Abstract
The immune system plays a critical role in the modulation of atherogenesis at all stages of the disease. However, there are many technical difficulties when studying the immune system within murine aortas. Common techniques such as PCR and immunohistochemistry have answered many questions about the presence of immune cells and mediators of inflammation within the aorta yet many questions remain unanswered due to the limitations of these techniques. On the other hand, cumulatively the flow cytometry approach has propelled the immunology field forward but it has been challenging to apply this technique to aortic tissues. Here, we describe the methodology to isolate and characterize the immune cells within the murine aorta and provide examples of functional assays for aortic leukocytes using flow cytometry. The method involves the harvesting and enzymatic digestion of the aorta, extracellular and intracellular protein staining, and a subsequent flow cytometric analysis.
Keywords: Atherosclerosis, Mouse Aorta, Flow Cytometry, Leukocytes
1. Introduction
Atherosclerosis is characterized by the formation of calcified plaques in the large and medium sized blood vessels due to changes in lipid metabolism, the activation of vascular wall components, and an increased pro-inflammatory immune response within the aortic wall. Mice are common models for atherosclerotic studies because of the ease of genetic manipulation and relatively quick progression of disease, where atherosclerotic plaques can be detected in aged or high fat diet-fed animals (1). However, the murine model also poses several challenges for immunological analysis due the size and the number of cells obtained from the aorta.
The aorta contains a heterogeneous population of endothelial cells, smooth muscles cells, and leukocytes. Leukocytes play a pivotal role in modulating disease pathogenesis and inflammation. Several leukocyte populations and their corresponding subsets including macrophages, dendritic cells, neutrophils, mast cells, and T and B lymphocytes reside within the aortic intima, media, and adventitia (2). Interestingly, small numbers of leukocytes are found within healthy, non-inflamed aortas but this number increases with the progression of atherosclerosis (3). The change in aortic leukocyte populations is due to altered influx and efflux rates of leukocytes as well as changes in the proliferation and apoptotic rates of specific leukocyte subsets within the aorta (2). Thus, it is not only difficult to identify these small numbers of cells within minimal amounts of available tissue, but understanding the dynamic phenotype and functions of these leukocytes is another challenge.
Techniques such as polymerase chain reaction (PCR) and immunohistochemistry (IHC) offered a firm foundation for our understanding of the immunological composition of the aorta. However, both of these techniques have several limitations that are further highlighted when studying atherosclerosis in the murine model. The small quantities of material needed for analysis makes PCR useful; however, to study the immune composition several markers are often needed to identify one cell type. On the other hand, immunohistochemistry offers the ability to use several cell markers simultaneously to identify single cell populations, their location within the aortic tissue, and any interacting partners. However, when performing immunohistochemistry on aortic tissue samples, functions of a specific cell population cannot be explored, and the data is only semi-quantitative, which may be difficult to analyze when addressing questions focused on small numbers of cells.
With the advancements of flow cytometry, studying the immune composition of mouse aortas has become increasingly feasible. Flow cytometry provides a method to identify and quantify subpopulations within a heterogeneous sample. Additionally, flow cytometry can be used to analyze certain functional properties of cells such as proliferation, apoptotic state, or cytokine production. Flow cytometry has been successfully used to analyze the immune response with several solid tissues including kidney, skin, and heart (4–6). Several investigators attempted to use flow cytometry to analyze the aorta, but remnant tissue debris, high auto-fluorescence, and loss of cells (7–9) made the analysis challenging. Recently, Galkina et al. developed a flow cytometry-based method using a combination of multiple enzymes to analyze aortic leukocytes (3). The use of CD45, a common leukocyte marker, significantly improved the quality of samples permitting the exclusion of highly-auto-fluorescent non-CD45 vascular cells from leukocyte analysis. Since then, several modifications for this protocol and varying enzyme combinations were proposed for flow cytometry analysis (10–13).
Despite the benefits of flow cytometry, this technique also has limitations, some of which are common with PCR and IHC. The amount of material required for quantification of aortic leukocytes by flow cytometry is larger than PCR, but like PCR, the specific location of analyzed cells cannot be identified. Furthermore, the enzymatic digestion of samples can alter the expression of surface markers and may increase auto-fluorescence that must be accounted for during analysis (9). Despite these limitations, flow cytometry allows the simultaneous use of multiple markers to identify a cell type resulting in a more detailed analysis of the aortic sample. Additionally, flow cytometry provides quantitative and qualitative results.
