Abstract
d-Serine, synthesized endogenously in the brain, is an important modulator of glutamatergic neurotransmission. Since colonic bacteria produce d-serine, we asked the question whether there are transport mechanisms in the colon that might make this exogenously produced d-serine available to the host. Here we identify for the first time an amino acid transporter in the intestine for high-affinity active transport of d-serine. This transporter, called ATB0,+, is a Na+- and Cl−-coupled transporter for L-enantiomers of neutral and cationic amino acids. Here we demonstrate that ATB0,+ is also capable of mediating the Na+- and Cl−-coupled transport of d-serine. The affinity of ATB0,+ for l-serine and d-serine is similar, the Kt value for the two enantiomers being ~150 μM. In addition to d-serine, ATB0,+ transports d-alanine, d-methionine, d-leucine, and d-tryptophan. However, several other neutral and cationic amino acids that are transportable substrates for ATB0,+ as L-enantiomers are not transported when presented as D-enantiomers. ATB0,+ is expressed in the intestinal tract, interestingly not in the proximal intestine but in the distal intestine. Expression is most predominant in the colon where the transporter is localized to the luminal membrane of colonocytes, making this transporter uniquely suitable for absorption of bacteria-derived d-serine.
D-Amino acids are generally considered foreign to metabolic pathways in mammals. Almost all mammalian enzymes are selective for L-amino acids with the notable exception of D-amino acid oxidase. D-amino acids are found in the plasma, but they are believed to originate from the diet or from the intestinal microbial flora (1-3). Since D-amino acids do not participate in metabolic pathways in mammals, the biological significance of these amino acids has been questioned. There is evidence however for beneficial effects of certain D-amino acids (2, 3). There is also evidence of a physiological function for d-serine in the modulation of glutamatergic neurotransmission by activation of glutamate signaling via the N-methyl-d-aspartate (NMDA) receptor (4, 5). Interestingly, this D-amino acid is generated endogenously in the brain by racemization of l-serine mediated by serine racemase (6, 7).
D-amino acids are abundant in bacteria (2) and therefore are expected to be present in significant quantities in the colon and ileum where bacterial colonization is prevalent. Since some of the D-amino acids are now known to possess important physiological and pharmacological functions, the issue of intestinal absorption of bacteria-derived and dietary D-amino acids becomes important. If there are effective mechanisms in the intestinal tract for the entry of luminal D-amino acids into blood, it is possible for exogenous D-amino acids to exert biological effects. Several transporters that mediate the intestinal absorption of amino acids have been cloned recently (8). To date, only two amino acid transporters have been shown to transport D-amino acids to any significant extent (9, 10). They are system L1 and system asc1. Both are facilitative transporters that mediate amino acid exchange across the plasma membrane. They are not active because their transport function is not coupled to any driving force. System L1 and system asc1 are expressed in the intestine. Interestingly, these transporters are heterodimeric, both containing the heavy chain of the 4F2 cell surface antigen (4F2hc) as a common subunit. Since 4F2hc is localized exclusively to the basolateral membrane of the absorptive cells of the kidney and intestine (11), system L1 and system asc1 are likely to participate in the efflux of amino acids from the intestinal mucosal cells into the blood. It is not known at present if there are transport systems in the brush border membrane of the intestinal epithelial cells that might mediate the entry of D-amino acids from the lumen into the cells.
The present study was undertaken to compare various amino acid transporters that are expressed in the intestine for their ability to transport d-serine. Here we report for the first time the identification of an amino acid transporter in the intestine for high-affinity active transport of d-serine.
