Abstract
Desmoplastic small round cell tumor is a rare, aggressive tumor primarily affecting young males. It is considered a childhood cancer, and is characterized by a unique chromosomal translocation which leads to failure to suppress tumor growth. It is classified as a soft tissue sarcoma, sharing some features with other small round cell tumors such as Ewing’s Sarcoma and primitive neuroectodermal tumor. Typical imaging findings include multiple heterogeneous, lobular abdominal masses, which can grow very large. Often there is a dominant mass with additional peritoneal, omental, retroperitoneal and retrovesical masses. Prognosis is relatively poor with a 3 year survival rate of 50% in those treated aggressively with surgical resection, chemotherapy, and radiation therapy. The clinical presentation, imaging characteristics and pathology are discussed in regards to a recent case.
Keywords: soft tissue sarcoma, abdominal mass, chromosomal translocation, desmoplastic small round cell tumor, round cell tumor
CASE REPORT
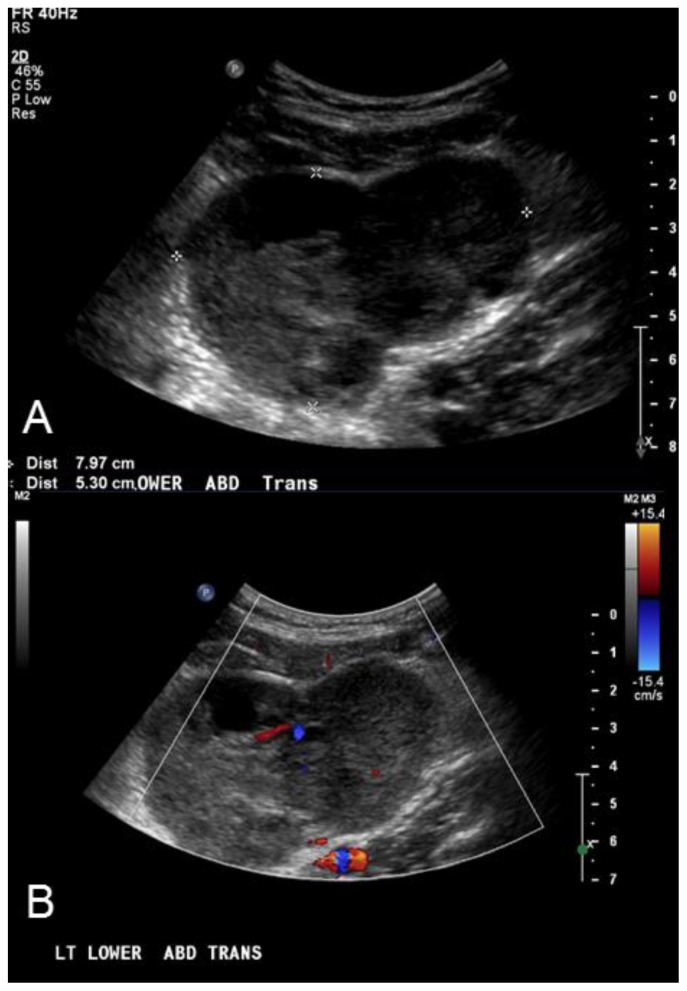
A 27 year old male presented to the emergency room with chief complaints of abdominal pain and palpable abdominal mass just to the left of his umbilicus for one week. Clinically, hernia was suspected and the patient underwent an ultrasound (US). Ultrasound demonstrated no evidence of hernia. A heterogeneous 8 × 5.3 × 6.6 cm lobular mass was detected (Figure 1,1B) and the patient was referred for further evaluation by computed tomography (CT). CT demonstrated 5 hypodense, lobular, intraperitoneal and retroperitoneal masses as well as a transient small bowel intussusception (Figures 2,3,4,5,6). The largest was a soft tissue density mass measuring 10 × 5.4 × 11.1 cm in the left lower quadrant, abutting but not obstructing several small bowel loops. A second mass in the retroperitoneum measured up 2.4 cm in length and encased the splenic artery, without evidence for thrombosis. Retroperitoneal lymphadenopathy was also noted in left para-aortic lymph nodes measuring 3.1 × 1.9 cm. The primary initial differential diagnosis was lymphoma or metastatic disease, so fluorodeoxyglucose positron emission tomography/computed tomography (FDG PET/CT) was performed. These masses were hypermetabolic on FDG PET/CT with a maximal SUV measurement of 10.2 (Figure 7). Following an uncomplicated CT-guided biopsy of the largest mass in the left lower quadrant, pathologic evaluation including histology, immunohistochemical and cytogenetic analysis was performed. Histology revealed small nests of poorly differentiated malignant cells having relatively high nuclear cytoplasmic ratios set in a desmoplastic stromal background (Figure 8). Immunohistochemical stains revealed positivity for cytokeratin CAM 5.2, AE1.3, CK7 and desmin (Figures 9a,9b,9c,9d). Cytogenetic analysis demonstrated an abnormal cell line positive for EWSRI. The pathologic findings were consistent with a desmoplastic small round cell tumor (DSRCT).
