Abstract
Per- and polyfluoroalkyl substances (PFASs), used in food packaging and stain-resistant coatings, are suspected developmental toxicants that are ubiquitous and persistent in the environment. We measured plasma PFAS concentrations during early pregnancy (median = 9.7 weeks gestation) among 1645 women in the Boston-area Project Viva cohort, recruited during 1999–2002. We used multivariable linear regression to estimate associations of sociodemographic and perinatal predictors, including measures of pregnancy physiology (albumin, glomerular filtration rate (GFR)), with log-transformed plasma PFAS concentrations. Geometric mean concentrations for the four main PFASs, perfluorooctanesulfonate (PFOS), perfluorooctanoate (PFOA), perfluorohexanesulfonate (PFHxS) and perfluorononanoate (PFNA) were 25.4, 5.7, 2.5, and 0.6 ng/mL, respectively, comparable with general U.S. population concentrations during those years. Higher early pregnancy PFAS concentrations were associated with younger age (except PFNA), less educational attainment, nulliparity, no history of breastfeeding and higher prepregnancy body mass index in adjusted models. In addition, lower GFR was associated with 3–4% higher PFAS concentrations and higher albumin was associated with 4–6% higher PFAS concentrations. Our results show associations consistent (parity and breastfeeding) and less consistent (age and education) with previous studies. We also report associations with GFR and albumin, which were strongly related to PFAS concentrations and thus could confound estimates of PFAS–outcome associations in epidemiologic studies.
Introduction
Per- and polyfluoroalkyl substances (PFASs) are synthetic chemicals that have been widely used in manufacturing of industrial and consumer products, such as food packaging, and stain-resistant coatings, since their introduction in the 1950s. General population exposure occurs by inhalation and ingestion of PFASs from a combination of dietary and indoor environmental sources.1,2 While drinking water can play a substantial role in water-contaminated areas,3 exposure to PFASs occurs primarily through nonindustrial sources. The factors that drive variability in PFAS concentrations among individuals, which include primarily exposure to consumer products that contain these chemicals, have not been well described. Exposure may be to PFAS compounds or precursors that can be metabolically converted into the compounds detected in blood.4–6 Strong carbon–fluorine bonds make many PFASs resistant to degradation and thus very persistent not only in the environment, but also in the human body. In addition, longer chain PFASs bioaccumulate more than shorter chain PFASs.7 Human half-lives for common PFASs are approximately 2–5 years.8 Unlike many persistent organic compounds, PFASs are proteinophilic rather than lipophilic; blood is the principal accumulation site and PFASs bind primarily to albumin.9 PFASs are universally present at varying concentrations in the U.S. population, as reported in the National Health and Nutrition Examination Survey (NHANES).10,11
PFAS production increased steadily since the 1950s with peak production in the 1990s. Human concentrations of one of the most commonly studied PFASs, perfluorooctanesulfonate (PFOS), have been on the decline since their peak in 1999–2000 due to their voluntary phase out by industry, a trend also observed for perfluorohexanesulfonate (PFHxS).12 Concentrations of (PFOA) also decreased after 1999–2000, but remained stable during 2003–2008. Concentrations of perfluorononanoate (PFNA) increased during 1999–2008.12
Animal and human studies suggest that PFASs are developmental toxicants and that the prenatal period may be a particularly sensitive time window for impacts on growth and development.13–15 Several epidemiologic studies have reported predictors of serum or plasma PFAS concentrations during pregnancy.16–21 In three studies, lower PFAS concentrations were associated with higher parity and history of breastfeeding.17,18,20 Previous studies also showed differences in PFAS concentrations for sociodemographic factors, including maternal age, race, and income, but patterns were not consistent across different studies or individual PFASs.16,19–21
Most previous studies of PFAS predictors have not examined measures of pregnancy physiology, which could confound associations of PFASs with developmental endpoints.22,23 For example, maternal glomerular filtration rate (GFR), a measure of flow rate of filtered fluid through the kidney, has been shown to be associated with infant birth weight.24 If GFR is also associated with PFAS concentrations, this could produce a spurious association between PFASs and birth weight. Better characterization of the relationship between maternal physiologic factors and PFAS concentrations would help determine whether these factors should be considered as potential confounders of associations of PFASs with child health outcomes.
We examined sociodemographic and perinatal factors in relation to plasma concentrations of PFASs measured in early pregnancy in a large, well-characterized birth cohort of women who were pregnant during 1999–2002. We also considered markers of pregnancy physiology, including GFR and plasma albumin.
Materials and Methods
Study Population
Pregnant women were enrolled in Project Viva 1999–2002 at their first prenatal visit at one of 8 obstetric clinics of Atrius Harvard Vanguard Medical Associates, a multispecialty group practice in eastern Massachusetts.25 Eligible mothers were fluent in English, had singleton gestations, were <22 weeks gestation, and had no plans to move away from the study area. Of 2128 mothers with a live birth between November 1999 and February 2003, 1668 (78%) provided an early pregnancy blood sample. The Human Subjects Committees of participating institutions approved all study protocols and all participating mothers provided written informed consent.
Plasma PFAS Measurement
Plasma samples were stored in non-PFAS containing cryovial tubes in liquid nitrogen freezers.
Of the 1668 early pregnancy samples (median = 9.7 weeks gestation; range = 4.8–21.4 weeks), 1645 had sufficient volume for PFAS measurements. Samples were thawed, aliquoted and sent to the Division of Laboratory Sciences at the CDC. Detailed analytic methods were described previously;26 briefly, the CDC used online solid-phase extraction coupled with isotope dilution high-performance liquid chromatography-tandem mass spectrometry to report plasma concentrations for PFOA, PFOS, PFHxS, PFNA, 2-(N-ethyl-perfluorooctane sulfonamido) acetate (Et-PFOSA-AcOH), 2-(N-methyl-perfluorooctane sulfonamido) acetate (Me-PFOSA-AcOH), perfluorodecanoate (PFDeA) and perfluorooctane sulfonamide (PFOSA). The reported concentrations are for the sum of linear and branched isomers of PFOS and PFOA. Low-concentration quality control materials (QCs) and high-concentration QCs, prepared from a calf serum pool, were analyzed with the study samples, analytical standards, and with reagent and serum blanks to ensure the accuracy and reliability of the data. Limits of detection (LOD) were 0.2 ng/mL for PFOS and 0.1 for all other PFASs (Table 1). Values below the LOD were not reported by the CDC and we therefore imputed these values as the LOD (0.1 or 0.2) divided by the square root of 2.
Table 1. Summary Statistics for PFASs (all in ng/mL) Measured in 1645 Project Viva Plasma Samples Collected during Early Pregnancy, 1999-2002.
| PFASa | % detect | LODb | geometric mean | 25th | 50th | 75th |
|---|---|---|---|---|---|---|
| PFOS | 100 | 0.2 | 25.4 | 18.9 | 25.7 | 34.9 |
| PFOA | 100 | 0.1 | 5.7 | 4.1 | 5.8 | 7.9 |
| PFHxS | 99 | 0.1 | 2.5 | 1.6 | 2.4 | 3.8 |
| PFNA | 99 | 0.1 | 0.6 | 0.5 | 0.7 | 0.9 |
| Et-PFOSA-AcOH | 100 | 0.1 | 1.2 | 0.7 | 1.2 | 1.9 |
| Me-PFOSA-AcOH | 100 | 0.1 | 1.9 | 1.3 | 1.9 | 3.1 |
| PFDeA | 45 | 0.1 | 0.1 | 0.1 | 0.1 | 0.3 |
| PFOSA | 10 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 |
PFAS = per- and polyfluoroalkyl substance; PFOS = perfluorooctane sulfonate; PFOA = perfluorooctanoate; PFHxS = perfluorohexanesulfonate; PFNA = perfluorononanoate; Et-PFOSA-AcOH = (2-(N-ethyl-perfluorooctane sulfonamido) acetate); Me-PFOSA-AcOH = (2-(N-methyl-perfluorooctane sulfonamido) acetate; PFDeA= perfluorodecanoate; PFOSA = perfluorooctane sulfonamide.
