Abstract
Two decades of studies in multiple model organisms have established the Hippo pathway as a key regulator of organ size and tissue homeostasis. By inhibiting YAP and TAZ transcription co-activators, the Hippo pathway regulates cell proliferation, apoptosis, and stemness in response to a wide range of extracellular and intracellular signals, including cell-cell contact, cell polarity, mechanical cues, ligands of G-protein coupled receptors, and cellular energy status. Dysregulation of the Hippo pathway exerts a significant impact on cancer development. Further investigation of the functions and regulatory mechanisms of this pathway will help uncovering the mystery of organ size control and identify new targets for cancer treatment.
The emergence of multicellular organisms is an evolutionary milestone. Among the most fundamental mechanisms supporting multicellularity, are those that ensure the proper size and shape of tissues and organs to meet the need of functionality. However, despite intensive investigations into the underlying principles behind a “preset” size of organs, we are far from having a clear picture to this basic question in developmental biology. Nevertheless, investigations of the Hippo pathway on organ size control have shed light into this mystery (Halder and Johnson, 2011; Pan, 2010; Yu and Guan, 2013).
In 1995, two studies in Drosophila discovered that deletion of Warts (Wts) gene resulted in dramatic overgrowth of multiple tissues (Justice et al., 1995; Xu et al., 1995). Several years later, a flurry of studies showed that Salvador (Sav) (Kango-Singh et al., 2002; Tapon et al., 2002), Hippo (hpo) (Harvey et al., 2003; Jia et al., 2003; Pantalacci et al., 2003; Udan et al., 2003; Wu et al., 2003), and Mob as tumor suppressor (Mats) (Lai et al., 2005) mutant Drosophila phenocopy Wts mutants with regards to tissue overgrowth. Hpo, Sav, Wts, and Mats interact genetically and physically, and the remarkable organ size phenotype elicited by their mutation is unprecedented in other established developmental pathways, thus they were grouped into a new signaling module — the Hippo pathway — named after the enormous size of Hpo mutant organs which resemble that of a hippopotamus. Yorkie (yki), the key functional effector of the Hippo pathway in organ size regulation, was soon discovered in a screen for Wts-interacting proteins (Huang et al., 2005).
The Hippo pathway is highly conserved in mammals. The mammalian orthologs of Hpo, Sav, Wts, Mats, and Yki are Mammalian sterile 20-like 1 /2 (MST1/2, also called STK4/3), Salvador (SAV1), Large tumor suppressor homolog 1/2 (LATS1/2), MOB kinase activator 1A/B (MOB1a/b), and Yes-associated protein (YAP) / Transcriptional co-activator with PDZ binding motif (TAZ, also called WWTR1), respectively (Halder and Johnson, 2011; Pan, 2010; Yu and Guan, 2013) (Table 1). The Hippo pathway quickly attracted broad attention due to its remarkable potency in regulating organ size, as well as its apparent relevance to tissue regeneration and cancer. Here, with an emphasis on recent developments, we review the current understanding of the organization, regulation, and function of the Hippo pathway and discuss some key open questions.
Table 1.
Hippo pathway components and major functions.
| Drosophila | Mammals | Major functions in Hippo pathway |
|---|---|---|
| Hippo (Hpo) | MST1/2 | Phosphorylate LATS1/2, MOB1, and SAV1, leading to LATS1/2 activation |
| Salvador (Sav) | SAV1 | Interacts with MST1/2, promotes phosphorylation of LATS1/2 by MST1/2 |
| Warts (Wts) | LATS1/2 | Phosphorylate and inactivate YAP/TAZ |
| Mats | MOB1A/B | Scaffold protein of LATS1/2 |
| Yorkie (Yki) | YAP/TAZ | Transcription co-activator, major effectors of the Hippo pathway |
| Scalloped (Sd) | TEAD1-4 | Transcription factors mediate the effect of YAP/TAZ |
| Tgi | VGLL4 | Competes with YAP/TAZ for TEADs, inhibits YAP/TAZ functions |
| misshapen (Msn) | MAP4K4 | Activates LATS1/2 |
| Merlin (Mer) | Merlin/NF2 | May form a complex and mediates upstream signals (from plasma membrane) to MST1/2. NF2 may bring LATS1/2 to plasma membrane and facilitate its activation by MST1/2 |
| Kibra | KIBRA | |
| Expanded (Ex) | FRMD6? | |
| AMOT | Sequesters YAP/TAZ to cell junctions, binding and indirectly activates LATS1/2; a substrate of LATS1/2 |
YAP/TAZ and Yki as Major Effectors of Hippo Signaling
In Drosophila, deleting Yki suppresses the overgrowth phenotypes of Hpo, Sav, or Wts mutants (Huang et al., 2005). In mice, deleting YAP also diminishes the overgrowth phenotypes caused by deficiency of MST1/2 or other upstream regulators (Zhang et al., 2010; Zhou et al., 2011). Thus, Yki and YAP/TAZ are the evolutionarily-conserved key effectors of the Hippo pathway.
Yki and YAP/TAZ are believed to mediate the biological functions of the Hippo pathway by regulating gene transcription. As transcriptional co-activators, Yki and YAP/TAZ cannot bind DNA directly, and they must interact with DNA-binding transcription factors to regulate target gene expression. In Drosophila, Scalloped (Sd) is a transcription factor and key partner of Yki which mediates the function of Yki in tissue growth (Goulev et al., 2008; Wu et al., 2008; Zhang et al., 2008; Zhao et al., 2008). Similarly, in mammalian cells, the TEAD family transcription factors (TEAD1-4, orthologs of Sd) are key partners for YAP (Zhao et al., 2008). Several lines of evidence indicate that TEADs are the major partners of YAP in transcriptional regulation. For instance, a TEAD-binding deficient YAP mutant lost its ability to induce transcription of known YAP target genes (Zhao et al., 2008), and knock-in of the TEAD-binding deficient YAP (Y94A mutant) phenotypically mimics YAP knockout in the skin and heart (Schlegelmilch et al., 2011; von Gise et al., 2012). In addition, the majority of YAP and TEAD occupied sites in the genome are shared (Stein et al., 2015; Zanconato et al., 2015; Zhao et al., 2008), and when TEAD is fused with a VP16 transactivation domain, it produces a gene expression profile similar to that induced by active YAP (Ota and Sasaki, 2008). TEAD1-4 or Sd can bind to a consensus motif similar to the GTIIC sequence (TGGAATGT or ACATTCCA), and transcription reporters under control of GTIIC concatemers are now widely used to measure Hippo pathway activity (Dupont et al., 2011; Mohseni et al., 2014; Ota and Sasaki, 2008). Besides TEAD1-4, YAP/TAZ have also been shown to interact with other transcription factors including Smad, RUNX1/2, p63/p73, and ErbB4 (reviewed in (Varelas, 2014)), although the functional significance of these transcription factors in Hippo pathway is less clear.
A strong transcriptional activation domain is present in YAP/TAZ, but absent in Yki. Nevertheless, human YAP can rescue the lethality resulting from Hippo pathway hyperactivation in Drosophila, indicating a functional conservation (Huang et al., 2005). Genome-wide assessment of chromatin binding status reveals that, in addition to occupancy at proximal promoters of target genes, YAP and TEAD largely exert their transcriptional activity by interacting with distal enhancers, suggesting that YAP, and probably TAZ and Yki, may regulate transcription via multiple mechanisms including recruitment of general transcription factors, modification of epigenetic markers, and modulation of chromatin structure (Lian et al., 2010; Stein et al., 2015; Zanconato et al., 2015) (Figure 1). Indeed, recent evidence shows that Yki can interact with the Brahma complex, GAGA factors, nuclear receptor coactivator 6 (NCoA6, a subunit of the Trithorax-related histone methyltransferase), and the Mediator complex (Jin et al., 2013; Oh et al., 2013; Qing et al., 2014), and TAZ can interact with the chromatin-remodeling complex SWI/SNF (Skibinski et al., 2014).
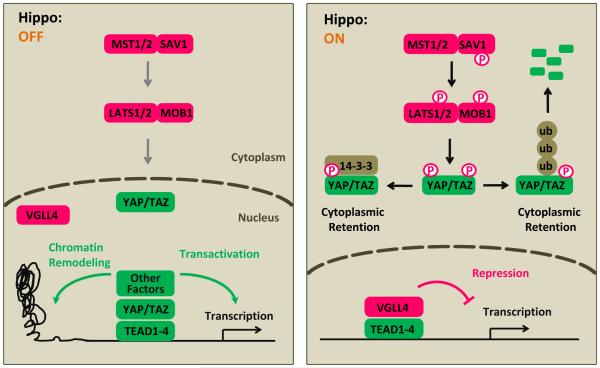
Figure 1.
Inhibition of YAP/TAZ transcriptional coactivators by LATS1/2. (A) When Hippo signaling is off, YAP/TAZ enters the nucleus, competes with VGLL4 for TEADs, and recruits other factors to induce gene transcription. YAP/TAZ may bind proximal promoters or distal enhancers of target genes to induce transcription. (B) When Hippo signaling is on, YAP/TAZ is phosphorylated by LATS1/2 on multiple sites, resulting interaction with 14-3-3 and cytoplasmic retention; phosphorylation also leads to YAP/TAZ poly-ubiquitination and degradation. VGLL4 interacts with TEADs and represses target gene transcription.
The transcriptional activity of Yki and YAP/TAZ is regulated in the nucleus by Tondu-domain-containing growth inhibitor (Tgi) and Vestigial-like family member 4 (VGLL4, an ortholog of Tgi). Tgi can directly compete with Yki for Sd binding, resulting in inhibition of Yki-regulated transcription (Guo et al., 2013; Koontz et al., 2013). When Hippo signaling is on, Tgi and Sd form a complex, leading to transcriptional repression; on the contrary, when Hippo signaling is off, Yki enters the nucleus and displaces Tgi from Sd, leading to expression of Yki target genes and tissue growth (Koontz et al., 2013). In mammals, VGLL4 similarly competes with YAP/TAZ for TEAD binding (Jiao et al., 2014; Zhang et al., 2014b) (Figure 1). However, whether YAP/TAZ functions simply by relieving a default repression of TEAD by VGLL4 has not been demonstrated. Interestingly, the expression of VGLL4 appears to be repressed by miR-130a—a microRNA (miRNA) that is directly induced by YAP, leading to amplification of YAP activity (Shen et al., 2015). A similar mechanism is also present in Drosophila, in which Bantam—a well-known Yki-induced miRNA (Nolo et al., 2006; Thompson and Cohen, 2006), can repress expression of Tgi (Shen et al., 2015).
Although their role as transcriptional co-activators is widely appreciated, YAP/TAZ and Yki may also repress expression of certain genes when bound to specific factors. For instance, YAP/TAZ can interact with the nucleosome remodeling and histone deacetylase (NuRD) complex, resulting in transcriptional repression (Kim et al., 2015). Moreover, YAP has been identified as a regulator for global miRNA biogenesis via modulation of miRNA processing enzymes Microprocessor or Dicer complex, suggesting a transcription-independent role for YAP (Chaulk et al., 2014; Mori et al., 2014). Hence YAP/TAZ and Yki may regulate gene expression via multiple mechanisms.
Gene expression signatures under YAP/TAZ and Yki hyperactivation (or ectopic expression) in Drosophila and different mammalian cell types have been profiled by independent studies. However, the overlap between these different gene profiling studies is not high, suggesting that YAP/TAZ and Yki may regulate target gene expression in a tissue or cell type-specific manner. In Drosophila, some common Yki target genes are Bantam, Diap1 and cyclin E, which may mediate the effect of Yki in inhibiting cell death and promoting cell proliferation (Huang et al., 2005; Nolo et al., 2006; Tapon et al., 2002; Thompson and Cohen, 2006). In mammals, connective tissue growth factor (CTGF) is a commonly used marker of YAP activation (Zhao et al., 2008), and many genes involved in cell proliferation, cell adhesion, and cell migration have also been identified as YAP targets (Stein et al., 2015). It is likely that a panel of genes work together to exert biological functions of YAP/TAZ.
MST1/2 and LATS1/2 Kinases Restrict YAP Activity
In the Hippo pathway, LATS1/2 directly phosphorylate and inhibit YAP/TAZ. Interestingly, YAP has five LATS1/2 target consensus motifs (HXRXXS), four of which are conserved in TAZ (Zhao et al., 2010). Phosphorylation of YAP on serine 127 (S127) generates a 14-3-3 binding site, and binding with 14-3-3 sequesters YAP in the cytoplasm (Dong et al., 2007; Zhao et al., 2007). In addition, phosphorylation of YAP on serine 381 (S381) triggers a subsequent phosphorylation by casein kinase 1 (CK1δ/ε) and activation of a phosphodegron, resulting in recruitment of SCFbeta-TRCP E3 ligase, ubiquitination, and proteasomal degradation of YAP (Zhao et al., 2010). Thus, through regulating both YAP subcellular localization and protein stability, LATS1/2 ensures a spatial and temporal control of YAP activity (Figure 1). TAZ is regulated by LATS1/2 in a similar fashion, although degradation plays a more prominent role in TAZ regulation possibly due to an additional phosphodegron at its N-terminus (Lei et al., 2008; Liu et al., 2010a). The subcellular localization of Yki is regulated similarly by Wts phosphorylation and 14-3-3 binding. However, the phosphodegron and the phosphorylation-mediated degradation mechanisms are not conserved in Yki (Huang et al., 2005; Oh and Irvine, 2008). In addition, YAP and Yki have also been shown to be degraded via the autolysosomal pathway (Kwon et al., 2013; Liang et al., 2014), suggesting a potential role of YAP and Yki in vesicular membrane dynamics and related cellular processes such as autophagy.
LATS1/2 are activated by MST1/2 through several mechanisms. MST1/2 phosphorylate LATS1/2 at the C-terminal hydrophobic motif, which promotes LATS1/2 autophosphorylation at its activation loop. Furthermore, phosphorylation of MOB1 by MST1/2 enhances MOB1 interaction with the autoinhibitory domain of LATS1/2, leading to full activation of LATS1/2 (Callus et al., 2006; Chan et al., 2005; Praskova et al., 2004; Wu et al., 2003). In addition, SAV1 is also phosphorylated by MST1/2, and SAV1 functions as a partner of MST1/2 in promoting LATS1/2 phosphorylation (Callus et al., 2006; Tapon et al., 2002) (Figure 1). In Drosophila, Wts is regulated by Hpo, Sav, and Mats by a similar mechanism (Wehr et al., 2013).
Merlin (Mer), Expanded (Ex), and Kibra, three cell cortex-localized and cytoskeleton-interacting proteins, may function as a scaffold for core Hippo components at the apical domain for activation, as Sav, Hpo, and Wts have been shown to physically interact with Ex/Mer/Kibra (Baumgartner et al., 2010; Genevet et al., 2010; Hamaratoglu et al., 2006; Yu et al., 2010) (Table 1). In addition, the effect of Ex/Mer/Kibra on the Hippo pathway may be mediated by Tao kinase 1 (Tao-1), which can directly phosphorylate and activate Hpo (Boggiano et al., 2011; Poon et al., 2011). In mammalian cells, Neurofibromin 2 (NF2, Mer ortholog) appears to play a more direct role in regulating LATS1/2 activity: it can directly interact with LATS1/2 and recruit LATS1/2 to the plasma membrane for activation by MST1/2 (Yin et al., 2013; Zhang et al., 2010) (Figures 2 and 3).
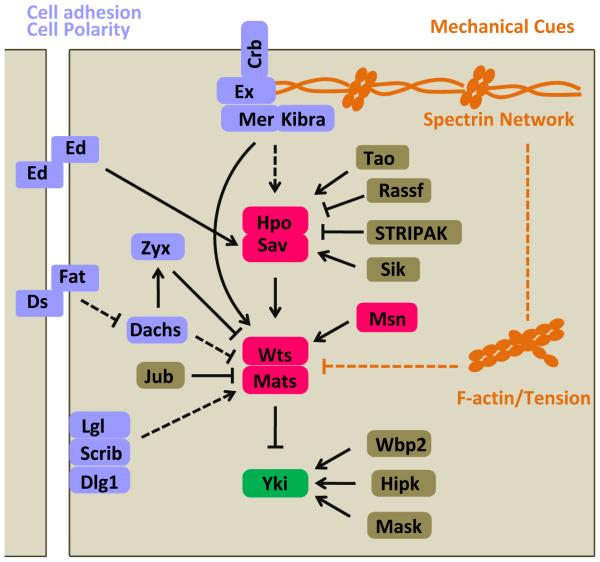
Figure 2.
