Abstract
Objectives
Adult body mass (MB) empirically scales as height (Ht) squared (MB ∝ Ht2), but does regional body mass and body composition as a whole also scale as Ht2? This question is relevant to a wide range of biological topics, including interpretation of body mass index.
Methods
Dual-energy x-ray absorptiometry (DXA) was used to quantify regional body mass (head [MH], trunk, arms, legs) and whole-body composition (fat, lean soft tissue [LST], and bone mineral content [BMC]) in non-Hispanic (NH) white, NH black, Mexican American, and Korean adults participating in the National Health and Nutrition Examination Survey (NHANES; n=17,126) and Korean NHANES (n=8,942). Regression models were developed to establish Ht scaling powers for each measured component with adjustments for age and adiposity.
Results
Exploratory analyses revealed a consistent scaling pattern across men and women of the four race/ethnic groups: regional mass powers, head (~0.8-1) < arms and trunk (~1.8-2.3) < legs (~2.3-2.6); and body composition, LST (~2.0-2.3) < BMC (~2.1-2.4). Small sex and race/ethnic differences in scaling powers were also observed. As body mass scaled uniformly across the eight sex and race/ethnic groups as Ht~2, tall and short subjects differed in body shape (e.g., Mh/Mb ∝ Ht−~1) and composition.
Conclusions
Adult human body shape and relative composition are a function of body size as defined by stature, a finding that has important implications in multiple areas of biological research.
Keywords: Schuna, Heymsfield, Peterson, Thomas, Heo, Hong, Choi, Body shape, body composition, adiposity, allometric analysis, nutritional assessment
INTRODUCTION
Tall adult humans weigh more than their short counterparts and this difference in body mass (MB) as it relates to height (Ht) is captured by Quetelet’s Rule: Mb ∝ Ht2 (Quetelet et al., 1842). The observation that body mass scales to height with a power of 2 is the basis for body mass index (BMI, Mb/Ht2) now used as a global measure of adiposity and health (Antezana, 2000; Jensen et al., 2013; PiSunyer et al., 1998). While the height power of 2 has long been debated (Benn, 1971; Bogin, 2008; Burton, 2007; Cole, 1991; Diverse Populations Group, 2005; Garn et al., 1986; Green, 2009; Keys et al., 1972; Larsson et al., 2006; Samras, 2007; Smalley et al., 1990; Trefethan, 2013), we recently showed in two large United States (US) and Korean nationally representative samples that body mass scales to height narrowly around a power of 2 across non-Hispanic (NH) white, NH black, Mexican American, and Korean Asian men and women (Heymsfield et al., submitted publication).
The power of 2 observed when body mass is scaled to height across a sample of adults is an empirical observation that at present lacks a theoretical biological or physical basis. Questions have been raised why the power of height is not 3 as would be expected if body mass and height are geometrically related to each other (Burton, 2007; Garn et al., 1986; Samras, 2007; Trefethan, 2013). Several shape indices, including M/H3, were applied in adults with this reasoning prior to the widespread adoption of BMI (Cole, 1991; Keys et al., 1972).
Moreover, it is largely unknown if regional body mass and body composition as a whole also scale to height with powers of 2 even though there are reasons to hypothesize the existence of more variable powers. For example, brain mass is only weakly associated with stature and typically scales as Ht0.5-1 (Heymsfield et al., 2007, 2009, 2014). Brain mass is thus a smaller fraction of body mass in tall adults compared to their short counterparts (i.e., brain mass/Mb ∝ Ht−1). It is thus unlikely that head mass scales similar to body mass (i.e., as Ht2) unless brain mass is a much smaller fraction of head mass in tall people.
As another example, bone mass in mammals scales to body mass with powers greater than 1 (e.g., bone mass ∝ Mb1.05) and large animals thus have a greater fraction of their body mass as skeleton than do small animals (Calder, 1996). Does the same “rule” apply to adult humans and, if so, then it would seem likely that bone mass would scale to height with powers exceeding that observed for body mass. Analysis of regional body mass and body composition scaling to height may thus provide a new opportunity to improve our understanding and interpretation of shape measures such as BMI.
The aim of the present study was to examine regional body mass and whole-body composition scaling to height across NH white, NH black, Mexican American, and Korean Asian men and women. We hypothesized that Quetelet’s Rule (Mb ∝ Ht2) embraces more complex and possibly more informative scaling patterns at the regional mass and whole-body composition levels. Our inclusion of race/ethnic groups in these analyses derives from our longer term explorations of BMI as a globally applicable measure of adiposity and health.
METHODS
Study Design and Rationale
The simple allometric model, Y = αXβε, was applied in the current study in natural logarithmic form as
| (1) |
with Y dependent variable, X predictor variable, β the scaling exponent, α the proportionality constant, and ε the multiplicative error (ε). The model dependent variables included regional body mass and whole-body composition. Height was the main predictor variable with age and %fat as potential additional predictor variables (Heymsfield et al. submitted publication).
In an earlier report on the same subject sample as examined in the present study we provided an in-depth analysis of body mass scaling to height as defined by various forms of equation 1 (Heymsfield et al. submitted publication). That study provided a foundation for the current report and established that adult body mass scales to height with a power of ~2 across the US and Korean adult populations (Supplementary Table 1). Herein we report regional mass and whole-body composition scaling to height in the previously evaluated sample.
