. 2005;15:724–31. doi: 10.1016/j.cub.2005.02.055. [DOI] [PubMed] [Google Scholar]
- 58.Urven LE, Yabe T, Pelegri F. A role for non-muscle myosin II function in furrow maturation in the early zebrafish embryo. J Cell Sci. 2006;119:4342–52. doi: 10.1242/jcs.03197. [DOI] [PubMed] [Google Scholar]
- 59.Zang J-H, Cavet G, Sabry JH, Wagner P, Moores SL, Spudich JA. On the role of myosin-II in cytokinesis: Division of Dictyostelium cells under adhesive and nonadhesive conditions. Mol Biol Cell. 1997;8:2617–29. doi: 10.1091/mbc.8.12.2617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rosenblatt J, Cramer LP, Baum B, McGee KM. Myosin II-dependent cortical movement is required for centrosome separation and positioning during mitotic spindle assembly. Cell. 2004;117:361–72. doi: 10.1016/s0092-8674(04)00341-1. [DOI] [PubMed] [Google Scholar]
- 61.Janmey PA, Weitz DA. Dealing with mechanics: mechanisms of force transduction in cells. Trends Biochem Sci. 2004;29:364–70. doi: 10.1016/j.tibs.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 62.Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–89. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 63.Merkel R, Simson R, Simson DA, Hohenadl M, Boulbitch A, Wallraff E, Sackmann E. A micromechanic study of cell polarity and plasma membrane cell body coupling in Dictyostelium. Biophys J. 2000;79:707–19. doi: 10.1016/S0006-3495(00)76329-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Charras GT, Yarrow JC, Horton MA, Mahadevan L, Mitchison TJ. Non-equilibration of hydrostatic pressure in blebbing cells. Nature. 2005;435:365–9. doi: 10.1038/nature03550. [DOI] [PMC free article] [PubMed] [Google Scholar]