Abstract
Cough is a vital protective reflex that is triggered by both mechanical and chemical stimuli. The current experiments explored how chemosensory stimuli modulate this important reflex. Cough thresholds were measured using a single-inhalation capsaicin challenge. Experiment 1 examined the impact of sweet taste: Cough thresholds were measured after rinsing the mouth with a sucrose solution (sweet) or with water (control). Experiment 2 examined the impact of menthol: Cough thresholds were measured after inhaling headspace above a menthol solution (menthol vapor) or headspace above the mineral oil solvent (control). Experiment 3 examined the impact of rinsing the mouth with a (bitter) sucrose octaacetate solution. Rinsing with sucrose and inhaling menthol vapor significantly increased measured cough thresholds. Rinsing with sucrose octaacete caused a non-significant decrease in cough thresholds, an important demonstration of specificity. Decreases in cough reflex sensitivity from sucrose or menthol could help explain why cough syrups without pharmacologically active ingredients are often almost as effective as formulations with an added drug. Further, the results support the idea that adding menthol to cigarettes might make tobacco smoke more tolerable for beginning smokers, at least in part, by reducing the sensitivity of an important airway defense mechanism.
Keywords: chemosensory, anti-tussive, sucrose, capsaicin, irritation, cough-suppressant
1.1 Introduction
Like the sneeze, the cough reflex helps protect the sensitive respiratory tract tissues by creating bursts of high-flow expiration that expel mucus, harmful vapors, and other foreign matter [1–3]. Cough is triggered by airway sensory afferents that terminate primarily in the brainstem, where the cough response is believed to be generated [4–8]. Mechanical obstruction or invasion of airways triggers coughing via mechanical somatosensory receptors. Chemical tussive stimuli are detected in the airways by ion channels (e.g., TPRV1, TRPA1, ASICs), among other possible mechanisms, which also transduce the burning and stinging sensations of chemical irritants in the nose and mouth [9–13]. Thus, coughing is an airway defense against threats from both mechanical and chemical stimuli. Despite some important advances, cough sensitivity and how it is modulated in health and disease remains poorly understood, especially in humans.
We wished to determine whether cough sensitivity can be modulated by chemosensory stimuli, a topic that holds applied as well as basic interests. Clinical trials with cough syrups provide a potential lead to the modulation of coughing: Control formulations are often almost as effective as formulations with the anti-tussive pharmacological actives included [14,15]. Eccles (15) notes that most cough syrups contain sucrose or other sweeteners. Further, some sweet foods, including honey and chocolate, are thought to provide relief from coughing [16,17]. Cough syrup or honey might trigger salivation or modulate mucus production, soothing the throat by mechanical coating. Some sweet foods might also contain compounds with pharmacological, antioxidant, or anti-microbial activity [16,17].
It is also possible, however, that perception of the taste quality sweetness itself suppresses coughing [15]. Crystalline sugar placed on the tongue can inhibit hiccoughs, a respiratory reflex related to cough [18]. Further, sweet taste can induce analgesia [19–22]. Importantly, non-nutritive sweeteners can also have analgesic effects [23,24], whereas administration of sucrose directly to the stomach does not [25]. Thus, sweet taste per se seems to have analgesic properties, likely due, in part, to release of endogenous opioids [26–28]. As noted by others, there is a close relationship between the analgesic and antitussive properties of opioids [15,29]. Thus, extant data suggest that sweet test may modulate cough sensitivity, but no empirical studies have directly tested this hypothesis.
Menthol is another anti-tussive compound of interest. It is commonly added to cough syrups and lozenges, presumably to suppress coughing. High concentrations of menthol on the skin and tongue can have anesthetic, analgesic and/or desensitizing effects [30–34]. Objective studies of the impact of menthol on airway sensitivity have been limited. In mice, menthol reduced sensitivity to airway irritation, an effect that was blocked by a TRPM8 antagonist [35]. In humans, a moderate (predominantly cooling) concentration of menthol vapor reduced sensitivity to nasal irritation from acetic acid, but actually enhanced sensitivity to the TPRA1 agonist allyl isothiocyanate [36]. Regarding cough sensitivity, two studies, one in children and one in adults, suggest that menthol may reduce the number of coughs elicited by a fixed concentration of citric acid [37,38]. However, in both studies the TRPM8 agonist eucalyptol was used to mask menthol. The potential for interactions between eucalyptol and menthol at the sensory neurons makes interpretation of the findings difficult.
The current report explores chemosensory modulation of the cough response by examining the effect of sucrose and menthol on human cough reflex sensitivity. The impact of another salient sensory stimulus, the bitter compound sucrose octaacetate (SOA), was also examined to assess the chemosensory specificity of sucrose and menthol.
2.1 Experiment 1
2.1.1 Purpose
Cough reflex thresholds were measured after rinsing the mouth either with sucrose solution or with water (control) to test the hypothesis that sweet taste can suppress cough sensitivity.
2.1.2 Materials and Methods
2.1.2.1 Participants
Thirteen healthy adults (7 women, 5 men; age 23–36 y) participated. Twelve subjects were non-smokers. One subject reported smoking 3 cigarettes per day. Participants were recruited from Monell staff of from the surrounding community, and were paid for their time. Procedures were approved by an institutional review board (IRB) at the University of Pennsylvania. Subjects provided written, informed consent on IRB approved forms prior to testing.