METHODS
Functional expression of amino acid transporter cDNAs in mammalian cells
The functional expression of the transporter cDNAs was carried out in human retinal pigment epithelial (HRPE) cells using the vaccinia virus expression system (12, 13). The transporter clones used in these studies were originally isolated from different cDNA libraries. All of these clones have been shown to be functional in heterologous expression systems (8). Transport measurements were made at 37°C for 15 min with [d-3H]serine or [3H]glycine. The transport buffer was 25 mM Hepes/Tris (pH 7.5) containing 140 mM NaCl, 5.4 mM KCl, 1.8 mM CaCl2, 0.8 mM MgSO4, and 5 mM glucose. Endogenous transport activity was always determined in parallel using cells transfected with vector alone. The cDNA-specific transport activity was calculated by adjusting for the endogenous activity.
Functional expression of mouse ATB0,+ cDNA in Xenopus laevis oocytes
For the functional expression of ATB0,+ in oocytes, mature oocytes from Xenopus laevis were isolated by collagenase treatment (12, 13). Oocytes were manually defolliculated and then used for injection with mouse ATB0,+ cRNA or water. cRNA was synthesized using the mMesSAGE mMACHINE kit (Ambion, Austin, TX). The transport of D-amino acids via mouse ATB0,+ in oocytes was monitored electrophysiologically using the two-microelectrode voltage-clamp technique (12, 13). The membrane potential was held steady at −50 mV. Oocytes were perfused with different L- and D-enantiomers of amino acids and the induced current was monitored. The induced current was taken as the measure of transport rate. The composition of the perfusion buffer was 10 mM Hepes/Tris (pH 7.5), containing 100 mM NaCl, 2 mM KCl, 1 mM MgCl2, and 1 mM CaCl2.
Immunofluorescent localization of ATB0,+ protein in mouse colon
For immunofluorescent localization of ATB0,+ in mouse colon, a polyclonal antibody was raised against the peptide TDHEIPTISGSTKPE corresponding to the C-terminal region 624–637. The specificity of the antibody was tested in Western blot using HRPE cells transfected with either vector alone or mouse ATB0,+ cDNA. The antibody recognized a single protein (64 kDa) in cDNA-transfected cells. There was no immunoreactive protein in vector-transfected cells. This antibody was used to localize ATB0,+ in mouse colon by immunofluorescent confocal microscopy as described previously (14, 15).
RESULTS AND DISCUSSION
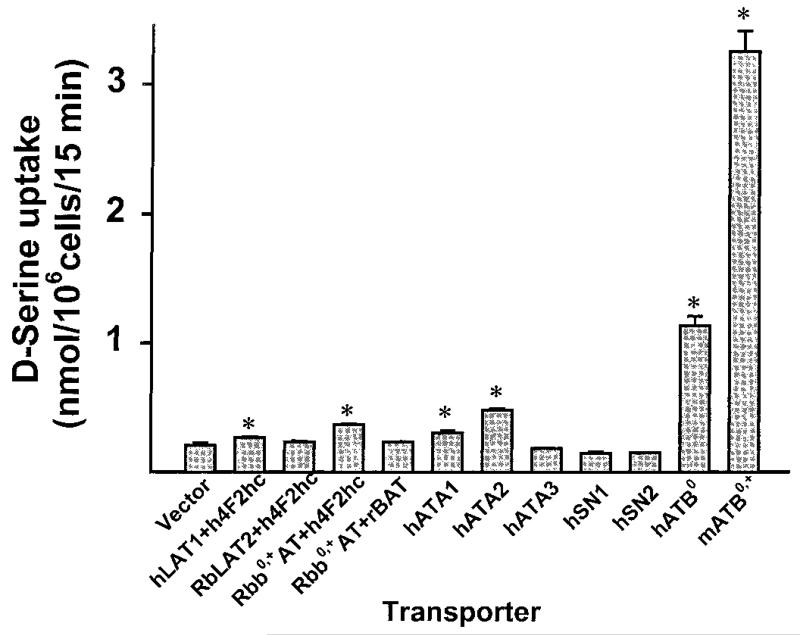