Figure 1.
27 year old male with Desmoplastic Small Round Cell Tumor (DSRCT).
Findings: A) Ultrasound imaging of the abdomen demonstrates a midline heterogeneous, hypoechoic, lobular 8 × 5.3 × 6.6 cm mass (outlined by white cursors). B) Color Doppler ultrasound of the mass in the left lower abdomen demonstrates the present of flow within the hypoechoic mass, indicating a solid lesion.
Technique: Abdominal Ultrasound with Curvilinear 5-2 MHz probe.
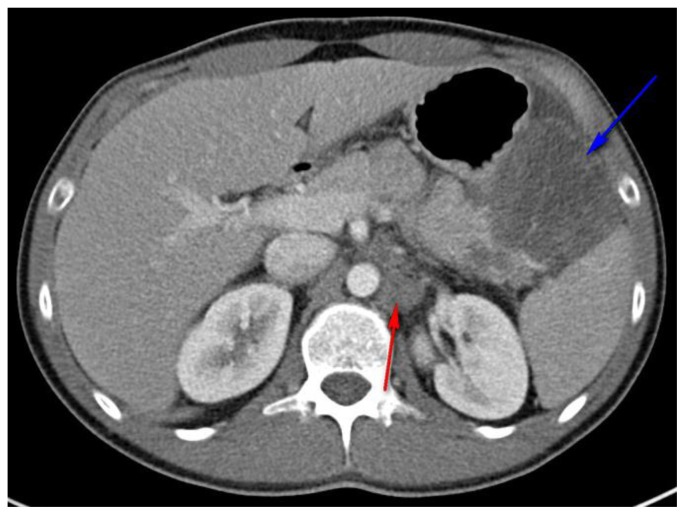
Figure 2.
27 year old male with Desmoplastic Small Round Cell Tumor (DSRCT).
Findings: Abdominal CT with hypodense, mesenteric mass (blue arrow) displacing the adjacent stomach (anteriorly) and spleen (posteriorly), and encasing the splenic artery. Left para-aortic lymphadenopathy was also present measuring 3.1 × 1.8 cm. (red arrow)
Technique: Axial, intravenous (IV) and enteric contrast enhanced Abdominal CT using a Siemens 64 slice scanner. 100 cc Isovue 370 was used at an injection rate of 2 ml/sec.
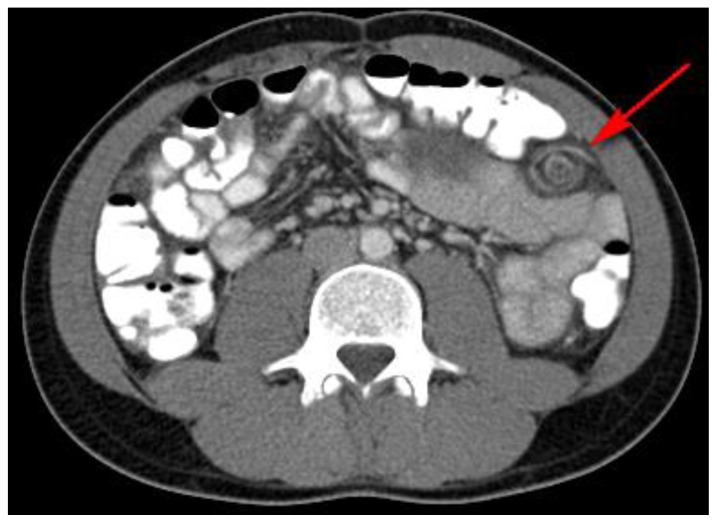
Figure 3.
27 year old male with Desmoplastic Small Round Cell Tumor (DSRCT).