Regulation of the Hippo pathway in Drosophila. The Hippo pathway is regulated by cell adhesion molecules (Ed), determinants of cell polarity (Crb, Fat/Ds, Scrib complex), and mechanical cues (spectrin, F-actin, or cellular tension). In addition, Hpo is regulated by Tao, salt induced kinase (Sik), Ras-associated factor (Rassf), striatin-interacting phosphatase and kinase (STRIPAK) complex; Wts is regulated by Zyxin (Zyx) and Jub; Yki is regulated by WW Domain Binding Protein 2 (Wbp2), Hipk, and Multiple ankyrin repeats single KH domain (Mask) (refer to (Varelas, 2014)). Arrows, blunt ends, and dashed lines indicate activation, inhibition, and indirect regulation, respectively.
The Drosophila Hpo or MST1/2 are not absolutely required for regulation of Wts or LATS1/2. It has been observed that in mouse embryonic fibroblast (MEF) cells, MST1/2 double knockout did not abolish YAP phosphorylation, suggesting the existence of additional Hippo-like activity (Zhou et al., 2009). Indeed, a recent study in Drosophila has identified Misshapen (Msn) as another kinase responsible for Wts activation. This mechanism is also conserved in mammals, as MAP4K4 (Msn ortholog) overexpression promotes phosphorylation of LATS1/2 (Li et al., 2014), and MAP4K4 knockdown induces activity of a YAP reporter (Mohseni et al., 2014). It is possible that additional kinases, especially some STE20 family members, may activate LATS1/2 in response to different upstream signals or in different tissue contexts (Figures 2 and 3).
YAP/TAZ have also been shown to be phosphorylated by many other kinases such as cyclin-dependent kinase 1 (CDK1), Jun N-terminal kinases (JNK), homeodomain interacting protein kinases (HIPK), ABL, and Src family tyrosine kinases (reviewed in (Varelas, 2014)), suggesting that YAP/TAZ can be regulated by mechanisms independent of Hippo pathway kinases.
Cell Polarity and Cell Adhesion Regulate Hippo Signaling
In searching for upstream regulators of the Hippo pathway, many proteins involved in cell polarity and cell adhesion have been identified. Echinoid (Ed), a cell adhesion molecule in Drosophila, can interact with and stabilize Sav, and leads to activation of Hpo (Yue et al., 2012). In mammalian cells, several proteins at adherens and tight junctions, such as Angiomotin (AMOT), protein tyrosine phosphatase non-receptor type 14 (PTPN14), and α-Catenin can sequester YAP/TAZ at cell junctions (reviewed in (Yu and Guan, 2013)). Therefore cell adhesion and formation of intercellular junctions serve as a mechanism to repress YAP/TAZ transcriptional activity (Figures 2 and 3).
Crumbs (Crb), a component of apical-basal polarity, interacts with Ex which is critical for the apical localization of Ex/Mer/Kibra complex (Chen et al., 2010; Ling et al., 2010; Robinson et al., 2010). In addition, Scribble (SCRIB) interacts with both MST1/2 and LATS1/2, thus promoting LATS1/2 activation (Cordenonsi et al., 2011; Mohseni et al., 2014). Fat, a protocadherin which plays a key role in planar cell polarity, also activates the Hippo pathway, possibly through regulating Ex protein levels and localization (Bennett and Harvey, 2006; Silva et al., 2006; Tyler and Baker, 2007; Willecke et al., 2006) (Figure 2). However, mammalian Fat orthologs do not seem to be major regulators of the Hippo pathway (Sharma and McNeill, 2013). It is noteworthy that the link between cell polarity and the Hippo pathway may be indirect, and some proteins, such as Fat, may regulate cell polarity and Hippo signaling via different mechanisms (Matakatsu and Blair, 2012).
Cell Contact and Mechanical Cues Regulate Hippo Signaling
Cells in solid tissues communicate with neighboring cells and their extracellular matrix, and perceive constant physical signals from their local environment. Cell-cell contact was discovered as the first signal regulating the Hippo pathway (Zhao et al., 2007). In a sparse culture, YAP and TAZ are primarily localized in the nucleus to promote target gene transcription and cell proliferation. On the contrary, at high cell density, YAP and TAZ are primarily cytoplasmic, corresponding to growth inhibition. It is known that a cell ceases to proliferate following physical contact with surrounding cells, and loss of cell contact inhibition is an indicator of oncogenic transformation. Thus, regulation of YAP/TAZ by cell density suggests a critical role for the Hippo pathway in contact inhibition, tissue growth, and tumorigenesis.
Mechanical cues, such as ECM stiffness and cell geometry, are also potent regulators of YAP/TAZ (Dupont et al., 2011) (Figure 3). When cells are grown on a stiff matrix or are spread across a large surface, YAP and TAZ are activated. In contrast, when cells are seeded on a soft matrix or are compressed into a small area, YAP and TAZ are inactivated. ECM stiffness and cell geometry are important for cell proliferation and differentiation, and YAP/ TAZ activity plays a role in these cellular processes. Cell geometry has also been proposed as a mechanism underlying Hippo pathway regulation by cell density: at low density, cells are flat and spread, leading to YAP activation; whereas at high density, cells adopt a round and compact geometry, resulting in YAP inactivation (Aragona et al., 2013; Wada et al., 2011). Further support for a role of YAP/TAZ in mechanosensing, mechanical strain and shear stress have been shown to stimulate YAP/TAZ, and YAP activation is required for mechanical strain-induced cell cycle reentry (Benham-Pyle et al., 2015; Codelia et al., 2014; Kim et al., 2014), and YAP also mediates changes in three dimensional body shape in response to tissue tension (Porazinski et al., 2015).
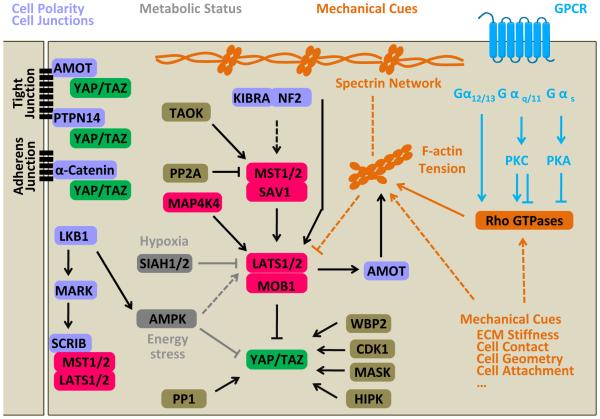
Figure 3.
Regulation of the Hippo pathway in mammals. The Hippo pathway is regulated by diverse signals: 1) Determinants of cell polarity and cell-cell junctions, such as SCRIB that interacts with MST1/2 and LATS1/2, AMOT, PTPN14, and α-Catenin, which can sequester YAP/TAZ to cell junctions; 2) Mechanical cues, such as stiffness, cell contact, cell geometry, and cell attachment status that regulate the Hippo pathway by modulating activity of Rho GTPases, remodeling the actin cytoskeleton, or altering cellular tension. Both apical and basolateral spectrin networks may function as sensors for mechanical cues in Hippo pathway regulation; 3) Soluble factors, especially ligands for GPCRs, regulate LATS1/2 likely through Rho GTPases and actin dynamics; 4) Metabolic status, such as cellular energy and oxygen stress, also regulate Hippo signaling. Many other proteins such as protein phosphatase 2A (PP2A), protein phosphatase 1 (PP1), WBP2, CDK1, MASK, and HIPK can also regulate activities of different Hippo pathway components (refer to (Varelas, 2014)). Arrows, blunt ends, and dashed lines indicate activation, inhibition, and indirect regulation, respectively.
The attachment of cells to the ECM is an important mechanism for maintaining cell survival, and cells normally undergo anoikis when detached from the ECM. The attachment status of a cell to its ECM can also regulate Hippo pathway activity. YAP is activated during the process of cell attachment but inactivated when cells are detached (Zhao et al., 2012). Expression of constitutively active YAP promotes survival of detached cells, suggesting that cancer cells with high YAP activity may escape anoikis and undergo metastasis. In support of this notion, LATS1/2 expression was found to be selectively reduced in metastatic but not primary prostate tumors (Zhao et al., 2012).
Soluble Factors Regulate Hippo Signaling
The primary function of YAP/TAZ is to promote growth, and many mitogenic hormones and growth factors act through G-protein coupled receptors (GPCR) to induce cell proliferation (Figure 3). GPCR ligands signal through Gα12/13 or Gαq/11, such as lysophosphatidic acid, thrombin, angiotensin II, and estrogen, can activate YAP/TAZ; in contrast, ligands signal through Gαs and protein kinase A (PKA), such as epinephrine and glucagon, can repress YAP/TAZ activity (Kim et al., 2013; Miller et al., 2012; Mo et al., 2012; Wennmann et al., 2014; Yu et al., 2013a; Yu et al., 2012; Zhou et al., 2015). Interestingly, activation of protein kinase C (PKC) by Gαq/11 can either activate or inhibit YAP, with conventional PKC activating YAP and novel PKC inhibiting YAP (Gong et al., 2015). The remarkable differential functions of PKC in YAP regulation provide a mechanism to explain some of the cell type-specific responses to PKC activation. GPCR is the largest family of membrane receptors mediating diverse physiological or pathological responses. The demonstration of Hippo regulation by GPCRs links the Hippo pathway to a wide range of upstream signals and biological functions.
The Wnt/β-catenin pathway is a key signaling cascade in development and carcinogenesis. A destruction complex including Axin, adenomatous polyposis coli (APC) and glycogen synthase kinase-3 (GSK3) causes constant degradation of β-catenin. Wnt stimulation disrupts the destruction complex and leads to accumulation of β-catenin. Interestingly, recent studies suggest that YAP/TAZ are also activated by diverse Wnt family ligands. YAP/TAZ have been shown to be components of the destruction complex, and are regulated by Wnt in a fashion similar to that of β-catenin (Azzolin et al., 2014; Azzolin et al., 2012). However, a recent study suggests that Wnt activates YAP/TAZ via Frizzled (a GPCR-like Wnt receptor), Gα12/13, Rho GTPases, and LATS1/2 (Park et al., 2015). In addition, APC has been shown to act as a scaffold protein for SAV1 and LATS1, and Apc deletion lead to YAP activation and tumorigenesis (Cai et al., 2015). More studies may be needed to verify the mechanism of YAP/TAZ activation by Wnt.
Epidermal growth factor (EGF) and insulin have also been shown to regulate YAP/Yki activity in cultured mammalian cells and Drosophila, and these signals are mediated by the Ras-Raf-MAPK signaling cascade or phosphoinositide-dependent kinase (PDK1) (Fan et al., 2013; Reddy and Irvine, 2013; Strassburger et al., 2012). TAZ is also stabilized upon PI3K activation, which is mediated by direct phosphorylation by GSK3 (Huang et al., 2012). However, no significant effect of EGF and IGF on YAP was observed in several other studies, and YAP activity appeared to be normal in the presence of inhibitors of PI3K or AKT, or in PDK1 null embryonic cells (Yu et al., 2012; Zhao et al., 2007). These discrepancies could be due to differences in experimental settings or cell types, and should be clarified by future studies.
Effect of Cellular Metabolic Status on Hippo Signaling
Recently, a link between cellular metabolic status and the Hippo pathway has been reported (Figure 3). Under energy deprivation, such as glucose starvation, the AMPK activated protein kinase (AMPK) can directly phosphorylate YAP at serine 61 (S61) and serine 94 (S94) (Mo et al., 2015; Wang et al., 2015). Phosphorylation of YAP S94 abolishes the interaction between YAP and TEADs, leading to inhibition of YAP activity. In addition, energy stress also inhibits YAP by increasing kinase activity of LATS1/2, either in an AMPK-dependent or -independent manner (Mo et al., 2015; Wang et al., 2015). AMPK can phosphorylate AMOTL1, which in turn facilitates YAP phosphorylation by LATS1/2 (DeRan et al., 2014). A similar mechanism is also present in Drosophila, in which Ampk inactivates Yki and affects cell proliferation in the larval central brain and central nerve cord (Gailite et al., 2015). It appears that during energy stress, both AMPK and LATS1/2 are unleashed to restrict YAP activity. These findings also suggest that the Hippo pathway may mediate the anticancer effect of metformin, which is known to activate AMPK (DeRan et al., 2014; Mo et al., 2015). Other than AMPK, glucose may also promote YAP/TAZ activity through phosphofructokinase, which stimulates the interaction between YAP/TAZ and TEADs (Enzo et al., 2015).
YAP/TAZ activity has also been linked to oxygen availability. Under hypoxic conditions, the hypoxia-inducible factor 1 (HIF1) stimulates expression of SIAH1/2, two E3 ubiquitin ligases. SIAH1/2 then promote ubiquitination and degradation of LATS2, leading to YAP/TAZ activation (Ma et al., 2015; Xiang et al., 2014) (Figure 3). In addition, HIF1 directly induces transcription of TAZ (Xiang et al., 2014), and YAP interacts with and stabilizes HIF1 to enhance transcription of HIF1 target genes (Ma et al., 2015).
YAP/TAZ are potent stimulators of cell growth and proliferation, which are energy-consuming processes. The regulation of Hippo signaling by AMPK suggests that metabolic status can function as a checkpoint for growth–promoting activity of YAP/TAZ. Under conditions of nutrient deprivation or energy crisis, YAP/TAZ activity needs to be restricted to prevent energy exhaustion caused by anabolic processes. Oxygen also plays a critical role in cellular metabolism, and hypoxia is involved in different pathological processes such as cancer. The link between hypoxia and YAP/TAZ activity indicates a role of YAP/TAZ in mediating oncogenic effect of hypoxia.
Actin Cytoskeleton Integrates Upstream Signals
The actin cytoskeleton and Rho GTPases are not only important in maintaining cell morphology, but also play important roles in regulating cell proliferation and differentiation (Jaffe and Hall, 2005). Manipulation of the actin cytoskeleton, such as overexpression of Rho GTPases or inhibition of Rho by C3 toxin, dramatically modulates YAP/TAZ activity (Dupont et al., 2011; Yu et al., 2012; Zhao et al., 2012). Rho GTPases and changes in actin cytoskeleton dynamics have been demonstrated to be key mediators of mechanical cues, GPCR ligands, and cell attachment in regulating the Hippo pathway (Figure 3). Consistently, deletion of different regulators of the actin cytoskeleton impacts YAP and Yki activity. For instance, loss of actin-capping proteins or the Capulet gene (inhibits actin polymerization) in Drosophila results in Yki activation and tissue overgrowth (Fernandez et al., 2011; Sansores-Garcia et al., 2011). Similarly, knockdown of actin-capping proteins or filamentous actin (F-actin) severing proteins (cofilin or gelsolin) in mammalian cells also leads to YAP activation (Aragona et al., 2013). In general, Rho GTPase activity and F-actin appear to activate YAP/TAZ, whereas destabilization of F-actin inhibits YAP/TAZ (Dupont et al., 2011; Miller et al., 2012; Wada et al., 2011; Yu et al., 2012; Zhao et al., 2012).
Spectrin proteins, in association with short actin filaments, are organized into an elastic polygonal meshwork which lines the intracellular side of the plasma membrane. In epithelial cells, localization of the spectrin network is usually polarized and present in both apical and basolateral domains. In addition to a supporting role for cell structure, the spectrin network may transmit diverse signals from cell microenvironment to regulate cellular functions (Bennett and Gilligan, 1993). Recently, three independent studies revealed a regulatory role of the spectrin network on the Hippo pathway, and disruption of the spectrin network in multiple Drosophila tissues leads to activation of Yki and tissue outgrowth, and a similar phenomenon is also observed in mammalian cells (Deng et al., 2015; Fletcher et al., 2015; Wong et al., 2015) (Figures 2 and 3). Therefore, spectrins or associated actin filaments may function as a major node for integrating upstream signals, such as mechanical cues.
Despite its apparent importance, it remains unclear how the actin cytoskeleton regulates activity of Hippo pathway core kinases. One possibility is that multiple Hippo pathway components are enriched at the apical domain via an actin-mediated mechanism which facilitates signal transduction, and actin remodeling may reinforce or disrupt the clustering of Hippo pathway components. In another scenario, the Hippo pathway could be regulated by contractile actomyosin and cellular tension (Figure 3). Inhibition of tension-related enzymes, such as non-muscle myosin (by Blebbistatin), Rho kinases (ROCK, by Y27632), and myosin light chain kinase (by ML-7) results in YAP/TAZ inhibition (Dupont et al., 2011; Wada et al., 2011). However, how tension is sensed by the Hippo pathway kinases is not fully understood. Treating cells with small molecules to inhibit cellular tension may also affect actin dynamics, so it is difficult to separate the effects of actin remodeling and tension generated by actomyosin. However, it has been proposed that in Drosophila, Ajuba (Jub) plays a critical role in tension-induced Yki regulation. In response to high tension, Jub recruits Wts to intercellular junctions via interactions with α-catenin, thereby inhibiting Wts activity (Rauskolb et al., 2014). In addition, JNK activation upon mechanical strain has also been shown to repress LATS1 (Codelia et al., 2014).