2.1.2.2 Stimulus Materials
The sweet pretreatment stimulus was sucrose (Sigma) dissolved in Millipore® filtered, de-ionized water (1 M). This concentration was selected to provide a moderately intense sweetness, comparable to a sweet hard candy, under the conditions in this experiment (see Procedures, below). The control pretreatment stimulus was pure filtered water. Sucrose solutions were prepared every 2–3 days. Solutions were stored under refrigeration, and samples were warmed to room temperature before presentation. The control pretreatment (pure water) was treated in the same way.
A dilution series of capsaicin was used to measure cough reflex thresholds. First, 30.5 mg of pharmaceutical grade capsaicin (Formosa Laboratories, Taiwan) was dissolved in 1 ml of ethanol (USP grade, 95%) and 1 ml polyoxyethylenesorbitan (Tween 80). The resulting solution was then dissolved in 8 ml sterile isotonic saline to form a stock solution, stored in an amber vial under refrigeration. This stock solution was further diluted with sterile isotonic saline to make 11 concentrations ranging from 0.98 to 1,000 μM (two-fold dilution steps). Dilution series were prepared on an as-needed basis. Blanks (see procedures, below) were prepared in parallel by mixing 1 ml of ethanol and 1 ml Tween 80 with 8 ml of saline, then diluting the resulting mixture down to the same concentration as in the intermediate capsaicin step.
2.1.2.3 Apparatus
A research nebulizer system delivered capsaicin solution. Similar systems have been described [39]. Capsaicin solutions were placed in standard medical nebulizer vessels (Model 646, DeVilbiss Health Care Inc., Somerset, PA, USA). Puffs of compressed air (medical grade) drove the solution onto a baffle to aerosolize it for inhalation. The position of the baffles was calibrated and fixed to provide consistent output. Air puffs were controlled by a digital dosimeter (KoKo Dosimeter, Ferraris Respiratory, Louisville, CO, USA), triggered by a pressure switch 0.5 s after the participant began to inhale. Puff duration was set at 0.6 s, which aerosolized approximately 0.015 ml of solution. Regulator valves were added to the nebulizer vessels to provide a controlled flow rate to help compensate for changes in inspiratory force.
2.1.2.4: Procedures
Cough reflex thresholds were measured using a modified, single inhalation cough challenge [39,40]. During each trial, the participant first exhaled naturally, then took a single inhalation from a nebulizer vessel. In the first two (warm-up) trials of a threshold run, the participant sampled pure saline (which never triggered cough). In subsequent trials, the participant inhaled ascending concentrations of capsaicin solution, with blanks randomly interspersed to increase the blindness of the test (the blanks never triggered cough). Concentration always started at the lowest step (0.98 μM) each threshold run. After inhalation of the lowest step, the experimenter counted the number of coughs that occurred in following 10 seconds (expulsive; throat clearing was not counted). If the lowest step triggered at least 3 coughs, testing ended. If not, concentration increased by a single 2-fold step. Testing continued in this fashion, with each concentration presented not more than once, until a particular concentration triggered at least 3 coughs. At this point, the threshold run ended and that concentration was defined as the threshold. A stopping criterion of 3coughs was selected as a compromise between the published criteria of either 2 or 5 coughs[39]. Preliminary studies suggested that three coughs provided greater reliability than two coughs with less discomfort than is elicited with five coughs. One minute elapsed between trials.
Before each inhalation, i.e., before each trial in the threshold run, the participant rinsed their mouth with 5 ml of liquid presented in a plastic medicine cup. The liquid, either sucrose solution or water (control), was fixed during a particular threshold run. Trays of medicine cups were prepared in advance by a different experimenter, so that the experimenter conducting the threshold run was blind to whether the pretreatment was sucrose or water.
Instructions for pretreatment were designed to help keep the condition of the throat as consistent as possible. Before rinsing the mouth on each trial, the participant first rinsed with de-ionized water and swallowed. After holding the pretreatment stimulus in the mouth for about 3 seconds, the participant spat into a sink and immediately inhaled from the nebulizer. The participant was instructed NOT to swallow after spitting. After the inhalation, subjects rinsed with water again and spit. The participant was instructed not to swallow again until the pre-rinse at the start of the next trial. This procedure helped to reduce the chances that effects of sucrose were due to coating of the throat.
Participants attended two sessions. The first session lasted about 1.5 hours. After a consent discussion, participants received basic instructions and completed several practice trials (using inhalation of pure saline after rinsing the mouth with water). Next, participants completed two cough threshold runs, separated by a break of 20 minutes (one run with sucrose pretreatment, one run with water pretreatment). The second session, which occurred 1 to 5 days later, lasted about 1 hour and also included a sucrose pretreatment run and a blank pretreatment run separated by a break of 20 minutes. The order of the pretreatment (sucrose vs. blank first) was counterbalanced: half the participants were randomly assigned to receive sucrose first in session 1 and the blank first in session 2, and the remaining participants were assigned the opposite orders.
2.1.2.5 Data analysis
The primary data were cough reflex thresholds, i.e., the minimum concentration of capsaicin that elicited at least 3 coughs. One subject, who failed to follow instructions, did not complete the study. Data from the remaining 12 subjects were analyzed. Since preliminary analyses found distributions of cough reflex thresholds to be positively skewed, thresholds were log-transformed prior to analysis. After transformation, cough thresholds were submitted to analysis of variance (ANOVA), conducted using Statistica software (Version 10, Statsoft). Data from the one light smoker did not distinguish themselves in any way and were included. Analyses that excluded this subject support the same conclusions and are not presented below. Absolute magnitude of prerinse effects, i.e., the degree to which sucrose decreased cough sensitivity, were calculated as a proportion increase in cough reflex thresholds.