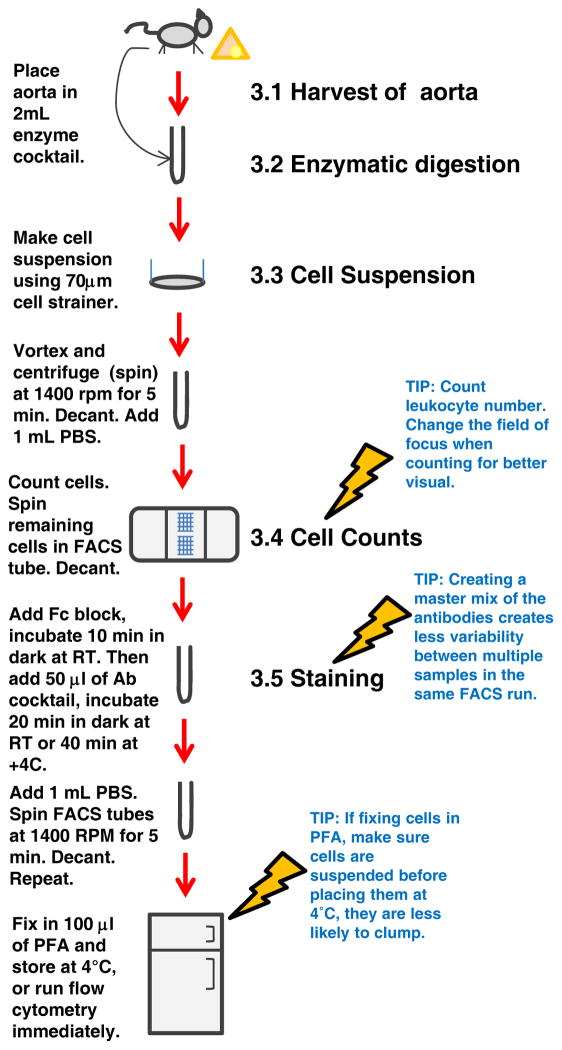
Here, we describe the method to prepare and complete flow cytometric analysis on leukocytes isolated from atherosclerotic Apolipoprotein E-null (Apoe−/−) murine aortas (Figure 1). This includes harvesting the aorta, treatment by enzymatic digestion, cell retrieval, flow cytometric preparation and sample collection, followed by a detailed description of the analysis performed to identify the aortic leukocyte populations and potential functional applications.
Figure 1.
Schematic describing aortic flow cytometry protocol.
2. Materials
2.1. Harvest Aorta
Microdissection tools.
Surgical microscope (e.g. Diagnostic Instruments Inc., Sterling Heights, Michigan).
1x Phosphate buffered saline (PBS): 2.7 mM potassium choloride, 1.5 mM potassium phosphate monobasic, 137.9 mM sodium chloride, 8.1 mM sodium phosphate dibasic.
2% heparin diluted in PBS.
12×75 mm, 5 mL polystyrene round bottom test tube (FACS tubes).
2.2 Enzymatic digestion
Collagenase, Type I (e.g. Worthington Biochemicals, Lakewood, New Jersey).
Collagenase, Type XI (e.g. Sigma Aldrich, St. Louis, Missouri).
Deoxyribonuclease I (DNase) from bovine pancreas, type II (e.g. Sigma Aldrich).
Hyaluronidase from bovine testes, type 1-s (e.g. Sigma Aldrich).
Water bath.
2.3. Cell suspension
70-μm nylon cell strainer.
Syringe without needle.
Table-top, swinging bucket centrifuge 5810R.
Red blood cell lysis buffer: 155 mM ammonium chloride (NH4Cl), 10 mM potassium bicarbonate (KHCO3), and 1.27 mM ethylenediaminetetraacetic acid (EDTA) diluted in distilled water, pH 7.4. Others are commercially available.
2.4. Cell Counting
0.4% solution Trypan blue.
Bright-line hemacytometer.
Light microscope.