LOD = limit of detection.
Predictor Data
Using interviews and questionnaires administered during early pregnancy, midpregnancy and at delivery, study staff obtained data on the mothers' sociodemographic, behavioral, and health history measures. Risk factors for this analysis included sociodemographic factors (maternal age at enrollment, marital status, race/ethnicity, and smoking status and parental educational attainment and household income) and perinatal factors (parity and prepregnancy body mass index (BMI)).
Data on history of breastfeeding prior to the current pregnancy were not collected in Project Viva. We created a binary variable to estimate breastfeeding history using information on parity and breastfeeding data for the child of the current pregnancy (which was collected following the birth). If the mother was parous (regardless of the number of previous births), and breastfed the child of the current pregnancy, history of breastfeeding was coded as “yes”, with the assumption that a mother who breastfed this child had a high likelihood of having breastfed an older child. If the mother was nulliparous or did not breastfeed the current child history of breastfeed was coded “no”.
To capture markers of pregnancy physiology, we measured plasma albumin and creatinine in the same samples used to measure PFASs. Albumin is the main binding site for PFASs as well as a marker of plasma volume expansion during pregnancy.27 GFR is a measure of the flow rate of filtered fluid through the kidney.24 We calculated GFR (mL/min per 1.73 m2) by plugging plasma creatinine into the Cockroft-Gault (GFR-CG) formula [GFR-CG = (140-age) × weight (kg) × 1.04/serum creatinine (μmol/L)].
Statistical Analysis
We estimated PFAS geometric means to account for the skewed distribution of PFAS concentrations in the population, and estimated unadjusted partial sum of square p-values (p-value across multiple categories) for each predictor using linear regression models. We fitted multivariable linear regression models to generate adjusted estimates and 95% confidence intervals (CI) for predictors of log-transformed PFAS concentrations. We calculated percent change in PFAS concentration for each predictor by exponentiating regression coefficients, subtracting 1 and multiplying by 100. We based our conclusions about whether or not a variable was an important predictor of our outcome on the magnitude and precision of the estimates.
Results
PFAS Concentrations
As shown in Table 1, we detected PFASs in 99–100% of plasma samples, with the exception of PFDeA and PFOSA, which were detected in 45% and 10% of samples, respectively. We did not include predictor analyses for these two analytes. PFAS concentrations were moderately to highly correlated with each other (range of Spearman correlation coefficients: 0.21–0.72, Table 2). Correlations were strongest for PFOS and PFOA.
Table 2. Spearman Correlation Coefficientsa between Pairs of PFASs in 1645 Project Viva Prenatal Plasma Samples Collected in Early Pregnancy, 1999-2002.
| PFOS | PFOA | PFHxS | PFNA | Et-PFOSA-AcOH | Me-PFOSA-AcOH | |
|---|---|---|---|---|---|---|
| PFOS | 1 | |||||
| PFOA | 0.72 | 1 | ||||
| PFHxS | 0.50 | 0.52 | 1 | |||
| PFNA | 0.61 | 0.52 | 0.42 | 1 | ||
| Et-PFOSA-AcOH | 0.52 | 0.40 | 0.21 | 0.19 | 1 | |
| Me-PFOSA-AcOH | 0.40 | 0.38 | 0.23 | 0.24 | 0.40 | 1 |
All p-values are <0.0001.
Figure 1 compares PFAS concentrations in Project Viva participants (from 1999 to 2002) with concentrations in 20–39 year old male and female NHANES participants between 1999 and 2008.12 Project Viva PFAS concentrations were comparable to concentrations reported in the 1999–2000 NHANES cycle, when concentrations of PFOS, PFOA, and PFHxS peaked in humans.
Figure 1.
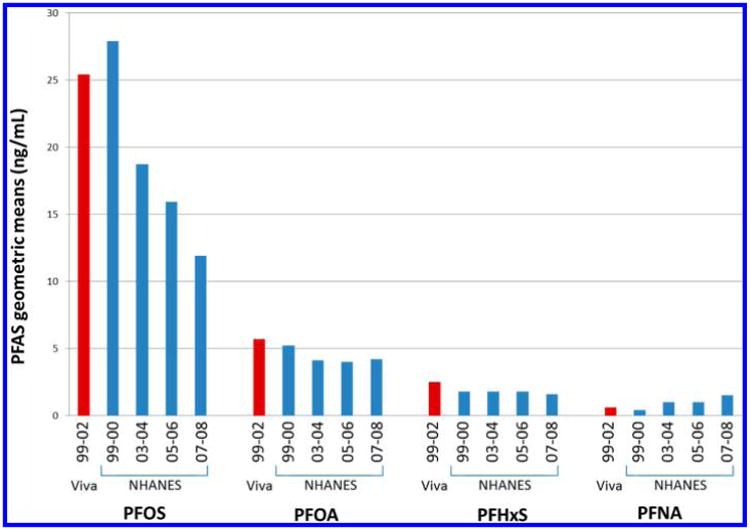
PFAS geometric means in NHANES cohorts12 and in Project Viva.
Sociodemographic Predictors
Table 3 shows unadjusted geometric means and interquartile ranges for the six PFASs across sociodemographic predictors for 1645 participants. All PFAS concentrations were lower for older women, with the exception of PFNA, which was higher. Concentrations were also lower for women with higher educational attainment, again with the exception of PFNA, which showed the opposite relationship, and PFHxS, which showed a null association. The pattern was the same for partner educational attainment, though there was quite a bit of missing data (∼11% missing) for this variable. Women who never smoked had the lowest PFAS concentrations. Concentrations of all six PFASs declined over the enrollment period. PFAS concentrations were not consistently different across categories of race/ethnicity, marital status or household income.
Table 3. Geometric Mean and Interquartile Range (in ng/mL), and Partial Sum of Square P-Values for PFASs Measured in 1645 Project Viva Prenatal Plasma Samples Collected in Early Pregnancy, 1999-2002, Across Participants' Sociodemographic and Perinatal Characteristics, Including Pregnancy Hemodynamics.