2.1.3 Results
Rinsing the mouth with sucrose solution decreased cough sensitivity, i.e., increased the concentration of capsaicin required to trigger cough, relative to rinsing the mouth with water. Cough reflex thresholds were submitted to a 3-way, mixed within-between subjects ANOVA: Prerinse condition (sucrose vs. water, within subjects) X Session (first measurement vs. second measurement, within subjects) X Prerinse order (sucrose first in session 1 vs. sucrose first in session 2, between subjects). The effect of prerinse condition reached significance, F(1,10) = 7.03, p = 0.02. The effect of session, prerinse order, and all interactions failed to reach significance (p > 0.27). The absolute magnitude of the prerinse effect was roughly comparable in the two experimental sessions. Rinsing the mouth with sucrose increased cough thresholds by 45% (Figure 1).
Figure 1. The effect of oral sucrose on cough thresholds.
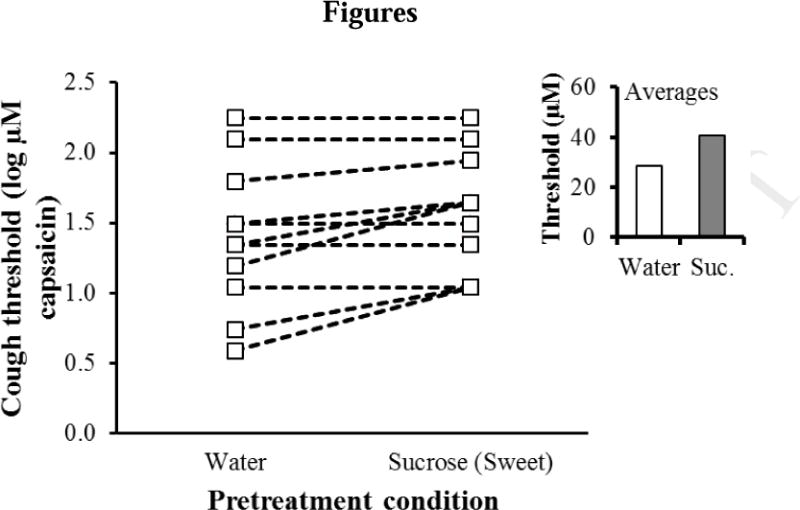
The minimum concentration of capsaicin required to elicit at least three coughs (Y-axis) by pre-treatment condition (X axis) for individual participants (average of two threshold measurements). The insert shows the average (geometric mean) threshold for the two pretreatment conditions.
Examining data for individual participants (Figure 1) shows that half the sample showed an increase in threshold for the sucrose rinse condition, and half did not. This finding could reflect individual differences in responsiveness to the treatment effect.
3.1 Experiment 2
3.1.1 Purpose
Cough thresholds were measured after inhalation of either headspace above a menthol solution or headspace above mineral oil (control) to test the hypothesis that menthol can suppress cough sensitivity.
3.1.2 Materials and methods
The design, materials, and methods largely match those of Experiment 1, with the following exceptions. First, since preliminary studies work suggested that the effect of menthol might be more subtle than that of sucrose, finer concentration steps of capsaicin were used. Concentrations ranged from 2.12 to 125 μM (11, 1.5-fold steps). Second, the pretreatment stimuli consisted of inhaled vapor rather than liquids.
One pretreatment was l-Menthol (Sigma–Aldrich) dissolved in filtered, light mineral oil (48.9 mg/ml). The menthol concentration was chosen in a sensory matching experiment [36] to have a moderate cooling intensity comparable in strength to cooling from a menthol cigarette. The other pretreatment (control) was pure solvent (mineral oil). Stimuli were presented in 250 ml glass bottles containing 10 ml of solution or solvent. The caps of the bottles had both an inlet tube that terminated about 1 cm above the surface of the solution, and another aperture to which a plastic mouth piece was connected.
Participants inhaled headspace from the bottles through the mouthpiece. Six menthol bottles and six blanks were prepared to avoid depletion of the solutions, and fresh solutions were prepared weekly. Participants pretreated before each trial in a threshold run, i.e., before each inhalation from the nebulizer, by taking three inhalations of headspace above either menthol solution or pure mineral oil. The inhalations lasted about 1 s each, with about 2 s elapsing between inhalations. Otherwise, the pretreatment procedure (including rinsing and swallowing) matched that of Experiment 1. Participants included 12 healthy non-smokers (9 women, 3 men, age 23–47 y). None had participated in Experiment 1.
3.1.3 Results
Inhalation of headspace above menthol solution decreased cough sensitivity, i.e., increased the concentration of capsaicin required to trigger cough, relative to inhalation of headspace above a solvent blank. Cough reflex thresholds were submitted to a 3-way, mixed within-between subjects ANOVA: Pretreatment condition (headspace above a menthol solution vs. headspace above a solvent blank, within subjects) X Session (first measurement vs. second measurement, within subjects) X Pretreatment order (sucrose first in session 1 vs. sucrose first in session 2, between subjects). The effect of pretreatment condition reached significance, F(1,10) = 5.67, p = 0.039. The effect of session, pretreatment order, and all interactions failed to reach significance (p > 0.15). Regarding the absolute magnitude of the pre-treatment effect, inhalation of menthol vapor increased cough thresholds by about 25% (Figure 2).
Figure 2. The effect of menthol vapor on cough thresholds.