2.5. Extracellular Staining
Fc blocking antibodies (CD16/CD32).
Antibodies applicable for flow cytometry.
Fixable Viability Dye (eBioscience, CA).
2% Paraformaldehyde (PFA).
2.6. Intracellular Staining
RPMI-1640 medium supplemented with 10% Fetal Bovine Serum, heat inactivated (FBS) and 1% Penicillin-Streptomycin.
Phorbol 12-myristate 13-acetate (PMA).
Ionomycin calcium salt from Streptomyces Conglobatus.
Golgi Plug solution containing brefeldin A (BD Biosciences).
Humidified incubator with CO2 tank.
Cytofix/Perm kit (BD Biosciences).
2.7 Aortic sample acquisition and analysis
Flow cytometry acquisition instrument (e.g. 8-color updated BD FACS Calibur, BD Biosciences/Cytek).
FACS tubes with cell strainer caps.
Flow cytometry analysis software (e.g. FlowJo).
3. Method
3.1. Harvest Aorta
After euthanizing the mouse, remove all blood via cardiac puncture (See Note 1).
Open the abdominal and chest cavities and gently perfuse the aorta with PBS containing 2% heparin to remove blood from the vessels.
Carefully remove any surrounding adipose tissue and dissect aorta (including branches within the arch), keeping the adventitia intact (See Note 2).
Place aorta in a FACS tube in 2 mL of PBS on ice.
Collect the spleen to prepare single control samples for flow cytometer parameter settings (See Step 4 in Section 3.3 Cell Suspension and Step 1 in Section 3.5 Extracellular staining for details).
3.2. Enzymatic digestion
-
Place 35 μL of enzyme digestion solution in the FACS tube containing the aorta and 2 mL of PBS. Aortas can be cut into small pieces to improve digestion of the tissues. Final enzyme digestion solution concentration (diluted in PBS) is:
450 U/mL collagenase type I
125 U/mL collagenase type XI
60 U/mL hyaluronidase type I-s
60 U/mL DNase-I
Incubate for 45–60 minutes at 37°C, cover tubes with caps (See Note 3).
3.3. Cell suspension
Use 70-μm cell strainer and syringe to release cells from digested aortic tissue returning cells to FACS tube.
Centrifuge at 368 rcf at 4°C for 5 minutes, then decant supernatant (See Notes 4 and 5).
Resuspend in 1 mL of PBS and place on ice.
For splenic tissue: Prepare single cell suspension using a 70-μm cell strainer, remove red blood cells using lysis buffer, wash cells twice with PBS, then centrifuge and decant as done with aortic samples. Resuspend in PBS.
3.4. Cell Counts
Pipet or vortex the cell suspension to ensure it is homogenous.
Count the number of aortic leukocytes using trypan blue to exclude non-viable cells.
Calculate the number of aortic leukocytes (See Note 6).
Centrifuge cells at 368 rcf, 4°C for 5 minutes then decant supernatant (See Note 5).
Following the same procedure, count the number of splenic leukocytes.
3.5 Extracellular Staining
See Note 7 for an explanation of the appropriate controls to use.
If interested in intracellular staining, see Section 3.6 Intracellular Cytokine Staining before proceeding with extracellular staining.
Perform staining on aortic cell suspension using a standard flow cytometry protocol. Briefly, incubate 1.0–1.5 × 106 cells with 10 μg/ml of Fc blocking buffer (See Note 8). (0.5–1.0 × 106 splenic cells should be used for each control sample).
Incubate for 10 minutes at room temperature to inhibit non-specific binding of antibodies.
Add antibodies of interest at appropriate dilutions to 100 μL of diluted Fc blocking buffer and briefly vortex (See Notes 8–12).
If completing the viability staining, incorporate with the extracellular antibody staining here.
Incubate samples in the dark for 20 minutes at room temperature or 40 minutes at 4°C.
After the antibody incubation, add 1 mL of PBS to wash.
Centrifuge at 368 rcf at 4°C for 5 minutes and decant the supernatant (See Note 5).
Repeat Steps 8 and 9.
If completing intracellular staining, return to Section 3.6 Intracellular Cytokine Staining.
After washing, fix cells in 100 μL of 2% PFA (See Note 13).