| PFOS | PFOA | PFHxS | PFNA | Et-PFOSA-AcOH | Me-PFOSA-AcOH | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n (%) | GM (25–75%ile) | pa | GM (25–75%ile) | pa | GM (25–75%ile) | pa | GM (25–75%ile) | pa | GM (25–75%ile) | pa | GM (25–75%ile) | pa | |
| Age at Enrollment (Years) | < 0.01 | < 0.01 | 0.04 | < 0.01 | 0.03 | < 0.01 | |||||||
| < 20 | 55 (3.3) | 28.1 (21.3, 38.1) | 6.6 (5.0, 8.4) | 3.1 (1.6, 5.2) | 0.5 (0.4, 0.7) | 1.5 (1.0, 2.2) | 3.1 (1.8, 4.9) | ||||||
| 20–34 | 1133 (68.9) | 26.1 (19.2, 35.6) | 5.8 (4.3, 8.0) | 2.5 (1.7, 3.9) | 0.6 (0.5, 0.9) | 1.2 (0.7, 1.9) | 2.0 (1.3, 3.2) | ||||||
| +35 | 457 (27.8) | 23.7 (17.8, 33.1) | 5.2 (3.7, 7.4) | 2.4 (1.6, 3.3) | 0.7 (0.5, 1.0) | 1.2 (0.7, 1.8) | 1.7 (1.1, 2.6) | ||||||
| Race/Ethnicity | 0.09 | < 0.01 | < 0.01 | < 0.01 | < 0.01 | 0.02 | |||||||
| black | 251 (15.4) | 27.6 (20.8, 36.7) | 5.1 (3.7, 7.1) | 2.1 (1.4, 3.0) | 0.6 (0.4, 0.9) | 1.3 (0.9, 2.0) | 2.2 (1.5, 3.6) | ||||||
| Hispanic | 117 (7.2) | 23.7 (17.5, 31.4) | 5.7 (4.1, 8.0) | 2.1 (1.5, 2.9) | 0.6 (0.5, 0.8) | 1.2 (0.8, 1.9) | 1.9 (1.2, 2.7) | ||||||
| Asian | 77 (4.7) | 25.4 (18.7, 36.2) | 4.8 (3.7, 6.3) | 2.1 (1.6, 3.0) | 1.0 (0.7, 1.5) | 0.9 (0.5, 1.5) | 1.6 (1.0, 2.7) | ||||||
| white | 1116 (68.6) | 25.2 (18.7, 34.4) | 5.9 (4.4, 8.1) | 2.7 (1.8, 4.1) | 0.7 (0.5, 0.9) | 1.2 (0.7, 1.9) | 1.9 (1.3, 3.0) | ||||||
| other | 66 (4.1) | 24.8 (19.1, 34.1) | 5.4 (4.0, 7.2) | 2.3 (1.4, 3.3) | 0.6 (0.4, 0.9) | 1.2 (0.7, 2.0) | 2.0 (1.2, 3.2) | ||||||
| Education | < 0.01 | < 0.01 | 0.19 | < 0.01 | < 0.01 | < 0.01 | |||||||
| < college | 576 (35.4) | 27.1 (20.1, 35.8) | 6.0 (4.4, 8.4) | 2.5 (1.6, 3.6) | 0.6 (0.5, 0.9) | 1.4 (0.9, 2.2) | 2.2 (1.5, 3.3) | ||||||
| college | 594 (36.5) | 25.4 (19.2, 35.2) | 5.7 (4.2, 7.8) | 2.6 (1.7, 4.0) | 0.7 (0.5, 0.9) | 1.2 (0.7, 1.9) | 2.0 (1.3, 3.2) | ||||||
| > college | 457 (28.1) | 23.5 (17.3, 32.5) | 5.3 (3.9, 7.4) | 2.4 (1.5, 3.7) | 0.7 (0.5, 1.0) | 1.1 (0.7, 1.7) | 1.7 (1.0, 2.6) | ||||||
| Partner Education | < 0.01 | < 0.01 | 0.02 | < 0.01 | < 0.01 | < 0.01 | |||||||
| < college | 523 (31.8) | 27.8 (20.5, 37.8) | 6.0 (4.4, 8.4) | 2.6 (1.7, 3.7) | 0.6 (0.5, 0.9) | 1.4 (0.9, 2.2) | 2.1 (1.4, 3.4) | ||||||
| college | 521 (31.7) | 25.3 (19.2, 33.7) | 5.8 (4.4, 8.0) | 2.7 (1.8, 4.2) | 0.7 (0.5, 0.9) | 1.2 (0.7, 1.8) | 1.9 (1.2, 3.1) | ||||||
| > college | 423 (25.7) | 23.0 (17.2, 32.5) | 5.2 (3.7, 7.4) | 2.4 (1.5, 3.6) | 0.7 (0.5, 1.0) | 1.0 (0.6, 1.6) | 1.7 (1.1, 2.7) | ||||||
| missing | 178 (10.8) | 25.4 (19.1, 35.5) | 5.5 (4.2, 7.3) | 2.3 (1.5, 3.5) | 0.6 (0.4, 0.9) | 1.4 (0.9, 2.1) | 2.2 (1.5, 3.3) | ||||||
| Married or Cohabitating | 0.59 | 0.44 | < 0.01 | < 0.01 | 0.04 | 0.01 | |||||||
| no | 143 (8.8) | 26.0 (19.3, 34.4) | 5.5 (4.2, 7.2) | 2.1 (1.4, 3.2) | 0.6 (0.4, 0.8) | 1.4 (0.8, 2.2) | 2.2 (1.5, 3.5) | ||||||
| yes | 1483 (91.2) | 25.4 (18.8, 34.9) | 5.7 (4.1, 8.0) | 2.6 (1.7, 3.8) | 0.7 (0.5, 0.9) | 1.2 (0.7, 1.8) | 1.9 (1.2, 3.1) | ||||||
| Annual household income | 0.04 | 0.12 | 0.01 | < 0.01 | < 0.01 | 0.05 | |||||||
| <$40K | 223 (15.3) | 24.3 (18.3, 34.1) | 5.3 (3.7, 7.3) | 2.3 (1.5, 3.2) | 0.6 (0.4, 0.9) | 1.3 (0.8, 2.2) | 2.0 (1.3, 3.4) | ||||||
| $40K-70K | 354 (24.2) | 26.9 (20.1, 36.4) | 5.7 (4.1, 8.1) | 2.4 (1.6, 3.6) | 0.6 (0.5, 0.9) | 1.3 (0.8, 2.3) | 2.0 (1.3, 3.2) | ||||||
| >$70K | 884 (60.5) | 24.9 (18.5, 33.8) | 5.7 (4.2, 7.9) | 2.6 (1.8, 3.9) | 0.7 (0.5, 1.0) | 1.1 (0.7, 1.7) | 1.8 (1.2, 2.8) | ||||||
| Smoking Status | 0.20 | < 0.01 | < 0.01 | 0.03 | < 0.01 | 0.01 | |||||||
| never | 1107 (68.0) | 25.0 (18.6, 34.4) | 5.4 (3.9, 7.6) | 2.4 (1.6, 3.6) | 0.6 (0.5, 0.9) | 1.2 (0.7, 1.8) | 1.9 (1.2, 3.1) | ||||||
| former | 307 (18.8) | 26.3 (19.1, 36.2) | 6.1 (4.4, 8.4) | 2.8 (1.8, 4.2) | 0.7 (0.5, 1.0) | 1.3 (0.8, 2.0) | 1.9 (1.3, 3.0) | ||||||
| during pregnancy | 215 (13.2) | 26.3 (19.6, 35.6) | 6.4 (4.9, 8.7) | 2.8 (1.8, 4.6) | 0.6 (0.5, 0.9) | 1.4 (0.9, 2.2) | 2.2 (1.4, 3.4) | ||||||
| Year of Enrollment | < 0.01 | < 0.01 | < 0.01 | < 0.01 | < 0.01 | < 0.01 | |||||||
