Abstract
CD8 T-cells are a critical brake on the initial development of tumors. In established tumors, the presence of CD8 T-cells is correlated with a positive patient prognosis, although immunosuppressive mechanisms limit their effectiveness and they are rarely curative without manipulation. Cancer immunotherapies aim to shift the balance back to dominant anti-tumor immunity through antibody blockade of immunosuppressive signaling pathways, vaccination, and adoptive transfer of activated or engineered T-cells. These approaches have yielded striking responses in small subsets of patients with solid tumors, most notably those with melanoma. Importantly, the subset of patients who respond to vaccination or immunosuppression blockade therapies are those with CD8 T-cells present in the tumor prior to initiating therapy. While current adoptive cell therapy approaches can be dramatically effective, they require infusion of extremely large numbers of T-cells, but the number that actually infiltrate the tumor is very small. Thus, poor representation of CD8 T-cells in tumors is a fundamental hurdle to successful immunotherapy, over and above the well-established barrier of immunosuppression. In this review, we discuss the factors that determine whether immune cells are present in tumors, with a focus on the representation of cytotoxic CD8 T-cells. We emphasize the critically important role of tumor-associated vasculature as a gateway that enables the active infiltration of both effector and naïve CD8 T-cells that exert anti-tumor activity. We also discuss strategies to enhance the gateway function and extend the effectiveness of immunotherapies to a broader set of cancer patients.
I. Prognostic significance of immune cell representation in tumors
A role for the immune system in cancer regression was suggested in the late 19th century by William Coley, who observed that spontaneous remission of tumors sometimes occurred in patients who contracted acute bacterial infections. He subsequently developed a mixture of bacterial toxins that he believed activated the immune system, and reported they were effective and even curative for some patients (Coley, 1893). Still, his method was controversial, and with the advent of chemo- and radiotherapy, fell out of favor (Wiemann and Starnes, 1994). It was not until the late 20th century that the importance of the immune system in tumor control was firmly established. In seminal studies examining the development of tumors in immunodeficient mice (Kaplan et al., 1998; Smyth et al., 2000, 2001; Shankaran et al., 2001), it was established that cytotoxic CD8 T-cells and NK cells controlled the incidence and severity of spontaneously occurring and chemically induced tumors. However, immune selective pressure also edited these tumors, enabling the expansion of tumor clones that had stopped expressing target antigens and making them less susceptible to immunological control. In addition, other immune elements, including regulatory T-cells (Treg) and several myeloid populations, were shown to suppress immunity, contributing to tumor outgrowth, angiogenesis, and metastasis (Coussens et al., 2000; Lin et al., 2001; Turk et al., 2004; L. Yang et al., 2004; De Palma et al., 2005). Nevertheless, early correlative studies of patients with many tumor types, including melanoma (Clark et al., 1989) and neurological tumors (Lauder and Aherne, 1972; Palma et al., 1978), demonstrated that the presence of intratumoral lymphocytes was associated with a positive prognosis and longer survival.
Different immune cell subsets have now been correlated with prevention of tumor establishment and outgrowth (Vesely et al., 2011) as well as a positive or negative prognosis in late stage tumors (Fridman et al., 2012). In fact, the same cell types are often beneficial at both stages of tumor development. Cells that are present in the tumor mass and most often linked to a positive prognosis include cytotoxic lymphocytes (CD8 T-cells and NK cells) and CD4 T-cells with a Th1 (interferon-γ, [IFNγ] producing) phenotype. Cells in the tumor mass that represent myeloid lineages, including neutrophils, macrophages, and myeloid derived suppressor cells, are most commonly associated with a negative prognosis. Other tumor-infiltrating cell types have not been consistently linked to a single prognostic outcome. In different studies, Th2 and Th17 cells, Treg, and NKT-cells have been linked to both positive and negative prognoses (Fridman et al., 2012). The reasons for these variable associations are unclear. For Treg, this could reflect the imprecision with which phenotypic markers (e.g. FoxP3) clearly identify true regulatory cells with suppressive function, as opposed to activated effector cells in humans (Tran et al., 2007; J. Wang et al., 2007). It has been proposed that Th17 cells might have different phenotypes or functions depending on the tumor type and therefore exert either pro- or anti-tumorigenic activity (Wilke et al., 2011; Bailey et al., 2014).