Store overnight at 4°C in the dark (See Note 14).
3.6 Intracellular Cytokine Staining
After counting cells, resuspend cells in FACS tube or cell culture plate with RPMI-1640 medium supplemented with 10% FBS and 1% Penicillin/Streptomycin.
Add to the medium, at a final concentration of 10 ng/mL PMA, 500 ng/mL Ionomycin C, and 500 ng/mL Golgi Plug to stimulate cytokine production.
Plate cells at a concentration of 1×106 cells/mL.
Incubate at 37°C with 5% CO2 for 5 hours.
If incubating in a cell culture plate, wash and remove cells, placing them in FACS tube.
Centrifuge at 368 rcf for 5 min in 4°C, and then decant the supernatant (See Note 5).
Wash the cells once with PBS.
Centrifuge at 368 rcf for 5 min in 4°C and decant the supernatant (See Note 5).
Stop here and complete Section 3.5 Extracellular Staining.
After completing extracellular staining without fixation, use BD CytoFix/Perm kit to prepare cells for intracellular cytokine staining (follow manufacturer’s protocol and See Note 8).
After BD protocol completion, fix cells in 2% PFA (See Note 14).
3.7 Aortic sample acquisition
Set up flow cytometer and settings according to manufacturer’s protocol (See Note 15) and filter the samples using the cell strainer.
Use unstained and single samples prepared from the spleen to set the appropriate forward (FSC) and side (SSC) scatter as well as gain values for the fluorescent channels to depict clear negative and positive populations for each fluorophore used. Then, begin collecting control samples (See Note 17).
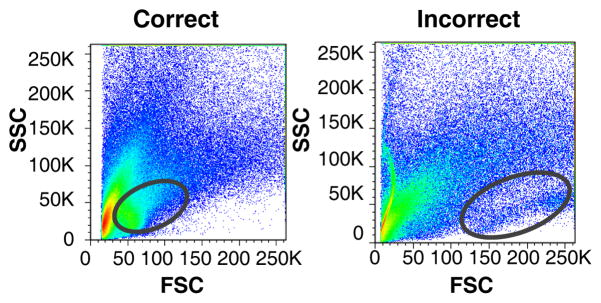
Run samples of interest (See Notes 16–19). For aortic samples, first ensure the FSC and SSC thresholds are set appropriately (Figure 2).
When sample volume reaches lower limit, add sheath fluid to sample, briefly vortex, and place back for collection. Continue collecting cells to ensure that all cells are recorded (See Note 19).
Figure 2. Setting of appropriate FSC and SSC for aortic tissue sample.
FSC and SSC can be slightly altered between different tissue samples. Depicted here are representative FACS plots of the FSC and SSC of aortic cell suspensions, in which different gains were used to set up FSC/SSC parameters. (Left) Correctly positioned leukocytes between 50–100K on FSC and between 25–50K on SSC versus (Right) incorrect positioning where the leukocyte population is shifted too much to the right.
3.8 Analysis
Using flow cytometric analysis software set the appropriate compensation values using the single controls.
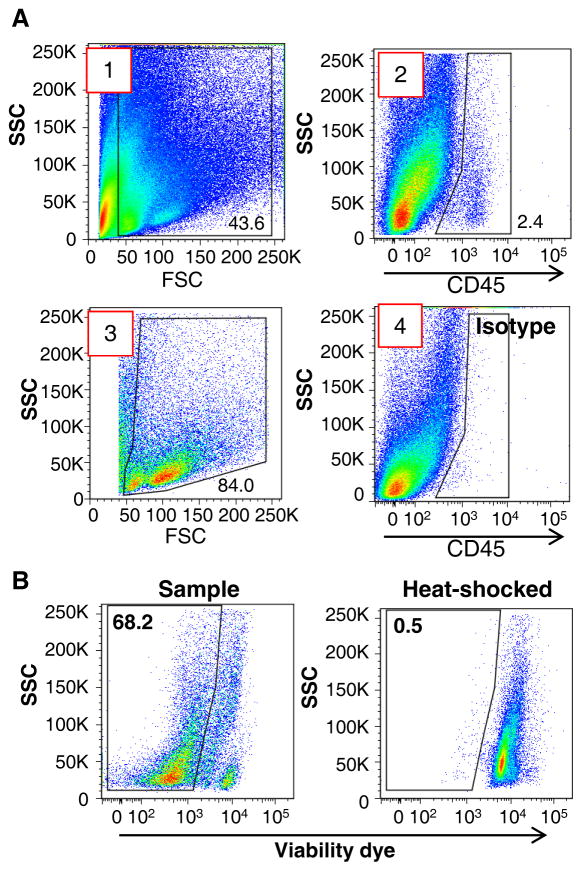
For the aortic samples, gate out cellular debris less than ~40K on the FSC axis (Figure 3A Plot #1).