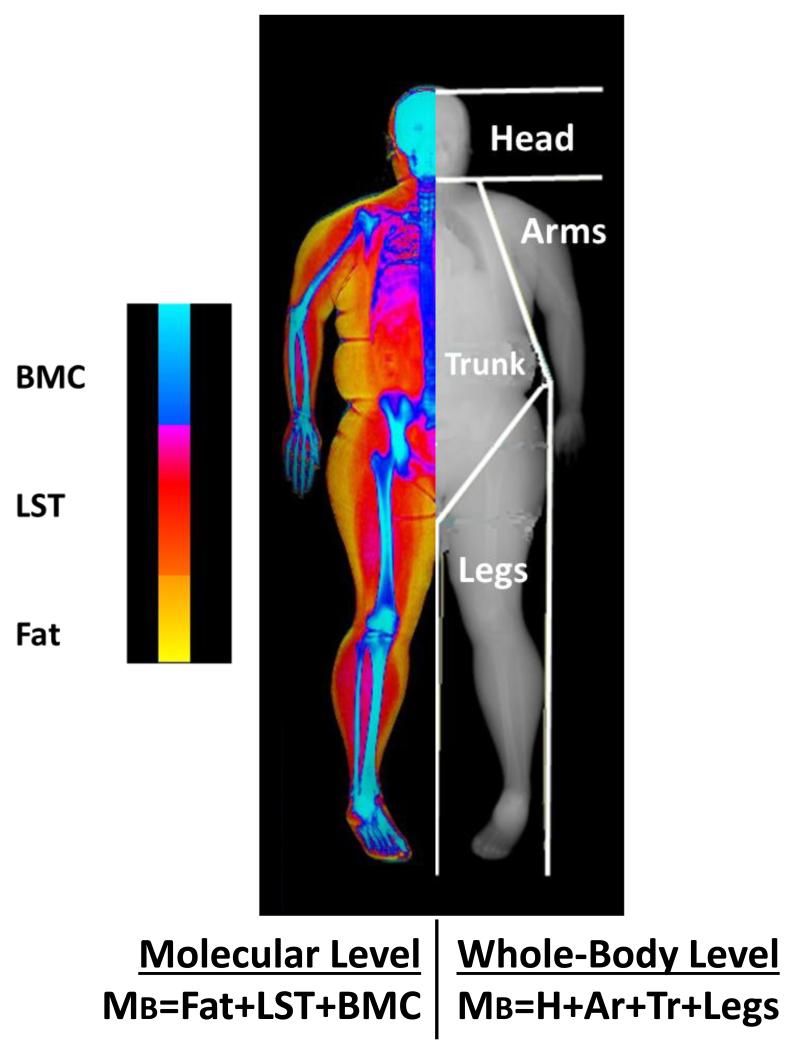
Dual-energy x-ray absorptiometry (DXA) was used to quantify body mass and the mass of four body regions and three body compartments (Figure 1). The pre-defined regional mass measurements included head, trunk, arms, and legs. The trunk region included the pelvis, ribs, and lumbar and thoracic spine. Total body mass was calculated as the sum of head, trunk, arm (right+left), and leg (right+left) mass. DXA also provided estimates of whole body fat, lean soft tissue (LST), and bone mineral content (BMC). Bone mineral content is a stable proportion of bone mass and as such represents a measure of skeletal weight (Pietrobelli et al., 1996). Thus, two parallel models of body mass (kg) were evaluated:
| (2) |
| (3) |
Allometric analyses, with age and adiposity adjustments (Heymsfield et al., submitted publication), included the four body mass (head, trunk, arms, and legs) and two body composition measures (LST and BMC).
Figure 1.
The evaluated regional body mass and whole-body composition regions are shown superimposed on a representative DXA scan. At the whole-body level, body mass (Mb) represents the sum of head (H), trunk (Tr), arm (Ar), and leg mass. At the molecular level, MB represents the sum of total body fat, lean soft tissue (LST), and bone mineral content (BMC) mass.
The study sample evaluated in primary analyses consisted of 8 subject groups (NH white, NH black, Mexican American, and Korean Asian men and women) drawn from the US National Health and Nutrition Examination Survey (NHANES) and the Korean NHANES (KNHANES)(Hong et al., 2011; Kelly et al., 2009; NHANES, 2003-4; Park, 2013).
The first analysis phase involved development of height powers (i.e., β±SE in equation 1) for the six body mass and composition measures across the eight groups with the aim of establishing if there are race/ethnic differences in scaling relations. The second analysis phase involved developing power estimates for the six measures across race/ethnicity-combined men and women with the aim of evaluating between-sex differences in scaling relations.
Subjects
Details of the NHANES and KNHANES sample are provided in previous reports (Heymsfield et al., submitted publication; Hong et al., 2011; Kelly et al., 2009; NCHS, 2011; NHANES, 2003-4; Park, 2013). Subjects were entered into the data set if they were age ≥18 years and had a DXA evaluation. The NHANES and KNHANES survey methodology and measurement protocols are provided in Hong et al. (2011), Kelly et al. (2009), Schenker et al. (2011), and Schoeller et al. (2005). A detailed accounting of the evaluated samples is summarized in Heymsfield, et al. (submitted publication) and in Supplementary Tables 2 and 3. NHANES procedures were approved by the National Center for Health Statistics, Centers for Disease Control and Prevention and the study had human subjects approval with all participants providing written informed consent. The KNHANES protocol was approved by the Korea Center for Disease Control and Prevention Institutional Review Board and all subjects provided written informed consent.
Body Composition Assessment
The same training protocols and DXA systems were used in NHANES and KNHANES as reported in Heymsfield et al. (submitted publication) and in Hong et al. (2011), Kelly et al. (2009), NCHS (2011), and NHANES (2003-4). Regional mass and whole-body composition analysis measurement methods were reported earlier by Heymsfield et al. (Heymsfield et al., submitted publication) and in Hong et al., (2011), Kelly et al. (2009), Schenker et al. (2011), and Schoeller et al. (2005).
Statistical Methods
We performed analyses to describe regional body mass and whole-body composition scaling relations to height that are generalizable to non-institutionalized portions of the US and Korean adult (≥18 yrs) populations. Analyses were conducted using procedures for sample survey data in SAS® (version 9.3; SAS Institute, Cary, NC) to produce nationally representative estimates while accounting for the complex, multistage probability designs of NHANES and KNHANES. Models included sample weights to adjust for non-coverage, non-response, and oversampling of some groups. Standard errors were derived using Taylor series linearization and statistical significance was defined as p <0.05 (two-tailed).
The first analysis phase included evaluation of baseline sample characteristics including total and regional body mass and body composition. Estimates for baseline characteristics were computed using PROC SURVEYMEANS and presented as mean ± SE. Scaling analyses as defined by equation 1 were conducted across the eight sex (men, women) and race/ethnic groups (NH white, NH black, Mexican American, and Korean) as moderated by adiposity and age. For each of the eight sex and race/ethnic groups, separate regression models were fitted using PROC SURVEYREG with regional body mass or whole-body composition component as the dependent variable, and height, age, and %fat as independent variables. All model variables were log-transformed (natural logarithm). Analyses were conducted using the same modeling strategy for NHANES and KNHANES; however, to account for the multiply-imputed structure of NHANES body composition data (5 imputed data sets), separate analyses were conducted for each imputed data set and the resulting estimates were averaged using PROC MIANALYZE. The scaling power (β) and corresponding standard error for height were extracted from each final model and presented as β ± SE (95% CI).
Between-race/ethnicity group differences in baseline characteristics and scaling powers for each sex were evaluated using Cochran’s Q Test for Heterogeneity with 3 degrees of freedom (df). Follow-up analyses of significant omnibus tests were performed by computing Cochran’s Q for all possible pairwise comparisons (6 comparisons with each evaluated at 1 df) while employing a Bonferroni-correction (p < 0.05/6 = 0.0083). Between-sex differences in regional body mass and whole-body composition scaling powers were evaluated using additional dummy-variable regressions while controlling for race/ethnicity.