Although it has been initially reported that YAP regulation by mechanical signals is independent of LATS1/2, recent studies suggest that LATS1/2 are involved in YAP/TAZ regulation by the actin cytoskeleton (Miller et al., 2012; Wada et al., 2011; Yu et al., 2012; Zhao et al., 2012; Zhao et al., 2007). This discrepancy may be due to incomplete LATS1/2 depletion in knockdown experiments reported in earlier studies. Supporting a role for LATS1/2, the phosphorylation status and in vitro kinase activity of LATS1/2 are clearly regulated upon actin cytoskeleton rearrangement, and the kinetics are similar to that of YAP/TAZ phosphorylation (Yu et al., 2012; Zhao et al., 2012). The regulation of Yki/YAP/TAZ by spectrins or cell attachment/detachment has been shown dependent on Wts and LATS1/2 (Deng et al., 2015; Fletcher et al., 2015; Zhao et al., 2012). Moreover, during the preimplantation stage of mouse embryo development, LATS1/2 are essential for YAP regulation by small molecules targeting the actin cytoskeleton (Kono et al., 2014). Collectively, mechanical signals most likely act through Wts and LATS1/2 to influence the activity and function of Yki and YAP/TAZ.
Negative Feedback Regulation and Crosstalk
High Yki or YAP/TAZ activity, especially over long-term, results in tissue overgrowth or cancer (see below). Thus the physiological fluctuation of Hippo signaling must be tightly controlled to avoid detrimental effects. In Drosophila, regulation of the Hippo pathway is fine-tuned by a built-in negative feedback loop in which activation of Yki turns on the expression of upstream reglators including Four-jointed, Ex, Mer, Kibra, and Wts (Cho et al., 2006; Genevet et al., 2010; Hamaratoglu et al., 2006; Jukam et al., 2013). Consistently, a similar negative feedback mechanism also exists in mammalian cells. YAP/TAZ directly induce the transcription of NF2, LATS2, and probably MST1, leading to LATS1/2 activation and YAP/TAZ inhibition (Chen et al., 2015b; Dai et al., 2015; Moroishi et al., 2015b). In addition, YAP/TAZ induce expression of angiomotin-like protein 2 (AMOTL2), a negative regulator of YAP (Mohseni et al., 2014; Zhao et al., 2008). This negative feedback loop is critical for maintaining the proper transient activation of YAP/TAZ upon stimulation. By these mechanisms, YAP and TAZ antagonize each other and may provide a buffer for fluctuations in Hippo pathway activity to ensure tissue homeostasis. When this negative feedback is disrupted, dysregulation of the Hippo pathway may lead to tumorigenesis.
Elucidating the Hippo pathway regulation and function is complicated by crosstalk between the Hippo pathway and many other developmental signaling pathways (reviewed in (Varelas, 2014)). Besides Wnt, YAP/TAZ may also be regulated by sonic hedgehog (SHH) signaling (Fernandez et al., 2009). On the other hand, YAP/TAZ has been shown to regulate the expression of ligands for Wnt, Shh, transforming growth factor beta (TGF-β), JAK-STAT, EGFR, and Notch pathways (reviewed in (Yu et al., 2015)). The extensive signaling crosstalk may create a microenvironment rich in different factors, which in turn regulates cell fate through autocrine or paracrine mechanisms in both cell autonomous and non-autonomous manners.
Hippo Pathway in Early Embryonic Development
YAP/TAZ are critical during early embryonic development. Although TAZ knockout mice are viable, YAP knockout mice die at E8.5, and blastomeres stop dividing before the morula (16-32 cells) stage when YAP and TAZ are both deleted (Hossain et al., 2007; Makita et al., 2008; Morin-Kensicki et al., 2006; Nishioka et al., 2009; Tian et al., 2007). Therefore, the role of YAP and TAZ in early development is partially overlapping.
The first cell-fate specification during embryogenesis occurs during preimplantation stage, in which the trophectoderm (TE) and inner cell mass (ICM) are formed. The TE consists of the outer cells of the blastocyst and forms extraembryonic tissues, while the ICM contains the inner cells of the blastocyst and give rise to the embryo proper and other tissues. The formation of the TE and ICM is mainly due to the position or polarity of cells in the morula, in which the inner and apolar cells form the ICM, while the outer and polar cells give rise to the TE (Sasaki, 2015). As early as the 16-cell stage, YAP/TAZ already show differential subcellular localization between inner and outer cells, and this difference lasts to the blastocyst stage. The different distribution of YAP/TAZ in the TE and ICM results in different gene expression signatures, especially the induction of TE-specific genes, such as Cdx2 in outer cells, thus directing cell fate specification (Nishioka et al., 2009). Indeed, mouse embryos with TEAD4 knockout failed to develop TE cells, with all cells transforming into ICM. On the other hand, depleting LATS1/2, NF2, or AMOT/AMOTL2 turns all cells into TE linage, and these embryos failed to develop ICM-derived tissues (Cockburn et al., 2013; Hirate et al., 2013; Lorthongpanich et al., 2013). These results suggest that the Hippo pathway plays a key role in early embryonic cell specification.
Hippo Signaling in Organ Size Control and Tissue Homeostasis
The effect on organ size is the best-known physiological function of the Hippo pathway. In Drosophila, mutation of Hippo pathway kinases (Hpo and Wts) or upstream regulators (Ex, Mer, Kibra, Ft, etc.) leads to overgrowth of organs such as eyes, wings, or other appendages, and transgenic expression of Yki results in a similar phenotype (reviewed in (Halder and Johnson, 2011; Pan, 2010)). The increased tissue/organ size is mainly due to Yki-induced cell proliferation and survival.
The effect of the Hippo pathway on organ size is highly conserved in mammals as revealed by many studies performed in mice (summarized in Table 2). For instance, liver specific transgenic Yap expression in mice can produce a dramatically enlarged liver. Remarkably, the liver returns to its normal size via apoptosis once Yap overexpression is turned off (Camargo et al., 2007; Dong et al., 2007). Similarly, liver specific knockout of Mst1/2, Sav1, or Nf2 also results in liver enlargement (Yin et al., 2013; Zhang et al., 2010; Zhou et al., 2009). The mouse embryonic heart is enlarged when Sav1, Mst1/2, or Lats1/2 is deleted, and proliferation or apoptosis of cardiomyocytes is sensitive to genetic manipulation of Yap (Del Re et al., 2013; Heallen et al., 2011; Lin et al., 2014; von Gise et al., 2012; Xin et al., 2013; Xin et al., 2011).
Table 2.
Physiological and pathological functions of Hippo pathway genes in mice.
| Organ | Phenotypes | References |
|---|---|---|
| Liver |
Yap inducible expression results in hepatomegaly in a reversible manner, and in long term, leads to development of liver tumors. |
(Camargo et al., 2007; Dong et al., 2007) |
|
Nf2, Sav1, or Mst1/2 deletion causes hepatomegaly and results in hepatocellular carcinoma, cholangiocarcinoma, or bile duct hamartoma. Mob1a/b deletion also causes liver cancer. |
(Benhamouche et al., 2010; Lee et al., 2010; Lu et al., 2010; McClatchey et al., 1998; Nishio et al., 2012; Song et al., 2010; Yin et al., 2013; Zhang et al., 2010; Zhou et al., 2009) |
|
|
Yap deletion causes bile duct defect, and YAP activity is involved in liver regereation upon tissue damage. |
(Bai et al., 2012; Su et al., 2015; Yimlamai et al., 2014; Zhang et al., 2010) |
|
|
Lkb1-deficiency-induced liver over growth is dependent on YAP activation. |
(Mohseni et al., 2014) | |
| Intestine | Yap transgenic expression causes intestinal dysplasia. | (Camargo et al., 2007) |
| Deletion of Sav1 or Mst1/2 in mouse intestine results in expansion of progenitor cells, colonic polyps or adenoma. |
(Cai et al., 2010; Lee et al., 2008; Zhou et al., 2011) |
|
| Deletion of Yap in mouse intestine shows no obvious phenotype, but affects regeneration upon tissue damage. |
(Cai et al., 2010) | |
|
Yap transgenic expression (intestine specific) results in rapid loss of intestinal crypts by repressing Wnt signaling. |
(Barry et al., 2013) | |
|
Apc deletion induced expansion of intestinal crypts in a Yap/Taz dependent manner. |
(Azzolin et al., 2014; Cai et al., 2015) |
|
| Skin | Deletion of Sav1 or transgenic expression of Yap leads to expansion of basal progenitor cells and skin thickening, long term activation of YAP results in squamous cell carcinoma. Mob1a/b deletion also causes skin cancer. |
(Camargo et al., 2007; Lee et al., 2008; Nishio et al., 2012) |
| Deletion of Mst1/2 shows no clear phenotype. Deletion of Ctnna1 (α-Catenin) leads to keratinocyte hyperproliferation and squamous cell carcinoma likely mediated by YAP activation. |
(Schlegelmilch et al., 2011) | |
|
Gnas (Gs) deletion causes Basal-cell carcinoma partially dependent on YAP activation. |
(Iglesias-Bartolome et al., 2015) | |
| Heart | Deletion of Sav1, Mst1/2, or Lats2 at embryonic stage, or transgenic expression of active Yap mutant, results in hyperproliferation of cardiomyocytes and enlargement of heart. |
(Del Re et al., 2013; Heallen et al., 2011; Lin et al., 2014; von Gise et al., 2012; Xin et al., 2013; Xin et al., 2011) |
| Deletion of Yap leads to heart hypoplasia, and more severe phenotype is observed when both Yap and Taz are deleted. |
(von Gise et al., 2012; Xin et al., 2011) |
|
| In adult heart, high YAP/TAZ activity enhances heart regeneration following cardiac damages such as myocardial infarction |
(Heallen et al., 2013; Lin et al., 2014; Xin et al., 2013) |
|
| Kidney |
Taz deletion causes polycystic kidney disease, whereas Yap
deletion leads to hypoplastic kidneys with severe defect in nephron morphogenesis. |
(Hossain et al., 2007; Makita et al., 2008; Reginensi et al., 2013; Tian et al., 2007) |
| Kidney with Mst1/2 or Sav1 deletion appears normal. | (Reginensi et al., 2013; Song et al., 2010) |
|
| Lung | Taz deleted mice display abnormal alveolar structures. | (Makita et al., 2008; Mitani et al., 2009; Tian et al., 2007) |
|
Mst1/2 deletion leads to disrupted lung structures, and neonatal lethality, which is dependent on high YAP/TAZ activity. Mst1/2 deletion in adult lung bronchiolar epithelial cells results in airway hyperplasia and altered differentiation. YAP appears critical in regulating proximal-distal patterning of the lung, and a decrease in YAP activity ensures epithelial cells differentiation. |
(Lange et al., 2015; Lin et al., 2015a; Mahoney et al., 2014) |
|
| Mob1a/b deletion causes lung tumor. | (Nishio et al., 2012) | |
| Forkhead box A2 (FOXA2) has also been shown critical in mediating the effect of Mst1/2 in lung development. |
(Chung et al., 2013) | |
| Pancreas | The effect of Hippo pathway is mainly in exocrine compartment of the pancreas. During postnatal stage, deletion of Mst1/2 increases ratio of ductal and acinar cells, leads to pancreatitis-like autodigestion and a reduced size of pancreas. |
(Gao et al., 2013; George et al., 2012) |
|
Kras (K12D) mutant induced Pancreatic ductal adenocarcinoma requires YAP activity. |
(Zhang et al., 2014a) | |
| Nervous System |
Nf2 deletion results in a reduction in hippocampus size, whereas the pool of Neural and neocortical progenitor cells. Nf2 deletion also affects development of corpus callosum, in which Yap mediated overexpression of SLIT2 disrupts callosal axon pathfinding. |
(Lavado et al., 2013; Lavado et al., 2014) |
| Mammary glands |
Yap and Sav1 are dispensable in mammary glands development. During pregnancy, Yap deletion results in hypoplasia and reduced alveolar structures, on the other hand Sav1 deletion or transgenic expression of Yap prevents terminal differentiation of mammary cells. |
(Chen et al., 2014) |
| Mob1a/b deletion causes breast tumor. | (Nishio et al., 2012) | |
|
Yap deletion delays mammary tumor growth induced by polyoma middle T antigen (PyMT). |
(Chen et al., 2014) | |
| Muscle |
Yap overexpression promotes proliferation of satellite cells and represses their differentiation. YAP down-regulation reduces basal skeletal muscle fibre size, and YAP activity is required to relief neurogenic muscle atrophy following injuries. |
(Judson et al., 2012; Watt et al., 2015) |
However, not all organs are equally sensitive to Hippo pathway mutations. For instance, Mst1/2 knockout results in dramatic overgrowth of liver, heart, stomach, and spleen, but not of kidney, lung, or limbs (Song et al., 2010). A possible explanation is that there are MST1/2-independent regulators of YAP/TAZ in these tissues. In the breast and intestine, tissue-specific deletion of Yap does not result in any defects in tissue structure or size (Cai et al., 2010; Chen et al., 2014). Yap knockout in the mouse liver leads to bile duct defects but not a general reduction of liver size. This lack of effect could be due to the presence of TAZ, which should be activated due to the loss of feedback inhibition upon Yap deletion. Alternatively, the Hippo pathway, or YAP/TAZ activity, may be negligible for the size control of some organs. Nevertheless, the organ specific effects of the Hippo pathway in size control may suggest that different principles are utilized for size regulation in different organs. For example, organ size could be determined by proliferation of differentiated cells or the number of progenitor cells in a pre-allocated pool.
Physiological signals upstream of the Hippo pathway important in organ size determination have yet to be identified. Mechanical force or tension may change as a result of organ growth and inhibit YAP/TAZ when the organ reaches its final size. Alternatively, organ size may be restricted/induced by a soluble factor via autocrine/paracrine mechanisms, and the concentration of this factor is controlled by organ size. These two models are not mutually exclusive, and further investigations are needed to address how the Hippo pathway senses physiological cues to modulate organ size.
High YAP/TAZ activity has been observed in the stem or progenitor cells of multiple tissues, suggesting a role for YAP/TAZ in stem cell maintenance. For example, YAP is highly nuclear in basal progenitor cells and in intestinal stem cells localized at the crypt base (Barry et al., 2013; Camargo et al., 2007; Schlegelmilch et al., 2011; Zhang et al., 2011). Activation of YAP, either by transgenic expression of YAP or deletion of upstream regulators, usually results in expansion of progenitor cells, impaired cell differentiation, and hyperplasia of target tissues such as intestine, liver, skin, and nervous system (Cai et al., 2010; Camargo et al., 2007; Cao et al., 2008; Lee et al., 2008; Lee et al., 2010; Lu et al., 2010; Zhou et al., 2011).
The role of YAP/TAZ on cell proliferation and stem cell expansion suggests a critical function of YAP in normal tissue development and homeostasis. Indeed, tissue-specific deletion of Yap results in abnormalities of the heart, skin, and kidney (Reginensi et al., 2013; Schlegelmilch et al., 2011; von Gise et al., 2012; Xin et al., 2011). However, mammary glands and the intestine remain relatively normal upon Yap deletion (Cai et al., 2010; Chen et al., 2014; Zhou et al., 2011). These findings suggest that YAP is required for development and homeostasis of some, but not all, tissues in mice. In human, TEAD1 mutations are found in Sveinsson chorioretinal atrophy, a disease characterized by chorioretinal degeneration, and Aicardi syndrome, a congenital neurodevelopmental disorder (Fossdal et al., 2004; Schrauwen et al., 2015). In addition, loss-of-function mutations of YAP have been identified in both isolated and syndromic optic fissure closure defects (Williamson et al., 2014). Hence, the loss of TEAD-mediated YAP transcriptional activity plays a role in some degeneration related disorders in humans.