From the gated cells, identify CD45+ cells as leukocytes (Figure 3A Plot #2) using the unstained aorta and/or the CD45 isotype control staining (Figure 3A Plot #4) as control references.
Once appropriately identified, check the FSC and SSC of the CD45+ gated cells and again gate out cellular debris less than 40K on the FSC axis (Figure 3A Plot #3).
If viability staining was used, identify all live cells (Figure 3B, left). A sample that was heat shocked at 70°C can be used as a positive control to set the appropriate gates (Figure 3B, right).
Begin analyzing the aortic immune composition by first establishing where the populations are located on the FACS plots using the isotype and FMO gates (Figure 4, right panels). Apply these gates directly to the samples of interest. Total leukocyte populations can be detected from the CD45+ population (Figure 5) and more specific analysis of leukocyte subset populations can be detected using additional markers (Figures 4 and 5) (See Note 20).
If cell types express various levels of a specific marker of interest, use the histogram feature to identify lo/intermediate/high populations (See Figure 4C).
For functional assays, the gating scheme will be dependent on the experimental design (See Figure 6 for examples of possible functions of leukocytes measured by flow cytometry).
Data can be represented as a percentage or cell number.
Figure 3. Initial gating scheme for aortic cell samples.
(A) (1) Gate out events that are less than 40K on the FSC axis. (2) Next, establish the gate for CD45 positive staining using the unstained aortic sample and/or isotype control staining for CD45 in aortic tissues (see Figure 3A (4) as an example). (3) From the established CD45+ population, gate out additional events that are less than 40K on the FSC axis. (B) To avoid high auto-fluorescent background and non-specific staining for aortic cells, a viability dye can be used. (Left) Representative FACS plot for aortic sample with Fixable Viability Dye ef506 demonstrates 68.2% of viable aortic cells within the analyzed sample. (Right) Gates for non-viable and viable cells can be determined by using heat shocked positive control sample.
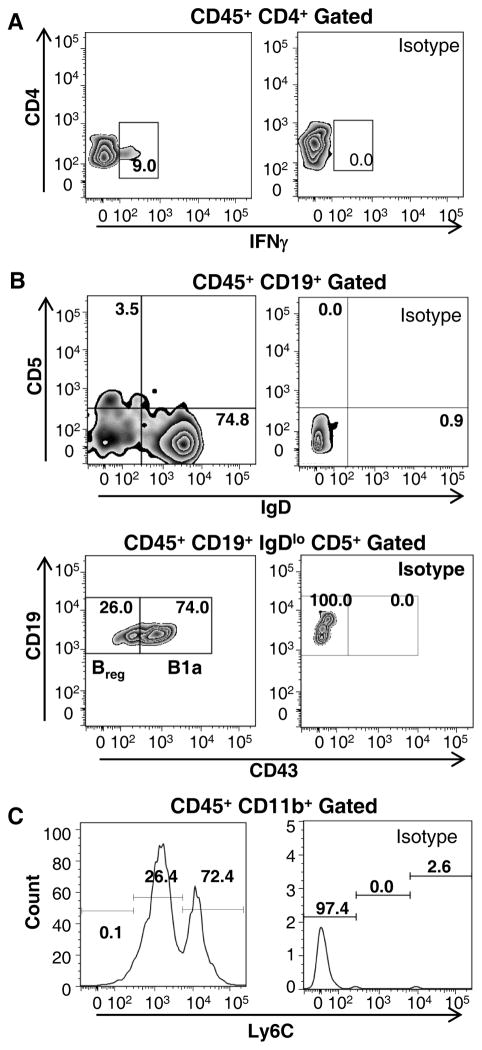
Figure 4. Characterization of several subpopulations of leukocytes within the murine aorta.