For NHANES data, confidence intervals for baseline characteristics and scaling powers and critical t-statistic values for testing differences between scaling powers were derived using df calculated according to the method of Barnard and Rubin (1999). The complete-data df used in this determination was 59 (number of primary sampling units – number of sampling strata). For KNHANES data, confidence intervals for baseline characteristics and scaling powers and critical t-statistic values for testing differences between scaling powers were derived using 243 df (number of primary sampling units).
RESULTS
Subjects
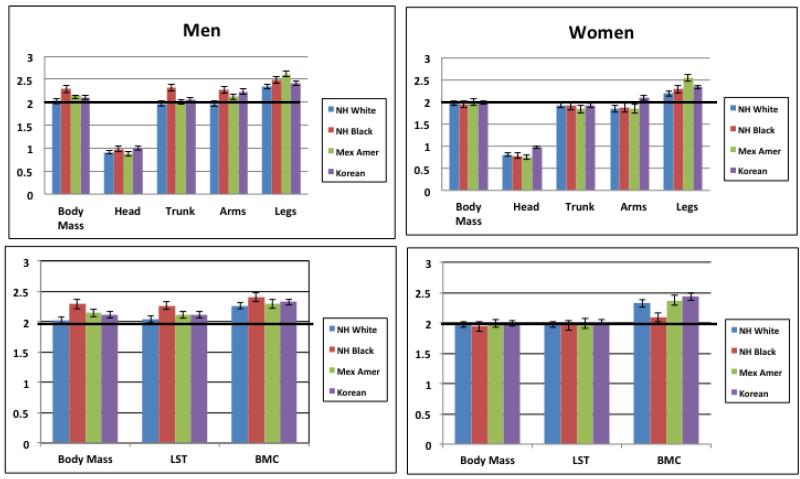
Regional body mass and whole-body composition results are presented in Table 1 for each of the study sex and race/ethnic groups. Total body mass ranged from a low of 56.6±0.2 kg in Korean women to a high of 89.4±0.4 kg in NH white men (Supplementary Table 3). Total body fat mass was smallest in the Korean men (15.6±0.1 kg) and largest in the NH black women (35.1±0.4 kg). The remaining regional mass and body composition means varied in ranking across the sex and race/ethnic groups. Between-group statistical comparisons are shown in the table.
Table 1.
Subject DXA Scan Regional Mass and Body Composition Results.
| Men | Women | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| NH white | NH black | Mex Amer | Korean | p | NH white | NH black | Mex Amer | Korean | p | |
| N | 4406 | 2035 | 2256 | 3851 | 4235 | 2045 | 2149 | 5091 | ||
| Body Mass(kg) | ||||||||||
| Total | 89.4±0.4a | 89.1±0.6a | 81.6±0.6b | 69.4±0.2c | < 0.001 | 74.7±0.4a | 83.9±0.5b | 72.8±0.6a | 56.6±0.2c | < 0.001 |
| Head | 5.19±0.01a | 5.43±0.02b | 5.08±0.02c | 5.44±0.02b | < 0.001 | 4.33±0.01a | 4.84±0.02b | 4.48±0.02c | 4.64±0.01d | < 0.001 |
| Trunk | 44.6±0.2a | 41.5±0.3b | 40.7±0.3b | 33.9±0.1c | < 0.001 | 36.6±0.2a | 39.0±0.3b | 36.5±0.3a | 27.9±0.1c | < 0.001 |
| Arms | 11.12±0.05a | 11.66±0.08b | 10.23±0.08c | 7.74±0.03d | < 0.001 | 8.23±0.05a | 9.78±0.07b | 8.17±0.08a | 5.75±0.02c | < 0.001 |
| Legs | 28.5±0.1a | 30.5±0.2b | 25.5±0.2c | 22.3±0.1d | < 0.001 | 25.5±0.1a | 30.2±0.2b | 23.7±0.2c | 18.2±0.1d | < 0.001 |
| Body Composition(kg) | ||||||||||
| Fat Mass | 26.0±0.2a | 23.8±0.3b | 23.4±0.3b | 15.6±0.1c | < 0.001 | 30.5±0.3a | 35.1±0.4b | 30.3±0.4a | 18.8±0.1c | < 0.001 |
| LST | 60.6±0.2a | 62.3±0.3b | 55.6±0.3c | 53.9±0.2d | < 0.001 | 42.0±0.1a | 46.4±0.2b | 40.5±0.2c | 37.7±0.1d | < 0.001 |
| BMC | 2.75±0.01a | 2.99±0.02b | 2.50±0.01c | 2.60±0.01d | < 0.001 | 2.11±0.01a | 2.33±0.01b | 2.03±0.01c | 2.01±0.01c | < 0.001 |
Results are mean±SE. Abbreviations: BMC, bone mineral content; NH, non-Hispanic; LST, lean soft tissue; Mex Amer, Mexican American. Between-race/ethnicity comparisons performed using Cochran’s Q Test for Heterogeneity. Values with different superscript letters are significantly different at p < 0.05/6 = 0.0083.
Scaling of Regional Body Mass to Height
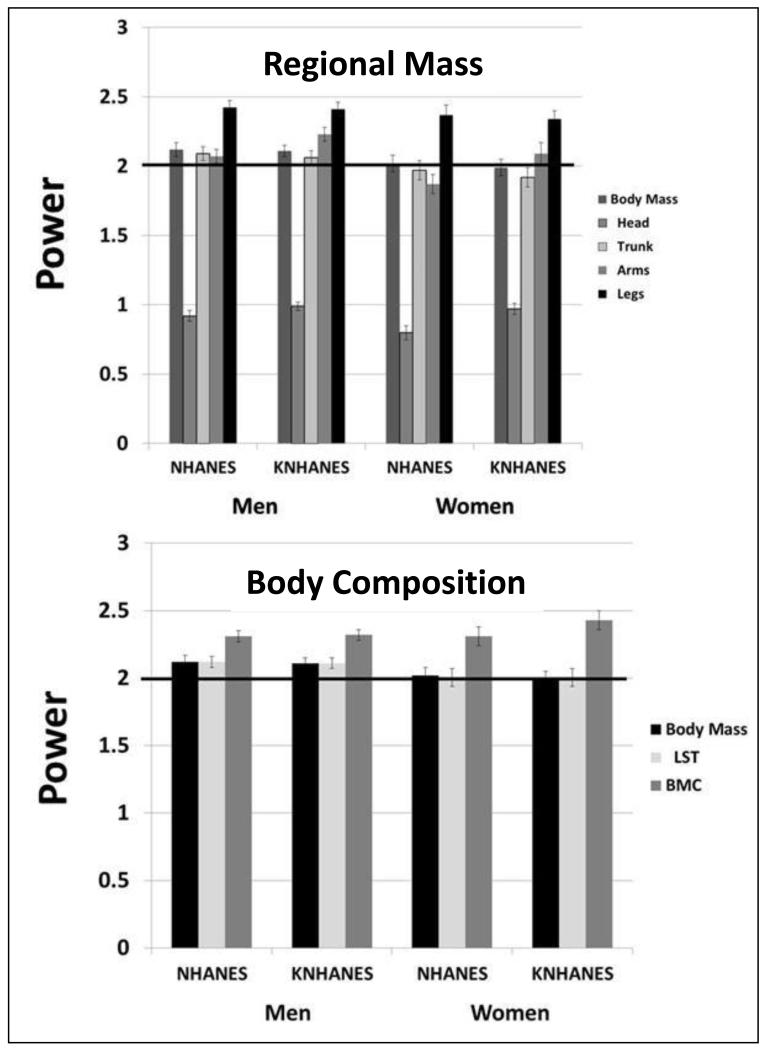
The powers (β±SE) observed for regional body mass scaled to height, adjusted for age and adiposity, are summarized for the eight sex-race/ethnic groups in Table 2. The general pattern across sex and race/ethnic groups was for head mass to scale with height at the lowest rounded powers of approximately 0.8-1.0 while the highest powers were consistently observed for leg mass scaled to height with mean rounded powers of approximately 2.3-2.6 (Figure 2). Trunk and arm mass scaled to height with powers of approximately 1.8-2.3, similar to that of total body mass.