Even though it is not required for development and normal homeostasis of some tissues, YAP activity is critical for tissue regeneration upon certain types of damage. For example, Yap deletion severely compromises pregnancy-induced mammary tissue growth, although virgin mammary development was normal (Chen et al., 2014). Likewise, in wild type mice, the intestines can effectively regenerate following colitis induced by dextran sulfate sodium (DSS) treatment; however, the regenerative capability is severely impeded in conditional Yap knockout mice (Cai et al., 2010). Similar results have also been observed in Drosophila midgut regeneration upon DSS-induced injury (Karpowicz et al., 2010; Ren et al., 2010; Shaw et al., 2010). Normally the liver regenerates efficiently following liver damage. For instance, after partial hepatectomy, hepatocytes start to proliferate to restore liver mass in a few days, and in this process, YAP activity is induced and is most likely required for complete liver regeneration (Grijalva et al., 2014; Su et al., 2015; Wu et al., 2013; Yimlamai et al., 2014). In contrast to the intestine and liver, tissue regeneration of the adult heart is very limited. However, inactivation of the Hippo pathway or transgenic expression of Yap restores some myocardial regenerative capability, although the efficiency is low. In contrast, cardiac-specific deletion of Yap impedes regeneration of the neonatal heart. (Heallen et al., 2013; Lin et al., 2014; Xin et al., 2013). Taken together, these results indicate that YAP plays a significant role in regeneration of multiple tissues.
Hippo Signaling in Cancer
Long-term YAP activation, such as transgenic expression of Yap in the mouse liver, results in cell transformation and tumor development (Dong et al., 2007), indicating the power of the Hippo pathway in cancer initiation and progression. Evidence of the Hippo pathway in tumorigenesis based on mouse models is summarized in Table 2, which generally supports an oncogenic role for YAP and TAZ, as well as a tumor suppressive function for Hippo pathway upstream components.
The tumor-promoting activity of YAP is largely dependent on a TEAD-mediated transcription program, as YAP-induced liver cancer is fully blocked by expression of a dominant negative TEAD which is able to sequester both YAP and TAZ (Liu-Chittenden et al., 2012). At the cellular level, YAP activation is important for cell proliferation, survival, migration, and invasion. High YAP or TAZ activity enables the cell to escape contact inhibition and anoikis, and support anchorage-independent growth (Chan et al., 2008; Zhao et al., 2012; Zhao et al., 2007). YAP induces expression of ZEB1/2 to stimulate the epithelial-to-mesenchymal transition (EMT), which is a key step for tumor metastasis (Gao et al., 2014; Lei et al., 2008; Liu et al., 2010b; Overholtzer et al., 2006). In addition, YAP is able to promote genomic instability (Fernandez et al., 2012), and TAZ is required to sustain self-renewal and the tumor-initiation capacity of breast cancer stem cells (Cordenonsi et al., 2011).
Accumulating evidence suggests that the Hippo pathway is dysregulated in many human cancers. Elevated YAP/TAZ expression or nuclear enrichment of YAP/TAZ has been observed in many types of cancers, including liver, breast, lung, colon, ovary, and others (Chan et al., 2008; Moroishi et al., 2015a; Steinhardt et al., 2008). However, the majority of cancers with high YAP/TAZ activity have not been linked to genetic mutations of the Hippo pathway, and the overall genetic alteration rate of Hippo pathway components in human cancer is relatively low (Table 3).
Table 3.
Genetic alterations of Hippo pathway genes in human cancers.
| Gene | Alteration | Cancer type | References |
|---|---|---|---|
| NF2 | Mutation or deletion | Mesothelioma neurofibromatosis type 2 (schwannoma, meningioma) |
(Sekido, 2011) (Rouleau et al., 1993) |
| LATS1/2 | Gene Fusion (LATS1-PSEN1) |
Mesothelioma | (Miyanaga et al., 2015) |
| LATS2 deletion | Mesothelioma | (Murakami et al., 2011) | |
| LATS/2 mutations | Sporadic in different cancers | (Yu et al., 2013b) | |
| YAP | Amplification | hepatocellular carcinoma medulloblastoma Esophageal squamous cell carcinoma |
(Fernandez et al., 2009) (Overholtzer et al., 2006) (Song et al., 2014) (Zender et al., 2006) |
| Mutation (R331W) | lung adenocarcinoma | (Chen et al., 2015a) | |
| Gene Fusion (YAP-TFE3, YAP-ESR1, YAP- C11orf95, and YAP-MAMLD1) |
epithelioid hemangioendothelioma luminal breast cancer Ependymal tumors |
(Antonescu et al., 2013) (Flucke et al., 2014) (Li et al., 2013) (Pajtler et al., 2015) (Parker et al., 2014) |
|
| Deletion | hematological cancer | (Cottini et al., 2014) | |
| TAZ | Gene Fusion (TAZ-CAMTA1, and TAZ-FOSB) |
epithelioid hemangioendothelioma |
(Errani et al., 2011) (Flucke et al., 2014) (Tanas et al., 2011) |
| GNAQ/GNA11 | Activating mutation | Uveal Melanoma | (Van Raamsdonk et al., 2009) (Van Raamsdonk et al., 2010) |
| vGPCR | Viral oncogene | Kaposi’s sarcoma | (Liu et al., 2015) |
One well-characterized example of a Hippo pathway mutation associated with cancer is in NF2, which causes neurofibromatosis 2 lesions including schwannomas and meningiomas (Xiao et al., 2003). Moreover, inactivating NF2 mutations are also observed in 40-50% of malignant mesothelioma (Sekido, 2011). Importantly, even heterozygous deletion of Yap completely blocks liver tumorigenesis induced by Nf2 knockout in mice, indicating that YAP activation is the major mechanism mediating the tumorigenic potential of Nf2 mutations (Zhang et al., 2010).
YAP gene amplification may contribute to a portion of hepatocellular carcinomas, medulloblastomas, and esophageal squamous cell carcinomas (Fernandez et al., 2009; Overholtzer et al., 2006; Song et al., 2014; Zender et al., 2006). Gene fusions involving YAP and TAZ have also been discovered in human cancers. Remarkably, virtually all epithelioid hemangioendotheliomas contain gene fusions of TAZ-CAMTA1, TAZ-FOSB, or YAP-TFE3 (Antonescu et al., 2013; Errani et al., 2011; Flucke et al., 2014; Tanas et al., 2011). In addition, YAP gene fusions with MAMLD1 or C11orf95 have been discovered in a subset of ependymal tumors (Pajtler et al., 2015; Parker et al., 2014). It is worth noting that in both epithelioid hemangioendotheliomas and ependymal tumors, all YAP/TAZ fusions proteins retain their N-terminal TEAD binding domain, but lose the C-terminal transactivation domain. These observations suggest that these YAP fusions may still bind to and activate the TEAD dependent transcriptional program to promote tumorigenesis. Indeed, neural stem cells carrying the YAP-C11orf95 fusion gene can effectively form brain tumors when grafted into mice (Parker et al., 2014). In addition, a familial YAP point mutation (R331W) has also been reported to correlate with a high incidence of lung adenocarcinomas (Chen et al., 2015a).
Aberrant GPCR signaling often results in tumorigenesis, so it is possible that GPCR dysregulation can cause cancer by activating YAP/TAZ. GNAQ or GNA11 (encoding Gαq or Gα11, respectively) activating mutations have been identified in approximately 80% of uveal melanomas and function as driver mutations (Van Raamsdonk et al., 2009; Van Raamsdonk et al., 2010). Recent studies showed that YAP is constitutively activated in mutant GNAQ or GNA11 mutant uveal melanomas and the high YAP activity contributes to tumor growth (Feng et al., 2014; Yu et al., 2014). In mice, deletion of GNAS (encoding GαS) in skin stem cells initiates basal-cell carcinogenesis, which is partially dependent on YAP (Iglesias-Bartolome et al., 2015). Moreover, expression of a viral GPCR induces tumorigenesis in Kaposi's sarcoma, where YAP/TAZ also play a critical role (Liu et al., 2015).
LATS1/2 mutations or gene fusion have been sporadically identified in different cancers which may lead to YAP/TAZ activation (Table 3). In addition, Crosstalk with other cancer-related signaling pathways also likely contributes to high YAP/TAZ activity in cancers that have no mutations of Hippo pathway components. For example, KRAS, APC, and LKB1 mutations have all been reported to activate YAP/TAZ (Azzolin et al., 2014; Gao et al., 2014; Mohseni et al., 2014; Zhang et al., 2014a).
YAP/TAZ activity is also linked to drug resistance and cancer relapse. Cultured breast cancer cells with high YAP/TAZ activity show resistance to drugs such as taxol, 5-fluorouracil, and doxorubicin (Cordenonsi et al., 2011; Lai et al., 2011; Touil et al., 2014). Furthermore, lung and colon cancer cells with high YAP activity are resistant to RAF- and MEK-targeted therapies (Lin et al., 2015b). Tamoxifen is commonly used to treat estrogen receptor (ER, a nuclear receptor) positive breast cancer; however, some ER positive breast cancers are insensitive to tamoxifen. Recently, tamoxifen has been shown to activate YAP/TAZ by stimulating the membrane estrogen receptor GPER, a GPCR. Therefore, activation of GPER by tamoxifen or estrogen may contribute to tumor growth and drug resistance (Zhou et al., 2015). Amplification of the YAP gene has been associated with cancer relapse in KRAS-driven colon and pancreatic cancers (Kapoor et al., 2014; Shao et al., 2014). Thus, inhibition of YAP/TAZ will not only target tumor initiation and progression, but also potentially sensitize cancer cells to chemotherapies and prevent cancer relapse.
Notably, in contrast to its oncogenic function in most solid tumors, YAP seems to play a tumor-suppressor role in hematological cancers. The YAP gene locus is frequently deleted in hematological cancer and expression of YAP or inhibition of MST1 leads to growth inhibition and increased apoptosis (Cottini et al., 2014). Currently, the underlying mechanism responsible for this tumor suppressor function of YAP in hematological cancers is not well understood.
Therapeutic Targeting of Hippo Signaling
The core Hippo pathway is a kinase cascade, and protein kinases are usually druggable. Thus, inhibitors for MAP4K4, MST1/2, or LATS1/2 may be developed to induce YAP/TAZ activity and facilitate the process of wound healing, tissue repair, or regeneration, and possibly for treating degenerative diseases (Figure 4). For example, temporal inhibition of MST1/2 or LATS1/2 may promote myocardial regeneration or survival that would be beneficial for heart attack patients. It is also possible that inhibitors of MST1/2 or LATS1/2 could be used for treating hematological cancers.
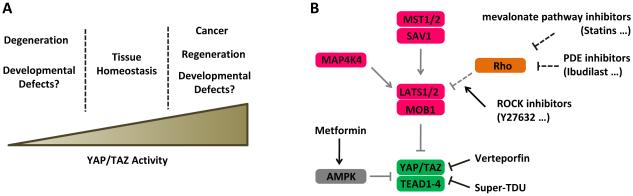
Figure 4.
Therapeutic targeting of the Hippo pathway. (A) Potential roles of YAP/TAZ activity in tissue development and diseases. A confined window of YAP/TAZ activity is required for normal tissue development and homeostasis. (B) Strategies for targeting YAP/TAZ activity. Inhibitors for MST1/2, MAP4K4, and LATS1/2 can activate YAP/TAZ. YAP/TAZ-TEAD interaction may be disrupted by small molecules directly (Verteporfin) or AMPK activators (Metformin). Small molecules inhibiting Rho family GTPases or ROCK can indirectly activate LATS1/2, leading to YAP/TAZ inhibition.
Generally, MST1/2 and LATS1/2 are tumor suppressors, and inhibition of MST1/2 or LATS1/2 may promote tumor growth in most instances. On the other hand, inhibiting YAP/TAZ activity would offer a new and attractive anti-cancer strategy (Park and Guan, 2013). The function of YAP/TAZ is primarily mediated by TEADs, so small molecules disrupting the YAP/TAZ-TEAD interaction will function as YAP/TAZ inhibitors. Indeed, porphyrin family molecules, especially verteporfin, are able to disrupt the interaction between YAP/TAZ and TEADs, and verteporfin can block transcription of YAP/TAZ target genes and suppress liver overgrowth induced by YAP over-expression or NF2 inactivation in mice (Liu-Chittenden et al., 2012). However, verteporfin has general cellular toxicity and low aqueous solubility. Based on structural information from the YAP-TEAD and VGLL4-TEAD complex, a polypeptide termed “super-TDU” has been designed to block YAP-TEAD interaction, and has been shown to suppress tumor growth in mouse models (Jiao et al., 2014).
It is challenging to design direct activators for protein kinases. However, LATS1/2 may be activated indirectly by molecules targeting their upstream regulators. The very first small molecule (dobutamine) identified with an inhibitory effect on YAP is an antagonist of a GPCR receptor (Bao et al., 2011). Since then, many indirect inhibitors for YAP/TAZ have been identified, including phosphodiesterase inhibitors rolipram and ibudilast (Yu et al., 2013a). The Rho family GTPases have a strong inhibitory effect on LATS1/2, and membrane localization is important for Rho cellular function. Indeed, mevalonate metabolic pathway inhibitor statins can block membrane translocation of Rho GTPases and indirectly inhibit YAP/TAZ activity (Mi et al., 2015; Sorrentino et al., 2014; Wang et al., 2014). It will be interesting to test whether these drugs are effective in suppressing tumor growth in mouse models and perform epidemiologic studies to determine whether patients using statins or rolipram have a lower incidence of cancer. Given the important function of the Hippo pathway in regulating cell proliferation and tissue homeostasis, it represents an exciting and previously unexplored field for cancer therapy.
Outstanding Questions.
Despite the rapid research progress in the Hippo pathway, some key questions remain unanswered and new questions are emerging. Listed below are some of the key questions in the Hippo field.
What are the molecular mechanisms regulating MST1/2 activity? Many upstream signals have been convincingly shown to regulate LATS1/2 phosphorylation and kinase activity. However, neither MST1/2 phosphorylation nor its kinase activity is strongly modulated by upstream signals. Drosophila misshapen (a member of MAP4K in mammals) acts upstream of Wts. An interesting question is whether MST1/2 and MAP4Ks mediate different or similar upstream signals to activate LATS1/2.
Where are Hippo pathway components localized in mammalian cells? This may be key to understanding how the Hippo pathway is regulated in response to upstream signals. It is obvious that phosphorylated YAP/TAZ are enriched in the cytoplasm and dephosphorylated YAP/TAZ in the nucleus. However, it is less clear where YAP/TAZ phosphorylation and dephosphorylation occur. A related question is what are the mechanisms underlying YAP/TAZ translocation between the nucleus and cytoplasm.
How are LATS1/2 regulated by actin remodeling and/or cellular tension? This is one of the key questions in understanding the biochemical mechanism of the Hippo kinase cascade regulation. Accumulating evidence suggests that the actin cytoskeleton and cellular tension play a key role in LATS1/2 regulation and appear to act downstream of many, if not most, upstream signals. The actin cytoskeleton and cellular tension are intertwined, thus the question is: which one plays a more direct role in regulating Hippo core components?
What is the mechanism of organ size sensing? Although many signals are reported to regulate the Hippo pathway in vitro, so far none have been demonstrated to play a key role in organ size control in vivo. Uncovering this magic signal will solve a key question in developmental biology.
How does YAP become deregulated in cancer? It is clear that YAP/TAZ activation is observed in a broad spectrum of human cancers, although mutations in Hippo pathway genes are rare. This interesting conundrum indicates that the Hippo pathway maybe regulated broadly by many other cancer driving pathways.