Flow cytometric analysis of aortas allows analyzing the distribution of leukocyte subpopulations within the murine aortas using both extracellular and intracellular markers. Aortic cell suspensions were prepared from a whole Apoe−/− aorta and stained with various antibodies against CD45, CD4, IFNγ, CD19, IgD, CD5, CD43, CD11b, and Ly6C. Cells were gated on CD45+ cells and debris was excluded based on the FSC/SSC profiles. (A) To distinguish the T helper 1 subset (Th1) of CD4+ cells within Apoe−/− aortas, PMA-stimulated CD4+ cells were stained with anti-IFNγ antibodies (Left) and analyzed based on isotype control staining (Right). (B) Detection of several B cell subsets within Apoe−/− aortas. Regulatory B cells are identified as CD19+CD5+IgDlo, then gated as CD43−, whereas B1a are CD43+ cells. Gating is determined by isotype staining (Right). (C) Distinction of monocyte subsets within CD11b+ population. Pro-inflammatory monocytes were identified as Ly6Chi myeloid cells and Ly6Clow myeloid cells as patrolling monocytes. Positive Ly6C staining is based on isotype control staining (14).
Figure 5. Variety of immune cells present within the murine aorta.
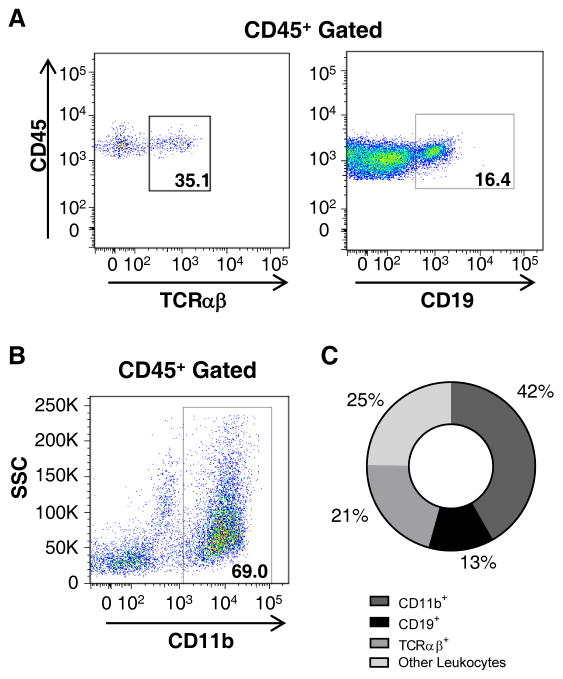
Identification of several leukocyte subpopulations and the characterization of the immune composition of the aorta can be provided by flow cytometry. Aortic cell suspensions were prepared from a whole Apoe−/− aorta (as we described in the text) and stained for CD45, TCRαβ CD19, and CD11b. Cells were gated on CD45+ leukocytes and debris was excluded based on the FSC/SCC profiles. Representative FACS plot of aortic cells isolated from Apoe−/− aortas stained with (A) anti-TCRαβ CD19, and (B) anti-CD11b Abs. Gates set up are based on FMO and isotype controls (not shown). (C) Composition of immune cells (%) in aortas of 40–50 week old Apoe−/− mice (n=12).
Figure 6. Functional applications of flow cytometry on aortic tissue.