We evaluated the function of 10 different amino acid transporter clones with respect to transport of d-serine in HRPE cells (Fig. 1). All of these transporters are expressed in the intestinal tract as evidenced from Northern blot. The transporters tested include three energy-independent, heterodimeric facilitative transporters (L1, L2, and b0,+), three subtypes of the Na+-coupled system A (ATA1, ATA2, and ATA3), two subtypes of the Na+- and H+-coupled system N (SN1 and SN2), the Na+-coupled system ATB0, and the Na+- and Cl−-coupled system ATB0,+. Among the facilitative transporters, system L1 (LAT1/4F2hc) and b0,+ (b0,+AT/4F2hc) showed significant ability to transport d-serine. The L2 (LAT2/4F2hc) and b0,+ (b0,+AT/rBAT) complexes did not exhibit detectable d-serine transport activity. Among the subtypes of system A, ATA1 and ATA2 showed d-serine transport activity, but ATA3 did not. SN1 and SN2 did not transport d-serine. ATB0 was able to transport d-serine to a marked extent, its transport activity being much higher than that of systems L1, b0,+, ATA1, and ATA2. The ability to transport d-serine was the highest, however, for the Na+- and Cl−-coupled transporter ATB0,+. ATB0,+ belongs to the gene family of neurotransmitter transporters whose transport function is energized by the Na+ gradient, Cl− gradient, and membrane potential (12, 13, 16). The functional activities of ATB0 and ATB0,+ have been demonstrated in the brush border membrane of the intestinal epithelial cells (17, 18), and therefore it is likely that these two transporters mediate the active absorption of d-serine from the lumen into the mucosal cells. Based on the energetics of these two transport systems, ATB0,+ is expected to play the leading role in this process.
FIG. 1.
Transport of d-serine (5 μM) by various amino acid transporters expressed in the intestine. The transporter cDNAs were expressed heterologously in HRPE cells. Where indicated by an asterisk, the transport was significantly (P < 0.05) higher than the corresponding transport in vector-transfected cells.
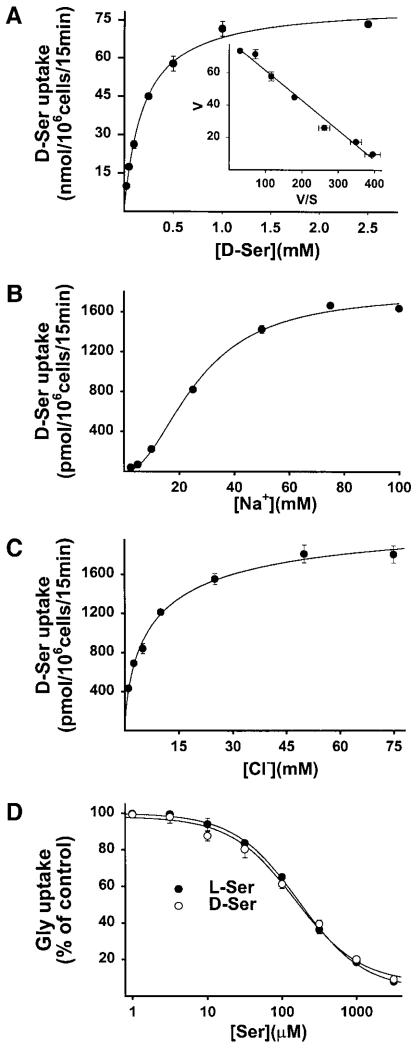
The characteristics of d-serine transport via ATB0,+ were studied in detail (Fig. 2). The transport process was saturable with a Michaelis–Menten constant of 180 ± 16 μM. It is a Na+- and Cl−-coupled process with a Na+:Cl−:d-serine stoichiometry of 2:1:1. ATB0,+ is known to transport several L-amino acids (12, 13, 16). To compare the relative affinity of the transporter for l-serine and d-serine, we assessed the ability of these two enantiomers to compete with glycine for transport via ATB0,+. Surprisingly, there was no difference between the inhibitory potencies of these two enantiomers (IC50 values for l-serine and d-serine were 168 ± 8 and 150 ± 11 μM, respectively). Thus, ATB0,+ does not differentiate between l-serine and d-serine for interaction and transport.