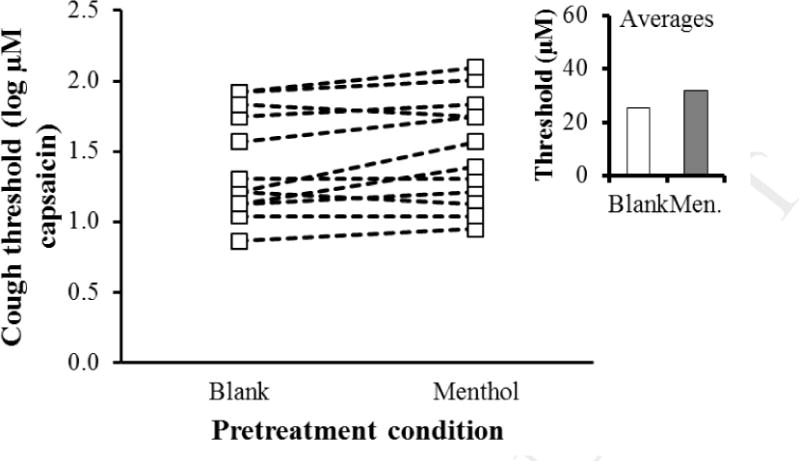
The minimum concentration of capsaicin required to elicit at least three coughs (Y-axis) by pre-treatment condition (X axis) for individual participants (average of two threshold measurements). The insert shows the average (geometric mean) threshold for the two pretreatment conditions.
As with the sucrose, an examination of data for individual participants (Figure 2) reveals that not all subjects showed a clear increase in threshold after inhalation of menthol. Again, this could indicate individual differences in the magnitude of the treatment effect.
4.1 Experiment 3
4.1.1 Purpose
Since cough can be suppressed voluntarily [41,42], it is possible that sucrose and menthol simply served as prompts to suppress coughing in the single-inhalation paradigm. If this hypothesis is correct, then any salient chemosensory stimulus might increase cough thresholds. To test whether cough suppression is stimulus-specific, an additional experiment was conducted to determine whether rinsing the mouth with a bitter stimulus affects cough thresholds.
4.1.2 Materials and methods
Methods largely matched those of Experiment 1, though 1.5-fold dilution steps of capsaicin were used to increase the chances of observing smaller effects. Instead of sucrose solutions, subjects rinsed with aqueous solutions (3.60 × 10−6 M) of the non-toxic bitter substance sucrose octaacetate (SOA). The concentration was selected based on preliminary studies to give a bitter sensation comparable in strength to the sweet sensation from the sucrose solutions used in Experiment 1. Participants included 12 healthy non-smokers (9 women, 3 men, age 24–61 y). Six had participated in Experiments 2 and 3. None had participated in Experiment 1.
4.1.3 Results
Rinsing the mouth with SOA solution had little or no effect on measured cough thresholds relative to rinsing the mouth with water. Cough reflex thresholds were submitted to a 3-way, mixed within-between subjects ANOVA: Prerinse condition (SOA vs. water, within subjects) X Session (first measurement vs. second measurement, within subjects) X Pretreatment order (sucrose first in session 1 vs. sucrose first in session 2, between subjects). None of the main effects or interactions reached significance (p > 0.41). Nominally, cough thresholds were slightly lower after rinsing with SOA (Figure 3).
Figure 3. The effect of oral sucrose octaacetate (bitter) on cough thresholds.
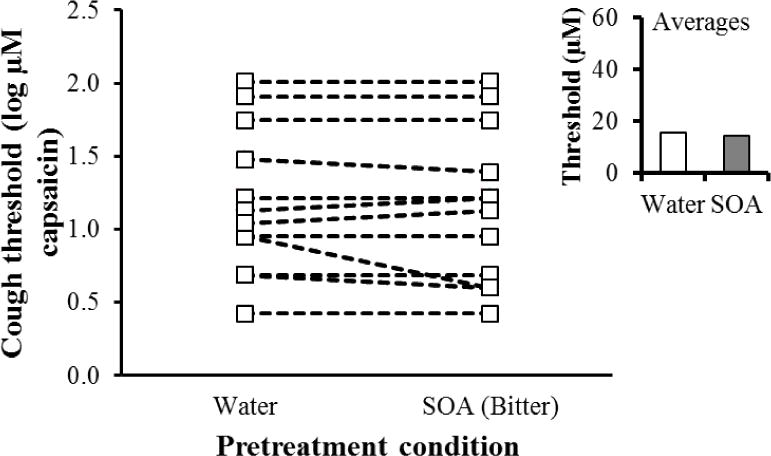
The minimum concentration of capsaicin required to elicit at least three coughs (Y-axis) by pre-treatment condition (X axis) for individual participants (average of two threshold measurements). The insert shows the average (geometric mean) threshold for the two pretreatment conditions.
5.1 Discussion
5.1.1 The effect of oral sucrose on cough thresholds
Rinsing the mouth with sucrose solution resulted in a modest but significant increase in the concentration of capsaicin required to trigger cough (Experiment 1). To the best of our knowledge, this experiment provides the first direct evidence that taste sensations can modulate cough reflex sensitivity [15]. The basis for the effect is currently unknown, though release of endogenous opioids in response to sweet taste is likely to play a role [27, 28, 43].
Instructions to subjects were intended to prevent coating of the throat from swallowing sucrose solutions or altered saliva. Subjects appeared to comply. Some physical effects on the throat, however, cannot be ruled out. It is also possible that nongustatory oral sensations, such as the mouth feel of sucrose solutions, played some role in the effect, since concentrated sucrose is more viscous than water.