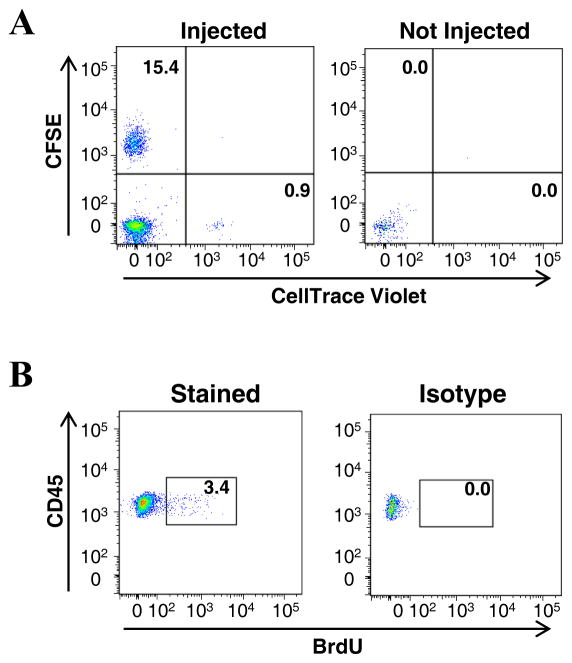
(A) L-selectin-sufficient and -deficient splenic B cells were individually labeled with carboxyfluorescein succinimidyl ester (CFSE) and CellTrace Violet (Invitrogen), respectively, then intravenously injected via tail vein into a 50-week old Apoe−/− recipient fed chow diet. After 16 hours post-injection, aortas were harvested and stained with antibodies against CD45 and CD19. Migrated L-selectin-sufficient B cells were identified as CD45+CD19+CFSE+ and L-selectin–deficient B cells as CD45+CD19+CellTrace Violet+ (Left). The migration is analyzed by gates set from a non-injected mouse (Right). (B) 50-week old Apoe−/− mice were intraperitoneally injected with BrdU every 12 hours, aortas harvested 24 hours after the initial injection. Aortas were digested and aortic cell suspensions were prepared and stained with antibodies against CD45, CD19, and BrdU (3). Proliferating CD45+CD19+ B cells were identified by positive BrdU staining (Left) in comparison to the isotype control (Right).
Acknowledgments
We thank the EVMS Flow Cytometry Facility for their excellent technical support. This work was funded through NHLBI RO1HL107522 (to E.G.) and NHLBI HL112605 supplemental grant 02S1 (to P.T.M.).
Footnotes
Additional aortas may need to be harvested to prepare the appropriate amount of control samples (refer to Note 7 for details about controls).
For adventitial removal, the aorta must be incubated in an enzyme solution (10, 13) to loosen the adventitial layer prior to the enzymatic digestion referred to in Section 3.2. Avoid freeze-thawing the enzyme solutions as this reduces their activity.
Vortex occasionally throughout incubation. If aorta is not completely digested after 45–60 minute incubation, incubate for another 10 minutes and check again. When resuspending, it should not be difficult to break apart the aorta, if it is this is a sign that the digestion is not complete. Important: Do not over digest.
After centrifugation, there should be a light hazy pellet at the bottom of the tube.
Do not disrupt the pellet while decanting.
Typically, a 50-week old Apoe−/− mouse fed chow diet has 0.91 ± 0.08×106 total aortic leukocytes. This number is increased in comparison to C57BL/6 mice (3). If cell numbers are low, it may be beneficial to pool several aortas together to have sufficient numbers for running on the flow cytometer.
- Unstained splenic and aortic cell suspensions
- “Singles”: prepared from splenic cell suspension. Each tube contains only 1 fluorophore-conjugated antibody that is used in the antibody cocktail for the samples of interest
- “FMO” (fluorescent minus one): prepared from aortic cell suspension. This sample contains all the appropriate fluorophore-conjugated antibodies except one.
- “Isotypes” prepared from aortic cell suspension. This sample contains all the appropriate fluorophore-conjugated antibodies, but one is replaced by the appropriate isotype (containing the same fluorophore) which is recommended by the manufacturer of each antibody. Be sure to use the same amount of isotype antibody that is actually used in the sample of interest.
To reduce variability, make a master mix for all appropriate samples for the Fc block and for staining.
Optimization of the antibody concentration should be determined prior to staining using practice tissue.
Enzymatic digestion of the aorta does interfere with the antigen sensitivity of some antibodies (3). Determine if antigens of interest are affected by enzymatic digestion prior to experiment.
Prepare all controls (singles, FMOs, Isotypes) simultaneously with samples of interest.
Incorporating a red blood cell marker such as TER-119 will help to identify levels of blood contamination within the analyzed samples (3).
Some fluorophore-conjugated antibodies are not stable upon PFA fixation; test during optimization.
If running within several hours after staining, resuspend cells in PBS rather than PFA.
For our acquisition, we use an 8-color updated BD FACS Calibur.
FSC, SSC, and antibody intensity will likely be different between tissues (Figure 2).