Table 2.
Results of Allometric Analyses.
| Men | Women | |||||||
|---|---|---|---|---|---|---|---|---|
| NH White | NH Black | Mex Amer | Korean | NH White | NH Black | Mex Amer | Korean | |
| Body Mass | 2.02±0.06 | 2.29±0.09 | 2.14±0.07 | 2.11±0.06 | 1.97±0.06 | 1.94±0.08 | 1.99±0.09 | 1.99±0.06 |
| 1.91-2.14 | 2.10-2.48 | 2.01-2.27 | 1.99-2.23 | 1.85-2.09 | 1.77-2.10 | 1.82-2.17 | 1.87-2.10 | |
| Head | 0.91±0.04 | 0.98±0.07 | 0.87±0.06 | 1.00±0.05 | 0.81±0.04a | 0.78±0.07ab | 0.75±0.05a | 0.97±0.04b |
| 0.82-1.00 | 0.84-1.11 | 0.74-0.99 | 0.90-1.09 | 0.72-0.90 | 0.64-0.91 | 0.65-0.85 | 0.89-1.06 | |
| Trunk | 1.97±0.06a | 2.32±0.10b | 2.01±0.08ab | 2.06±0.07ab | 1.93±0.07 | 1.91±0.10 | 1.84±0.10 | 1.92±0.07 |
| 1.85-2.10 | 2.12-2.52 | 1.85-2.16 | 1.92-2.21 | 1.79-2.07 | 1.70-2.12 | 1.62-2.05 | 1.79-2.05 | |
| Arms | 1.96±0.06a | 2.27±0.12ab | 2.12±0.09ab | 2.23±0.07b | 1.84±0.08 | 1.87±0.11 | 1.84±0.11 | 2.09±0.08 |
| 1.83-2.09 | 2.03-2.50 | 1.94-2.29 | 2.09-2.38 | 1.68-2.00 | 1.63-2.10 | 1.62-2.06 | 1.94-2.24 | |
| Legs | 2.34±0.06a | 2.49±0.10ab | 2.61±0.07b | 2.41±0.07ab | 2.29±0.06 | 2.21±0.08 | 2.54±0.10 | 2.34±0.06 |
| 2.22-2.46 | 2.29-2.70 | 2.46-2.75 | 2.28-2.54 | 2.17-2.41 | 2.04-2.39 | 2.33-2.74 | 2.22-2.46 | |
|
Body
Composition |
||||||||
| LST | 2.04±0.05 | 2.26±0.09 | 2.11±0.06 | 2.11±0.06 | 1.97±0.05 | 1.96±0.08 | 1.99±0.09 | 2.01±0.06 |
| 1.93-2.14 | 2.07-2.44 | 1.98-2.24 | 1.99-2.23 | 1.86-2.08 | 1.79-2.13 | 1.81-2.17 | 1.90-2.13 | |
| BMC | 2.26±0.05 | 2.40±0.10 | 2.29±0.08 | 2.32±0.07 | 2.32±0.05a | 2.09±0.06b | 2.37±0.07a | 2.43±0.07a |
| 2.16-2.37 | 2.19-2.61 | 2.13-2.45 | 2.19-2.46 | 2.21-2.42 | 1.96-2.22 | 2.23-2.52 | 2.30-2.57 | |
Powers are expressed as β±SE with 95% CI below. Abbreviations: BMC, bone mineral content; NH, non-Hispanic; LST, lean soft tissue; Mex Amer, Mexican American. Between-race/ethnicity comparisons performed using Cochran’s Q Test for Heterogeneity. Values with different superscript letters are significantly different at p < 0.05/6 = 0.0083.
Figure 2.
Results for regional mass scaling powers (upper panel) and body composition powers (lower panel) observed for NHANES and KNHANES participants. Results are mean±SE. Powers for body mass scaling are from Heymsfield et al. (submitted publication; Supplementary Table 1). A horizontal line is shown in each figure at a power of 2. Abbreviations: BMC, bone mineral content; LST, lean soft tissue; Mex, Mexican; NH, non-Hispanic. Statistical comparisons between the groups are shown in Table 2.
For men, significant differences in scaling powers for trunk, arm, and leg mass were observed between the four race/ethnic groups. In addition, several significant differences in scaling powers for regional body mass components were observed between race/ethnic groups among women (Table 2). There were no discernable patterns in these power differences across the four race/ethnic groups of women. The Korean women had the highest powers among the women for head and arms, an identical but non-significant pattern was observed for head mass in men.