Recognizing that multiple subsets of immune cells are often present in tumors at the same time, their relative representations and function may be as important as their simple presence, as these create a balance between positive and negative influences. The ratio of CD8 T-cells to Treg or total CD4 T-cells has been shown to be prognostically important in ovarian, colorectal, and pancreatic cancer (Diederichsen et al., 2003; Ino et al., 2013; Preston et al., 2013; Sato et al., 2005). In addition, although high levels of CD8 T-cells in tumors have been linked to positive clinical outcomes more commonly than for any other cell type and in a number of different tumors, their functional status in the tumor is also relevant. For example, CD8 T-cells were only associated with longer survival of renal cell carcinoma patients if they were actively proliferating (Nakano et al., 2001). CD8 T-cells that expressed CD45RO, a marker of antigen experience, were associated with both enhanced expression of cytotoxicity genes and positive prognosis in colorectal cancer patients (Galon et al., 2006; Pagès et al., 2009). Gene signatures associated with cytotoxicity and IFNγ signaling as markers of effector CD8 T-cells have similarly been associated with a positive prognosis in many other tumor types (Galon et al., 2013).
Interestingly, the precise localization of CD8 T-cells within the tumor also alters their prognostic significance. The density and location of CD8 T-cells in colorectal carcinomas, encompassed as an analysis termed the ‘Immunoscore’, was shown to exceed traditional histopathological staging in prognostic power (Angell and Galon, 2013; Galon et al., 2006). CD8 T-cell presence in both the center of the tumor and the invasive margins was associated with a better outcome than presence in only one location (Pagès et al., 2009). The presence of CD8 T-cells was associated with improved survival if they were localized to intraepithelial, but not stromal regions of ovarian carcinoma tumors (Sato et al., 2005). In metastatic melanoma, three distinct ‘immunotypes’ have been defined based on the presence and intratumoral distribution of immune cells (Erdag et al., 2012). Immunotype A tumors were poorly or negligibly infiltrated by immune cells, and these patients had the poorest prognosis. Immunotype B tumors contained CD8 T-cells that remained perivascular. This phenotype was associated with an intermediate prognosis. Immunotype C tumors contained CD8 T-cells that extended well away from blood vessels throughout the tumor and were associated with the best overall prognosis. Therefore, even among patients with the same histological tumor type, there is a remarkable heterogeneity in overall CD8 T-cell representation and intratumoral distribution. Most importantly, this heterogeneity indicates that fundamental processes controlling T-cell infiltration into and migration within tumors vary.
II. CD8 T-cell representation in tumors as a predictive marker of responsiveness to therapy
The emergence of clinically evident cancers reveals that despite the presence and activity of CD8 T-cells, tumors can escape their control (Vesely et al., 2011). Numerous strategies to harness and/or enhance the anti-tumor properties of CD8 T-cells have been developed, and in recent years, have led to encouraging successes. Melanoma has historically been the most studied tumor for immunotherapies, in part because it is also the most responsive to a wide spectrum of such therapies. However, efficacy of certain treatments has also been shown in renal cell carcinoma, lung cancer, and bladder cancer. Regardless, only a fraction of patients with any of these cancers respond to these therapies. Efforts to identify the basis for responsiveness have suggested that a major determinant is the presence of effector CD8 T-cells in tumors prior to initiating therapy.
High dose IL-2 has consistently been shown to elicit clinical responses, including complete responses, in a small fraction of patients (Rosenberg et al., 1994; Schwartzentruber et al., 2011). Gene expression profiling of pre-treatment tumor biopsies has revealed that a pre-existing immune related gene signature, indicating elevated representation of CD4 and CD8 T-cells and elevated expression of T-cell derived cytokines and T-cell attracting chemokines, is associated with clinical responses to IL-2 (Sullivan et al., 2009; Wang et al., 2002; Weiss et al., 2011). A similar association has also been observed with clinical responses to other immunotherapies, including therapeutic cancer vaccines (Gajewski et al., 2010; Ulloa-Montoya et al., 2013), and treatment with the checkpoint blockade antibodies anti-CTLA-4 (Ji et al., 2012), and anti-PD-1/anti-PD-L1 (Herbst et al., 2014; Tumeh et al., 2014). The pre-treatment presence of effector T-cells is also associated with enhanced responsiveness to some chemotherapy and radiotherapy treatments in colorectal cancer (Halama et al., 2011, 2009; Morris et al., 2008; Yasuda et al., 2011), breast cancer (Denkert et al., 2010; Loi et al., 2013; West et al., 2011) head and neck cancer (Balermpas et al., 2014), and non-small cell lung cancer (Liu et al., 2012).