The Possibility of individual differences in the sucrose effect is an interesting topic for further research. If cough suppression from sucrose is related to sweet analgesia, we might expect individual differences to mirror those seen for sweet taste induced analgesia. For example, we expect differences in the duration of effects between men and women [22,44], or a relationship between sweet taste preference and degree of cough suppression [21].
5.1.2 The effect of inhaled menthol vapor on cough thresholds
Inhalation of menthol vapor also resulted in a significant increase in the concentration of capsaicin required to trigger cough (Experiment 2). Two other studies have examined the effect of menthol vapor on the number of coughs triggered by a fixed concentration of citric acid [37,38]. Both studies concluded that menthol can reduce cough reflex sensitivity. However, in both studies the presence of menthol vapor was masked with a relatively high concentration of eucalyptol. A recent animal study found that eucalyptol and menthol both suppress sensitivity to airway irritation in mice, and that the counter-irritant effects of both compounds are blocked by an antagonist of the cool receptor TRPM8 [35]. The present study supports and extends these previous findings to show that menthol alone does indeed suppress cough sensitivity.
Human work on perceived irritation from chemicals suggests that the effects of menthol vapor may be compound-specific: Menthol vapor decreased sensitivity to nasal irritation from acetic acid, but actually increased sensitivity to allyl isothiocyanate [36]. Sensory cooling (and, as noted above, the counter-irritant effect) of menthol depends at least in part on stimulation of TRPM8 [45,46]. However, menthol also acts on TRPA1, either directly [47–49] or indirectly, as enzymes in the respiratory mucosa metabolize menthol into one or more TRPA1 agonists [35]. Thus, the effects of menthol on irritation from TRPA1 agonists like allyl isothiocyanate [50–52] might be complicated. In contrast, the model irritant in the current study acts on the noxious heat/capsaicin receptor TRPV1 rather than on TRPA1 [53]. It is also possible that menthol not only acts directly on ion channel receptors, but may also affect responsiveness through effects on the tissue surrounding the receptors [54,55]. Future research using a wider array of model irritants and a range of menthol concentrations will help elucidate the effects of menthol as a counter-irritant and cough suppressant.
5.1.2 The effect of Oral SOA on cough thresholds
Rinsing the mouth with a bitter solution did not produce a measurable change in cough thresholds (Experiment 3). Further, cough thresholds were nominally lower after rinsing with SOA, which is inconsistent with the direction of the treatment effect in first two experiments. This negative finding with an additional salient stimulus makes it unlikely that chemosensory stimulation was simply a cue to suppress coughing, or influenced the degree of suppression by changes in attention.
Another consideration is the influence of taste and oral chemesthetic stimulation on swallowing [56,57]. Though subjects were instructed not to swallow before inhalations of capsaicin, it is possible that taste stimuli and menthol influenced the urge to swallow. Little is known regarding coordination of cough and swallow, but control of the two responses may well be linked [58]. It is plausible that sweet and bitter tastes might differ in this regard, though bitter and sweet stimuli seem to have similar effects on cortical motor pathways involved in swallowing [59].
5.1.3 Practical Implications
The chemosensory modulation of cough sensitivity has helpful implications for cough suppressants when applied to medicines, and potentially harmful implications when applied to cigarettes. Further work would be needed to evaluate the therapeutic potential of chemosensory stimuli in patients with active disease; the present study only examined healthy adults. However, since it is likely that sucrose and menthol act via separate receptor mechanisms, additive or synergistic effects are possible. If sucrose and menthol are shown to have therapeutic potential in actual patient populations, evidence-based optimization of cough suppressants might be achieved via systematic variation in relative concentration of the two compounds.
Regarding smoking behavior, the tobacco industry has long purported that menthol added to cigarettes can reduce the harshness of cigarette smoke [60]. Others have suggested that menthol can ease young people into a smoking habit by making cigarettes easier to tolerate [61]. Though empirical support for this idea is not conclusive [62], the current report provides further evidence that menthol vapor can actually reduce the sensitivity of an important airway defense mechanism. In light of objective evidence that menthol vapor modulates sensitivity to airway irritation [35,36], it is increasingly likely that menthol can affect the somatosensory impact of cigarette smoke, which could well play a role in initiation and dependence [62,63].
5.1.4 Conclusions
The current report provides the first empirical evidence that sweet taste can suppress cough sensitivity, and supports and extends previous work by showing that menthol alone (as opposed to menthol mixed with eucalyptol) can suppress cough sensitivity. Further, the finding that bitter taste had no effect on cough thresholds shows that the effects of sucrose and menthol were stimulus specific. Though the exact pathways underlying the effects remain unclear, further investigation of chemosensory stimuli as cough modulators seems promising.
Acknowledgments
The authors thank Rebecca Lapinski and Jennifer Louie for technical assistance, Dr. Julie Mennella for a useful discussion of sweet taste analgesia, and Dr. Peter Dicpinigaitis for valuable advice regarding the set-up of the apparatus. The work was funded in part by NIH grants DC02995 (PASB) and DC03794 (PHD), and by Kraft foods (Monell Term Chair in Chemosensory Pyschophysics (PMW)).