Findings: Incidental transient intussusception involving the small bowel, likely related to bowel inflammation or non-visualized peritoneal involvement. (red arrow)
Technique: Axial, intravenous (IV) and enteric contrast enhanced Abdominal CT using a Siemens 64 slice scanner. 100 cc Isovue 370 was used at an injection rate of 2 ml/sec.
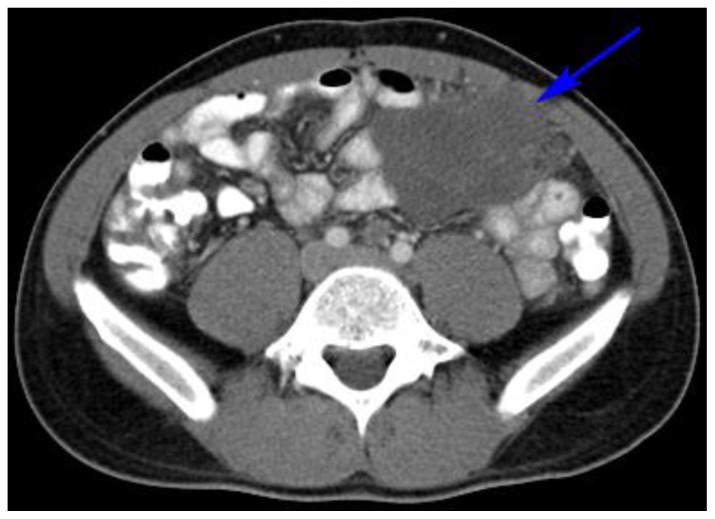
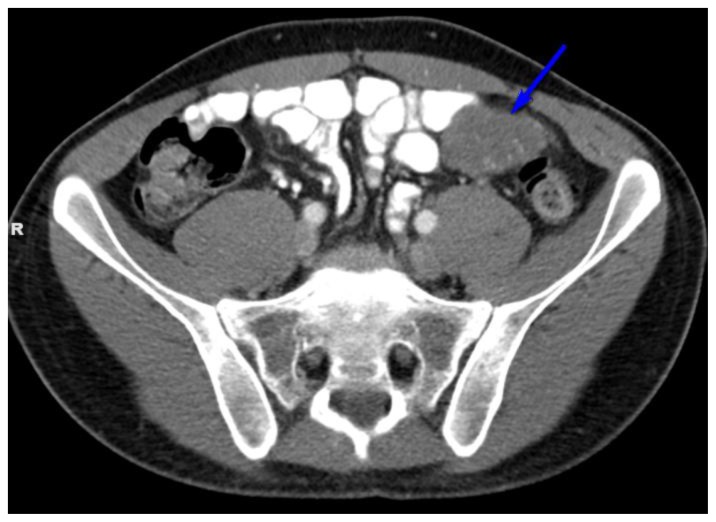
Figure 4.
27 year old male with Desmoplastic Small Round Cell Tumor (DSRCT).
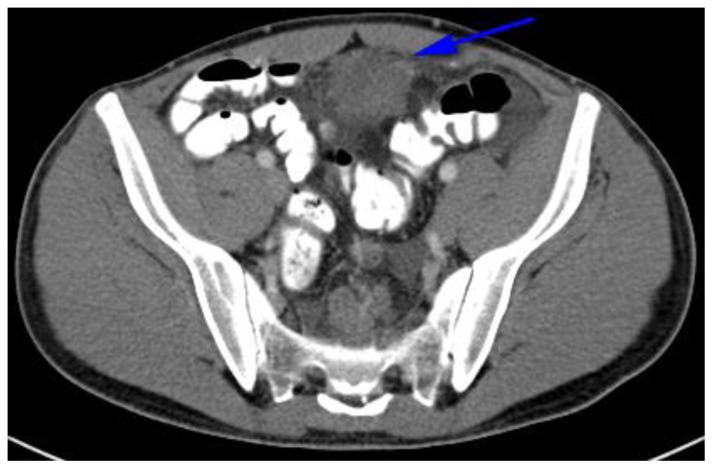
Findings: Hypodense, lobular, pelvic peritoneal or omental mass in the left lower quadrant measuring 10 × 5.4 × 11.1 cm and demonstrating bowel displacement. (blue arrow)
Technique: Axial, intravenous (IV) and enteric contrast enhanced Abdominal CT using a Siemens 64 slice scanner. 100 cc Isovue 370 was used at an injection rate of 2 ml/sec.
Figure 5.
27 year old male with Desmoplastic Small Round Cell Tumor (DSRCT).