Given that the immunotherapies and traditional therapies outlined augment immunity and/or inhibit tumor growth through diverse and distinct mechanisms of action, the reason(s) that a pre-existing infiltrate of CD8 T-cells in the tumor is associated with clinical responses are not entirely clear. The connection is most easily explained for anti-PD-1/PD-L1 therapy and IL-2 therapies. IL-2 may act to expand the intratumoral effector T cell population or rescue it from an anergic phenotype (Beverly et al., 1992; Margolin, 2000). PD-1 expression on effector T-cells, and engagement with its ligand PD-L1, inhibits their secretion of effector cytokines, blunts their cytotoxic function, and promotes cell death, thereby protecting peripheral tissues from excessive immune-mediated damage (Keir et al., 2008). In tumors, the ligands PD-L1 and PD-L2 can be expressed on tumor and stromal cells and likewise restrict immune attack and drive T-cell apoptosis (Blank et al., 2004; Dong et al., 2002). In keeping with this, patients whose tumors or stroma express PD-L1 are more likely to respond to anti-PD-1 (Topalian et al., 2012; Tumeh et al., 2014). The pre-existing accumulation of effector CD8 T-cells in the tumor therefore represents the immediate target of anti-PD-1 therapy.
Anti-CTLA-4 is proposed to act primarily in the lymph node (LN) to enhance T-cell priming rather than locally in the tumor (Pardoll, 2012). This is supported by the emergence of a broader TCR repertoire among tumor infiltrating lymphocytes upon treatment, likely reflecting expansion of lower-affinity T-cells due to a lowered threshold of activation with CTLA-4 blockade (Cha et al., 2014; Kvistborg et al., 2014). In mouse models, anti-CTLA-4 can also deplete Treg (Bulliard et al., 2013; Simpson et al., 2013). Although this mechanism has not been demonstrated in humans, responsiveness to anti-CTLA-4 has been associated with the pre-existing level of intratumoral FoxP3+ cells (Hamid et al., 2011). IL-2, in addition to local effects in the tumor, could also support a more robust proliferative response in the LN. Therapeutic vaccination likewise aims to enhance the response to specific tumor antigens that occurs in tumor-draining LN. Finally, chemotherapies or radiotherapies may promote the immunogenic death of tumor cells acting as a natural vaccination to promote T-cell priming against antigens from the killed cells (Casares et al., 2005; Formenti and Demaria, 2013; Lugade et al., 2005; Zitvogel et al., 2011). Radiotherapy is also associated with abscopal effects, whereby local irradiation leads to regression of distant metastases. This phenomenon is poorly understood, but requires effector CD8 T-cells, indicating it is immune-mediated (Demaria et al., 2005, 2004; Postow et al., 2012).
Thus, for a variety of therapies, the presence and distribution of effector CD8 T-cells in the tumor microenvironment prior to therapy predicts a positive response. This is true even for interventions that do not seem to be directly targeting the CD8 T-cells already in the tumor. In all of these instances, we suggest that the prognostic significance of pre-existing CD8 T-cells in the tumor reflects a microenvironment, and a tumor associated vasculature, that is permissive to CD8 T-cell accumulation. As a consequence, new CD8 effectors generated in local secondary lymphoid tissue by any of these treatments may more readily enter the tumor and control it.
The adoptive transfer of ex vivo expanded lymphocytes has also shown clinical efficacy in a substantial percentage of treated patients with melanoma (Restifo et al., 2012). The pre-existing presence of CD8 T-cells in the tumor is naturally a prerequisite for treatment, as that is the source of the cells that are harvested, expanded, and reinfused. New adoptive transfer therapies using T-cells derived from the blood rather than the tumor, which are genetically engineered to recognize tumor antigens, have recently been shown to have some anti-tumor activity in solid tumors (Beatty et al., 2014). It would be expected that the presence of endogenous T-cells in the responding patients’ tumors is required, but this remains to be investigated.