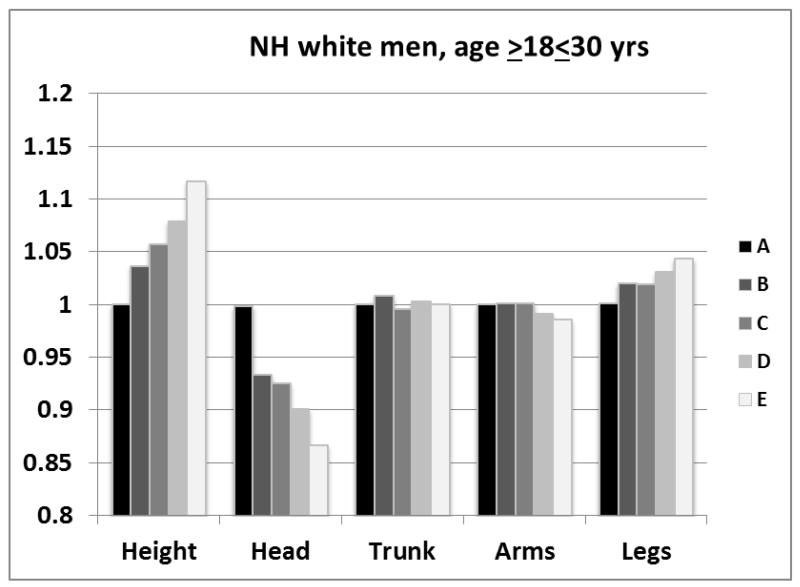
The striking range of regional powers above and below the approximate mean power for body mass scaled to height (i.e., ~2) implies that body proportions differ between short and tall subjects. This effect is shown in Figure 3 for young NH white men (age ≥18<30 yrs). Subjects were divided into five height groups (A-E) and the lowest fifth (A) used as the reference. With greater height head mass progressively becomes a smaller fraction of the lowest fifth reference group while leg mass becomes a larger fraction of the reference group. Small decreases with greater group height are present for arm mass. Relative body mass distribution thus differed across subjects varying in stature. Since body mass scales to height with a power of 2, these findings indicate that short subjects with the same BMI as tall subjects have a relatively larger head mass and smaller leg mass.
Figure 3.
Fraction of body mass as each of four regions (head, trunk, arms, legs) across non-Hispanic (NH) white men ≥18<30 years of age ranked by height divided into fifths (A-E; n=918) and expressed as the ratio to the lowest fifth (A).
The global height scaling power of ~2 for body mass observed across the groups represents the individual regional mass powers scaled to height. For example, removing leg mass from total body mass for the NH white men substantially reduced the remaining mass scaling power (±SE) from ~2.02±0.06 to 1.87±0.06 while a corresponding increase in power to 2.09±0.06 is present when head mass is removed from the analysis. Smaller effects on power are present for removal of trunk (2.07±0.06) and arm mass (2.03±0.06) from total body mass.
Scaling of Body Composition to Height
The results of body composition scaling to height are presented in Table 2 and Figure 2 for the eight sex and race/ethnic groups. Lean soft tissue mass scaled to height with powers of approximately 2, similar to corresponding powers for body weight scaled to height. By contrast, the powers for BMC scaled to height across the eight groups were substantially higher, ranging from about 2.1 to 2.4 and were in all cases larger than those for LST. In contrast to the observed regional mass scaling relations, there were no significant between-group differences in whole-body composition scaling powers among the race/ethnic groups in men but some significant BMC power differences in women.
Since BMC scaled to height with powers exceeding 2, bone was as larger fraction of body mass even in subjects with the same BMI.
Comparisons Between Men and Women
The scaling powers of height for regional mass and whole-body composition observed for race/ethnicity-combined US and Korean men and women are presented in Table 3 and Figure 4. With respect to the scaling of regional mass to height, a very similar pattern was again present in both men and women: head mass scaled with the lowest rounded powers (~0.8-1.0), legs with the largest rounded powers (~2.3-2.4), and trunk and arms with intermediate powers (~1.9-2.2) similar to that of body mass (Supplementary Table 1). Powers also again tended to be larger in men than women with significant or borderline significant sex differences present for head (NHANES), trunk (NHANES and KNHANES), and arms (NHANES and KNHANES).
Table 3.
Nationally Representative Scaling Powers.
| NHANES | KNHANES | |||||
|---|---|---|---|---|---|---|
| Men | Women | Δ † | Men | Women | Δ † | |
| Sample N | 9406 | 9192 | 3851 | 50918 | ||
| Regional Mass | ||||||
| Head | 0.92 ± 0.04 | 0.80 ± 0.03 | 0.12 ± 0.05* | 0.99 ± 0.05 | 0.97 ± 0.04 | 0.02 ± 0.06 |
| 0.84-1.00 | 0.73-0.87 | 0.02-0.22 | 0.90-1.09 | 0.89-1.06 | −0.09-0.13 | |
| Trunk | 2.09 ± 0.05 | 1.97 ± 0.05 | 0.12 ± 0.07 | 2.06 ± 0.07 | 1.92 ± 0.07 | 0.14 ± 0.09 |
| 1.99-2.19 | 1.87-2.07 | −0.02-0.26 | 1.92-2.21 | 1.79-2.05 | −0.04-0.33 | |
| Arms | 2.07 ± 0.05 | 1.87 ± 0.06 | 0.19 ± 0.08* | 2.23 ± 0.07 | 2.09 ± 0.08 | 0.15 ± 0.10 |
| 1.96-2.17 | 1.76-1.99 | 0.03-0.35 | 2.09-2.38 | 1.94-2.24 | −0.04-0.34 | |
| Legs | 2.42 ± 0.05 | 2.37 ± 0.05 | 0.06 ± 0.07 | 2.41 ± 0.07 | 2.34 ± 0.06 | 0.08 ± 0.09 |
| 2.32-2.52 | 2.27-2.46) | −0.09-0.20 | 2.28-2.54) | 2.22-2.46 | −0.10-0.25 | |
| Body Composition | ||||||
| LST | 2.12 ± 0.04 | 2.01 ± 0.04 | 0.10 ± 0.06 | 2.11 ± 0.06 | 2.01 ± 0.06 | 0.10 ± 0.08 |
| 2.03-2.20 | 1.93-2.10 | −0.02-0.23 | 1.99-2.23 | 1.90-2.13 | −0.06-0.25 | |
| BMC | 2.31 ± 0.04 | 2.31 ± 0.04 | 0.00 ± 0.06 | 2.32 ± 0.07 | 2.43 ± 0.07 | −0.11 ± 0.09 |
| 2.22-2.39 | 2.22-2.40 | −0.12-0.12 | 2.19-2.46 | 2.30-2.5 | −0.30-0.08 | |
Δ, difference between men and women. Results are β±SE with 95% CI.
p<0.05.
Figure 4.
Nationally representative (NHANES and KNHANES) results for regional mass scaling powers (mean±SE) in the upper panel and body composition scaling powers (mean±SE) in the lower panel. Powers for body mass scaling are from Heymsfield et al. (submitted publication; Supplementary Table 1). Horizontal lines are shown in the figure at a power of 2. Statistical comparisons between the groups are shown in Table 3. Abbreviations: BMC, bone mineral content; LST, lean soft tissue.