Findings: Extension of lobular, hypodense, omental pelvic mass down the midline of the abdomen. (blue arrow)
Technique: Axial, intravenous (IV) and enteric contrast enhanced Abdominal CT using a Siemens 64 slice scanner. 100 cc Isovue 370 was used at an injection rate of 2 ml/sec.
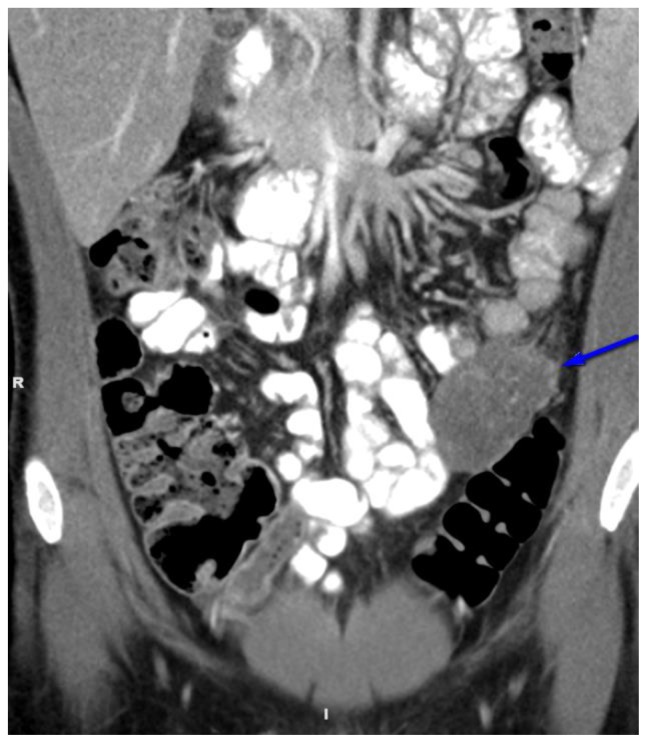
Figure 6.
27 year old male with Desmoplastic Small Round Cell Tumor (DSRCT).
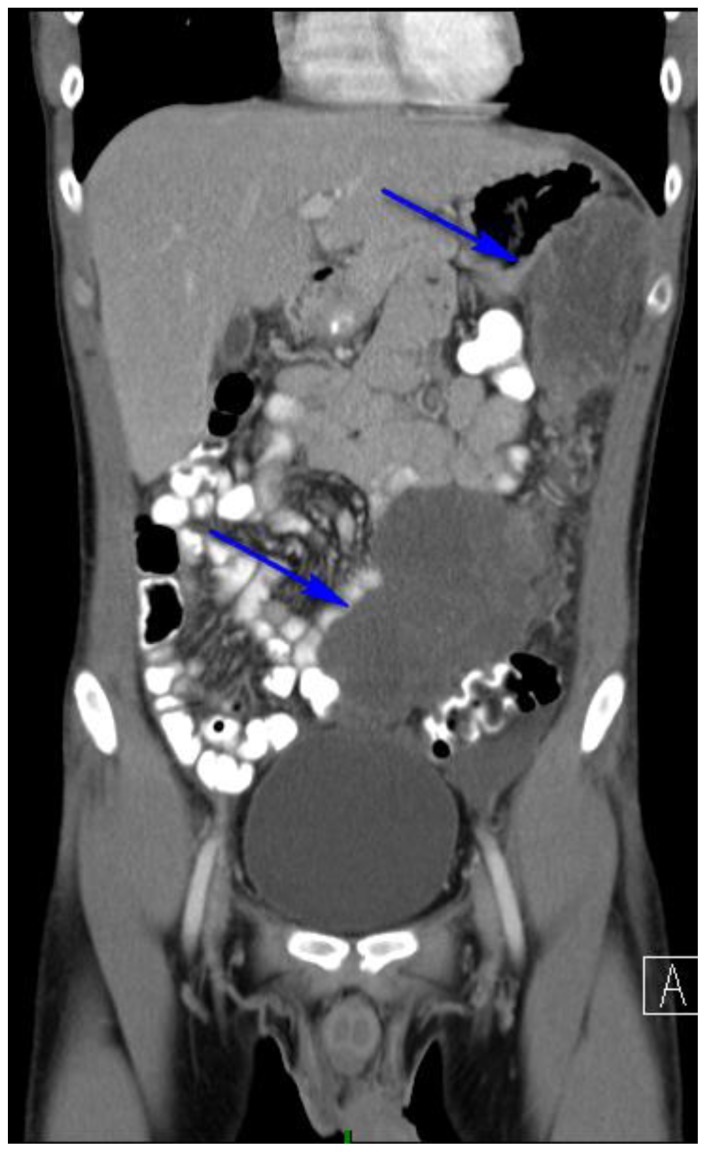
Findings: Abdominal CT revealing at least two large hypodense, lobular abdominopelvic omental masses. (blue arrows). The more superior mass was eventually biopsied under CT guidance allowing for pathologic diagnosis.