Acknowledgements
KLG is supported by grants from NIH (CA196878, CA132809, DE015964, EY022611, GM51586). FXY is supported by grants from "Thousand Youth Talents", Shanghai "Oriental Scholar", and NSFC (31571479). BZ is supported by grants from NSFC (31422036, 31271508, 31471316), State Key Development Program for Basic Research of China (2013CB945303), and the NSF of Zhejiang (LR12C07001). We thank Jean Guan, Hyun Woo Park, and Steven Plouffe for critical reading of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Antonescu CR, Le Loarer F, Mosquera JM, Sboner A, Zhang L, Chen CL, Chen HW, Pathan N, Krausz T, Dickson BC, et al. Novel YAP1-TFE3 fusion defines a distinct subset of epithelioid hemangioendothelioma. Gene Chromosome Canc. 2013;52:775–784. doi: 10.1002/gcc.22073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aragona M, Panciera T, Manfrin A, Giulitti S, Michielin F, Elvassore N, Dupont S, Piccolo S. A mechanical checkpoint controls multicellular growth through YAP/TAZ regulation by actin-processing factors. Cell. 2013;154:1047–1059. doi: 10.1016/j.cell.2013.07.042. [DOI] [PubMed] [Google Scholar]
- Azzolin L, Panciera T, Soligo S, Enzo E, Bicciato S, Dupont S, Bresolin S, Frasson C, Basso G, Guzzardo V, et al. YAP/TAZ Incorporation in the beta-Catenin Destruction Complex Orchestrates the Wnt Response. Cell. 2014;158:157–170. doi: 10.1016/j.cell.2014.06.013. [DOI] [PubMed] [Google Scholar]
- Azzolin L, Zanconato F, Bresolin S, Forcato M, Basso G, Bicciato S, Cordenonsi M, Piccolo S. Role of TAZ as mediator of Wnt signaling. Cell. 2012;151:1443–1456. doi: 10.1016/j.cell.2012.11.027. [DOI] [PubMed] [Google Scholar]
- Bai H, Zhang N, Xu Y, Chen Q, Khan M, Potter JJ, Nayar SK, Cornish T, Alpini G, Bronk S, et al. Yes-associated protein regulates the hepatic response after bile duct ligation. Hepatology. 2012;56:1097–1107. doi: 10.1002/hep.25769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao YJ, Nakagawa K, Yang Z, Ikeda M, Withanage K, Ishigami-Yuasa M, Okuno Y, Hata S, Nishina H, Hata Y. A cell-based assay to screen stimulators of the Hippo pathway reveals the inhibitory effect of dobutamine on the YAP-dependent gene transcription. J Biochem. 2011;150:199–208. doi: 10.1093/jb/mvr063. [DOI] [PubMed] [Google Scholar]
- Barry ER, Morikawa T, Butler BL, Shrestha K, de la Rosa R, Yan KS, Fuchs CS, Magness ST, Smits R, Ogino S, et al. Restriction of intestinal stem cell expansion and the regenerative response by YAP. Nature. 2013;493:106–110. doi: 10.1038/nature11693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumgartner R, Poernbacher I, Buser N, Hafen E, Stocker H. The WW domain protein Kibra acts upstream of Hippo in Drosophila. Dev Cell. 2010;18:309–316. doi: 10.1016/j.devcel.2009.12.013. [DOI] [PubMed] [Google Scholar]
- Benham-Pyle BW, Pruitt BL, Nelson WJ. Cell adhesion. Mechanical strain induces E-cadherin-dependent Yap1 and beta-catenin activation to drive cell cycle entry. Science. 2015;348:1024–1027. doi: 10.1126/science.aaa4559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benhamouche S, Curto M, Saotome I, Gladden AB, Liu CH, Giovannini M, McClatchey AI. Nf2/Merlin controls progenitor homeostasis and tumorigenesis in the liver. Genes Dev. 2010;24:1718–1730. doi: 10.1101/gad.1938710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett FC, Harvey KF. Fat cadherin modulates organ size in Drosophila via the Salvador/Warts/Hippo signaling pathway. Curr Biol. 2006;16:2101–2110. doi: 10.1016/j.cub.2006.09.045. [DOI] [PubMed] [Google Scholar]
- Bennett V, Gilligan DM. The spectrin-based membrane skeleton and micron-scale organization of the plasma membrane. Annu Rev Cell Biol. 1993;9:27–66. doi: 10.1146/annurev.cb.09.110193.000331. [DOI] [PubMed] [Google Scholar]
- Boggiano JC, Vanderzalm PJ, Fehon RG. Tao-1 phosphorylates Hippo/MST kinases to regulate the Hippo-Salvador-Warts tumor suppressor pathway. Dev Cell. 2011;21:888–895. doi: 10.1016/j.devcel.2011.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai J, Maitra A, Anders RA, Taketo MM, Pan D. beta-Catenin destruction complex-independent regulation of Hippo-YAP signaling by APC in intestinal tumorigenesis. Genes Dev. 2015;29:1493–1506. doi: 10.1101/gad.264515.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai J, Zhang N, Zheng Y, de Wilde RF, Maitra A, Pan D. The Hippo signaling pathway restricts the oncogenic potential of an intestinal regeneration program. Genes Dev. 2010;24:2383–2388. doi: 10.1101/gad.1978810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callus BA, Verhagen AM, Vaux DL. Association of mammalian sterile twenty kinases, Mst1 and Mst2, with hSalvador via C-terminal coiled-coil domains, leads to its stabilization and phosphorylation. FEBS J. 2006;273:4264–4276. doi: 10.1111/j.1742-4658.2006.05427.x. [DOI] [PubMed] [Google Scholar]
- Camargo FD, Gokhale S, Johnnidis JB, Fu D, Bell GW, Jaenisch R, Brummelkamp TR. YAP1 increases organ size and expands undifferentiated progenitor cells. Curr Biol. 2007;17:2054–2060. doi: 10.1016/j.cub.2007.10.039. [DOI] [PubMed] [Google Scholar]
- Cao X, Pfaff SL, Gage FH. YAP regulates neural progenitor cell number via the TEA domain transcription factor. Genes Dev. 2008;22:3320–3334. doi: 10.1101/gad.1726608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan EH, Nousiainen M, Chalamalasetty RB, Schafer A, Nigg EA, Sillje HH. The Ste20-like kinase Mst2 activates the human large tumor suppressor kinase Lats1. Oncogene. 2005;24:2076–2086. doi: 10.1038/sj.onc.1208445. [DOI] [PubMed] [Google Scholar]
- Chan SW, Lim CJ, Guo K, Ng CP, Lee I, Hunziker W, Zeng Q, Hong W. A role for TAZ in migration, invasion, and tumorigenesis of breast cancer cells. Cancer Res. 2008;68:2592–2598. doi: 10.1158/0008-5472.CAN-07-2696. [DOI] [PubMed] [Google Scholar]
- Chaulk SG, Lattanzi VJ, Hiemer SE, Fahlman RP, Varelas X. The Hippo Pathway Effectors TAZ/YAP Regulate Dicer Expression and MicroRNA Biogenesis through Let-7. J Biol Chem. 2014;289:1886–1891. doi: 10.1074/jbc.C113.529362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CL, Gajewski KM, Hamaratoglu F, Bossuyt W, Sansores-Garcia L, Tao C, Halder G. The apical-basal cell polarity determinant Crumbs regulates Hippo signaling in Drosophila. Proc Natl Acad Sci U S A. 2010;107:15810–15815. doi: 10.1073/pnas.1004060107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen HY, Yu SL, Ho BC, Su KY, Hsu YC, Chang CS, Li YC, Yang SY, Hsu PY, Ho H, et al. R331W Missense Mutation of Oncogene YAP1 Is a Germline Risk Allele for Lung Adenocarcinoma With Medical Actionability. J Clin Oncol. 2015a;33:2303–2310. doi: 10.1200/JCO.2014.59.3590. [DOI] [PubMed] [Google Scholar]
- Chen Q, Zhang N, Gray RS, Li H, Ewald AJ, Zahnow CA, Pan D. A temporal requirement for Hippo signaling in mammary gland differentiation, growth, and tumorigenesis. Genes Dev. 2014;28:432–437. doi: 10.1101/gad.233676.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q, Zhang N, Xie R, Wang W, Cai J, Choi KS, David KK, Huang B, Yabuta N, Nojima H, et al. Homeostatic control of Hippo signaling activity revealed by an endogenous activating mutation in YAP. Genes Dev. 2015b;29:1285–1297. doi: 10.1101/gad.264234.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho E, Feng Y, Rauskolb C, Maitra S, Fehon R, Irvine KD. Delineation of a Fat tumor suppressor pathway. Nat Genet. 2006;38:1142–1150. doi: 10.1038/ng1887. [DOI] [PubMed] [Google Scholar]
- Chung C, Kim T, Kim M, Kim M, Song H, Kim TS, Seo E, Lee SH, Kim H, Kim SK, et al. Hippo-Foxa2 signaling pathway plays a role in peripheral lung maturation and surfactant homeostasis. Proc Natl Acad Sci U S A. 2013;110:7732–7737. doi: 10.1073/pnas.1220603110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cockburn K, Biechele S, Garner J, Rossant J. The Hippo pathway member Nf2 is required for inner cell mass specification. Curr Biol. 2013;23:1195–1201. doi: 10.1016/j.cub.2013.05.044. [DOI] [PubMed] [Google Scholar]
- Codelia VA, Sun GP, Irvine KD. Regulation of YAP by Mechanical Strain through Jnk and Hippo Signaling. Curr Biol. 2014;24:2012–2017. doi: 10.1016/j.cub.2014.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordenonsi M, Zanconato F, Azzolin L, Forcato M, Rosato A, Frasson C, Inui M, Montagner M, Parenti AR, Poletti A, et al. The Hippo transducer TAZ confers cancer stem cell-related traits on breast cancer cells. Cell. 2011;147:759–772. doi: 10.1016/j.cell.2011.09.048. [DOI] [PubMed] [Google Scholar]
- Cottini F, Hideshima T, Xu C, Sattler M, Dori M, Agnelli L, ten Hacken E, Bertilaccio MT, Antonini E, Neri A, et al. Rescue of Hippo coactivator YAP1 triggers DNA damage-induced apoptosis in hematological cancers. Nat Med. 2014;20:599–606. doi: 10.1038/nm.3562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai X, Liu H, Shen S, Guo X, Yan H, Ji X, Li L, Huang J, Feng XH, Zhao B. YAP activates the Hippo pathway in a negative feedback loop. Cell Res. 2015 doi: 10.1038/cr.2015.101. doi: 10.1038/cr.2015.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Re DP, Yang Y, Nakano N, Cho J, Zhai P, Yamamoto T, Zhang N, Yabuta N, Nojima H, Pan D, et al. Yes-associated protein isoform 1 (Yap1) promotes cardiomyocyte survival and growth to protect against myocardial ischemic injury. J Biol Chem. 2013;288:3977–3988. doi: 10.1074/jbc.M112.436311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng H, Wang W, Yu J, Zheng Y, Qing Y, Pan D. Spectrin regulates Hippo signaling by modulating cortical actomyosin activity. Elife. 2015;4:e06567. doi: 10.7554/eLife.06567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeRan M, Yang J, Shen CH, Peters EC, Fitamant J, Chan P, Hsieh M, Zhu S, Asara JM, Zheng B, et al. Energy stress regulates hippo-YAP signaling involving AMPK-mediated regulation of angiomotin-like 1 protein. Cell Rep. 2014;9:495–503. doi: 10.1016/j.celrep.2014.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong J, Feldmann G, Huang J, Wu S, Zhang N, Comerford SA, Gayyed MF, Anders RA, Maitra A, Pan D. Elucidation of a universal size-control mechanism in Drosophila and mammals. Cell. 2007;130:1120–1133. doi: 10.1016/j.cell.2007.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupont S, Morsut L, Aragona M, Enzo E, Giulitti S, Cordenonsi M, Zanconato F, Le Digabel J, Forcato M, Bicciato S, et al. Role of YAP/TAZ in mechanotransduction. Nature. 2011;474:179–183. doi: 10.1038/nature10137. [DOI] [PubMed] [Google Scholar]
- Enzo E, Santinon G, Pocaterra A, Aragona M, Bresolin S, Forcato M, Grifoni D, Pession A, Zanconato F, Guzzo G, et al. Aerobic glycolysis tunes YAP/TAZ transcriptional activity. EMBO J. 2015;34:1349–1370. doi: 10.15252/embj.201490379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Errani C, Zhang L, Sung YS, Hajdu M, Singer S, Maki RG, Healey JH, Antonescu CR. A Novel WWTR1-CAMTA1 Gene Fusion Is a Consistent Abnormality in Epithelioid Hemangioendothelioma of Different Anatomic Sites. Gene Chromosome Canc. 2011;50:644–653. doi: 10.1002/gcc.20886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan R, Kim NG, Gumbiner BM. Regulation of Hippo pathway by mitogenic growth factors via phosphoinositide 3-kinase and phosphoinositide-dependent kinase-1. Proc Natl Acad Sci U S A. 2013;110:2569–2574. doi: 10.1073/pnas.1216462110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng XD, Degese MS, Iglesias-Bartolome R, Vaque JP, Molinolo AA, Rodrigues M, Zaidi MR, Ksander BR, Merlino G, Sodhi A, et al. Hippo-Independent Activation of YAP by the GNAQ Uveal Melanoma Oncogene through a Trio-Regulated Rho GTPase Signaling Circuitry. Cancer Cell. 2014;25:831–845. doi: 10.1016/j.ccr.2014.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez BG, Gaspar P, Bras-Pereira C, Jezowska B, Rebelo SR, Janody F. Actin-Capping Protein and the Hippo pathway regulate F-actin and tissue growth in Drosophila. Development. 2011;138:2337–2346. doi: 10.1242/dev.063545. [DOI] [PubMed] [Google Scholar]
- Fernandez LA, Northcott PA, Dalton J, Fraga C, Ellison D, Angers S, Taylor MD, Kenney AM. YAP1 is amplified and up-regulated in hedgehog-associated medulloblastomas and mediates Sonic hedgehog-driven neural precursor proliferation. Genes Dev. 2009;23:2729–2741. doi: 10.1101/gad.1824509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez LA, Squatrito M, Northcott P, Awan A, Holland EC, Taylor MD, Nahle Z, Kenney AM. Oncogenic YAP promotes radioresistance and genomic instability in medulloblastoma through IGF2-mediated Akt activation. Oncogene. 2012;31:1923–1937. doi: 10.1038/onc.2011.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher GC, Elbediwy A, Khanal I, Ribeiro PS, Tapon N, Thompson BJ. The Spectrin cytoskeleton regulates the Hippo signalling pathway. EMBO J. 2015;34:940–954. doi: 10.15252/embj.201489642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flucke U, Vogels RJ, de Saint Aubain Somerhausen N, Creytens DH, Riedl RG, van Gorp JM, Milne AN, Huysentruyt CJ, Verdijk MA, van Asseldonk MM, et al. Epithelioid Hemangioendothelioma: clinicopathologic, immunhistochemical, and molecular genetic analysis of 39 cases. Diagn Pathol. 2014;9:131. doi: 10.1186/1746-1596-9-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fossdal R, Jonasson F, Kristjansdottir GT, Kong A, Stefansson H, Gosh S, Gulcher JR, Stefansson K. A novel TEAD1 mutation is the causative allele in Sveinsson's chorioretinal atrophy (helicoid peripapillary chorioretinal degeneration) Hum Mol Genet. 2004;13:975–981. doi: 10.1093/hmg/ddh106. [DOI] [PubMed] [Google Scholar]
- Gailite I, Aerne BL, Tapon N. Differential control of Yorkie activity by LKB1/AMPK and the Hippo/Warts cascade in the central nervous system. Proc Natl Acad Sci U S A. 2015 doi: 10.1073/pnas.1505512112. doi: 10.1073/pnas.1505512112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao T, Zhou D, Yang C, Singh T, Penzo-Mendez A, Maddipati R, Tzatsos A, Bardeesy N, Avruch J, Stanger BZ. Hippo signaling regulates differentiation and maintenance in the exocrine pancreas. Gastroenterology. 2013;144:1543–1553. doi: 10.1053/j.gastro.2013.02.037. 1553 e1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y, Zhang W, Han X, Li F, Wang X, Wang R, Fang Z, Tong X, Yao S, Li F, et al. YAP inhibits squamous transdifferentiation of Lkb1-deficient lung adenocarcinoma through ZEB2-dependent DNp63 repression. Nat Commun. 2014;5:4629. doi: 10.1038/ncomms5629. [DOI] [PubMed] [Google Scholar]