Lean soft tissue mass scaled to height with powers similar to those of body mass (~2.0-2.1) while BMC scaled to height with substantially larger powers (~2.3-2.4) in the men and women (Figure 4). The powers of LST scaled to height were larger in men than in women, approaching statistical significance (p<0.1) in the NHANES sample.
DISCUSSION
Body mass index is now used as a global measure of adiposity and health, although not without controversy surrounding the mathematical construct as first stimulated by Quetelet’s 1842 observation that adult body mass scales as height squared (Benn, 1971; Bogin, 2008; Burton, 2007; Cole, 1991; Diverse Populations Group, 2005; Garn et al., 1986; Green, 2009; Keys et al., 1972; Larsson et al., 2006; Samras, 2007; Smalley et al., 1990; Trefethan, 2013). A comprehensive earlier report from our group showed that in the carefully evaluated and analyzed large NHANES and KNHANES samples body mass does indeed scale to height across adult sex and race/ethnic groups with powers very close to 2 (Heymsfield et al., submitted publication). The current report describes the next stage in exploring scaling relations on the pathway to evaluating race/ethnic differences in body shape and composition while additionally seeking an overall mechanistic understanding of why adult human body mass scales to height with a power of 2.
Body Shape
Our findings, the first of their type, show that regional mass height scaling powers display a distinct pattern across the eight evaluated sex and race/ethnic groups: head < trunk and arms < legs with respective rounded powers of approximately 1, 2, 2, and 2.5. As body mass scales as Ht2, tall adult humans have heads that are relatively smaller in mass and legs that are relatively larger in mass than in subjects who are short; arms and trunk remain approximately stable proportions of body mass. At the same BMI, tall adults differ in regional mass proportions from their shorter counterparts. At the whole-body composition level, LST scaled to height with powers similar to that of body mass while BMC, a representation of skeletal mass, scaled with powers larger than 2 (i.e., ~2.1-2.4). Accordingly, skeletal mass is a larger fraction of body mass in tall adults compared to their short counterparts. These scaling patterns were remarkably consistent across all evaluated sex and race/ethnic groups.
The varied regional mass scaling relations are “masked” by the global scaling of body mass to height with a power of approximately 2. If tall “large” adults are isometric copies of short “small” adults then body mass would scale approximately as Ht3 as if often implied or assumed (Burton, 2007; Samras, 2007; Trefethan, 2013). Our findings firmly establish that BMI, based on body mass scaling as height squared, reflects a previously unforeseen heterogeneous regional body mass and composition scaling pattern.
As in our previous report (Heymsfield et al., submitted publication) and those of others (Burton, 2007), regional mass and body composition height scaling powers in women generally were smaller in magnitude than those observed in men, although the statistical significance of these differences was inconsistent. One explanation for these sex differences is that the greater anabolic stimulus males experience during puberty may lead to greater relative body mass at any level of stature compared to women (Rogol, 2003). While this sex difference pattern was consistent across all evaluated compartments, the estimated power differences tended to be small and did not detract from the larger aforementioned regional mass and body composition scaling patterns that were consistent across sex and race/ethnic groups.
Power differences across race groups tended to be relatively small and did not appear to fall into any consistent discernable patterns. The one finding that appears to represent a subtle specific race/ethnic effect was that head and arm mass scaled to height with larger powers in Korean women than in other race/ethnic groups of women. Additional in-depth analyses are needed to establish the significance of these race/ethnic differences in body shape.
Mechanistic Associations
We have now established that with greater body size, as defined by stature, adult humans have a relatively smaller fraction of their mass as head and larger fractions as legs and skeleton; arms, trunk, and LST remain relatively stable proportions of body mass independent of height. Analysis of the basis for this pattern can take many directions and focus at different organizational levels.
An example is presented for the distinct scaling pattern observed for head mass (Mh) as ∝ Ht~1. Earlier studies report that brain mass also scales to height across adults with powers of 1 or less (Heymsfield et al., 2004, 2007, 2009). These observations suggest that brain and head mass scale similarly to height and are concordant with the vast anthropological literature linking brain volume to skull features (Lieberman, 2011; Vannucci et al., 2011). Moreover, recent studies of microcephalic humans and Zebrafish with induced counterpart mutations show that skull development mirrors reduced brain growth (Dauber et al., 2012). Skull and head mass grow to accommodate an enlarged intracranial volume with early-life hydrocephaly features (Lieberman, 2011). Changes in head mass as a function of body size thus appear concordant with corresponding findings for brain mass and molecular/biomechanical mechanisms may account for this association.
Human growth progresses in a cephalocaudal direction with major head and brain growth both reaching peak levels during the first decade of life while substantial somatic growth continues and accelerates during adolescence (Keenan, 2002). Different molecular and hormonal control mechanisms, at least in part, thus regulate growth of the head and other body regions. The observed scaling pattern with head and that was observed earlier for brain (Heymsfield et al., 2004, 2007, 2009) is consistent with the morphogenic pattern for humans depicted by Stratz (1909) and Scammon (1930) in which head size and neurological tissue progressively become smaller fractions of body mass with growth from birth onward. Our findings based on static allometric relations in fully grown adults suggest that the head is part of this continuum with greater body size accompanied by relatively heavier, and presumably longer, legs and a proportionally smaller head mass.
Brain is a high-energy expenditure organ, consuming 240 kcal/kg/day or about 20% of adult resting energy expenditure (REE; Gallagher et al., 1996). Since brain mass scales as Ht~1 and REE as Ht~1.5, tall adults have a lower mass-specific REE relative to their short counterparts (i.e., Ht−0.5). Low energy expenditure relative to body mass, after adjusting for adiposity, may convey metabolic advantages such as greater fasting endurance to tall subjects (Heymsfield et al., 2009). Had brain and related head mass scaled as Ht2 or even Ht3, the additional energy and thus dietary needs would be substantial. Since human brain growth largely ceases at least one decade before somatic growth, it is not surprising that brain mass contributes independently to adult REE even after controlling for the metabolically active fat-free mass compartment (Heymsfield et al., 2014). Head scaling to body size with a power of 1 can thus be connected through a series of potential linkages to human energy requirements.
While these analytical threads may be tenuous, we show here how Quetelet’s Rule embraces another rule (Mh ∝ Ht~1) and that rule can be associated with an array of related biological processes.