Technique: Coronal, intravenous (IV) and enteric contrast enhanced Abdominal CT using a Siemens 64 slice scanner. 100 cc Isovue 370 was used at an injection rate of 2 ml/sec.
Figure 7.
27 year old male with Desmoplastic Small Round Cell Tumor (DSRCT).
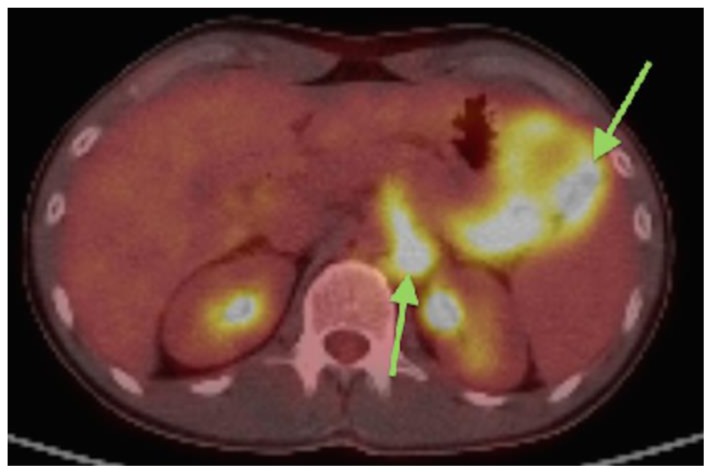
Findings: PET/CT with FDG avid metabolic foci in the peritoneum and retroperitoneum correlating to the masses and lymphadenopathy seen on axial CT. (green arrows) SUV measurements are 10.2 and 9.8 respectively.
Technique: PET/CT performed 66 minutes after the intravenous administration of 11.2 mCi of 18F-Fluorodeoxyglucose.
Figure 8.
27 year old male with Desmoplastic Small Round Cell Tumor (DSRCT).
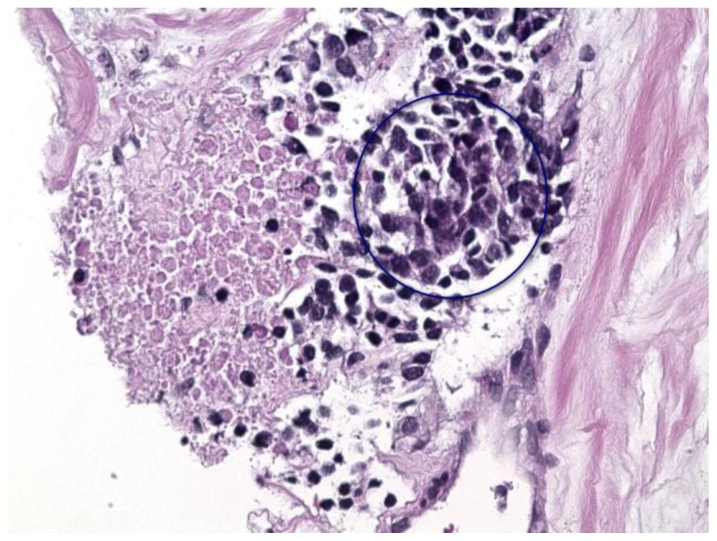
Findings: H&E stain, 20× magnification section demonstrating small nests of poorly differentiated malignant cells with high nuclear cytoplasmic ratios (blue circle) set in a background of desmoplastic stroma.
Technique: Histology section.