FIG. 2.
Characteristics of ATB0,+-mediated d-serine transport in HRPE cells. The transport of d-serine (A–C) and glycine (D) was measured and the cDNA-specific transport was calculated by subtracting transport in vector-transfected cells from transport in cDNA-transfected cells.
To determine whether ATB0,+ is capable of transporting other D-amino acids, two different approaches were used (Table 1). In the first approach, the transport function of ATB0,+ was measured in HRPE cells using glycine as the substrate for the transporter. To compare the interaction of ATB0,+ with L- and D-enantiomers of various amino acids, the ability of these amino acids to inhibit ATB0,+-mediated glycine transport was assessed. All neutral and cationic amino acids tested were potent inhibitors of ATB0,+-mediated glycine transport when present as L-enantiomers. In the case of D-enantiomers, only alanine, serine, methionine, leucine and tryptophan were potent inhibitors. The extent of inhibition was comparable between L-enantiomers and D-enantiomers for these five amino acids. In contrast, the D-enantiomers of threonine, histidine, phenylalanine, and glutamine were much less effective than the corresponding L-enantiomers as inhibitors. Asparagine, lysine, arginine, valine, and isoleucine were almost totally ineffective as inhibitors when present as D-enantiomers even though the corresponding L-enantiomers were potent inhibitors. Since the inhibition does not necessarily mean that the inhibitors are translocated across the membrane via the transporter, a second approach was used in which the transport of L- and D-enantiomeric forms of various amino acids was assessed directly in X. laevis oocytes expressing ATB0,+ heterologously. This was done using the two-microelectrode voltage-clamp technique and monitoring the amino acid-induced inward currents. The L-enantiomers of all amino acids tested induced inward currents, indicating their transport via ATB0,+. In the case of D-enantiomers, only alanine, serine, methionine, leucine, and tryptophan induced currents. Threonine, histidine, phenylalanine, and glutamine produced small but significant currents whereas the remaining amino acids did not produce currents. These results with the X. laevis oocyte expression system corroborate the results with the mammalian cell expression system. These data show that ATB0,+ is capable of transporting all neutral and cationic amino acids when presented as the L-enantiomers. In contrast, the transporter recognizes only alanine, serine, methionine, leucine, and tryptophan in their D-enantiomeric form as transportable substrates.
TABLE 1.
Relative Transport of L-Amino Acids versus D-Amino Acids via ATB0,+
| ATB0,+-specific [3H]glycine uptake in HRPE cells (% control) |
ATB0,+-specific inward current in X. laevis oocytes (nA) |
|||
|---|---|---|---|---|
| Amino acid | L-isomer | D-isomer | L-isomer | D-isomer |
| Control | 100 ± 6 | 100 ± 6 | ||
| Alanine | 6 ± 1 | 10 ± 1 | 650 ± 86 | 402 ± 39 |
| Serine | 10 ± 1 | 14 ± 1 | 470 ± 42 | 267 ± 23 |
| Methionine | 3 ± 0 | 14 ± 1 | 278 ± 26 | 213 ± 15 |
| Leucine | 3 ± 1 | 13 ± 1 | 187 ± 14 | 161 ± 25 |
| Tryptophan | 4 ± 1 | 7 ± 1 | 112 ± 4 | 157 ± 23 |
| Threonine | 19 ± 2 | 47 ± 3 | 233 ± 12 | 41 ± 5 |
| Histidine | 9 ± 1 | 35 ± 2 | 410 ± 64 | 29 ± 6 |
| Phenylalanine | 3 ± 0 | 26 ± 2 | 275 ± 58 | 24 ± 24 |
| Glutamine | 13 ± 1 | 77 ± 4 | 453 ± 53 | 16 ± 11 |
| Asparagine | 15 ± 1 | 95 ± 7 | 394 ± 67 | 3 ± 2 |
| Lysine | 18 ± 1 | 86 ± 5 | 321 ± 34 | 9 ± 9 |
| Arginine | 25 ± 1 | 93 ± 6 | 339 ± 35 | 6 ± 1 |
| Valine | 7 ± 1 | 87 ± 4 | 310 ± 24 | 4 ± 3 |
| Isoleucine | 4 ± 1 | 85 ± 5 | 117 ± 16 | 4 ± 4 |
Note. Human retinal pigment epithelial (HRPE) cells were transfected with either vector alone or mouse ATB0,+ cDNA and the functional expression was carried out by the vaccinia virus technique. Transport of [3H]glycine (10 μM) was measured in the presence and absence of various amino acids (2.5 mM). Data represent only cDNA-specific transport. Mouse ATB0,+ was also expressed in Xenopus laevis oocytes and amino acid (1 mM)-induced currents were monitored using the two-microelectrode voltage-clamp technique.