- Genevet A, Wehr MC, Brain R, Thompson BJ, Tapon N. Kibra is a regulator of the Salvador/Warts/Hippo signaling network. Dev Cell. 2010;18:300–308. doi: 10.1016/j.devcel.2009.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George NM, Day CE, Boerner BP, Johnson RL, Sarvetnick NE. Hippo signaling regulates pancreas development through inactivation of Yap. Mol Cell Biol. 2012;32:5116–5128. doi: 10.1128/MCB.01034-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong R, Hong AW, Plouffe SW, Zhao B, Liu G, Yu FX, Xu Y, Guan KL. Opposing roles of conventional and nove PKC in the Hippo-YAP pathway regulation. Cell Res. 2015;25:985–988. doi: 10.1038/cr.2015.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goulev Y, Fauny JD, Gonzalez-Marti B, Flagiello D, Silber J, Zider A. SCALLOPED interacts with YORKIE, the nuclear effector of the hippo tumor-suppressor pathway in Drosophila. Curr Biol. 2008;18:435–441. doi: 10.1016/j.cub.2008.02.034. [DOI] [PubMed] [Google Scholar]
- Grijalva JL, Huizenga M, Mueller K, Rodriguez S, Brazzo J, Camargo F, Sadri-Vakili G, Vakili K. Dynamic alterations in Hippo signaling pathway and YAP activation during liver regeneration. Am J Physiol Gastrointest Liver Physiol. 2014;307:G196–204. doi: 10.1152/ajpgi.00077.2014. [DOI] [PubMed] [Google Scholar]
- Guo T, Lu Y, Li PX, Yin MX, Lv DK, Zhang WJ, Wang HZ, Zhou ZC, Ji HB, Zhao Y, et al. A novel partner of Scalloped regulates Hippo signaling via antagonizing Scalloped-Yorkie activity. Cell Res. 2013;23:1201–1214. doi: 10.1038/cr.2013.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halder G, Johnson RL. Hippo signaling: growth control and beyond. Development. 2011;138:9–22. doi: 10.1242/dev.045500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamaratoglu F, Willecke M, Kango-Singh M, Nolo R, Hyun E, Tao C, Jafar-Nejad H, Halder G. The tumour-suppressor genes NF2/Merlin and Expanded act through Hippo signalling to regulate cell proliferation and apoptosis. Nat Cell Biol. 2006;8:27–36. doi: 10.1038/ncb1339. [DOI] [PubMed] [Google Scholar]
- Harvey KF, Pfleger CM, Hariharan IK. The Drosophila Mst ortholog, hippo, restricts growth and cell proliferation and promotes apoptosis. Cell. 2003;114:457–467. doi: 10.1016/s0092-8674(03)00557-9. [DOI] [PubMed] [Google Scholar]
- Heallen T, Morikawa Y, Leach J, Tao G, Willerson JT, Johnson RL, Martin JF. Hippo signaling impedes adult heart regeneration. Development. 2013;140:4683–4690. doi: 10.1242/dev.102798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heallen T, Zhang M, Wang J, Bonilla-Claudio M, Klysik E, Johnson RL, Martin JF. Hippo pathway inhibits Wnt signaling to restrain cardiomyocyte proliferation and heart size. Science. 2011;332:458–461. doi: 10.1126/science.1199010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirate Y, Hirahara S, Inoue K, Suzuki A, Alarcon VB, Akimoto K, Hirai T, Hara T, Adachi M, Chida K, et al. Polarity-dependent distribution of angiomotin localizes Hippo signaling in preimplantation embryos. Curr Biol. 2013;23:1181–1194. doi: 10.1016/j.cub.2013.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hossain Z, Ali SM, Ko HL, Xu J, Ng CP, Guo K, Qi Z, Ponniah S, Hong W, Hunziker W. Glomerulocystic kidney disease in mice with a targeted inactivation of Wwtr1. Proc Natl Acad Sci U S A. 2007;104:1631–1636. doi: 10.1073/pnas.0605266104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J, Wu S, Barrera J, Matthews K, Pan D. The Hippo signaling pathway coordinately regulates cell proliferation and apoptosis by inactivating Yorkie, the Drosophila Homolog of YAP. Cell. 2005;122:421–434. doi: 10.1016/j.cell.2005.06.007. [DOI] [PubMed] [Google Scholar]
- Huang W, Lv X, Liu C, Zha Z, Zhang H, Jiang Y, Xiong Y, Lei QY, Guan KL. The N-terminal phosphodegron targets TAZ/WWTR1 protein for SCFbeta-TrCP-dependent degradation in response to phosphatidylinositol 3-kinase inhibition. J Biol Chem. 2012;287:26245–26253. doi: 10.1074/jbc.M112.382036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iglesias-Bartolome R, Torres D, Marone R, Feng X, Martin D, Simaan M, Chen M, Weinstein LS, Taylor SS, Molinolo AA, et al. Inactivation of a Galphas-PKA tumour suppressor pathway in skin stem cells initiates basal-cell carcinogenesis. Nat Cell Biol. 2015;17:793–803. doi: 10.1038/ncb3164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe AB, Hall A. Rho GTPases: biochemistry and biology. Annu Rev Cell Dev Biol. 2005;21:247–269. doi: 10.1146/annurev.cellbio.21.020604.150721. [DOI] [PubMed] [Google Scholar]
- Jia JH, Zhang WS, Wang B, Trinko R, Jiang J. The Drosophila Ste20 family kinase dMST functions as a tumor suppressor by restricting cell proliferation and promoting apoptosis. Gene Dev. 2003;17:2514–2519. doi: 10.1101/gad.1134003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao S, Wang HZ, Shi ZB, Dong AM, Zhang WJ, Song XM, He F, Wang YC, Zhang ZZ, Wang WJ, et al. A Peptide Mimicking VGLL4 Function Acts as a YAP Antagonist Therapy against Gastric Cancer. Cancer Cell. 2014;25:166–180. doi: 10.1016/j.ccr.2014.01.010. [DOI] [PubMed] [Google Scholar]
- Jin Y, Xu J, Yin MX, Lu Y, Hu L, Li P, Zhang P, Yuan Z, Ho MS, Ji H, et al. Brahma is essential for Drosophila intestinal stem cell proliferation and regulated by Hippo signaling. Elife. 2013;2:e00999. doi: 10.7554/eLife.00999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Judson RN, Tremblay AM, Knopp P, White RB, Urcia R, De Bari C, Zammit PS, Camargo FD, Wackerhage H. The Hippo pathway member Yap plays a key role in influencing fate decisions in muscle satellite cells. J Cell Sci. 2012;125:6009–6019. doi: 10.1242/jcs.109546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jukam D, Xie B, Rister J, Terrell D, Charlton-Perkins M, Pistillo D, Gebelein B, Desplan C, Cook T. Opposite feedbacks in the Hippo pathway for growth control and neural fate. Science. 2013;342:1238016. doi: 10.1126/science.1238016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Justice RW, Zilian O, Woods DF, Noll M, Bryant PJ. The Drosophila tumor suppressor gene warts encodes a homolog of human myotonic dystrophy kinase and is required for the control of cell shape and proliferation. Genes Dev. 1995;9:534–546. doi: 10.1101/gad.9.5.534. [DOI] [PubMed] [Google Scholar]
- Kango-Singh M, Nolo R, Tao C, Verstreken P, Hiesinger PR, Bellen HJ, Halder G. Shar-pei mediates cell proliferation arrest during imaginal disc growth in Drosophila. Development. 2002;129:5719–5730. doi: 10.1242/dev.00168. [DOI] [PubMed] [Google Scholar]
- Kapoor A, Yao W, Ying H, Hua S, Liewen A, Wang Q, Zhong Y, Wu CJ, Sadanandam A, Hu B, et al. Yap1 activation enables bypass of oncogenic Kras addiction in pancreatic cancer. Cell. 2014;158:185–197. doi: 10.1016/j.cell.2014.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karpowicz P, Perez J, Perrimon N. The Hippo tumor suppressor pathway regulates intestinal stem cell regeneration. Development. 2010;137:4135–4145. doi: 10.1242/dev.060483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KM, Choi YJ, Hwang JH, Kim AR, Cho HJ, Hwang ES, Park JY, Lee SH, Hong JH. Shear Stress Induced by an Interstitial Level of Slow Flow Increases the Osteogenic Differentiation of Mesenchymal Stem Cells through TAZ Activation. Plos One. 2014;9:e92427. doi: 10.1371/journal.pone.0092427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M, Kim T, Johnson RL, Lim DS. Transcriptional Co-repressor Function of the Hippo Pathway Transducers YAP and TAZ. Cell Rep. 2015;11:270–282. doi: 10.1016/j.celrep.2015.03.015. [DOI] [PubMed] [Google Scholar]
- Kim M, Lee S, Kuninaka S, Saya H, Lee H, Lim DS. cAMP/PKA signalling reinforces the LATS-YAP pathway to fully suppress YAP in response to actin cytoskeletal changes. EMBO J. 2013;32:1543–1555. doi: 10.1038/emboj.2013.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kono K, Tamashiro DA, Alarcon VB. Inhibition of RHO-ROCK signaling enhances ICM and suppresses TE characteristics through activation of Hippo signaling in the mouse blastocyst. Dev Biol. 2014;394:142–155. doi: 10.1016/j.ydbio.2014.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koontz LM, Liu-Chittenden Y, Yin F, Zheng YG, Yu JZ, Huang B, Chen Q, Wu S, Pan DJ. The Hippo Effector Yorkie Controls Normal Tissue Growth by Antagonizing Scalloped-Mediated Default Repression. Dev Cell. 2013;25:388–401. doi: 10.1016/j.devcel.2013.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon Y, Vinayagam A, Sun XY, Dephoure N, Gygi SP, Hong PY, Perrimon N. The Hippo Signaling Pathway Interactome. Science. 2013;342:737–740. doi: 10.1126/science.1243971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai D, Ho KC, Hao YW, Yang XL. Taxol Resistance in Breast Cancer Cells Is Mediated by the Hippo Pathway Component TAZ and Its Downstream Transcriptional Targets Cyr61 and CTGF. Cancer Res. 2011;71:2728–2738. doi: 10.1158/0008-5472.CAN-10-2711. [DOI] [PubMed] [Google Scholar]
- Lai ZC, Wei X, Shimizu T, Ramos E, Rohrbaugh M, Nikolaidis N, Ho LL, Li Y. Control of cell proliferation and apoptosis by mob as tumor suppressor, mats. Cell. 2005;120:675–685. doi: 10.1016/j.cell.2004.12.036. [DOI] [PubMed] [Google Scholar]
- Lange AW, Sridharan A, Xu Y, Stripp BR, Perl AK, Whitsett JA. Hippo/Yap signaling controls epithelial progenitor cell proliferation and differentiation in the embryonic and adult lung. J Mol Cell Biol. 2015;7:35–47. doi: 10.1093/jmcb/mju046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavado A, He Y, Pare J, Neale G, Olson EN, Giovannini M, Cao X. Tumor suppressor Nf2 limits expansion of the neural progenitor pool by inhibiting Yap/Taz transcriptional coactivators. Development. 2013;140:3323–3334. doi: 10.1242/dev.096537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavado A, Ware M, Pare J, Cao X. The tumor suppressor Nf2 regulates corpus callosum development by inhibiting the transcriptional coactivator Yap. Development. 2014;141:4182–4193. doi: 10.1242/dev.111260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JH, Kim TS, Yang TH, Koo BK, Oh SP, Lee KP, Oh HJ, Lee SH, Kong YY, Kim JM, et al. A crucial role of WW45 in developing epithelial tissues in the mouse. EMBO J. 2008;27:1231–1242. doi: 10.1038/emboj.2008.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KP, Lee JH, Kim TS, Kim TH, Park HD, Byun JS, Kim MC, Jeong WI, Calvisi DF, Kim JM, et al. The Hippo-Salvador pathway restrains hepatic oval cell proliferation, liver size, and liver tumorigenesis. Proc Natl Acad Sci U S A. 2010;107:8248–8253. doi: 10.1073/pnas.0912203107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei QY, Zhang H, Zhao B, Zha ZY, Bai F, Pei XH, Zhao S, Xiong Y, Guan KL. TAZ promotes cell proliferation and epithelial-mesenchymal transition and is inhibited by the hippo pathway. Mol Cell Biol. 2008;28:2426–2436. doi: 10.1128/MCB.01874-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Li S, Mana-Capelli S, Roth Flach RJ, Danai LV, Amcheslavsky A, Nie Y, Kaneko S, Yao X, Chen X, et al. The conserved misshapen-warts-Yorkie pathway acts in enteroblasts to regulate intestinal stem cells in Drosophila. Dev Cell. 2014;31:291–304. doi: 10.1016/j.devcel.2014.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li SQ, Shen D, Shao JY, Crowder R, Liu WB, Prat A, He XP, Liu SY, Hoog J, Lu C, et al. Endocrine-Therapy-Resistant ESR1 Variants Revealed by Genomic Characterization of Breast-Cancer-Derived Xenografts. Cell Rep. 2013;4:1116–1130. doi: 10.1016/j.celrep.2013.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lian I, Kim J, Okazawa H, Zhao J, Zhao B, Yu J, Chinnaiyan A, Israel MA, Goldstein LS, Abujarour R, et al. The role of YAP transcription coactivator in regulating stem cell self-renewal and differentiation. Genes Dev. 2010;24:1106–1118. doi: 10.1101/gad.1903310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang N, Zhang C, Dill P, Panasyuk G, Pion D, Koka V, Gallazzini M, Olson EN, Lam H, Henske EP, et al. Regulation of YAP by mTOR and autophagy reveals a therapeutic target of tuberous sclerosis complex. J Exp Med. 2014;211:2249–2263. doi: 10.1084/jem.20140341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin C, Yao E, Chuang PT. A conserved MST1/2-YAP axis mediates Hippo signaling during lung growth. Dev Biol. 2015a;403:101–113. doi: 10.1016/j.ydbio.2015.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin L, Sabnis AJ, Chan E, Olivas V, Cade L, Pazarentzos E, Asthana S, Neel D, Yan JJ, Lu X, et al. The Hippo effector YAP promotes resistance to RAF- and MEK-targeted cancer therapies. Nat Genet. 2015b;47:250–256. doi: 10.1038/ng.3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Z, von Gise A, Zhou P, Gu F, Ma Q, Jiang J, Yau AL, Buck JN, Gouin KA, van Gorp PR, et al. Cardiac-specific YAP activation improves cardiac function and survival in an experimental murine MI model. Circ Res. 2014;115:354–363. doi: 10.1161/CIRCRESAHA.115.303632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling C, Zheng Y, Yin F, Yu J, Huang J, Hong Y, Wu S, Pan D. The apical transmembrane protein Crumbs functions as a tumor suppressor that regulates Hippo signaling by binding to Expanded. Proc Natl Acad Sci U S A. 2010;107:10532–10537. doi: 10.1073/pnas.1004279107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu-Chittenden Y, Huang B, Shim JS, Chen Q, Lee SJ, Anders RA, Liu JO, Pan D. Genetic and pharmacological disruption of the TEAD-YAP complex suppresses the oncogenic activity of YAP. Genes Dev. 2012;26:1300–1305. doi: 10.1101/gad.192856.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu CY, Zha ZY, Zhou X, Zhang H, Huang W, Zhao D, Li T, Chan SW, Lim CJ, Hong W, et al. The hippo tumor pathway promotes TAZ degradation by phosphorylating a phosphodegron and recruiting the SCF{beta}-TrCP E3 ligase. J Biol Chem. 2010a;285:37159–37169. doi: 10.1074/jbc.M110.152942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu G, Yu FX, Kim YC, Meng Z, Naipauer J, Looney DJ, Liu X, Gutkind JS, Mesri EA, Guan KL. Kaposi sarcoma-associated herpesvirus promotes tumorigenesis by modulating the Hippo pathway. Oncogene. 2015;34:3536–3546. doi: 10.1038/onc.2014.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Xin Y, Ye F, Wang W, Lu Q, Kaplan HJ, Dean DC. Taz-tead1 links cell-cell contact to zeb1 expression, proliferation, and dedifferentiation in retinal pigment epithelial cells. Invest Ophthalmol Vis Sci. 2010b;51:3372–3378. doi: 10.1167/iovs.09-4321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorthongpanich C, Messerschmidt DM, Chan SW, Hong W, Knowles BB, Solter D. Temporal reduction of LATS kinases in the early preimplantation embryo prevents ICM lineage differentiation. Genes Dev. 2013;27:1441–1446. doi: 10.1101/gad.219618.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L, Li Y, Kim SM, Bossuyt W, Liu P, Qiu Q, Wang Y, Halder G, Finegold MJ, Lee JS, et al. Hippo signaling is a potent in vivo growth and tumor suppressor pathway in the mammalian liver. Proc Natl Acad Sci U S A. 2010;107:1437–1442. doi: 10.1073/pnas.0911427107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma B, Chen Y, Chen L, Cheng H, Mu C, Li J, Gao R, Zhou C, Cao L, Liu J, et al. Hypoxia regulates Hippo signalling through the SIAH2 ubiquitin E3 ligase. Nat Cell Biol. 2015;17:95–103. doi: 10.1038/ncb3073. [DOI] [PubMed] [Google Scholar]