Abbreviations
- SOA
sucrose octaacetate
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors maybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Coleridge HM, Coleridge JC. Pulmonary reflexes: Neural mechanisms of pulmonary defense. AnnuRev Physiol. 1994;56:69–91. doi: 10.1146/annurev.ph.56.030194.000441. [DOI] [PubMed] [Google Scholar]
- 2.Fontana GA, Lavorini F. Cough motor mechanisms. Respir Phys Neurobiol. 2006;152:266–81. doi: 10.1016/j.resp.2006.02.016. [DOI] [PubMed] [Google Scholar]
- 3.Brooks SM. Perspective on the human cough reflex. Cough. 2011 Nov 10; doi: 10.1186/1745-9974-7-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bolser DC, Poliacek I, Jakus J, Fuller DD, Davenport PW. Neurogenesis of cough, other airway defensive behaviors and breathing: A holarchical system? Respir Physiol Neurobiol. 2006;152:255–65. doi: 10.1016/j.resp.2006.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Canning BJ, Mori N, Mazzone SB. Vagal afferent nerves regulating the cough reflex. Respir Physiol Neurobiol. 2006;152:223–42. doi: 10.1016/j.resp.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 6.Bonham AC, Sekizawa SI, Chen CY, Joad JP. Plasticity of brainstem mechanisms of cough. Respir Physiol Neurobiol. 2006;152:312–19. doi: 10.1016/j.resp.2006.02.010. [DOI] [PubMed] [Google Scholar]
- 7.Kubin L, Alheid GF, Zuperku EJ, McCrimmon DR. Central pathways of pulmonary and lower airway vagal afferents. J Appl Physiol. 2006;101:618–27. doi: 10.1152/japplphysiol.00252.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Widdicombe JG. Afferent receptors in the airways and cough. Respir Physiol Neurobiol. 1998;114:5–15. doi: 10.1016/s0034-5687(98)00076-0. [DOI] [PubMed] [Google Scholar]
- 9.Bessac BF, Jordt S-E. Breathtaking TRP channels: TRPA1 and TRPV1 in airway chemosensation and reflex control. Physiology. 2008;23:360–70. doi: 10.1152/physiol.00026.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kollarik M, Undem B. Sensory transduction in cough-associated nerves. Respir Physiol Neurobiol. 2006;152:243–54. doi: 10.1016/j.resp.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 11.Kollarik M, Ru F, Undem BJ. Acid-sensitive vagal sensory pathways and cough. Pulm Pharmacol Ther. 2007;20:402–11. doi: 10.1016/j.pupt.2006.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee LY, Ni D, Hayes D, Lin RL. TRPV1 as a cough sensor and its temperature-sensitive properties. Pulm Pharmacol Ther. 2011;24:280–5. doi: 10.1016/j.pupt.2010.12.003. [DOI] [PubMed] [Google Scholar]
- 13.Grace MS, Belvisi MG. TRPA1 receptors in cough. Pulm Pharmacol Ther. 2011;24:286–8. doi: 10.1016/j.pupt.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 14.Schroder K, Fahey T. Systematic review of randomised controlled trials of over the counter cough medicines for acute cough in adults. Br Med J. 2002;324:1–6. doi: 10.1136/bmj.324.7333.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eccles R. Mechanisms of the placebo effect of sweet cough syrups. Respir Phys Neurobiol. 2006;152:340–8. doi: 10.1016/j.resp.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 16.Halfdanarson TR, Jatoi A. Chocolate as a cough suppressant: Rationale and justification for an upcoming clinical trial. Support Cancer Ther. 2007;4:119–22. doi: 10.3816/SCT.2007.n.006. [DOI] [PubMed] [Google Scholar]
- 17.Paul IM, Beiler J, McMonagle A, Shaffer ML, Duda L, Berlin CM., Jr Effect of honey, dextromethorphan, and no treatment on nocturnal cough and sleep quality for coughing children and their parents. Arch Pediatr Adolesc Med. 2007;161:1140–6. doi: 10.1001/archpedi.161.12.1140. [DOI] [PubMed] [Google Scholar]
- 18.Engleman EG, Lankton J, Lankton B. Granulated sugar as treatment for hiccups in conscious patients. N Engl J Med. 1971;285:1489. [PubMed] [Google Scholar]
- 19.Anseloni VC, Weng HR, Terayama R, Letizia D, Davis BJ, Ren K, et al. Age dependency of analgesia elicited by intraoral sucrose in acute and persistent pain models. Pain. 2002;97:93–103. doi: 10.1016/s0304-3959(02)00010-6. [DOI] [PubMed] [Google Scholar]
- 20.Bhattacharjee M, Mathur R. Antinociceptive effect of sucrose ingestion in the human. Indian J Physiol Pharmacol. 2005;49:383–94. [PubMed] [Google Scholar]
- 21.Pepino MY, Mennella JA. Sucrose-induced analgesia is related to sweet preferences in children but not adults. Pain. 2005;19:210–18. doi: 10.1016/j.pain.2005.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kakeda T, Ishikawa T. Gender differences in pain modulation by a sweet stimulus in adults: A randomized study. Nurs Health Sci. 2011;13:34–40. doi: 10.1111/j.1442-2018.2010.00573.x. [DOI] [PubMed] [Google Scholar]