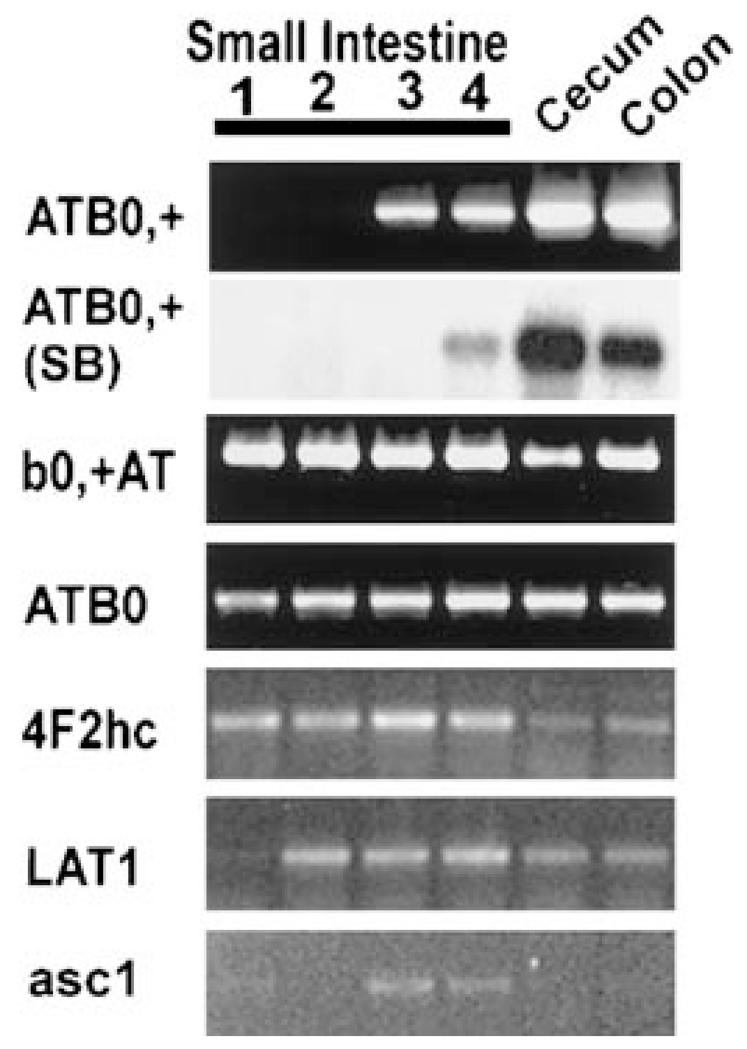
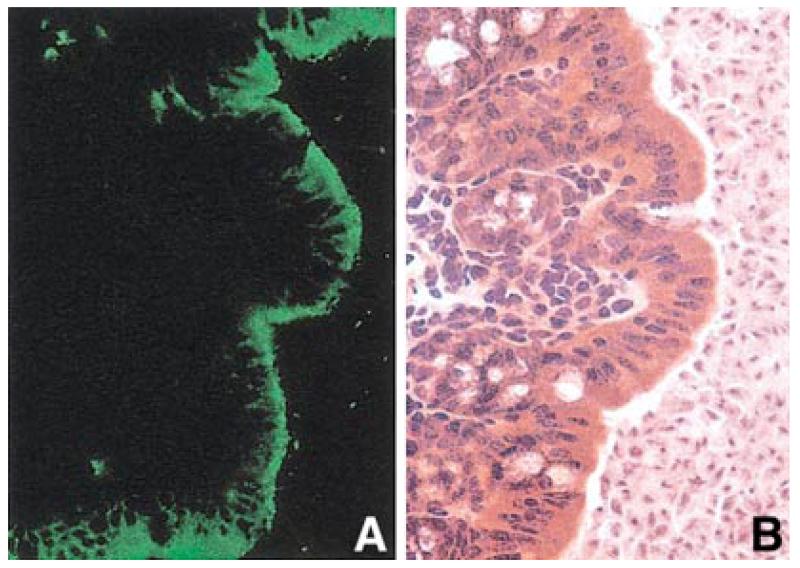
To evaluate the physiological relevance of these findings to the intestinal absorption of d-serine and other D-amino acids via ATB0,+, we assessed the expression pattern of this transporter along the longitudinal axis in the entire intestinal tract in the mouse (Fig. 3). ATB0,+ mRNA is detectable by RT-PCR and semiquantitative RT-PCR only in the cecum, colon, and the terminal part of the small intestine. This expression pattern is unique among the known amino acid transporters. Other transporters (b0,+, ATB0, and L1) are expressed along almost all the intestinal tract. Transcripts for system asc1 are found in the terminal part of the small intestine, cecum and colon. Immunofluorescent confocal microscopic analysis shows that ATB0,+ is localized to the luminal membrane of the colonic epithelial cells (Fig. 4).
FIG. 3.
Expression pattern of various transporters or transporter components along the longitudinal axis of the mouse intestinal tract. The small intestine was divided into four equal segments, segment 1 representing the most proximal part of the small intestine and segment 4 representing the most distal part of the small intestine. RT-PCR was carried out using mRNA from different regions of the intestinal