- Mahoney JE, Mori M, Szymaniak AD, Varelas X, Cardoso WV. The hippo pathway effector Yap controls patterning and differentiation of airway epithelial progenitors. Dev Cell. 2014;30:137–150. doi: 10.1016/j.devcel.2014.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makita R, Uchijima Y, Nishiyama K, Amano T, Chen Q, Takeuchi T, Mitani A, Nagase T, Yatomi Y, Aburatani H, et al. Multiple renal cysts, urinary concentration defects, and pulmonary emphysematous changes in mice lacking TAZ. Am J Physiol Renal Physiol. 2008;294:F542–553. doi: 10.1152/ajprenal.00201.2007. [DOI] [PubMed] [Google Scholar]
- Matakatsu H, Blair SS. Separating planar cell polarity and Hippo pathway activities of the protocadherins Fat and Dachsous. Development. 2012;139:1498–1508. doi: 10.1242/dev.070367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClatchey AI, Saotome I, Mercer K, Crowley D, Gusella JF, Bronson RT, Jacks T. Mice heterozygous for a mutation at the Nf2 tumor suppressor locus develop a range of highly metastatic tumors. Gene Dev. 1998;12:1121–1133. doi: 10.1101/gad.12.8.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mi W, Lin Q, Childress C, Sudol M, Robishaw J, Berlot CH, Shabahang M, Yang W. Geranylgeranylation signals to the Hippo pathway for breast cancer cell proliferation and migration. Oncogene. 2015;34:3095–3106. doi: 10.1038/onc.2014.251. [DOI] [PubMed] [Google Scholar]
- Miller E, Yang J, Deran M, Wu C, Su AI, Bonamy GM, Liu J, Peters EC, Wu X. Identification of Serum-Derived Sphingosine-1-Phosphate as a Small Molecule Regulator of YAP. Chem Biol. 2012;19:955–962. doi: 10.1016/j.chembiol.2012.07.005. [DOI] [PubMed] [Google Scholar]
- Mitani A, Nagase T, Fukuchi K, Aburatani H, Makita R, Kurihara H. Transcriptional coactivator with PDZ-binding motif is essential for normal alveolarization in mice. Am J Respir Crit Care Med. 2009;180:326–338. doi: 10.1164/rccm.200812-1827OC. [DOI] [PubMed] [Google Scholar]
- Miyanaga A, Masuda M, Tsuta K, Kawasaki K, Nakamura Y, Sakuma T, Asamura H, Gemma A, Yamada T. Hippo pathway gene mutations in malignant mesothelioma: revealed by RNA and targeted exon sequencing. J Thorac Oncol. 2015;10:844–851. doi: 10.1097/JTO.0000000000000493. [DOI] [PubMed] [Google Scholar]
- Mo JS, Meng Z, Kim YC, Park HW, Hansen CG, Kim S, Lim DS, Guan KL. Cellular energy stress induces AMPK-mediated regulation of YAP and the Hippo pathway. Nat Cell Biol. 2015;17:500–510. doi: 10.1038/ncb3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mo JS, Yu FX, Gong R, Brown JH, Guan KL. Regulation of the Hippo-YAP pathway by protease activated receptor PAR. Genes Dev. 2012;29:2138–2143. doi: 10.1101/gad.197582.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohseni M, Sun J, Lau A, Curtis S, Goldsmith J, Fox VL, Wei C, Frazier M, Samson O, Wong KK, et al. A genetic screen identifies an LKB1-MARK signalling axis controlling the Hippo-YAP pathway. Nat Cell Biol. 2014;16:108–117. doi: 10.1038/ncb2884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori M, Triboulet R, Mohseni M, Schlegelmilch K, Shrestha K, Camargo FD, Gregory RI. Hippo signaling regulates microprocessor and links cell-density-dependent miRNA biogenesis to cancer. Cell. 2014;156:893–906. doi: 10.1016/j.cell.2013.12.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morin-Kensicki EM, Boone BN, Howell M, Stonebraker JR, Teed J, Alb JG, Magnuson TR, O'Neal W, Milgram SL. Defects in yolk sac vasculogenesis, chorioallantoic fusion, and embryonic axis elongation in mice with targeted disruption of Yap65. Mol Cell Biol. 2006;26:77–87. doi: 10.1128/MCB.26.1.77-87.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moroishi T, Hansen CG, Guan KL. The emerging roles of YAP and TAZ in cancer. Nat Rev Cancer. 2015a;15:73–79. doi: 10.1038/nrc3876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moroishi T, Park HW, Qin B, Chen Q, Meng Z, Plouffe SW, Taniguchi K, Yu FX, Karin M, Pan D, et al. A YAP/TAZ-induced feedback mechanism regulates Hippo pathway homeostasis. Genes Dev. 2015b;29:1271–1284. doi: 10.1101/gad.262816.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami H, Mizuno T, Taniguchi T, Fujii M, Ishiguro F, Fukui T, Akatsuka S, Horio Y, Hida T, Kondo Y, et al. LATS2 is a tumor suppressor gene of malignant mesothelioma. Cancer Res. 2011;71:873–883. doi: 10.1158/0008-5472.CAN-10-2164. [DOI] [PubMed] [Google Scholar]
- Nishio M, Hamada K, Kawahara K, Sasaki M, Noguchi F, Chiba S, Mizuno K, Suzuki SO, Dong Y, Tokuda M, et al. Cancer susceptibility and embryonic lethality in Mob1a/1b double-mutant mice. J Clin Invest. 2012;122:4505–4518. doi: 10.1172/JCI63735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishioka N, Inoue K, Adachi K, Kiyonari H, Ota M, Ralston A, Yabuta N, Hirahara S, Stephenson RO, Ogonuki N, et al. The Hippo signaling pathway components Lats and Yap pattern Tead4 activity to distinguish mouse trophectoderm from inner cell mass. Dev Cell. 2009;16:398–410. doi: 10.1016/j.devcel.2009.02.003. [DOI] [PubMed] [Google Scholar]
- Nolo R, Morrison CM, Tao C, Zhang X, Halder G. The bantam microRNA is a target of the hippo tumor-suppressor pathway. Curr Biol. 2006;16:1895–1904. doi: 10.1016/j.cub.2006.08.057. [DOI] [PubMed] [Google Scholar]
- Oh H, Irvine KD. In vivo regulation of Yorkie phosphorylation and localization. Development. 2008;135:1081–1088. doi: 10.1242/dev.015255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh H, Slattery M, Ma L, Crofts A, White KP, Mann RS, Irvine KD. Genome-wide association of Yorkie with chromatin and chromatin-remodeling complexes. Cell Rep. 2013;3:309–318. doi: 10.1016/j.celrep.2013.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ota M, Sasaki H. Mammalian Tead proteins regulate cell proliferation and contact inhibition as transcriptional mediators of Hippo signaling. Development. 2008;135:4059–4069. doi: 10.1242/dev.027151. [DOI] [PubMed] [Google Scholar]
- Overholtzer M, Zhang J, Smolen GA, Muir B, Li W, Sgroi DC, Deng CX, Brugge JS, Haber DA. Transforming properties of YAP, a candidate oncogene on the chromosome 11q22 amplicon. Proc Natl Acad Sci U S A. 2006;103:12405–12410. doi: 10.1073/pnas.0605579103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pajtler KW, Witt H, Sill M, Jones DTW, Hovestadt V, Kratochwil F, Wani K, Tatevossian R, Punchihewa C, Johann P, et al. Molecular Classification of Ependymal Tumors across All CNS Compartments, Histopathological Grades, and Age Groups. Cancer Cell. 2015;27:728–743. doi: 10.1016/j.ccell.2015.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan D. The hippo signaling pathway in development and cancer. Dev Cell. 2010;19:491–505. doi: 10.1016/j.devcel.2010.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantalacci S, Tapon N, Leopold P. The Salvador partner Hippo promotes apoptosis and cell-cycle exit in Drosophila. Nat Cell Biol. 2003;5:921–927. doi: 10.1038/ncb1051. [DOI] [PubMed] [Google Scholar]
- Park HW, Guan KL. Regulation of the Hippo pathway and implications for anticancer drug development. Trends Pharmacol Sci. 2013;34:581–589. doi: 10.1016/j.tips.2013.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park HW, Kim YC, Yu B, Moroishi T, Mo JS, Plouffe SW, Meng Z, Lin KC, Yu FX, Alexander CM, et al. Alternative Wnt Signaling Activates YAP/TAZ. Cell. 2015;162:780–794. doi: 10.1016/j.cell.2015.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker M, Mohankumar KM, Punchihewa C, Weinlich R, Dalton JD, Li Y, Lee R, Tatevossian RG, Phoenix TN, Thiruvenkatam R, et al. C11orf95-RELA fusions drive oncogenic NF-kappaB signalling in ependymoma. Nature. 2014;506:451–455. doi: 10.1038/nature13109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poon CL, Lin JI, Zhang X, Harvey KF. The sterile 20-like kinase Tao-1 controls tissue growth by regulating the Salvador-Warts-Hippo pathway. Dev Cell. 2011;21:896–906. doi: 10.1016/j.devcel.2011.09.012. [DOI] [PubMed] [Google Scholar]
- Porazinski S, Wang H, Asaoka Y, Behrndt M, Miyamoto T, Morita H, Hata S, Sasaki T, Krens SF, Osada Y, et al. YAP is essential for tissue tension to ensure vertebrate 3D body shape. Nature. 2015;521:217–221. doi: 10.1038/nature14215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Praskova M, Khoklatchev A, Ortiz-Vega S, Avruch J. Regulation of the MST1 kinase by autophosphorylation, by the growth inhibitory proteins, RASSF1 and NORE1, and by Ras. Biochem J. 2004;381:453–462. doi: 10.1042/BJ20040025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qing Y, Yin F, Wang W, Zheng Y, Guo P, Schozer F, Deng H, Pan D. The Hippo effector Yorkie activates transcription by interacting with a histone methyltransferase complex through Ncoa6. Elife. 2014;3:e02564. doi: 10.7554/eLife.02564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauskolb C, Sun S, Sun G, Pan Y, Irvine KD. Cytoskeletal tension inhibits Hippo signaling through an Ajuba-Warts complex. Cell. 2014;158:143–156. doi: 10.1016/j.cell.2014.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy BV, Irvine KD. Regulation of Hippo signaling by EGFR-MAPK signaling through Ajuba family proteins. Dev Cell. 2013;24:459–471. doi: 10.1016/j.devcel.2013.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reginensi A, Scott RP, Gregorieff A, Bagherie-Lachidan M, Chung C, Lim DS, Pawson T, Wrana J, McNeill H. Yap- and Cdc42-dependent nephrogenesis and morphogenesis during mouse kidney development. PLoS Genet. 2013;9:e1003380. doi: 10.1371/journal.pgen.1003380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren F, Wang B, Yue T, Yun EY, Ip YT, Jiang J. Hippo signaling regulates Drosophila intestine stem cell proliferation through multiple pathways. Proc Natl Acad Sci U S A. 2010;107:21064–21069. doi: 10.1073/pnas.1012759107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson BS, Huang J, Hong Y, Moberg KH. Crumbs regulates Salvador/Warts/Hippo signaling in Drosophila via the FERM-domain protein Expanded. Curr Biol. 2010;20:582–590. doi: 10.1016/j.cub.2010.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouleau GA, Merel P, Lutchman M, Sanson M, Zucman J, Marineau C, Hoangxuan K, Demczuk S, Desmaze C, Plougastel B, et al. Alteration in a New Gene Encoding a Putative Membrane-Organizing Protein Causes Neurofibromatosis Type-2. Nature. 1993;363:515–521. doi: 10.1038/363515a0. [DOI] [PubMed] [Google Scholar]
- Sansores-Garcia L, Bossuyt W, Wada K, Yonemura S, Tao C, Sasaki H, Halder G. Modulating F-actin organization induces organ growth by affecting the Hippo pathway. EMBO J. 2011;30:2325–2335. doi: 10.1038/emboj.2011.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki H. Position- and polarity-dependent Hippo signaling regulates cell fates in preimplantation mouse embryos. Semin Cell Dev Biol. 2015 doi: 10.1016/j.semcdb.2015.05.003. doi:10.1016/j.semcdb.2015.1005.1003. [DOI] [PubMed] [Google Scholar]
- Schlegelmilch K, Mohseni M, Kirak O, Pruszak J, Rodriguez JR, Zhou D, Kreger BT, Vasioukhin V, Avruch J, Brummelkamp TR, et al. Yap1 acts downstream of alpha-catenin to control epidermal proliferation. Cell. 2011;144:782–795. doi: 10.1016/j.cell.2011.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrauwen I, Szelinger S, Siniard AL, Corneveaux JJ, Kurdoglu A, Richholt R, De Both M, Malenica I, Swaminathan S, Rangasamy S, et al. A De Novo Mutation in TEAD1 Causes Non-X-Linked Aicardi Syndrome. Invest Ophthalmol Vis Sci. 2015;56:3896–3904. doi: 10.1167/iovs.14-16261. [DOI] [PubMed] [Google Scholar]
- Sekido Y. Inactivation of Merlin in malignant mesothelioma cells and the Hippo signaling cascade dysregulation. Pathol Int. 2011;61:331–344. doi: 10.1111/j.1440-1827.2011.02666.x. [DOI] [PubMed] [Google Scholar]
- Shao DD, Xue W, Krall EB, Bhutkar A, Piccioni F, Wang X, Schinzel AC, Sood S, Rosenbluh J, Kim JW, et al. KRAS and YAP1 converge to regulate EMT and tumor survival. Cell. 2014;158:171–184. doi: 10.1016/j.cell.2014.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma P, McNeill H. Fat and Dachsous cadherins. Prog Mol Biol Transl Sci. 2013;116:215–235. doi: 10.1016/B978-0-12-394311-8.00010-8. [DOI] [PubMed] [Google Scholar]
- Shaw RL, Kohlmaier A, Polesello C, Veelken C, Edgar BA, Tapon N. The Hippo pathway regulates intestinal stem cell proliferation during Drosophila adult midgut regeneration. Development. 2010;137:4147–4158. doi: 10.1242/dev.052506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen S, Guo X, Yan H, Lu Y, Ji X, Li L, Liang T, Zhou D, Feng XH, Zhao JC, et al. A miR-130a-YAP positive feedback loop promotes organ size and tumorigenesis. Cell Res. 2015;25:997–1012. doi: 10.1038/cr.2015.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva E, Tsatskis Y, Gardano L, Tapon N, McNeill H. The tumor-suppressor gene fat controls tissue growth upstream of expanded in the hippo signaling pathway. Curr Biol. 2006;16:2081–2089. doi: 10.1016/j.cub.2006.09.004. [DOI] [PubMed] [Google Scholar]
- Skibinski A, Breindel JL, Prat A, Galvan P, Smith E, Rolfs A, Gupta PB, Labaer J, Kuperwasser C. The Hippo transducer TAZ interacts with the SWI/SNF complex to regulate breast epithelial lineage commitment. Cell Rep. 2014;6:1059–1072. doi: 10.1016/j.celrep.2014.02.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song H, Mak KK, Topol L, Yun K, Hu J, Garrett L, Chen Y, Park O, Chang J, Simpson RM, et al. Mammalian Mst1 and Mst2 kinases play essential roles in organ size control and tumor suppression. Proc Natl Acad Sci U S A. 2010;107:1431–1436. doi: 10.1073/pnas.0911409107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Y, Li L, Ou Y, Gao Z, Li E, Li X, Zhang W, Wang J, Xu L, Zhou Y, et al. Identification of genomic alterations in oesophageal squamous cell cancer. Nature. 2014;509:91–95. doi: 10.1038/nature13176. [DOI] [PubMed] [Google Scholar]
- Sorrentino G, Ruggeri N, Specchia V, Cordenonsi M, Mano M, Dupont S, Manfrin A, Ingallina E, Sommaggio R, Piazza S, et al. Metabolic control of YAP and TAZ by the mevalonate pathway. Nat Cell Biol. 2014;16:357–366. doi: 10.1038/ncb2936. [DOI] [PubMed] [Google Scholar]
- Stein C, Bardet AF, Roma G, Bergling S, Clay I, Ruchti A, Agarinis C, Schmelzle T, Bouwmeester T, Schubeler D, et al. YAP1 Exerts Its Transcriptional Control via TEAD-Mediated Activation of Enhancers. PLoS Genet 11, e1005465. 2015 doi: 10.1371/journal.pgen.1005465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinhardt AA, Gayyed MF, Klein AP, Dong J, Maitra A, Pan D, Montgomery EA, Anders RA. Expression of Yes-associated protein in common solid tumors. Hum Pathol. 2008;39:1582–1589. doi: 10.1016/j.humpath.2008.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strassburger K, Tiebe M, Pinna F, Breuhahn K, Teleman AA. Insulin/IGF signaling drives cell proliferation in part via Yorkie/YAP. Dev Biol. 2012;367:187–196. doi: 10.1016/j.ydbio.2012.05.008. [DOI] [PubMed] [Google Scholar]