III. Determinants of CD8 T-cell representation in tumors and other tissues
The overall representation of T-cells in tissues, including tumors, is determined by the balance of several fundamental processes: cells entering tissues from the blood vasculature, cells leaving through draining lymphatics, and cells proliferating and dying in situ. To accumulate in a tumor at a peripheral site, however, typically effector T-cells are first activated by specific antigen in the draining LN (see Figure 1). Tumors that are poorly infiltrated by effector T-cells may be poorly immunogenic due to a paucity of antigens. Consistent with this, we have observed that murine B16 melanoma tumors transfected to express ovalbumin as a strong neoantigen are infiltrated by larger numbers of effector CD8 T-cells than the parental B16 line, which is poorly antigenic (Peske et al., 2015). Similar results have been reported for other implantable murine tumors (Yu et al., 2005). Responses to anti-CTLA-4 or anti-PD-1/PD-L1, which are associated with a pre-existing CD8 T-cell infiltrate, have also been associated with mutational burden (Champiat et al., 2014; Snyder et al., 2014), providing a link between T-cell representation and tumor antigenicity.
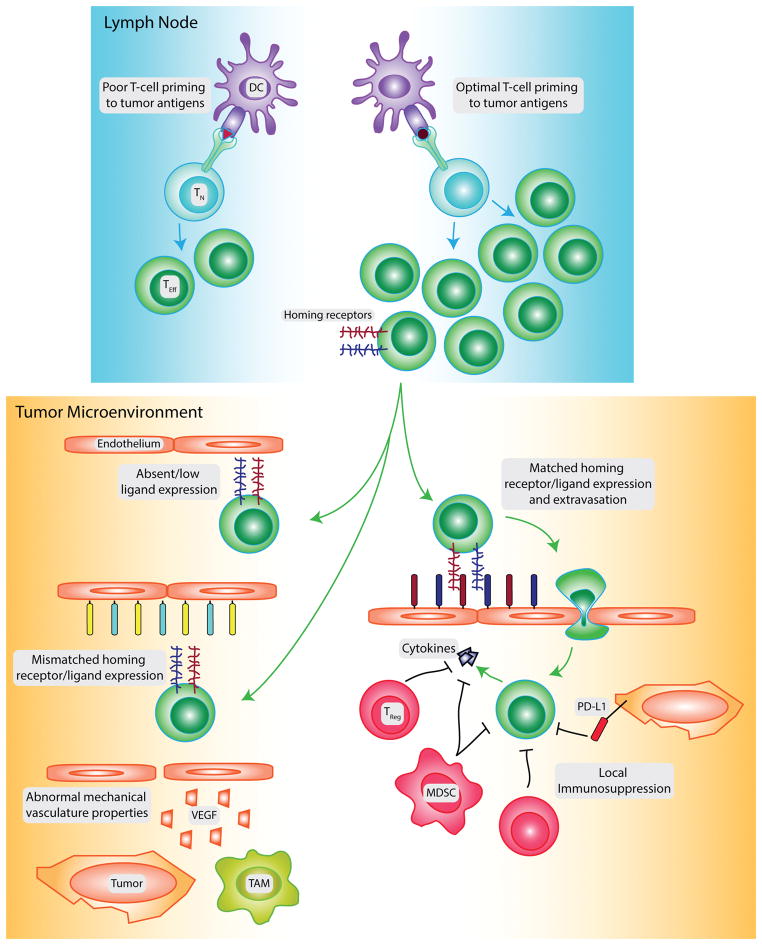
Figure 1. Factors controlling CD8 T-cell presence in tumors.
The infiltration of CD8 T-cells into tumors is controlled by many factors. Initial naïve T-cell (TN) priming to tumor antigens may be limited by poor tumor antigenicity or poor dendritic cell (DC) trafficking/maturation. If a large population of effector CD8 T-cells (TEff) is generated by spontaneous priming to tumor antigens, vaccination, or adoptive transfer, additional barriers may prevent the infiltration of these cells into the tumor. The tumor vasculature can express low levels of cognate ligands for the homing receptors expressed on the CD8 T-cell surface, or express ligands for homing receptors not expressed by the T-cells. High levels of VEGF produced by tumor and stromal cells such as tumor-associated macrophages (TAM) promote dysregulated angiogenesis, generating vasculature with abnormal mechanical properties that poorly supports CD8 T-cell infiltration. Activated T-cells that overcome these barriers and extravasate into the tumor can be suppressed by the tumor and other stromal populations, including regulatory T-cells (TReg) and myeloid-derived suppressor cells (MDSC). This limits their expression of inflammatory cytokines and inhibits further induction of ligand expression on the tumor vasculature.