tract. In the case of ATB0,+, the expression of mRNA was checked also by semiquantitative RT-PCR in which the PCR cycle number was low and the RT-PCR product was detected by Southern blot hybridization and quantified (SB).
FIG. 4.
Localization of ATB0,+ in mouse colon. (A) Immunofluorescence; (B) hematoxylin–eosin staining.
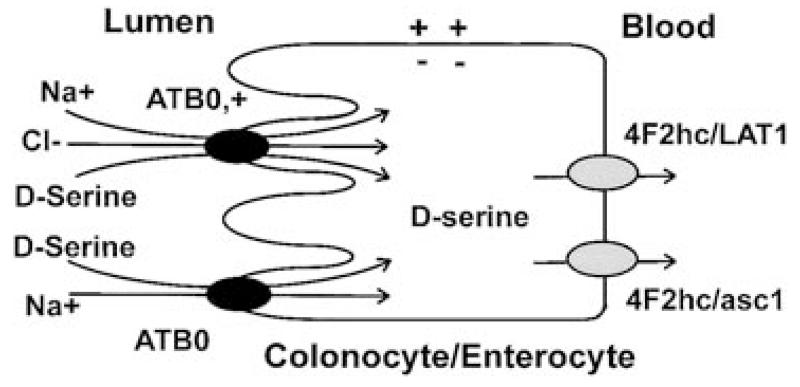
The observation that the expression of ATB0,+ is restricted only to the distal small intestine and the large intestine suggests that this transporter is uniquely suitable to function in the intestinal absorption of bacteria-derived d-serine and other D-amino acids. The sites in the intestinal tract where the expression of ATB0,+ is seen are the same sites where bacterial colonization occurs. Based on these data, we propose a model for the absorption of D-amino acids in the ileum and colon in which D-amino acids are actively absorbed into ileal and colonic epithelial cells via ATB0,+ in a Na+- and Cl−-coupled manner (Fig. 5). ATB0 is also expected to play a role, though to a much lesser extent than ATB0,+, in this process. Thus, D-amino acids enter the intestinal mucosal cells via an energy-coupled process whereas the exit of these amino acids across the basolateral membrane occurs via the facilitative transport systems L1 and asc1.