- Su T, Bondar T, Zhou X, Zhang C, He H, Medzhitov R. Two-signal requirement for growth-promoting function of Yap in hepatocytes. Elife. 2015;4:e02948. doi: 10.7554/eLife.02948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanas MR, Sboner A, Oliveira AM, Erickson-Johnson MR, Hespelt J, Hanwright PJ, Flanagan J, Luo Y, Fenwick K, Natrajan R, et al. Identification of a disease-defining gene fusion in epithelioid hemangioendothelioma. Sci Transl Med. 2011;3:98ra82. doi: 10.1126/scitranslmed.3002409. [DOI] [PubMed] [Google Scholar]
- Tapon N, Harvey KF, Bell DW, Wahrer DC, Schiripo TA, Haber DA, Hariharan IK. salvador Promotes both cell cycle exit and apoptosis in Drosophila and is mutated in human cancer cell lines. Cell. 2002;110:467–478. doi: 10.1016/s0092-8674(02)00824-3. [DOI] [PubMed] [Google Scholar]
- Thompson BJ, Cohen SM. The Hippo pathway regulates the bantam microRNA to control cell proliferation and apoptosis in Drosophila. Cell. 2006;126:767–774. doi: 10.1016/j.cell.2006.07.013. [DOI] [PubMed] [Google Scholar]
- Tian Y, Kolb R, Hong JH, Carroll J, Li D, You J, Bronson R, Yaffe MB, Zhou J, Benjamin T. TAZ promotes PC2 degradation through a SCFbeta-Trcp E3 ligase complex. Mol Cell Biol. 2007;27:6383–6395. doi: 10.1128/MCB.00254-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touil Y, Igoudjil W, Corvaisier M, Dessein AF, Vandomme J, Monte D, Stechly L, Skrypek N, Langlois C, Grard G, et al. Colon Cancer Cells Escape 5FU Chemotherapy-Induced Cell Death by Entering Stemness and Quiescence Associated with the c-Yes/YAP Axis. Clin Cancer Res. 2014;20:837–846. doi: 10.1158/1078-0432.CCR-13-1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler DM, Baker NE. Expanded and fat regulate growth and differentiation in the Drosophila eye through multiple signaling pathways. Dev Biol. 2007;305:187–201. doi: 10.1016/j.ydbio.2007.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udan RS, Kango-Singh M, Nolo R, Tao C, Halder G. Hippo promotes proliferation arrest and apoptosis in the Salvador/Warts pathway. Nat Cell Biol. 2003;5:914–920. doi: 10.1038/ncb1050. [DOI] [PubMed] [Google Scholar]
- Van Raamsdonk CD, Bezrookove V, Green G, Bauer J, Gaugler L, O'Brien JM, Simpson EM, Barsh GS, Bastian BC. Frequent somatic mutations of GNAQ in uveal melanoma and blue naevi. Nature. 2009;457:599–602. doi: 10.1038/nature07586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Raamsdonk CD, Griewank KG, Crosby MB, Garrido MC, Vemula S, Wiesner T, Obenauf AC, Wackernagel W, Green G, Bouvier N, et al. Mutations in GNA11 in uveal melanoma. N Engl J Med. 2010;363:2191–2199. doi: 10.1056/NEJMoa1000584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varelas X. The Hippo pathway effectors TAZ and YAP in development, homeostasis and disease. Development. 2014;141:1614–1626. doi: 10.1242/dev.102376. [DOI] [PubMed] [Google Scholar]
- von Gise A, Lin Z, Schlegelmilch K, Honor LB, Pan GM, Buck JN, Ma Q, Ishiwata T, Zhou B, Camargo FD, et al. YAP1, the nuclear target of Hippo signaling, stimulates heart growth through cardiomyocyte proliferation but not hypertrophy. Proc Natl Acad Sci U S A. 2012;109:2394–2399. doi: 10.1073/pnas.1116136109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada K, Itoga K, Okano T, Yonemura S, Sasaki H. Hippo pathway regulation by cell morphology and stress fibers. Development. 2011;138:3907–3914. doi: 10.1242/dev.070987. [DOI] [PubMed] [Google Scholar]
- Wang W, Xiao ZD, Li X, Aziz KE, Gan B, Johnson RL, Chen J. AMPK modulates Hippo pathway activity to regulate energy homeostasis. Nat Cell Biol. 2015;17:490–499. doi: 10.1038/ncb3113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Wu Y, Wang H, Zhang Y, Mei L, Fang X, Zhang X, Zhang F, Chen H, Liu Y, et al. Interplay of mevalonate and Hippo pathways regulates RHAMM transcription via YAP to modulate breast cancer cell motility. Proc Natl Acad Sci U S A. 2014;111:E89–98. doi: 10.1073/pnas.1319190110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watt KI, Turner BJ, Hagg A, Zhang X, Davey JR, Qian H, Beyer C, Winbanks CE, Harvey KF, Gregorevic P. The Hippo pathway effector YAP is a critical regulator of skeletal muscle fibre size. Nat Commun. 2015;6:6048. doi: 10.1038/ncomms7048. [DOI] [PubMed] [Google Scholar]
- Wehr MC, Holder MV, Gailite I, Saunders RE, Maile TM, Ciirdaeva E, Instrell R, Jiang M, Howell M, Rossner MJ, et al. Salt-inducible kinases regulate growth through the Hippo signalling pathway in Drosophila. Nat Cell Biol. 2013;15:61–71. doi: 10.1038/ncb2658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wennmann DO, Vollenbroker B, Eckart AK, Bonse J, Erdmann F, Wolters DA, Schenk LK, Schulze U, Kremerskothen J, Weide T, et al. The Hippo pathway is controlled by Angiotensin II signaling and its reactivation induces apoptosis in podocytes. Cell Death Dis. 2014;5:e1519. doi: 10.1038/cddis.2014.476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willecke M, Hamaratoglu F, Kango-Singh M, Udan R, Chen CL, Tao C, Zhang X, Halder G. The fat cadherin acts through the hippo tumor-suppressor pathway to regulate tissue size. Curr Biol. 2006;16:2090–2100. doi: 10.1016/j.cub.2006.09.005. [DOI] [PubMed] [Google Scholar]
- Williamson KA, Rainger J, Floyd JA, Ansari M, Meynert A, Aldridge KV, Rainger JK, Anderson CA, Moore AT, Hurles ME, et al. Heterozygous loss-of-function mutations in YAP1 cause both isolated and syndromic optic fissure closure defects. Am J Hum Genet. 2014;94:295–302. doi: 10.1016/j.ajhg.2014.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong KK, Li W, An Y, Duan Y, Li Z, Kang Y, Yan Y. beta-Spectrin regulates the hippo signaling pathway and modulates the basal actin network. J Biol Chem. 2015;290:6397–6407. doi: 10.1074/jbc.M114.629493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H, Xiao Y, Zhang S, Ji S, Wei L, Fan F, Geng J, Tian J, Sun X, Qin F, et al. The Ets transcription factor GABP is a component of the hippo pathway essential for growth and antioxidant defense. Cell Rep. 2013;3:1663–1677. doi: 10.1016/j.celrep.2013.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S, Huang J, Dong J, Pan D. hippo encodes a Ste-20 family protein kinase that restricts cell proliferation and promotes apoptosis in conjunction with salvador and warts. Cell. 2003;114:445–456. doi: 10.1016/s0092-8674(03)00549-x. [DOI] [PubMed] [Google Scholar]
- Wu S, Liu Y, Zheng Y, Dong J, Pan D. The TEAD/TEF family protein Scalloped mediates transcriptional output of the Hippo growth-regulatory pathway. Dev Cell. 2008;14:388–398. doi: 10.1016/j.devcel.2008.01.007. [DOI] [PubMed] [Google Scholar]
- Xiang L, Gilkes DM, Hu H, Takano N, Luo W, Lu H, Bullen JW, Samanta D, Liang H, Semenza GL. Hypoxia-inducible factor 1 mediates TAZ expression and nuclear localization to induce the breast cancer stem cell phenotype. Oncotarget. 2014;5:12509–12527. doi: 10.18632/oncotarget.2997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao GH, Chernoff J, Testa JR. NF2: The wizardry of Merlin. Gene Chromosome Canc. 2003;38:389–399. doi: 10.1002/gcc.10282. [DOI] [PubMed] [Google Scholar]
- Xin M, Kim Y, Sutherland LB, Murakami M, Qi X, McAnally J, Porrello ER, Mahmoud AI, Tan W, Shelton JM, et al. Hippo pathway effector Yap promotes cardiac regeneration. Proc Natl Acad Sci U S A. 2013;110:13839–13844. doi: 10.1073/pnas.1313192110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin M, Kim Y, Sutherland LB, Qi X, McAnally J, Schwartz RJ, Richardson JA, Bassel-Duby R, Olson EN. Regulation of insulin-like growth factor signaling by Yap governs cardiomyocyte proliferation and embryonic heart size. Sci Signal. 2011;4:ra70. doi: 10.1126/scisignal.2002278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu T, Wang W, Zhang S, Stewart RA, Yu W. Identifying tumor suppressors in genetic mosaics: the Drosophila lats gene encodes a putative protein kinase. Development. 1995;121:1053–1063. doi: 10.1242/dev.121.4.1053. [DOI] [PubMed] [Google Scholar]
- Yimlamai D, Christodoulou C, Galli GG, Yanger K, Pepe-Mooney B, Gurung B, Shrestha K, Cahan P, Stanger BZ, Camargo FD. Hippo pathway activity influences liver cell fate. Cell. 2014;157:1324–1338. doi: 10.1016/j.cell.2014.03.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin F, Yu J, Zheng Y, Chen Q, Zhang N, Pan D. Spatial organization of Hippo signaling at the plasma membrane mediated by the tumor suppressor Merlin/NF2. Cell. 2013;154:1342–1355. doi: 10.1016/j.cell.2013.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu FX, Guan KL. The Hippo pathway: regulators and regulations. Gene Dev. 2013;27:355–371. doi: 10.1101/gad.210773.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu FX, Luo J, Mo JS, Liu GB, Kim YC, Meng ZP, Zhao L, Peyman G, Ouyang H, Jiang W, et al. Mutant Gq/11 Promote Uveal Melanoma Tumorigenesis by Activating YAP. Cancer Cell. 2014;25:822–830. doi: 10.1016/j.ccr.2014.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu FX, Meng Z, Plouffe SW, Guan KL. Hippo pathway regulation of gastrointestinal tissues. Annu Rev Physiol. 2015;77:201–227. doi: 10.1146/annurev-physiol-021014-071733. [DOI] [PubMed] [Google Scholar]
- Yu FX, Zhang Y, Park HW, Jewell JL, Chen Q, Deng Y, Pan D, Taylor SS, Lai ZC, Guan KL. Protein kinase A activates the Hippo pathway to modulate cell proliferation and differentiation. Gene Dev. 2013a;27:1223–1232. doi: 10.1101/gad.219402.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu FX, Zhao B, Panupinthu N, Jewell JL, Lian I, Wang LH, Zhao J, Yuan H, Tumaneng K, Li H, et al. Regulation of the Hippo-YAP pathway by G-protein-coupled receptor signaling. Cell. 2012;150:780–791. doi: 10.1016/j.cell.2012.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J, Zheng Y, Dong J, Klusza S, Deng WM, Pan D. Kibra functions as a tumor suppressor protein that regulates Hippo signaling in conjunction with Merlin and Expanded. Dev Cell. 2010;18:288–299. doi: 10.1016/j.devcel.2009.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu T, Bachman J, Lai ZC. Evidence for a tumor suppressor role for the large tumor suppressor genes LATS1 and LATS2 in human cancer. Genetics. 2013b;195:1193–1196. doi: 10.1534/genetics.113.156372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue T, Tian A, Jiang J. The cell adhesion molecule echinoid functions as a tumor suppressor and upstream regulator of the Hippo signaling pathway. Dev Cell. 2012;22:255–267. doi: 10.1016/j.devcel.2011.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanconato F, Forcato M, Battilana G, Azzolin L, Quaranta E, Bodega B, Rosato A, Bicciato S, Cordenonsi M, Piccolo S. Genome-wide association between YAP/TAZ/TEAD and AP-1 at enhancers drives oncogenic growth. Nat Cell Biol. 2015;17:1218–1227. doi: 10.1038/ncb3216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zender L, Spector MS, Xue W, Flemming P, Cordon-Cardo C, Silke J, Fan ST, Luk JM, Wigler M, Hannon GJ, et al. Identification and validation of oncogenes in liver cancer using an integrative oncogenomic approach. Cell. 2006;125:1253–1267. doi: 10.1016/j.cell.2006.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Pasolli HA, Fuchs E. Yes-associated protein (YAP) transcriptional coactivator functions in balancing growth and differentiation in skin. Proc Natl Acad Sci U S A. 2011;108:2270–2275. doi: 10.1073/pnas.1019603108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Ren F, Zhang Q, Chen Y, Wang B, Jiang J. The TEAD/TEF family of transcription factor Scalloped mediates Hippo signaling in organ size control. Dev Cell. 2008;14:377–387. doi: 10.1016/j.devcel.2008.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang N, Bai H, David KK, Dong J, Zheng Y, Cai J, Giovannini M, Liu P, Anders RA, Pan D. The Merlin/NF2 tumor suppressor functions through the YAP oncoprotein to regulate tissue homeostasis in mammals. Dev Cell. 2010;19:27–38. doi: 10.1016/j.devcel.2010.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Nandakumar N, Shi Y, Manzano M, Smith A, Graham G, Gupta S, Vietsch EE, Laughlin SZ, Wadhwa M, et al. Downstream of mutant KRAS, the transcription regulator YAP is essential for neoplastic progression to pancreatic ductal adenocarcinoma. Sci Signal. 2014a;7:ra42. doi: 10.1126/scisignal.2005049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang WJ, Gao YJ, Li PX, Shi ZB, Guo T, Li F, Han XK, Feng Y, Zheng C, Wang ZY, et al. VGLL4 functions as a new tumor suppressor in lung cancer by negatively regulating the YAP-TEAD transcriptional complex. Cell Res. 2014b;24:331–343. doi: 10.1038/cr.2014.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao B, Li L, Tumaneng K, Wang CY, Guan KL. A coordinated phosphorylation by Lats and CK1 regulates YAP stability through SCF(beta-TRCP) Genes Dev. 2010;24:72–85. doi: 10.1101/gad.1843810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao B, Li L, Wang L, Wang CY, Yu J, Guan KL. Cell detachment activates the Hippo pathway via cytoskeleton reorganization to induce anoikis. Genes Dev. 2012;26:54–68. doi: 10.1101/gad.173435.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao B, Wei X, Li W, Udan RS, Yang Q, Kim J, Xie J, Ikenoue T, Yu J, Li L, et al. Inactivation of YAP oncoprotein by the Hippo pathway is involved in cell contact inhibition and tissue growth control. Genes Dev. 2007;21:2747–2761. doi: 10.1101/gad.1602907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao B, Ye X, Yu J, Li L, Li W, Li S, Yu J, Lin JD, Wang CY, Chinnaiyan AM, et al. TEAD mediates YAP-dependent gene induction and growth control. Genes Dev. 2008;22:1962–1971. doi: 10.1101/gad.1664408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou D, Conrad C, Xia F, Park JS, Payer B, Yin Y, Lauwers GY, Thasler W, Lee JT, Avruch J, et al. Mst1 and Mst2 maintain hepatocyte quiescence and suppress hepatocellular carcinoma development through inactivation of the Yap1 oncogene. Cancer Cell. 2009;16:425–438. doi: 10.1016/j.ccr.2009.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou D, Zhang Y, Wu H, Barry E, Yin Y, Lawrence E, Dawson D, Willis JE, Markowitz SD, Camargo FD, et al. Mst1 and Mst2 protein kinases restrain intestinal stem cell proliferation and colonic tumorigenesis by inhibition of Yes-associated protein (Yap) overabundance. Proc Natl Acad Sci U S A. 2011;108:E1312–1320. doi: 10.1073/pnas.1110428108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, Wang S, Wang Z, Feng X, Liu P, Lv XB, Li F, Yu FX, Sun Y, Yuan H, et al. Estrogen regulates Hippo signaling via GPER in breast cancer. J Clin Invest. 2015;125:2123–2135. doi: 10.1172/JCI79573. [DOI] [PMC free article] [PubMed] [Google Scholar]