| 1999 | 407 (24.7) | 28.8 (20.8, 38.5) | 5.8 (4.3, 7.7) | 2.7 (1.8, 4.1) | 0.7 (0.5, 1.0) | 1.6 (1.0, 2.3) | 2.4 (1.6, 3.7) | ||||||
| 2000 | 606 (36.8) | 26.6 (19.8, 36.2) | 6.0 (4.5, 8.6) | 2.7 (1.8, 4.1) | 0.7 (0.5, 0.9) | 1.3 (0.8, 2.0) | 2.1 (1.4, 3.2) | ||||||
| 2001 | 576 (35.0) | 22.7 (16.9, 31.3) | 5.4 (3.8, 7.6) | 2.3 (1.5, 3.4) | 0.6 (0.5, 0.9) | 1.0 (0.6, 1.6) | 1.6 (1.1, 2.4) | ||||||
| 2002 | 56 (3.4) | 21.2 (16.5, 29.3) | 4.8 (3.6, 6.6) | 2.2 (1.4, 3) | 0.6 (0.5, 0.9) | 0.6 (0.4, 0.9) | 1.3 (0.7, 2.1) | ||||||
| Parity | < 0.01 | < 0.01 | < 0.01 | < 0.01 | 0.63 | 0.38 | |||||||
| 0 | 800 (48.6) | 27.9 (20.8, 37.5) | 6.6 (5.1, 8.7) | 2.8 (1.9, 4.3) | 0.7 (0.5, 1.0) | 1.2 (0.7, 1.9) | 1.9 (1.2, 3.2) | ||||||
| 1+ | 845 (51.4) | 23.3 (17.6, 32.2) | 4.9 (3.6, 6.9) | 2.3 (1.5, 3.3) | 0.6 (0.5, 0.9) | 1.2 (0.7, 1.9) | 2.0 (1.3, 3.1) | ||||||
| Breastfed before Current Pregnancyb | < 0.01 | < 0.01 | < 0.01 | < 0.01 | 0.12 | 0.50 | |||||||
| no | 927 (61.3) | 27.5 (20.7, 36.8) | 6.5 (5.0, 8.6) | 2.7 (1.9, 4.2) | 0.7 (0.5, 1.0) | 1.2 (0.8, 1.9) | 2.0 (1.3, 3.2) | ||||||
| yes | 586 (38.7) | 22.2 (16.8, 29.9) | 4.6 (3.3, 6.4) | 2.2 (1.5, 3.1) | 0.6 (0.4, 0.8) | 1.2 (0.7, 1.8) | 1.9 (1.2, 2.9) | ||||||
| Prepregnancy BMI, kg/m2 | 0.04 | 0.12 | 0.80 | 0.04 | < 0.01 | 0.24 | |||||||
| < 18.5 | 56 (3.4) | 23.1 (16.4, 36.0) | 4.9 (3.3, 7.6) | 2.4 (1.4, 3.5) | 0.6 (0.4, 0.9) | 0.9 (0.6, 1.5) | 1.7 (1.0, 2.5) | ||||||
| 18.5–24.9 | 944 (57.8) | 25.2 (18.9, 34.3) | 5.7 (4.3, 7.8) | 2.6 (1.7, 3.9) | 0.7 (0.5, 1.0) | 1.2 (0.7, 1.8) | 2.0 (1.3, 3.2) | ||||||
| 25–29.9 | 366 (22.4) | 25.2 (18.0, 36.2) | 5.6 (3.9, 8.2) | 2.5 (1.6, 3.8) | 0.6 (0.5, 0.9) | 1.2 (0.8, 1.9) | 1.9 (1.2, 3.2) | ||||||
| 30+ | 267 (16.4) | 27.6 (20.3, 35.4) | 5.9 (4.3, 8.0) | 2.4 (1.7, 3.5) | 0.6 (0.5, 0.9) | 1.4 (0.8, 2.1) | 2.0 (1.4, 2.8) | ||||||
| Gestational Age at Blood Draw (Weeks)c | <.001 | < 0.01 | 0.02 | < 0.01 | < 0.01 | < 0.01 | |||||||
| quartile 1 | 399 (24.3) | 28.4 (21.2, 38.3) | 6.1 (4.6, 8.3) | 2.7 (1.8, 3.9) | 0.7 (0.5, 1.0) | 1.4 (0.8, 2.1) | 2.2 (1.5, 3.4) | ||||||
| quartile 2 | 421 (25.6) | 25.4 (18.6, 34.9) | 5.7 (4.1, 8.0) | 2.4 (1.6, 3.6) | 0.6 (0.5, 0.9) | 1.2 (0.8, 1.9) | 2.0 (1.3, 3.2) | ||||||
| quartile 3 | 415 (25.2) | 24.9 (18.4, 34.6) | 5.7 (4.2, 7.9) | 2.7 (1.7, 4.2) | 0.7 (0.5, 0.9) | 1.2 (0.7, 1.9) | 2.0 (1.3, 3.1) | ||||||
| quartile 4 | 410 (24.9) | 23.4 (17.6, 31.5) | 5.2 (3.8, 7.3) | 2.3 (1.5, 3.4) | 0.6 (0.4, 0.9) | 1.1 (0.7, 1.7) | 1.7 (1.1, 2.6) | ||||||
| Prenatal GFR (mL/min per 1.73 m2)d | < 0.01 | < 0.01 | < 0.01 | < 0.01 | 0.05 | < 0.01 | |||||||
| quartile 1 | 407 (25.0) | 29.1 (21.5, 38.5) | 6.3 (4.8, 8.7) | 3.0 (1.9, 4.3) | 0.8 (0.6, 1.1) | 1.3 (0.8, 2.0) | 2.2 (1.4, 3.6) | ||||||
| quartile 2 | 408 (25.0) | 26.0 (19.4, 35.4) | 5.7 (4.2, 7.8) | 2.7 (1.7, 4.1) | 0.7 (0.5, 0.9) | 1.3 (0.8, 2.0) | 2.1 (1.3, 3.3) | ||||||
| quartile 3 | 408 (25.0) | 24.3 (18.2, 32.8) | 5.5 (4.0, 7.6) | 2.3 (1.5, 3.2) | 0.6 (0.5, 0.9) | 1.2 (0.7, 1.8) | 1.8 (1.2, 3.0) | ||||||
| quartile 4 | 407 (25.0) | 22.8 (16.7, 30.4) | 5.2 (3.9, 7.2) | 2.2 (1.5, 3.5) | 0.5 (0.4, 0.8) | 1.1 (0.7, 1.8) | 1.7 (1.1, 2.5) | ||||||
| Plasma Albumin (g/dL)e | < 0.01 | < 0.01 | < 0.01 | < 0.01 | < 0.01 | < 0.01 | |||||||
| quartile 1 | 396 (25.0) | 22.7 (17.0, 30.7) | 5.0 (3.7, 7.3) | 2.2 (1.4, 3.3) | 0.5 (0.4, 0.8) | 1.1 (0.7, 1.8) | 1.7 (1.1, 2.7) | ||||||
| quartile 2 | 396 (25.0) | 24.9 (18.9, 34.8) | 5.4 (4.0, 7.6) | 2.5 (1.6, 3.5) | 0.6 (0.5, 0.9) | 1.2 (0.7, 1.9) | 2.0 (1.3, 3.2) | ||||||
| quartile 3 | 396 (25.0) | 27.1 (19.8, 36.6) | 6.1 (4.3, 8.4) | 2.6 (1.7, 3.9) | 0.7 (0.5, 1.0) | 1.2 (0.7, 2.0) | 1.9 (1.2, 3.2) | ||||||
| quartile 4 | 396 (25.0) | 27.5 (20.3, 36.4) | 6.3 (4.7, 8.4) | 2.8 (1.9, 4.2) | 0.7 (0.6, 1.0) | 1.3 (0.8, 2.0) | 2.2 (1.4, 3.3) | ||||||
Partial sum of square p-values (global p-value across multiple categories of a predictor) for each predictor from linear regression models.