FIG. 5.
A model for the intestinal absorption of d-serine from the lumen into the blood.
The human intestine, especially the colon, contains the largest number of bacteria in the body. These microbes and humans coexist with a symbiotic relationship. The bacteria participate actively in modulating the intestinal function (19). The expression of a number of genes that are critical for normal intestinal function is altered significantly by bacterial colonization. The present study suggests that bacteria-derived metabolites may play a role also in shaping human biology. d-serine, produced by the bacteria in the colon and ileum and actively absorbed through the transporter ATB0,+, may exert significant influence on glutamatergic neurotransmission in the brain. d-Serine present in the systemic circulation has access to the brain because the amino acid transport system L1 (LAT1/4F2hc), which has the ability to transport d-serine, is expressed in the blood–brain barrier (20). Furthermore, glutamate signaling via the NMDA receptor occurs in several non-neuronal tissues as diverse as bone, pancreas, and skin (21). d-Serine present in the systemic circulation may have modulatory role in glutamate signaling in these tissues as well.
ATB0,+ is capable of transporting several neutral and cationic amino acids. However, transport of these amino acids may not be the physiological function of ATB0,+ in the intestine. The absorption of protein digestion products occurs almost entirely in the small intestine (22). Protein digestion products rarely enter the colon under physiological conditions. Since ATB0,+ is expressed predominantly in the colon, it is unlikely that the transporter encounters significant amounts of amino acids arising from protein digestion. The physiologic function of ATB0,+ in the colon may be to mediate the absorption of bacteria-derived d-serine and other D-amino acids which have the potential of modulating various biological functions in humans. d-Serine is an established neuromodulator and it is interesting that the most likely physiological function of ATB0,+, a member of the neurotransmitter transporter gene family, is to mediate the intestinal transport of this neuromodulator.
ACKNOWLEDGMENTS
This work was supported by National Institutes of Health Grant GM54122 and Chugai Pharmaceutical Company, Japan.