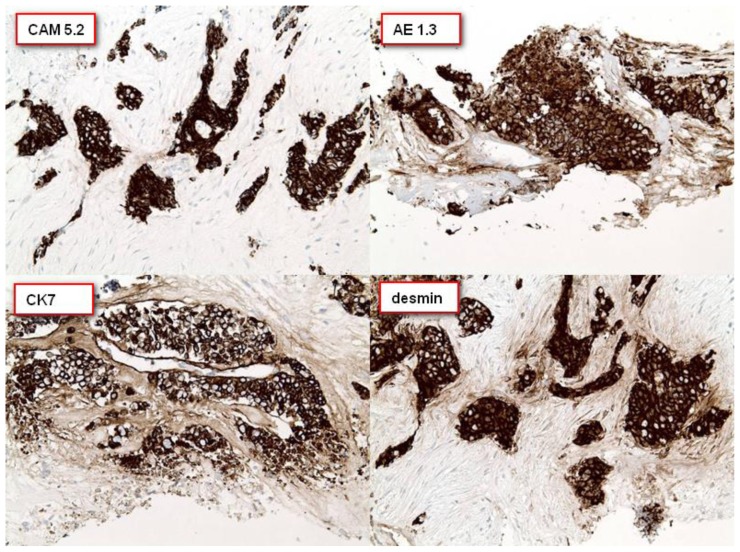
Figure 9.
27 year old male with Desmoplastic Small Round Cell Tumor (DSRCT).
Findings: A panel of immunohistochemical stains were performed which revealed positive staining of the malignant cells for cytokeratins CAM 5.2, AE1.3 and CK7. The tumor cells are also strongly positive for desmin.
Technique: Immunohistochemical panel consisting of CAM 5.2, AE1.3, CK7 and desmin.
Following diagnosis, the patient was treated with systemic chemotherapy with vincristine, adriamycin, and cyclophosphamide. This was alternated with ifosfamide and etoposide. The patient was subsequently treated with surgery including, distal pancreatectomy, splenectomy, omentectomy and tumor debulking. Follow-up CT abdomen/pelvis with contrast demonstrated significant decrease in size of the abdominal masses (Figures 10,11). At 8 months from diagnosis the patient is currently doing well with normal functional capacity.
Figure 10.
27 year old male with Desmoplastic Small Round Cell Tumor (DSRCT).
Findings: Abdominal CT revealing a low density soft tissue mass (blue arrow) in the left midabdomen abutting but not obstructing the small bowel. This mass has decreased in size, currently measuring 4.4 × 2.9 × 5.1 cm. Previously it measured 10 × 5.4 × 11.1 cm as demonstrated in figure 4. High density foci posteriorly within the mass represent calcification and suggest treatment response.
Technique: Axial, intravenous (IV) and enteric contrast enhanced Abdominal CT using a Siemens 64 slice scanner. 100 cc Isovue 370 was used at an injection rate of 2 ml/sec.
Figure 11.
27 year old male with Desmoplastic Small Round Cell Tumor (DSRCT).
Findings: Abdominal CT revealing a low density soft tissue mass (blue arrow) in the left midabdomen abutting but not obstructing the small bowel. This mass has decreased in size, currently measuring 4.4 × 2.9 × 5.1 cm. Previously it measured 10 × 5.4 × 11.1 cm as demonstrated in figure 6. Technique: Coronal, intravenous (IV) and enteric contrast enhanced Abdominal CT using a Siemens 64 slice scanner. 100 cc Isovue 370 was used at an injection rate of 2 ml/sec.
DISCUSSION
Etiology & Demographics
Desmoplastic small round cell tumor is a rare, aggressive malignancy of unknown etiology [1,2,3]. Estimated incidence is between 0.2 and 0.5 people per million. It is considered a childhood cancer, and is characterized by a unique chromosomal translocation which leads to failure to suppress tumor growth [4]. It is classified as a soft tissue sarcoma, sharing some features with other small round cell tumors such as Ewing’s Sarcoma and primitive neuroectodermal tumor (PNET) [4].
Clinical & Imaging Findings
DSRCT usually presents as abdominal pain, predominantly affecting boys and young men. Typical imaging findings include multiple heterogeneous, lobular abdominal masses, which can grow very large. Often there is a dominant mass with additional peritoneal, omental, retroperitoneal and retrovesical masses [2,4,5,6]. On CT, soft tissue masses with patchy hypodense foci are common [5,6]. Tumor calcifications occur in up to 55% of cases. On magnetic resonance imaging (MRI), lesions are variable demonstrating heterogeneity with patchy areas of hypointensity, hyperintensity, isointensity or mixed intensities. In a study by Thomas et al. MRI typically demonstrated heterogeneous lesions with primarily T1 hypointensity to isointensity and T2 hyperintensity with heterogeneous contrast enhancement [3]. Lymphadenopathy is common. PET/CT is typically used for staging and demonstrates multiple foci of FDG avid metabolic activity within the tumor masses [5,6,7].