Estimated using information on parity and breastfeeding data for the child of the current pregnancy,
Gestational age at blood draw quartile ranges (weeks) are Quartile 1: 4.8–8.6; Quartile 2: 8.7–9.7; Quartile 3: 9.7–10.9; Quartile 4: 10.9–21.4.
GFR (mL/min per 1.73 m2) computed using the Cockroft-Gault (GFR-CG) formula; quartile ranges are Quartile 1: 39.3–82.0; Quartile 2: 82.0–101.4; Quartile 3: 101.5–126.1; Quartile 4: 126.1–968.4.
Plasma albumin (g/dL) quartile ranges are Quartile 1: 0.1–7.0; Quartile 2: 7.0–8.3; Quartile 3: 8.3–9.4; Quartile 4: 9.4–68.4.
In multivariable linear regression models (Table 4) adjusting for all sociodemographic and perinatal predictors, PFAS concentrations were lower with age, except for PFNA. PFAS concentrations were also lower with higher educational attainment; associations were substantially weaker for PFNA and for PFHxS with partner educational attainment. Annual household income showed the opposite trend as education, with lower PFOS, PFOA and PFNA concentrations at the lowest income level (<40k/year) vs. the highest (>70k/year). With the exception of PFNA, all PFAS concentrations declined over the enrollment period, and most strongly for Et-PFOSA-AcOH and Me-PFOSA-AcOH. Associations were attenuated for prenatal smoking, though concentrations still tended to be highest among former and current smokers.
Table 4. Adjusteda Associations from Multivariable Linear Regression Models for Sociodemographic and Perinatal Predictors and Log-Transformed Prenatal PFAS Concentrations (ng/mL) in Plasma Collected in Early Pregnancy,1999-2002, in Project Viva (n = 1195).
| PFOS | PFOA | PFHxS | PFNA | Et_PFOSA_AcOH | Me_PFOSA_AcOH | |
|---|---|---|---|---|---|---|
|
|
|
|
|
|
|
|
| % change (95% CI) | % change (95% CI) | % change (95% CI) | % change (95% CI) | % change (95% CI) | % change (95% CI) | |
| Age at Enrollment (per 5 Years) | −1.4 (−4.8, 2.1) | −4.5 (−7.5, −1.5) | −6.5 (−11.1, −1.6) | 3.7 (0.2, 7.4) | −3.5 (−8.3, 1.6) | −13.9 (−17.7,−10.0) |
| Race/Ethnicity | ||||||
| black | 6.5 (−3.9, 18.1) | −14.8 (−22.2, −6.7) | −18.4 (−29.6, −5.4) | 1.8 (−7.9, 12.5) | 2.2 (−11.8, 18.4) | −0.7 (−12.8, 13.1) |
| other | −6.2 (−13.8, 2.0) | −9.5 (−16.0, −2.4) | −23.0 (−31.8,−13.1) | 13.9 (5.0, 23.7) | −14.6 (−24.4, −3.6) | −10.1 (−19.2, 0.0) |
| white | 0.0 (ref) | 0.0 (ref) | 0.0 (ref) | 0.0 (ref) | 0.0 (ref) | 0.0 (ref) |
| Maternal Education | ||||||
| < college | 11.8 (2.3, 22.2) | 13.0 (4.5, 22.3) | 18.9 (4.8, 35.1) | -1.4 (-9.5, 7.5) | 13.0 (−0.4, 28.3) | 9.0 (−2.5, 21.9) |
| college | 3.9 (−2.9, 11.2) | 2.0 (−4.0, 8.3) | 10.5 (0.2, 21.8) | −2.9 (−9.1, 3.7) | 0.2 (−9.1, 10.5) | 10.8 (1.6, 20.7) |
| > college | 0.0 (ref) | 0.0 (ref) | 0.0 (ref) | 0.0 (ref) | 0.0 (ref) | 0.0 (ref) |
| Paternal Education | ||||||
| < college | 13.3 (3.9, 23.5) | 14.7 (6.3, 23.8) | 3.9 (−8.2, 17.5) | 4.0 (−4.3, 13.2) | 24.6 (10.1, 41.0) | 14.2 (2.4, 27.3) |
| college | 5.5 (−1.7, 13.1) | 8.3 (1.8, 15.3) | 5.8 (−4.4, 17.0) | 1.0 (−5.7, 8.1) | 10.2 (−0.4, 21.9) | 8.5 (−0.7, 18.6) |
| > college | 0.0 (ref) | 0.0 (ref) | 0.0 (ref) | 0.0 (ref) | 0.0 (ref) | 0.0 (ref) |
| married or cohabitating | ||||||
| no | −14.0 (−50.6, 49.5) | −2.5 (−40.2, 59.1) | −19.8 (−63.7, 77.2) | 6.6 (−37.7, 82.5) | −30.2 (−68.4, 54.1) | −41.6 (−70.9, 17.3) |
| yes | 0.0 (ref) | 0.0 (ref) | 0.0 (ref) | 0.0 (ref) | 0.0 (ref) | 0.0 (ref) |
| Annual Household Income | ||||||
| < $40K | −9.8 (−18.9, 0.3) | −11.1 (−19.1, −2.4) | −0.7 (−14.7, 15.7) | −18.5 (−26.5, −9.7) | 2.2 (−12.2, 18.9) | −5.2 (−17.1, 8.4) |
| $40–70 K | 3.2 (−3.8, 10.6) | −4.5 (−10.3, 1.5) | −9.4 (−18.0, 0.1) | −10.4 (−16.3, −4.1) | 7.4 (−2.8, 18.7) | −0.6 (−9.0, 8.6) |
| >$70K | 0.0 (ref) | 0.0 (ref) | 0.0 (ref) | 0.0 (ref) | 0.0 (ref) | 0.0 (ref) |
| Smoking Status | ||||||
| during pregnancy | 1.1 (−7.8, 10.8) | 10.5 (1.9, 19.9) | 0.6 (−11.8, 14.8) | 0.3 (−8.3, 9.6) | 7.6 (−5.6, 22.8) | 4.1 (−7.3, 16.9) |
| former | 6.8 (−0.4, 14.6) | 10.5 (3.8, 17.5) | 8.7 (−1.7, 20.1) | 8.8 (1.6, 16.4) | 11.2 (0.6, 22.9) | −0.5 (−8.9, 8.7) |
| never | 0.0 (ref) | 0.0 (ref) | 0.0 (ref) | 0.0 (ref) | 0.0 (ref) | 0.0 (ref) |
| Year of Enrollment | ||||||
| 1999 | 17.7 (0.3, 38.2) | 12.0 (−2.8, 29.1) | 12.6 (−10.5, 41.7) | −2.1 (−16.2, 14.3) | 117.7 (73.1,173.8) | 72.9 (41.3,111.6) |
| 2000 | 19.5 (2.3, 39.7) | 23.1 (7.3, 41.3) | 19.5 (−4.4, 49.4) | −1.3 (−15.2, 14.8) | 99.2 (59.4,149.1) | 61.4 (32.6, 96.5) |
| 2001 | 6.6 (−8.7, 24.3) | 13.9 (−0.6, 30.6) | 5.7 (−15.3, 31.8) | −3.4 (−16.8, 12.2) | 53.8 (23.3, 91.8) | 34.0 (10.3, 62.7) |
| 2002 | 0.0 (ref) | 0.0 (ref) | 0.0 (ref) | 0.0 (ref) | 0.0 (ref) | 0.0 (ref) |
| Parity | ||||||
| 0 | 0.0 (ref) | 0.0 (ref) | 0.0 (ref) | 0.0 (ref) | 0.0 (ref) | 0.0 (ref) |
| 1+ | −4.6 (−14.1, 5.9) | −5.0 (−13.4, 4.3) | −13.1 (−25.2, 1.0) | −13.5 (−21.9, −4.2) | 17.7 (1.3, 36.8) | 21.8 (6.7, 39.0) |
| Breastfed before Current Pregnancyb | ||||||
| no | 19.8 (7.9, 33.1) | 37.5 (25.3, 50.8) | 6.8 (−8.1, 24.2) | 10.0 (−0.7, 21.8) | 16.8 (0.5, 35.7) | 8.0 (−5.4, 23.3) |
| yes | 0.0 (ref) | 0.0 (ref) | 0.0 (ref) | 0.0 (ref) | 0.0 (ref) | 0.0 (ref) |
| Prepregnancy BMI (per 5 kg/m2) | 11.2 (7.8, 14.7) | 11.6 (8.6, 14.7) | 8.7 (4.0, 13.7) | 8.3 (5.0, 11.6) | 11.9 (7.0, 17.0) | 9.8 (5.6, 14.2) |
| Gestational age at blood draw (per week) | −1.9 (−3.1, −0.5) | −0.7 (−1.9, 0.4) | 0.0 (−1.9, 1.9) | −1.5 (−2.8, −0.2) | −1.9 (−3.7, 0.0) | −1.4 (−3.0, 0.3) |