Study Limitations
While DXA provides an important approach for measuring regional body mass, the question remains how these estimates correspond to body lengths. Bone lengths can be measured directly from the DXA scan and it would be possible in future biomechanics studies to establish how our regional mass-related findings integrate with the many anthropological length ratios that are in widespread use (Bogin and Varela-Silva, 2008, 2010, 2012). A second concern and proviso is that our results may not be generalizable to all race/ethnic groups. For example, Korean Asians may differ in body shape and composition from their counterparts in other Asian nations. Lastly, many earlier comprehensive studies link adult body proportions to environmental influences experienced during the growth period (Bogin and Varela-Silva, 2010, 2012). To the extent factors such as socioeconomic status and nutritional adequacy influence the evaluated scaling relations remains unknown and a topic worthy of future study.
Summary and Conclusions
At the outset our goal was to further explore BMI as a measure of adiposity and health across sex and race/ethnic groups amid the controversy surrounding body mass scaling to height with a power of 2 and that the empirical relation MB ∝ Ht2 lacks a theoretical context. Further, even after adjustment for stature, body mass appears to be only an approximate metric of an individual’s body composition across sex and race/ethnic groups (Gallagher et a., 1996). Accordingly, we laid out a series of investigations centered on the unique NHANES and KNHANES data sets that address these concerns. Our first report firmly established that with greater body size, as defined by stature, body mass empirically scales to adult height across sex and race/ethnic groups narrowly around a power of 2 (Heymsfield et al., submitted publication). The current study extended these earlier efforts and establishes that Quetelet’s Rule represents a series of related structural and body composition rules that can initially be framed in a broader biological context. The newly discovered static allometric scaling pattern was consistent across all evaluated sex and race/ethnic groups. With these collective observations as a foundation, the next phase of research addressing BMI as a universal health marker is two-fold: exploring potential mechanisms for the newly established adult body size scaling pattern; and in the context of BMI, conducting in-depth analyses of race/ethnic differences in body shape and composition, an important opportunity provided by the available methodologically-rigorous NHANES-KNHANES database.
Supplementary Material
ACKNOWLEDGEMENTS
The authors acknowledge Emily F. Mire, MS for her help with database acquisition and development and Kevin E. Wilson at Hologic Inc. (Bedford, MA) for his facilitation of investigator collaborations.
Funding Sources: CMP is funded by a Roadmap Scholars Fellowship from the Louisiana Clinical and Translational Science (LA CaTS) Center.
Abbreviations
- BMI
body mass index
- BMC
bone mineral content
- CI
confidence interval
- df
degrees of freedom
- DXA
dual-energy x-ray absorptiometry
- Ht
height
- KNHANES
Korean National Health and Nutrition Survey
- Mb
body mass
- Mex Amer
(Mexican American)
- NH
non-Hispanic
- NHANES
National Health and Nutrition Survey
- SD
standard deviation
- SE
standard error
- US
United States
Footnotes
The authors did not have financial or personal associations with a company or organization sponsoring the study at the time the research was done.
REFERENCES
- Antezana FS. Obesity: preventing and managing the global epidemic. Report of a WHO Consultation. World Health Organization; Geneva: 2000. Introducer for the WHO Technical Report Series 894. [PubMed] [Google Scholar]
- Barnard J, Rubin D. Miscellanea. Small-sample degrees of freedom with multiple imputation. Biometrika. 1999;86:948–55. [Google Scholar]
- Benn RT. Some properties of weight-for-height indices when used as a measure of adiposity. Br J Prev Soc Med. 1971;25:42–50. doi: 10.1136/jech.25.1.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogin B, Varela-Silva MI. Fatness biases the use of estimated leg length as an epidemiological marker for adults in the NHANES III sample. Int J Epidemiol. 2008;37:201–9. doi: 10.1093/ije/dym254. [DOI] [PubMed] [Google Scholar]
- Bogin B, Varela-Silva MI. Leg Length, Body Proportion, and Health: A Review with a Note on Beauty. Int. J. Environ. Res. Public Health. 2010;7:1047–1075. doi: 10.3390/ijerph7031047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogin B, Varela-Silva MI. The Body Mass Index: the Good, the Bad, and the Horrid. Bulletin der Schweizerischen Gesellschaft für Anthropologie. 2012;18:5–11. [Google Scholar]
- Burton RF. Why is the body mass index calculated as mass/height2, not as mass/height3? Ann Hum Biol. 2007;34:656–63. doi: 10.1080/03014460701732962. [DOI] [PubMed] [Google Scholar]
- Calder WA., III . Size, Function, and Life History. Harvard University Press; Cambridge: 1984. [Google Scholar]
- Cole T. Weight-Stature Indices to Measure Underweight, Overweight, and Obesity. In: Himes JE, editor. Anthropometric Assessment of Nutritional Status. Wiley-Liss; 1991. pp. 83–111. [Google Scholar]
- Dauber A, Lafranchi SH, Maliga Z, Lui JC, Moon JE, McDeed C, Henke K, Zonana J, Kingman GA, Pers TH, Baron J, Rosenfeld RG, Hirschhorn JN, Harris MP, Hwa V. Novel microcephalic primordial dwarfism disorder associated with variants in the centrosomal protein ninein. J Clin Endocrinol Metab. 2012;97:E2140–51. doi: 10.1210/jc.2012-2150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diverse Populations Collaborative Group Weight-height relationships and body mass index: some observations from the Diverse Populations Collaboration. Am J Phys Anthropol. 2005;128:220–9. doi: 10.1002/ajpa.20107. [DOI] [PubMed] [Google Scholar]
- Gallagher D, Visser M, Sepulveda D, Pierson RN, Harris T, Heymsfield SB. How useful is body mass index for comparison of body fatness across age, sex, and ethnic groups? Am J Epidemiol. 1996;143:228–39. doi: 10.1093/oxfordjournals.aje.a008733. [DOI] [PubMed] [Google Scholar]
- Garn SM, Leonard WR, Hawthorne VM. Three limitations of the body mass index. Am J Clin Nutr. 1986;44:996–7. doi: 10.1093/ajcn/44.6.996. [DOI] [PubMed] [Google Scholar]
- Grande F. Nutrition and energy balance in body composition studies. In: Brozek JHA, editor. Techniques for Measuring Body Composition. National Academy of Sciences-National Research Council; Washington, DC: 1961. [Google Scholar]