Differential Diagnosis
In young patients, the imaging differential diagnosis usually includes neuroblastoma, rhabdomyosarcoma, PNET and peritoneal leiomyosarcomatosis. In older patients, the differential primarily includes lymphoma and metastasis, or less commonly mesothelioma.
Neuroblastoma
Neuroblastoma on US demonstrates heterogeneous, hypoechoic masses similar to DSRCT [7]. On CT they appear as a large mass with low attenuation and heterogeneous enhancement. PET/CT is not commonly used in the evaluation of neuroblastoma. MRI typically demonstrates heterogeneity with variable enhancement, hypointensity on T1 with hyperintensity on T2 weighted imaging.
Pelvic Rhabdomyosarcoma
Pelvic rhabdomyosarcoma is the most common malignant sarcoma of childhood, commonly presenting before age 3 [8]. In older children, rhabdomyosarcoma is the most common soft tissue malignancy. In both cases it presents as a well-defined, inhomogeneous lobular mass which is hypoechoic on US and relatively hypodense on CT. Masses tend to be smaller in size than DSRCT [8]. PET/CT is not typically used in evaluation of rhabdomyosarcomas. MRI demonstrates non-specific findings with intermediate T1 and intermediate to hyperintensity on T2 weighted imaging.
PNET
PNET can occur like DSCRT in the abdominopelvis or retroperitoneum with similar US, CT, MRI and FDG PET/CT findings, but fine tumor calcifications are rare [6].
Peritoneal leiomyosarcomatosis
Peritoneal leiomyosarcomatosis has a similar imaging appearance but tends to occur in older patients, presenting as multiple peritoneal nodules or mesenteric masses with well-defined margins on US, CT and MRI [6].
Pathology
Pathologically, this is a rare aggressive neoplasm characterized by a unique chromosomal translocation (t11;22) (p13:q12) and cytogenetic gene fusion transcript (EWS-WT1) that are diagnostic [4]. The tumor tends to grow very rapidly and can metastasize hematologically or via lymph nodes, typically to the liver or lungs [2,9]. Patients may present with multifocal disease with hundreds of tumors studding the peritoneal cavity. Histology typically features clusters of small, round, or spindled cells of variable size surrounded by desmoplastic stroma [9]. In a study reviewing 32 tumors, immunohistochemical was commonly positive for desmin, WTI, keratin, and neuron-specific enolase.
Treatment and Prognosis
Prognosis is relatively poor with a 3 year survival rate of 50% in those treated aggressively with surgical resection, chemotherapy, and radiation therapy [5]. Treatment with tumor debulking only, rather than a combination of surgery, chemotherapy and radiation therapy, had a significantly worse prognosis in a study by Zhang et al.
It is important to consider DSRCT in the setting of multiple abdominal masses in a young male, so that he may be referred to a specialist for treatment. Long term control of the disease may be achieved with complete surgical resection, including peritoneal implants [9]. Hyperthermic intraperitoneal chemotherapy is an option, and radiation therapy, chemotherapy and stem cell transplants have all been used with varying degrees of success.
TEACHING POINT
Desmoplastic small round cell tumor (DSRCT) is an aggressive, rare cancer most often presenting as multiple masses in abdomen of young males. The characteristic clinical presentation of abdominal pain and mass, with radiology featuring multifocal abdominal masses, and pathology demonstrating unique chromosomal translocation (t11;22) (p13:q12) and EWSRI positivity, is diagnostic for DSRCT. Typical imaging findings include multiple heterogeneous, lobular abdominal masses, which can grow very large. Often there is a dominant mass with additional peritoneal, omental, retroperitoneal and retrovesical masses.
Table 1.
Summary table for Desmoplastic Small Round Cell Tumor (DSRCT).
| Etiology | Unknown |
| Incidence | Rare; 0.2 – 0.5 cases / million. |
| Gender ratio | Predominantly affects males |
| Age predilection | Boys and young men |
| Risk factors | No known risk factors |
| Treatment | Surgical resection, chemotherapy, and radiation |
| Prognosis | Poor |
| Imaging findings | Multiple, lobular abdominal masses, which can grow very large, typically with a dominant lesion and multifocal peritoneal/omental/retroperitoneal masses, often involving the retrovesical region. |
Table 2.