- 23.Barr RG, Pantel MS, Young SN, Wright JH, Hendricks LA, Gravel R. The response of crying newborns to sucrose: is it a “sweetness” effect? Physiol Behav. 1999;66:409–17. doi: 10.1016/s0031-9384(98)00294-7. [DOI] [PubMed] [Google Scholar]
- 24.Bucher HU, Baumgartner R, Bucher N, Seiler M, Fauchere JC. Artificial sweetener reduces nociceptive reaction in term newborn infants. Early Hum Dev. 2000;59:51–60. doi: 10.1016/s0378-3782(00)00085-2. [DOI] [PubMed] [Google Scholar]
- 25.Ramenghi LA, Evans DJ, Levene MI. “Sucrose analgesia”: absorptive mechanism or taste perception? Arch Dis Child Fetal Neonatal Ed. 1999;80:F146–7. doi: 10.1136/fn.80.2.f146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pelchat ML. Of human bondage: food craving, obsession, compulsion, and addiction. Physiol Behav. 2002;76:347–52. doi: 10.1016/s0031-9384(02)00757-6. [DOI] [PubMed] [Google Scholar]
- 27.Jain R, Mukherjee K, Singh R. Influence of sweet tasting solutions on opioid withdrawal. Brain Res Bull. 2004;64:319–22. doi: 10.1016/j.brainresbull.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 28.Lewkowski MD, Young SN, Ghosh S, Ditto B. Effects of opioid blockade on the modulation of pain and mood by sweet taste and blood pressure in young adults. Pain. 2008;135:75–81. doi: 10.1016/j.pain.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 29.Eddy NB, Friebel H, Hahn KJ, Halbach H. Codeine and its alternates for pain and cough relief. Bull World Health Organ. 1969;40:425–54. [PMC free article] [PubMed] [Google Scholar]
- 30.Cliff MA, Green BG. Sensitization and desensitization to capsaicin and menthol in the oral cavity: Interactions and individual differences. Physiol Behav. 1996;59:487–94. doi: 10.1016/0031-9384(95)02089-6. [DOI] [PubMed] [Google Scholar]
- 31.Galeotti N, Di Cesare Mannelli L, Mazzanti G, Bartolini A, Ghelardini C. Menthol: A natural analgesic compound. Neurosci Lett. 2002;322:145–8. doi: 10.1016/s0304-3940(01)02527-7. [DOI] [PubMed] [Google Scholar]
- 32.Green BG, McAuliffe BL. Menthol desensitization of capsaicin irritation. Evidence of a short-term anti-nociceptive effect. Physiol Behav. 2000;68:631–9. doi: 10.1016/s0031-9384(99)00221-8. [DOI] [PubMed] [Google Scholar]
- 33.Klein AH, Sawyer CM, Carstens MI, Tsagareli MG, Tsiklauri N, Carstens E. Topical application of l-menthol induces heat analgesia, mechanical allodynia, and a biphasic effect on cold sensitivity in rats. Behav Brain Res. 2010;212:179–86. doi: 10.1016/j.bbr.2010.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Klein AH, Carstens MI, Zanotto KL, Sawyer CM, Ivanov M, Cheung S, et al. Self-and cross-desensitization of oral irritation by menthol and cinnamaldehyde (CA) via peripheral interactions at trigeminal sensory neurons. Chem Senses. 2011;36:199–208. doi: 10.1093/chemse/bjq115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Willis DN, Liu B, Ha MA, Jordt SE, Morris JB. Menthol attenuates respiratory irritation responses to multiple cigarette smoke irritants. FASEB J. 2011;25:4434–44. doi: 10.1096/fj.11-188383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wise PM, Preti G, Eades J, Wysocki CJ. The effect of menthol vapor on nasal sensitivity to chemical irritation. Nicotine Tob Res. 2011;13:989–97. doi: 10.1093/ntr/ntr107. [DOI] [PubMed] [Google Scholar]
- 37.Morice AH, Marshall AE, Higgins KS, Grattan TJ. Effect of inhaled menthol on citric acid induced cough in normal subjects. Thorax. 49:1024–6. doi: 10.1136/thx.49.10.1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kenia P, Houghton T, Beardsmore C. Does inhaling menthol affect nasal patency or cough? Pediatr Pulmonol. 2008;43:532–7. doi: 10.1002/ppul.20797. [DOI] [PubMed] [Google Scholar]
- 39.Dicpinigaitis PV. Inhalation cough challenge. Curr Resp Med Rev. 2010;6:142–7. [Google Scholar]
- 40.Dicpinigaitis PV. Experimentally induced cough. Pulm Pharmacol Ther. 2007;20:319–24. doi: 10.1016/j.pupt.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 41.Hutchings HA, Morris S, Eccles R, Jawad M. Voluntary suppression of cough induced by inhalation of capsaicin in healthy volunteers. Respir Med. 1993;87:379–82. doi: 10.1016/0954-6111(93)90052-2. [DOI] [PubMed] [Google Scholar]
- 42.Widdicombe J, Eccles R, Fontana G. Supramedullary influences on cough. Respir Physiol Neurobiol. 2006;152:320–8. doi: 10.1016/j.resp.2006.02.018. [DOI] [PubMed] [Google Scholar]
- 43.Rebouças EC, Segato EN, Kishi R, Freitas RL, Savoldi M, Morato S, et al. Effect of the blockade of mu1-opioid and 5HT2A-serotonergic/alpha1-noradrenergic receptors on sweet-substance-induced analgesia. Psychopharmacology (Berl) 2005;179:349–55. doi: 10.1007/s00213-004-2045-x. [DOI] [PubMed] [Google Scholar]
- 44.Bhattacharjee M, Bhatia R, Mathur R. Gender specificity of sucrose induced analgesia in human adults. Indian J Physiol Pharmacol. 2007;51:410–14. [PubMed] [Google Scholar]