| GFR (per 10 mL/min per 1.73 m2) | −3.7 (−4.4, −3.0) | −3.6 (−4.2, −3.0) | −4.3 (−5.3, −3.3) | −4.2 (−4.8, −3.5) | −3.3 (−4.3, −2.3) | −3.6 (−4.4, −2.7) |
| Plasma Albumin (per 1 g/dL) | 4.8 (3.6, 6.0) | 5.1 (4.0, 6.2) | 5.3 (3.6, 7.1) | 5.7 (4.5, 6.9) | 4.5 (2.8, 6.3) | 4.3 (2.8, 5.9) |
| model R2 | 0.20 | 0.30 | 0.13 | 0.22 | 0.18 | 0.17 |
Multivariable models were adjusted for all variables included in the table.
Estimated using information on parity and breastfeeding data for the child of the current pregnancy.
Perinatal and Physiologic Predictors
Table 3 reports higher unadjusted PFAS concentrations among women who were nulliparous and among women we estimated did not breastfeed prior to the current pregnancy, with the exception of Et-PFOSA-AcOH and Me-PFOSA-AcOH. In addition, PFOS, PFOA, and Et-PFOSA-AcOH concentrations were highest among women who were obese prior to pregnancy (BMI ≥ 30 kg/m2). For markers of pregnancy hemodynamics, all PFAS concentrations were higher for women with blood drawn earlier in pregnancy, lower prenatal GFR and higher prenatal plasma albumin levels. GFR and plasma albumin were moderately correlated (correlation coefficient = 0.2).
Fully adjusted multivariable models (Table 4) showed lower PFAS concentrations among nulliparous vs. parous women, though we observed the opposite trend for Et-PFOSA-AcOH and Me-PFOSA-AcOH. All PFAS concentrations were higher among women who never breastfed prior to the current pregnancy, especially PFOS and PFOA. PFAS concentrations were also higher for mothers with higher prepregnancy BMI (though not shown in Table 4, associations increased monotonically across BMI categories). PFAS concentrations were inversely associated with GFR and positively associated with plasma albumin (these associations were predominantly linear across quartiles of GFR and albumin). However, associations were weaker for gestational age at blood draw in multivariable models.
R2 for fully adjusted models reported in Table 4 showed that depending on the PFAS, predictors included explained between 13 and 30% of the variance in PFAS concentrations.
Discussion
In models adjusted for all predictors we found that overall PFAS concentrations were higher among pregnant women who were younger, less educated (but higher income), had less educated partners, were nulliparous, did not breastfeed prior to the current pregnancy, and had higher prepregnancy BMI. In addition, overall PFAS concentrations declined over the enrollment period, and were lower for participants with no history of smoking, higher GFR and lower plasma albumin levels.
PFAS concentrations in Project Viva were comparable to NHANES 1999–2000 concentrations, reflecting general population concentrations during these peak production years. As with NHANES, Viva PFAS concentrations declined over time, with the phase out of long-chained PFAS, including PFOS and PFOA. PFNA concentrations, on the other hand, were highest in the final Project Viva enrollment year (2002), consistent with higher PFNA concentrations over time in NHANES from 1999 to 2008.
Previous studies of prenatal PFAS concentrations have found inconsistent relationships for maternal age, with some studies reporting lower PFOA and PFOS with older age,18,28 as we report in the current study, and other studies reporting the opposite trend17 or no consistent pattern.16,20,21 NHANES reported higher concentrations of these compounds in women of older age.12 We found a positive association between PFNA and age, similar to a few other pregnancy studies17,20 and NHANES,12 but other studies found null PFNA-age associations.18,21 The differing trends of PFOS/PFOA and PFNA may partly reflect usage patterns of these compounds or their precursors.9 Differences with respect to age across pregnancy studies may also reflect differing year of sampling. PFASs were still very much in use during the Project Viva enrollment period. In general, age trends of persistent organic pollutants depend on year since peak emission, environmental persistence and biological half-life.29
Patterns of PFAS concentrations were not uniform across socioeconomic status indicators. While PFAS concentrations were lower for higher maternal and partner educational attainment, concentrations of most PFASs were higher with higher household income. We are unable to explain this inconsistency between education and income in our results, which was not reported in two other pregnancy studies, which found higher PFAS concentrations for more educated and higher income women.18,20 Two additional studies reported no associations for socioeconomic indicators and PFAS concentrations.16,28
PFOS, PFOA, PFHxS, and PFNA concentrations were higher for nulliparous women, which has been well documented in previous studies.16,17,20,21 Lower PFAS concentrations in parous women are likely a function of placental transfer of PFASs during previous pregnancies as well as deposition of these chemicals in breast milk. We found the opposite association for parity and Et-PFOSA-AcOH and Me-PFOSA-AcOH, that is, higher concentrations among parous women. It is unclear why these two analytes showed different associations. As this is the first study to report predictors of Et-PFOSA-AcOH and Me-PFOSA-AcOH, these findings require replication.