REFERENCES
- 1.Corrigan JJ. Science. 1969;164:142–149. doi: 10.1126/science.164.3876.142. [DOI] [PubMed] [Google Scholar]
- 2.Friedman M. J. Agric. Food Chem. 1999;47:3457–3479. doi: 10.1021/jf990080u. [DOI] [PubMed] [Google Scholar]
- 3.Man EH, Bada JL. Annu. Rev. Nutr. 1987;7:209–225. doi: 10.1146/annurev.nu.07.070187.001233. [DOI] [PubMed] [Google Scholar]
- 4.Matsui T, Sekiguchi M, Hashimoto A, Tomita U, Nishikawa T, Wada K. J. Neurochem. 1995;65:454–458. doi: 10.1046/j.1471-4159.1995.65010454.x. [DOI] [PubMed] [Google Scholar]
- 5.Mothet JP, Parent AT, Wolosker H, Brady RO, Jr., Linden DJ, Ferris CD, Rogawski MA, Snyder SH. Proc. Natl. Acad. Sci. USA. 2000;97:4926–4931. doi: 10.1073/pnas.97.9.4926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wolosker H, Blackshaw S, Snyder SH. Proc. Natl. Acad. Sci. USA. 1999;96:13409–13414. doi: 10.1073/pnas.96.23.13409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wolosker H, Sheth KN, Takahashi M, Mothet JP, Brady RO, Jr., Ferris CD, Snyder SH. Proc. Natl. Acad. Sci. USA. 1999;96:721–725. doi: 10.1073/pnas.96.2.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ganapathy V, Ganapathy ME, Leibach FH. Current Topics in Membranes. Vol. 50. Academic Press; San Diego: 2001. pp. 379–412. [Google Scholar]
- 9.Fukasawa Y, Segawa H, Kim JY, Chairoungdua A, Kim DK, Matsuo H, Cha SH, Endou H, Kanai Y. J. Biol. Chem. 2000;275:9690–9698. doi: 10.1074/jbc.275.13.9690. [DOI] [PubMed] [Google Scholar]
- 10.Kanai Y, Segawa H, Miyamoto K, Uchino H, Takeda E, Endou H. J. Biol. Chem. 1998;273:23629–23632. doi: 10.1074/jbc.273.37.23629. [DOI] [PubMed] [Google Scholar]
- 11.Palacin M, Estevez R, Bertran J, Zorzano A. Physiol. Rev. 1998;78:969–1054. doi: 10.1152/physrev.1998.78.4.969. [DOI] [PubMed] [Google Scholar]
- 12.Hatanaka T, Nakanishi T, Huang W, Leibach FH, Prasad PD, Ganapathy V, Ganapathy ME. J. Clin. Invest. 2001;107:1035–1043. doi: 10.1172/JCI12060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nakanishi T, Hatanaka T, Huang W, Prasad PD, Leibach FH, Ganapathy ME, Ganapathy V. J. Physiol. (London) 2001;532:297–304. doi: 10.1111/j.1469-7793.2001.0297f.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bridges CC, Kekuda R, Huang W, Prasad PD, Kuhnel JM, Sirotnak FM, Roon P, Ganapathy V, Smith SB. J. Biol. Chem. 2000;275:20676–20684. doi: 10.1074/jbc.M002328200. [DOI] [PubMed] [Google Scholar]
- 15.Ling R, Bridges CC, Sugawara M, Fujita T, Leibach FH, Prasad PD, Ganapathy V. Biochim. Biophys. Acta. 2001;1512:15–21. doi: 10.1016/s0005-2736(01)00310-8. [DOI] [PubMed] [Google Scholar]
- 16.Sloan JL, Mager S. J. Biol. Chem. 1999;274:23740–23745. doi: 10.1074/jbc.274.34.23740. [DOI] [PubMed] [Google Scholar]
- 17.Munck LK. Biochim. Biophys. Acta. 1995;1241:195–213. doi: 10.1016/0304-4157(95)00005-c. [DOI] [PubMed] [Google Scholar]
- 18.Stevens BR. Mammalian Amino Acid Transport: Mechanisms and Control. Plenum; New York: 1992. pp. 149–164. [Google Scholar]
- 19.Hooper LV, Wong MH, Thelin A, Hansson L, Falk PG, Gordon JI. Science. 2001;291:881–884. doi: 10.1126/science.291.5505.881. [DOI] [PubMed] [Google Scholar]
- 20.Boado RJ, Li JY, Nagaya M, Zhang C, Pardridge WM. Proc. Natl. Acad. Sci. USA. 1999;96:12079–12084. doi: 10.1073/pnas.96.21.12079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Skerry TM, Genever PG. Trends Pharmacol. Sci. 2001;22:174–181. doi: 10.1016/s0165-6147(00)01642-4. [DOI] [PubMed] [Google Scholar]
- 22.Ganapathy V, Leibach FH. Textbook of Gastroenterology. Lippincott & Williams; Philadelphia, PA: 1999. pp. 456–467. [Google Scholar]