- 45.McKemy DD, Neuhausser WM, Julius D. Identification of a cold receptor reveals a general role for TRP channels in thermosensation. Nature. 2002;416:52–8. doi: 10.1038/nature719. [DOI] [PubMed] [Google Scholar]
- 46.Peier AM, Moqrich A, Hergarden AC, Reeve AJ, Andersson DA, Story GM, et al. A TRP channel that senses cold stimuli and menthol. Cell. 2002;108:705–715. doi: 10.1016/s0092-8674(02)00652-9. [DOI] [PubMed] [Google Scholar]
- 47.Macpherson LJ, Hwang SW, Miyamoto T, Dubin AE, Patapoutian A, Story GM. More than cool: promiscuous relationships of menthol and other sensory compounds. Mol Cell Neurosci. 2006;32:335–43. doi: 10.1016/j.mcn.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 48.Karashima Y, Damann N, Prenen J, Talavera K, Segal A, Voets T, et al. Bimodal action of menthol on the transient receptor potential channel TRPA1. J Neurosci. 2007;27:9874–84. doi: 10.1523/JNEUROSCI.2221-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xiao B, Dubin AE, Bursulaya B, Viswanath V, Jegla TJ, Patapoutian A. Identification of transmembrane domain 5 as a critical molecular determinant of menthol sensitivity in mammalian TRPA1 channels. J Neurosci. 2008;28:9640–51. doi: 10.1523/JNEUROSCI.2772-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Andrè E, Campi B, Materazzi S, Trevisani M, Amadesi S, Massi D, et al. Cigarette smoke-induced neurogenic inflammation is mediated by alpha, beta-unsaturated aldehydes and the TRPA1 receptor in rodents. J Clin Invest. 2008;118:2574–82. doi: 10.1172/JCI34886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bessac BF, Jordt SE. Sensory detection and responses to toxic gases: mechanisms, health effects, and countermeasures. Proc Am Thorac Soc. 2010;7:269–77. doi: 10.1513/pats.201001-004SM. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Talavera K, Gees M, Karashima Y, Meseguer VM, Vanoirbeek JA, Damann N, et al. Nicotine activates the chemosensory cation channel TRPA1. Nature Neuroscience. 2009;12:1293–9. doi: 10.1038/nn.2379. [DOI] [PubMed] [Google Scholar]
- 53.Peyrot des Gachons C, Uchida K, Bryant B, Shima A, Sperry JB, Dankulich-Nagrudny L, et al. Unusual pungency from extra-virgin olive oil is attributable to restricted spatial expression of the receptor of oleocanthal. J Neurosci. 2011;31:999–1009. doi: 10.1523/JNEUROSCI.1374-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ginzel KH. The importance of sensory nerve endings as sites of drug action. Naunyn Schmiedebergs Arch Pharmacol. 1975;288:29–56. doi: 10.1007/BF00501812. [DOI] [PubMed] [Google Scholar]
- 55.Squier CA, Mantz MJ, Wertz PW. Effect of menthol on the penetration of tobacco carcinogens and nicotine across porcine oral mucosa ex vivo. Nicotine Tob Res. 2010;12:763–7. doi: 10.1093/ntr/ntq084. [DOI] [PubMed] [Google Scholar]
- 56.Pelletier CA, Dhanaraj GE. The effect of taste and palatability on lingual swallowing pressure. Dysphagia. 2006;21:121–8. doi: 10.1007/s00455-006-9020-0. [DOI] [PubMed] [Google Scholar]
- 57.Yamamura K, Kitagawa J, Kurose M, Sugino S, Takatsuji H, Mostafeezur RM, et al. Neural mechanisms of swallowing and effects of taste and other stimuli on swallow initiation. Biol Pharm Bull. 2010;33:1786–90. doi: 10.1248/bpb.33.1786. [DOI] [PubMed] [Google Scholar]
- 58.Pitts TE, Morris K, Lindsey B, Davenport PW, Poliacek I, Bolser DC. Coordination of cough and swallow in vivo and in silico. Exp Physiol. doi: 10.1113/expphysiol.2011.063362/pdf. Epub Dec 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mistry S, Rothwell JC, Thompson DG, Hamdy S. Modulation of human cortical swallowing motor pathways after pleasant and aversive taste stimuli. Am J Physiol Gastrointest Liver Physiol. 2006;291:G666–71. doi: 10.1152/ajpgi.00573.2005. [DOI] [PubMed] [Google Scholar]
- 60.Wood AJ. ([Report prepared for Ted Bates & Company] 1959 Bates No.: 679213552-679214020).Kool cigarettes: Its image and place in the menthol market. Available at: http://tobaccodocuments.org/bw/456658.html.
- 61.Kreslake JM, Wayne GF, Connolly GN. The menthol smoker: Tobacco industry research on consumer sensory perception of menthol cigarettes and its role in smoking behavior. Nicotine Tob Res. 2008;10:705–15. doi: 10.1080/14622200801979134. [DOI] [PubMed] [Google Scholar]
- 62.Rising J, Wasson-Blader K. Menthol and initiation of cigarette smoking. Tob Induc Dis. 9(Suppl 1):S4. doi: 10.1186/1617-9625-9-S1-S4. [Internet]. [cited 2011 May 23] Available from: http://www.tobaccoinduceddiseases.com/content/9/S1/S4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ahijevych K, Garrett BE. The role of menthol in cigarettes as a reinforcer of smoking behavior. Nicotine Tob Res. 2010;12(Suppl 2):S110–6. doi: 10.1093/ntr/ntq203. [DOI] [PMC free article] [PubMed] [Google Scholar]