Vortex each sample prior to running on flow cytometer to ensure homogeneity.
When starting to collect the first aortic sample, make sure that the distribution of leukocytes on FSC and SSC looks appropriate. Ideally this is done by using an unstained aortic sample. Lymphocyte populations are typically set at the following values: FSC: 50K, SSC: 25K (See Figure 2).
Occasionally run water between samples to clean the instrument.
In some cases it may be necessary to concatenate several files or analyzed samples to increase the number of events seen. This will generate a clearer larger population and is often useful when studying a small leukocyte population.
References
- 1.Getz G, Reardon C. Animal Models of Atherosclerosis. Arterioscler Thromb Vasc Biol. 2012;32:1104–1115. doi: 10.1161/ATVBAHA.111.237693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Galkina E, Ley K. Immune and Inflammatory Mechanisms of Atherosclerosis *. Annu Rev Immunol. 2009;27:165–197. doi: 10.1146/annurev.immunol.021908.132620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Galkina E, Kadl A, Sanders J, et al. Lymphocyte recruitment into the aortic wall before and during development of atherosclerosis is partially L-selectin dependent. J Exp Med. 2006;203:1273–1282. doi: 10.1084/jem.20052205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Afanasyeva M, Georgakopoulos D, Belardi F, et al. Quantitative Analysis of Myocardial Inflammation by Flow Cytometry in Murine Autoimmune Myocarditis. Am J Pathol. 2004;164:807–815. doi: 10.1016/S0002-9440(10)63169-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hammond T. Analysis and isolation of renal tubular cells by flow cytometry. Kidney Int. 1992;42:997–1005. doi: 10.1038/ki.1992.379. [DOI] [PubMed] [Google Scholar]
- 6.Wu H, Smith M, Millenson M, et al. Contribution of Flow Cytometry in the Diagnosis of Cutaneous Lymphoid Lesions. J Invest Dermatol. 2003;121:1522–1530. doi: 10.1046/j.1523-1747.2003.12631.x. [DOI] [PubMed] [Google Scholar]
- 7.Mattsson L, Bondjers G, Wiklund O. Isolation of cell populations from arterial tissue, using monoclonal antibodies and magnetic microspheres. Atherosclerosis. 1991;89:25–34. doi: 10.1016/0021-9150(91)90004-m. [DOI] [PubMed] [Google Scholar]
- 8.Bonanno E, Mauriello A, Partenzi A, et al. Flow cytometry analysis of atherosclerotic plaque cells from human carotids: a validation study. Cytometry. 2000;39:158–165. doi: 10.1002/(sici)1097-0320(20000201)39:2<158::aid-cyto9>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 9.Liu-Wu Y, Svenningsson A, Stemme S, et al. Identification and analysis of macrophage-derived foam cells from human atherosclerotic lesions by using a “mock” FL3 channel in flow cytometry. Cytometry. 1997;29:155–164. [PubMed] [Google Scholar]
- 10.Butcher M, Herre M, Ley K, et al. Flow Cytometry Analysis of Immune Cells Within Murine Aortas. J Vis Exp. 2011;53 doi: 10.3791/2848. pii 2848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weber C, Meiler S, Döring Y, et al. CCL17-expressing dendritic cells drive atherosclerosis by restraining regulatory T cell homeostasis in mice. J Clin Invest. 2011;121:2898–2910. doi: 10.1172/JCI44925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Koltsova E, Chodaczek G, Landau M, et al. Dynamic T cell–APC interactions sustain chronic inflammation in atherosclerosis. J Clin Invest. 2012;122:3114–3126. doi: 10.1172/JCI61758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smith E, Prasad K, Butcher M, et al. Blockade of Interleukin-17A Results in Reduced Atherosclerosis in Apolipoprotein E-Deficient Mice. Circulation. 2010;121:1746–1755. doi: 10.1161/CIRCULATIONAHA.109.924886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Taghavie-Moghadam P, Butcher M, Galkina E. The dynamic lives of macrophage and dendritic cell subsets in atherosclerosis. Ann N Y Acad Sci. 2014;1319:19–37. doi: 10.1111/nyas.12392. [DOI] [PMC free article] [PubMed] [Google Scholar]