Differential table for Desmoplastic Small Round Cell Tumor (DSRCT).
| Ultrasound | CT | PET/CT | MRI | Enhancement Pattern | |
|---|---|---|---|---|---|
| Desmoplastic Small Round Cell Tumor | Heterogeneous, lobular mass | Heterogeneous, lobular masses, tumor calcification occurs in 55% | Multiple nodular foci of metabolic activity in masses | Heterogeneous of variable signal intensity | Heterogeneous |
| Neuroblastoma | Heterogeneous, hypoechoic mass | Low attenuation, heterogeneously enhancing mass, causes displacement | FDG avid mass | Heterogeneous, variably enhancing with low T1 and high T2 signal intensity | Heterogeneous, depending on degree of hemorrhage/necrosis |
| Rhabdomyosarcoma | Heterogeneous mass | Heterogeneous, lobulated mass | FDG avid mass | T1 intermediate with T2 intermediate to high signal intensity | Heterogeneous, depending on degree of hemorrhage/necrosis |
| Primitive Neuroectodermal Tumor | Heterogeneous, lobular mass | Heterogeneous, lobular masses, tumor, calcification is rare | Multiple nodular foci of metabolic activity in masses | Heterogeneous of variable signal intensity | Significant enhancement which can be homogeneous or heterogeneous |
| Peritoneal Leiomyo-sarcomatosis | Multiple, well defined masses | Multiple, well defined masses | Multiple nodular foci of metabolic activity in masses | Multiple, well defined masses. | Heterogeneous enhancement, lower in attenuation than normal myometrium. |
ABBREVIATIONS
- CT
Computed tomography
- DSRCT
Desmoplastic small round cell tumor
- FDG PET/CT
Fluorodeoxyglucose positron emission tomography/computed tomography
- IV
Intravenous
- MRI
Magnetic resonance imaging
- PNET
Primitive neuroectodermal tumor
- US
Ultrasound
REFERENCES
- 1.Arora VC, Price AP, Fleming S, Sohn MJ, Magnan H, Laquaglia MP, Abramson S. Characteristic imaging features of desmoplastic small round cell tumour. Pediatr Radiol. 2013;43:93–102. doi: 10.1007/s00247-012-2485-0. [DOI] [PubMed] [Google Scholar]
- 2.Li G, Wang H-T, Gao Y, Cui X-J, Zhang G-Z. Primary abdominopelvic desmoplastic small round cell tumor: CT and correlated clinicopathologic features. European Review for Medical and Pharmacological Sciences. 2014;18:2670–77. [PubMed] [Google Scholar]
- 3.Thomas R, Rajeswaran G, Thway K, Benson C, Shahabuddin K, Moskovic E. Desmoplastic small round cell tumour: the radiological, pathological and clinical features. Insights Imaging. 2013 Feb;4(1):111–18. doi: 10.1007/s13244-012-0212-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lae ME, Roche PC, Jin L, Lloyd RV, Nascimento AG. Desmoplastic small round cell rumor: a clinicopathologic, immunohistochemical and molecular study of 32 tumors. Am J Surg Pathol. 2002 Jul;26(7):823–35. doi: 10.1097/00000478-200207000-00001. [DOI] [PubMed] [Google Scholar]
- 5.Zhang G, Liu G, Zhao D, Cui X, Li G. Desmoplastic small round cell tumor of the abdomen and pelvis: clinicopathologic characteristics of 12 cases. Scientific World Journal. 2014;2014:549–612. [Google Scholar]
- 6.Bellah R, Suzuki-Bordalo L, Brecher E, Ginsberg JP, Maris J, Pawel BR. Desmoplastic small round cell tumor in the abdomen and pelvis: report of CT findings in 11 affected children and young adults. AJR. 2005;184(6):1910–14. doi: 10.2214/ajr.184.6.01841910. [DOI] [PubMed] [Google Scholar]
- 7.Pappaoiannou G, McHugh K. Neuroblastoma in childhood: review and radiological findings. Cancer Imaging. 2005;5(1):116–27. doi: 10.1102/1470-7330.2005.0104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Van Rijn R, Wilde JC, Bras J, Oldenburger F, McHugh K, Merk JHM. Imaging findings in noncraniofacial childhood rhabdomyosarcoma. Pediatr Radiol. 2008 Jun;38:617–34. doi: 10.1007/s00247-008-0751-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hayes-Jordan A, Anderson PM. The diagnosis and management of desmoplastic small round cell tumor: a review. Curr Opin Oncol. 2011 Jul;23(4):385–89. doi: 10.1097/CCO.0b013e3283477aab. [DOI] [PubMed] [Google Scholar]