We created a variable to represent breastfeeding prior to the current pregnancy, which we derived from parity and report of breastfeeding the child of the current pregnancy. While this derived variable likely serves as a proxy for parity, we did find independent associations for history of breastfeeding and parity in our multivariable models. History of breastfeeding has also been identified as a predictor of PFAS concentrations in previous studies.18,20
PFAS concentrations were higher among women with higher prepregnancy BMI, which is consistent with some previous studies that also reported suggestive associations;16,18,28 other studies have reported null associations for PFAS concentrations and prepregnancy BMI.20,21
We measured plasma creatinine and albumin levels in the same samples used for quantifying PFASs. In Viva we observed lower PFAS concentrations with higher GFR (estimated using creatinine) and lower albumin. This is the first epidemiologic study to show associations of PFASs with GFR in pregnant women, and we hypothesize that these associations may be due to higher flow rate of filtered fluid through the kidney. In addition, lower PFASs with lower albumin levels may be explained by hemodilution due to plasma volume expansion as pregnancy progresses. Plasma albumin was measured in males and females participating in NHANES and was related to higher PFOS and PFOA concentrations, but lower PFHxS concentrations.19 Strong associations of PFAS and albumin are also plausible due to the strong binding affinity of PFASs to plasma albumin.30,31 Normalizing for albumin in descriptive comparisons of PFASs should be considered in future studies, similar to the lipid normalization that is done for lipophilic compounds such as PCBs and PBDEs. It may also be appropriate to adjust for albumin, as well as GFR, in multivariable models when estimating associations for PFASs with health outcomes, depending on the relationship between these physiologic markers and the outcome.32 For example, a recent study that used a pharmacokinetic model to examine the impact of GFR on PFAS-birth weight associations reported results for simulations that suggested that associations were substantially attenuated due to confounding by GFR.33
A limitation of this study is that we did not have data on some potentially important predictors of PFASs, including history of breastfeeding and interpregnancy interval. While we used existing data on parity and breastfeeding the child of the current pregnancy to reconstruct a history of breastfeeding variable, misclassification is possible and results should be interpreted with caution. Another limitation of this analysis is that we did not adjust for diet. A number of studies have found certain foods, including seafood, read meat, and salty snacks, to be important sources of PFAS exposure.17,28,34 Project Viva collected high quality data on prenatal diet using validated food frequency questionnaires; however given the complexity of these data, and dietary sources of PFASs (e.g., food packaging is likely to be a source of dietary exposure), we chose to report dietary predictors of PFASs in future work. This may pose some limitations for our multivariable model, as these potentially important predictors were not accounted for, and may explain the low R2 in adjusted models (Table 4).
The limitations of this analysis are offset by some notable strengths, including the large sample size (this is the largest prospective study of prenatal PFASs to date), excellent measurement of prenatal factors during the relevant time window and during the period when human biomarker concentrations of PFOS and PFOA were at their peak, and data on pregnancy physiology, which has not yet been well characterized in studies of environmental biomarkers.
In summary, our data show that PFAS exposures were ubiquitous among a cohort of pregnant women living in eastern Massachusetts, an area not known to be proximal to any PFAS water-contaminated areas. Furthermore, concentrations exhibited variability across individuals, with higher PFAS concentrations in women who were younger, had lower educational attainment, were nulliparous, had no history of breastfeeding, were enrolled earlier in the study period, and had higher prepregnancy BMI. We also report associations with pregnancy physiology, including GFR and albumin, which were consistently related to PFAS concentrations. Future studies of PFASs and health outcomes should consider measuring and adjusting for these physiologic factors when estimating PFAS-outcome associations.
Acknowledgments
Work was supported by NIH grants R01 ES021447, K24 HD069408, P30 DK092924, and R37 HD034568. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Abbreviations
- BMI
body mass index
- CI
confidence Interval
- CDC
Centers for Disease Control and Prevention
- Et-PFOSA-AcOH
2-(N-ethyl-perfluorooctane sulfonamido) acetate
- GFR
glomerular filtration rate
- Me-PFOSA-AcOH
2-(N-methyl-perfluorooctane sulfonamido) acetate
- NHANES
National Health and Nutrition Examination Survey
- PFAS
per- and polyfluoroalkyl substances
- PFDeA
perfluorodecanoate
- PFHxS
perfluorohexanesulfonate
- PFNA
perfluorononanoate
- PFOA
perfluorooctanoate
- PFOS
perfluorooctanesulfonate
- PFOSA
perfluorooctane sulfonamide
Footnotes
Notes: The authors declare no competing financial interest.
References
- 1.Fraser AJ, Webster TF, Watkins DJ, Strynar MJ, Kato K, Calafat AM, Vieira VM, McClean MD. Polyfluorinated compounds in dust from homes, offices, and vehicles as predictors of concentrations in office workers' serum. Environ Int. 2013;60:128–36. doi: 10.1016/j.envint.2013.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Johansson JH, Berger U, Vestergren R, Cousins IT, Bignert A, Glynn A, Darnerud PO. Temporal trends (1999–2010) of perfluoroalkyl acids in commonly consumed food items. Environ Pollute. 2014;188:102–8. doi: 10.1016/j.envpol.2014.01.026. [DOI] [PubMed] [Google Scholar]
- 3.Frisbee SJ, Brooks AP, Jr, Maher A, Flensborg P, Arnold S, Fletcher T, Steenland K, Shankar A, Knox SS, Pollard C, Halverson JA, Vieira VM, Jin C, Leyden KM, Ducatman AM. The C8 health project: design, methods, and participants. Environ Health Perspect. 2009;117(12):1873–82. doi: 10.1289/ehp.0800379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fraser AJ, Webster TF, Watkins DJ, Nelson JW, Stapleton HM, Calafat AM, Kato K, Shoeib M, Vieira VM, McClean MD. Polyfluorinated compounds in serum linked to indoor air in office environments. Environ Sci Technol. 2012;46(2):1209–15. doi: 10.1021/es2038257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Steenland K, Jin C, MacNeil J, Lally C, Ducatman A, Vieira V, Fletcher T. Predictors of PFOA levels in a community surrounding a chemical plant. Environ Health Perspect. 2009;117(7):1083–8. doi: 10.1289/ehp.0800294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vestergren R, Berger U, Glynn A, Cousins IT. Dietaryexposure to perfluoroalkyl acids for the Swedish population in 1999, 2005 and 2010. Environ Int. 2012;49:120–7. doi: 10.1016/j.envint.2012.08.016. [DOI] [PubMed] [Google Scholar]
- 7.Conder JM, Hoke RA, DeWolf W, Russell MH, Buck RC. Are PFCAs bioaccumulative? A critical review and comparison with regulatory criteria and persistent lipophilic compounds. Environ Sci Technol. 2008;42(4):995–1003. doi: 10.1021/es070895g. [DOI] [PubMed] [Google Scholar]