Poor representation of T-cells in tumors might also result from interference with dendritic cell (DC) maturation or trafficking. For example, type I IFN signaling is required to generate DCs capable of inducing antigen-specific anti-tumor CD8 T cell responses in B16 tumor-bearing mice (Fuertes et al., 2011). Type I IFN is induced by tumor DNA that acts through the STING cytosolic DNA sensing pathway in DC (Deng et al., 2014; Woo et al., 2014). Thus defects in STING or type I IFN signaling might restrict DC activation and subsequent T-cell priming. Murine melanoma cells that failed to express chemokines that recruited cross-presenting DCs generated a poor T-cell response to an otherwise immunogenic tumor (Spranger et al., 2014). High STAT-3 signaling, another common pathway activated in melanoma and other tumors, can also inhibit DC maturation thus limiting CD8 T cell activation (Wang et al., 2004). In pancreatic tumors, the Kras driver mutation has been shown to prevent accumulation of T-cells by inducing the expression of GM-CSF, which promotes the infiltration of immunosuppressive myeloid derived suppressor cells (Gabrilovich et al., 2012; Pylayeva-Gupta et al., 2012; Vonderheide and Bayne, 2013).
In addition to the importance of these factors, there is also ample evidence to suggest that the tumor vasculature limits the representation of CD8 T-cells in tumors. Subcutaneous (SC) and intravenous (IV) routes of vaccination with bone marrow-derived DCs presenting tumor antigen both induce robust CD8 T-cell responses, but these differentially infiltrate and control tumors growing in SC sites or lungs (Mullins et al., 2003). The importance of vaccination route in enabling effective control of tumors growing in different anatomical locations has been confirmed with other vaccine modalities as well (Chang et al., 2004; Hangalapura et al., 2011; Sandoval et al., 2013). This was shown to be related to the ability of vaccine induced T cells to enter tumors. Many other studies have also shown that even when circulating antigen-specific T-cells are present in blood, their accumulation in the tumor can be minimal (Lurquin et al., 2005; Marincola et al., 2003; Mullins et al., 2003; Thurner et al., 1999; Weiss et al., 2012). In this regard, limited infiltration of T-cells into tumors prevented rejection after Treg depletion in a B16 melanoma model (Quezada et al., 2008). Similarly, an exceedingly small number of adoptively transferred tumor-specific effector T-cells get into tumors, in both human and mouse studies (Bernhard et al., 2008; Economou et al., 1996; Fisher et al., 1989; Ganss and Hanahan, 1998; Garbi et al., 2004; Griffith et al., 1989; Kershaw et al., 2006; Pockaj et al., 1994). Finally, adoptively transferred T-cells also controlled SC tumors but not gastric tumors in one murine study (Bourquin et al., 2010), suggesting that adoptively transferred T cells can also differentially enter tumors based on their anatomical location. Detailed understanding of the homing characteristics of effector T-cells and tumor vasculature may explain why, even with robust effector T-cells in circulation, entry into tumors is limited.
A. Trafficking of effector T-cells into tissues
Trafficking of leukocytes, including T-cells, from blood into lymphoid and peripheral tissues involves sequential interactions between homing receptors on leukocytes and corresponding ligands on vascular endothelial cells (Sackstein, 2005). An initial transient adhesion, in which leukocytes engage and roll slowly on the vascular surface, is followed by chemokine mediated activation of the high-affinity conformation of integrins, leading to firm adhesion and transmigration into the underlying tissue (Butcher, 1991; Ley et al., 2007). Effector differentiation in the LN up-regulates expression of new homing receptors that enable CD8 T-cell entry into peripheral tissues where the corresponding vascular ligands are expressed (Mora and von Andrian, 2006).