- Green DJ. Is body mass index really the best measure of obesity in individuals? J Am Coll Cardiol. 2009;53:526. doi: 10.1016/j.jacc.2008.08.078. author reply 7-8. [DOI] [PubMed] [Google Scholar]
- Heymsfield SB, Gallagher D, Mayer L, Beetsch J, Pietrobelli A. Scaling of human body composition to stature: new insights into body mass index. Am J Clin Nutr. 2007;86:82–91. doi: 10.1093/ajcn/86.1.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heymsfield SB, Chirachariyavej T, Rhyu IJ, Roongpisuthipong C, Heo M, Pietrobelli A. Differences between brain mass and body weight scaling to height: potential mechanism of reduced mass-specific resting energy expenditure of taller adults. J Appl Physiol. 2009;106:40–8. doi: 10.1152/japplphysiol.91123.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heymsfield SB, Heo M, Thomas D, Pietrobelli A. Scaling of body composition to height: relevance to height-normalized indexes. Am J Clin Nutr. 2011;93:736–40. doi: 10.3945/ajcn.110.007161. [DOI] [PubMed] [Google Scholar]
- Heymsfield SB, Gonzalez MC, Shen W, Redman L, Thomas D. Weight loss composition is one-fourth fat-free mass: a critical review and critique of this widely cited rule. Obes Rev. 2014;15:310–21. doi: 10.1111/obr.12143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong S, Oh HJ, Choi H, Kim JG, Lim SK, Kim EK, Pyo EY, Oh K, Kim YT, Wilson K, Choi WH. Characteristics of body fat, body fat percentage and other body composition for Koreans from KNHANES IV. J Korean Med Sci. 2011;26:1599–605. doi: 10.3346/jkms.2011.26.12.1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen MD, Ryan DH, Donato KA, Apovian CM, Ard JD, Comuzzie AG, Hu FB, Hubbard VS, Jakicic JM, Kushner RF, Loria CM, Millen BE, Nonas CA, Pi-Sunyer FX, Stevens J, Stevens VJ, Wadden TA, Wolfe BM, Yanovski SZ. Executive summary: Guidelines (2014) for the management of overweight and obesity in adults: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society Published by The Obesity Society and American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Based on a systematic review from the The Obesity Expert Panel, 2013. Obesity. 22(Suppl 2):S5–S39. doi: 10.1002/oby.20821. [DOI] [PubMed] [Google Scholar]
- Keenan T. Introduction to Child Development. Sage Publications; London: 2012. [Google Scholar]
- Kelly TL, Wilson KE, Heymsfield SB. Dual energy X-Ray absorptiometry body composition reference values from NHANES. PLoS One. 2009;4:e7038. doi: 10.1371/journal.pone.0007038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keys A, Fidanza F, Karvonen MJ, Kimura N, Taylor HL. Indices of relative weight and obesity. J Chronic Dis. 1972;25:329–43. doi: 10.1016/0021-9681(72)90027-6. [DOI] [PubMed] [Google Scholar]
- Larsson I, Henning B, Lindroos AK, Naslund I, Sjostrom CD, Sjostrom L. Optimized predictions of absolute and relative amounts of body fat from weight, height, other anthropometric predictors, and age 1. Am J Clin Nutr. 2006;83:252–9. doi: 10.1093/ajcn/83.2.252. [DOI] [PubMed] [Google Scholar]
- Lieberman DE. The Evolution of the Human Head. Belknap Press; Cambridge, MA: 2011. [Google Scholar]
- NHANES. National Center for Health Statisitic . Documentation, codebook, and frequencies: dual-energy x-ray absortiometry. National Health and Nutrition Examination Survey; 2003-2004. http://www.cdc.gov/nchs/data/nhanes/dxa/dxx_c.pdf. [Google Scholar]
- NCHS. National Center for Health Statisitic . Technical documentation for the 1999-2004 dual energy x-ray absorptiometry (DXA) multiple imupation data files. National Health and Nutrition Examination Survey; 2011. http://www.cdc.gov/nchs/data/nhanes/dxa/dxa_techdoc.pdf. [Google Scholar]
- Park HA. The Korea National Health and Nutrition Examination Survey as a Primary Data Source. Korean J Fam Med. 2013;34(2):79. doi: 10.4082/kjfm.2013.34.2.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietrobelli A, Formica C, Wang Z, Heymsfield SB. Dual-energy X-ray absorptiometry body composition model: review of physical concepts. Am J Physiol. 1996;271:E941–51. doi: 10.1152/ajpendo.1996.271.6.E941. [DOI] [PubMed] [Google Scholar]
- PiSunyer FXP. National Institutes of Health, National Heart, Lung, and Blood Institute, Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults: the evidence report. Obes Res. 1998;6(suppl 2):S51–S210. Chair. [PubMed] [Google Scholar]
- Quetelet LAJ, Knox R, Smibert T, Smibert T. A treatise on man and the development of his faculties, tr. (under the superintendence of R. Knox) 1842. People’s ed. [Google Scholar]
- Rogol AD. Growth, body composition and hormonal axes in children and adolescents. J Endocrinol Invest. 2003;26:855–60. doi: 10.1007/BF03345236. [DOI] [PubMed] [Google Scholar]
- Samaras T, editor. Human Body Size and the Laws of Scaling: Physiological, Performance, Growth, Longevity and Ecological Ramifications. Nova Science Publishers Inc; New York: 2007. [Google Scholar]
- Scammon RE, Harris LA, Jackson CM, Patterson DG, Scammon RE. The Measurement of Man. University of Minnesota Press; Minneapolis: 1930. The measurement of the body in childhood. [Google Scholar]
- Schenker N, Borrud LG, Burt VL, Curtin LR, Flegal KM, Hughes J, Johnson CL, Looker AC, Mirel L. Multiple imputation of missing dual-energy X-ray absorptiometry data in the National Health and Nutrition Examination Survey. Stat Med. 2011;30:260–76. doi: 10.1002/sim.4080. [DOI] [PubMed] [Google Scholar]
- Schoeller DA, Tylavsky FA, Baer DJ, Chumlea WC, Earthman CP, Fuerst T, Harris TB, Heymsfield SB, Horlick M, Lohman TG, et al. QDR 4500A dual-energy X-ray absorptiometer underestimates fat mass in comparison with criterion methods in adults. Am J Clin Nutr. 2005;81:1018–25. doi: 10.1093/ajcn/81.5.1018. [DOI] [PubMed] [Google Scholar]
- Smalley KJ, Knerr AN, Kendrick ZV, Colliver JA, Owen OE. Reassessment of body mass indices. Am J Clin Nutr. 1990;52:405–8. doi: 10.1093/ajcn/52.3.405. [DOI] [PubMed] [Google Scholar]
- Stratz CH. Der Körper des Kindes und seine Pflege : für Eltern, Erzieher, Ärzte, und Künstler F. Stuttgart; Enke: 1909. [Google Scholar]
- Trefethan N. 2013 http://people.maths.ox.ac.uk/trefethen/bmi.html.
- Vannucci RC, Barron TF, Holloway RL. Craniometric ratios of microcephaly and LB1, Homo floresiensis, using MRI and endocasts. Proc Natl Acad Sci U S A. 2011;108:14043–8. doi: 10.1073/pnas.1105585108. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.