- 8.Olsen GW, Burris JM, Ehresman DJ, Froehlich JW, Seacat AM, Butenhoff JL, Zobel L. R Half-life of serum elimination of perfluorooctanesulfonate, perfluorohexanesulfonate, and perfluorooctanoate in retired fluorochemical production workers. Environ Health Perspect. 2007;115(9):1298–305. doi: 10.1289/ehp.10009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bartell SM, Calafat AM, Lyu C, Kato K, Ryan PB, Steenland K. Rate of decline in serum PFOA concentrations aftergranular activated carbon filtration at two public water systems in Ohioand West Virginia. Environ Health Perspect. 2009;118(2):222–228. doi: 10.1289/ehp.0901252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Calafat AM, Kuklenyik Z, Reidy JA, Caudill SP, Tully JS, Needham LL. Serum concentrations of 11 polyfluoroalkyl compounds in the U.S. population: data from the national health and nutrition examination survey (NHANES) Environ Sci Technol. 2007;41(7):2237–42. doi: 10.1021/es062686m. [DOI] [PubMed] [Google Scholar]
- 11.Calafat AM, Wong LY, Kuklenyik Z, Reidy JA, Needham LL. Polyfluoroalkyl chemicals in the U.S. population: data from the National Health and Nutrition Examination Survey (NHANES) 2003– 2004 and comparisons with NHANES 1999–2000. Environ Health Perspect. 2007;115(11):1596–602. doi: 10.1289/ehp.10598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kato K, Wong LY, Jia LT, Kuklenyik Z, Calafat AM. Trends in exposure to polyfluoroalkyl chemicals in the U.S. Population: 1999–2008. Environ Sci Technol. 2011;45(19):8037–45. doi: 10.1021/es1043613. [DOI] [PubMed] [Google Scholar]
- 13.Lau C, Butenhoff JL, Rogers JM. The developmental toxicityof perfluoroalkyl acids and their derivatives. Toxicol Appl Pharmacol. 2004;198(2):231–241. doi: 10.1016/j.taap.2003.11.031. [DOI] [PubMed] [Google Scholar]
- 14.Olsen GW, Butenhoff JL, Zobel LR. Perfluoroalkyl chemicals and human fetal development: an epidemiologic review with clinical and toxicological perspectives. Reprod Toxicol. 2009;27(3–4):212–30. doi: 10.1016/j.reprotox.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 15.Johansson N, Eriksson P, Viberg H. Neonatal exposure to PFOS and PFOA in mice results in changes in proteins which are important for neuronal growth and synaptogenesis in the developing brain. Toxicol Sci. 2009;108(2):412–418. doi: 10.1093/toxsci/kfp029. [DOI] [PubMed] [Google Scholar]
- 16.Apelberg BJ, Goldman LR, Calafat AM, Herbstman JB, Kuklenyik Z, Heidler J, Needham LL, Halden RU, Witter FR. Determinants of fetal exposure to polyfluoroalkyl compounds in Baltimore, Maryland. Environ Sci Technol. 2007;41(11):3891–7. doi: 10.1021/es0700911. [DOI] [PubMed] [Google Scholar]
- 17.Berg V, Nost TH, Huber S, Rylander C, Hansen S, Veyhe AS, Fuskevag OM, Odland JO, Sandanger TM. Maternal serum concentrations of per- and polyfluoroalkyl substances and their predictors in years with reduced production and use. Environ Int. 2014;69:58–66. doi: 10.1016/j.envint.2014.04.010. [DOI] [PubMed] [Google Scholar]
- 18.Brantsaeter AL, Whitworth KW, Ydersbond TA, Haug LS, Haugen M, Knutsen HK, Thomsen C, Meltzer HM, Becher G, Sabaredzovic A, Hoppin JA, Eggesbo M, Longnecker MP. Determinants of plasma concentrations of perfluoroalkyl substances in pregnant Norwegian women. Environ Int. 2013;54:74–84. doi: 10.1016/j.envint.2012.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jain RB. Contribution of diet and other factors to the levels of selected polyfluorinated compounds: data from NHANES 2003–2008. Int J Hyg Environ Health. 2014;217(1):52–61. doi: 10.1016/j.ijheh.2013.03.008. [DOI] [PubMed] [Google Scholar]
- 20.Kato K, Wong LY, Chen A, Dunbar C, Webster GM, Lanphear BP, Calafat AM. Changes in serum concentrations of maternal poly- and perfluoroalkyl substances over the course of pregnancy and predictors of exposure in a multiethnic cohort of Cincinnati, Ohio pregnant women during 2003–2006. Environ Sci Technol. 2014;48(16):9600–8. doi: 10.1021/es501811k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ode A, Rylander L, Lindh CH, Kallen K, Jonsson BA, Gustafsson P, Olofsson P, Ivarsson SA, Rignell-Hydbom A. Determinants of maternal and fetal exposure and temporal trends of perfluorinated compounds. Environ Sci Pollute Res. 2013;20(11):7970–8. doi: 10.1007/s11356-013-1573-5. [DOI] [PubMed] [Google Scholar]
- 22.Savitz DA. Guest editorial: biomarkers of perfluorinated chemicals and birth weight. Environ Health Perspect. 2007;115(11):A528–9. doi: 10.1289/ehp.10923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Savitz DA. Invited commentary: interpreting associations between exposure biomarkers and pregnancy outcome. Am J Epidemiol. 2014;179(5):545–7. doi: 10.1093/aje/kwt314. [DOI] [PubMed] [Google Scholar]
- 24.Morken NH, Travlos GS, Wilson RE, Eggesbo M, Longnecker MP. Maternal glomerular filtration rate in pregnancy and fetal size. PLoS One. 2014;9(7):e101897. doi: 10.1371/journal.pone.0101897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oken E, Baccarelli AA, Gold DR, Kleinman KP, Litonjua AA, De Meo D, Rich-Edwards JW, Rifas-Shiman SL, Sagiv S, Taveras EM, Weiss ST, Belfort MB, Burris HH, Camargo CA, Jr, Huh SY, Mantzoros C, Parker MG, Gillman MW. Cohort Profile: Project Viva. Int J Epidemiol. 2015;44:37. doi: 10.1093/ije/dyu008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kato K, Basden BJ, Needham LL, Calafat AM. Improved selectivity for the analysis of maternal serum and cord serum for polyfluoroalkyl chemicals. J Chromatogr A. 2011;1218(15):2133–7. doi: 10.1016/j.chroma.2010.10.051. [DOI] [PubMed] [Google Scholar]
- 27.D'Eon JC, Simpson AJ, Kumar R, Baer AJ, Mabury S. A Determining the molecular interactions of perfluorinated carboxylic acids with human sera and isolated human serum albumin using nuclear magnetic resonance spectroscopy. Environ Toxicol Chem. 2010;29(8):1678–88. doi: 10.1002/etc.204. [DOI] [PubMed] [Google Scholar]
- 28.Halldorsson TI, Fei C, Olsen J, Lipworth L, McLaughlin JK, Olsen SF. Dietary predictors of perfluorinated chemicals: a study from the Danish National Birth Cohort. Environ Sci Technol. 2008;42(23):8971–7. doi: 10.1021/es801907r. [DOI] [PubMed] [Google Scholar]
- 29.Quinn CL, Wania F. Understanding differences in the body burden-age relationships of bioaccumulating contaminants based on population cross sections versus individuals. Environ Health Perspect. 2012;120(4):554–9. doi: 10.1289/ehp.1104236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Han X, Snow TA, Kemper RA, Jepson GW. Binding of perfluorooctanoic acid to rat and human plasma proteins. Chem Res Toxicol. 2003;16(6):775–81. doi: 10.1021/tx034005w. [DOI] [PubMed] [Google Scholar]
- 31.Zhang X, Chen L, Fei XC, Ma YS, Gao HW. Binding of PFOS to serum albumin and DNA: insight into the molecular toxicity of perfluorochemicals. BMC Mol Biol. 2009;10:16. doi: 10.1186/1471-2199-10-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schisterman EF, Whitcomb BW, Louis GM, Louis TA. Lipid adjustment in the analysis of environmental contaminants and human health risks. Environ Health Perspect. 2005;113(7):853–7. doi: 10.1289/ehp.7640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Verner MA, Loccisano AE, Morken NH, Yoon M, Wu H, Mc Dougall R, Maisonet M, Marcus M, Kishi R, Miyashita C, Chen MH, Hsieh WS, Andersen ME, Clewell HJ, 3rd, Longnecker MP. Associations of Perfluoroalkyl Substances (PFASs) with Lower Birth Weight: An Evaluation of Potential Confounding by Glomerular Filtration Rate Using a Physiologically Based Pharmacokinetic Model (PBPK) Environ Health Perspect. 2015 doi: 10.1289/ehp.1408837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rylander C, Sandanger TM, Froyland L, Lund E. Dietary patterns and plasma concentrations of perfluorinated compounds in 315 Norwegian women: the NOWAC Postgenome Study. Environ Sci Technol. 2010;44(13):5225–32. doi: 10.1021/es100224q. [DOI] [PubMed] [Google Scholar]