The specific homing receptors upregulated on effector CD8 T-cells depend on the location of the priming LN and in turn on the properties of DCs and LN stromal cells that vary depending on the local environment (Campbell et al., 2003; Dudda et al., 2005; Hammerschmidt et al., 2008; Johansson-Lindbom et al., 2003; Masopust and Schenkel, 2013; Mora et al., 2005, 2003; Sigmundsdottir et al., 2007). For example, T-cells activated in gut-associated LN, or by DCs from gut-associated lymphoid tissues, upregulate the integrin α4β7 and the chemokine CCR9 (Campbell and Butcher, 2002; Mora et al., 2003). Conversely, T-cells activated in skin-associated LN, or by the corresponding DCs, express E-Selectin Ligand (ESL), P-Selectin Ligand (PSL), and the chemokine CCR10 (Dudda et al., 2005; Johansson-Lindbom et al., 2003; Mora et al., 2005; Sigmundsdottir et al., 2007).
Similar results are observed when T-cells are activated in different LN beds by exogenously administered DCs. Intraperitoneal (IP) immunization with bone marrow derived DC activates T-cells in the mesenteric and mediastinal LN that upregulate α4β7 integrin (Ferguson and Engelhard, 2010; Sheasley-O’Neill et al., 2007). SC immunization activates T-cells in skin-draining LN, most of which upregulate ESL and PSL, while some express α4β1 integrin (Ferguson and Engelhard, 2010). IV immunization activates T-cells in the mediastinal LN and spleen that express α4β1 integrin without coexpression of either ESL or α4β7 integrin (Brinkman et al., 2008; Ferguson and Engelhard, 2010; Sheasley-O’Neill et al., 2007). Finally, some homing receptors are upregulated on murine CD8 T-cells regardless of the site of activation. CXCR3, the receptor for CXCL9, 10, and 11 is upregulated on a substantial fraction of effector CD8 T-cells. A subset of these cells also express PSL, indicating that PSL expression is not restricted to cells primed in skin-draining LNs. This PSL+ subset also co-expresses several other chemokine receptors (CCR3, 4, 5, and 6) (Ferguson and Engelhard, 2010). Collectively, these results define 3 major populations of effector CD8 T-cells based on expression of molecules that initiate slow rolling and confer tissue specificity. By contrast, broad based expression of chemokine receptors enables sensing of the presence of a variety of different inflammation induced chemokines for the purposes of further immobilization and tissue entry.
Expression of the corresponding vascular ligands also varies depending on the anatomical location and inflammatory state of the underlying tissues. In some cases this provides a basis for tissue selective T-cell trafficking. E- and P-selectin on skin vasculature facilitate slow-rolling interactions with ESL+ and PSL+ effector T-cells (Austrup et al., 1997; Berg et al., 1991; Borges et al., 1997; Fuhlbrigge et al., 2002, 1997). E-selectin is homeostatically expressed at low levels only on skin vasculature (Brinkman et al., 2013; Chong et al., 2004; Kupper and Fuhlbrigge, 2004). Transcription and translation leading to E-selectin expression within 4–6 hrs is enhanced by numerous inflammatory stimuli, including IL-1, tumor necrosis factor-α (TNFα), IFNγ, lipopolysaccharide (LPS), thrombin, and radiation (Hallahan et al., 1995; Kaplanski et al., 1997; Matsumoto et al., 2007, 2005; Yao et al., 1999; Zarbock et al., 2011). TNFα, IL-1, LPS, thrombin, histamine, and radiation also upregulate P-selectin within minutes as it is released from pre-formed stores in Weibel-Palade bodies on endothelial cells and alpha granules of platelets (Hariri et al., 2008; Yao et al., 1999). Inflammation induces transient expression of E- and P-selectin in a broad range of tissues (Barthel et al., 2007). Chemokines have also been implicated in selective T-cell trafficking to the skin. CCL27 and CCL17 are both homeostatically displayed on cutaneous venules and are upregulated by inflammatory stimuli (Homey et al., 2002; Kupper and Fuhlbrigge, 2004). CCL17 binds CCR4 on skin homing CD4 T-cells to mediate integrin activation and induce extravasation (Andrew et al., 2001; Campbell et al., 1999), while CCL27 binds to CCR10 and promotes movement into the epidermis (Homey et al., 2002; Morales et al., 1999; Sigmundsdottir et al., 2007).
MAdCAM-1, a major ligand for α4β7 integrin, and CCL25, the ligand for CCR9, are selectively expressed on the vasculature of gut-associated tissues (e.g. intestinal lamina propria) (Hammerschmidt et al., 2008; Rott et al., 1996). CCL25 is constitutively expressed at high levels on gut-associated vasculature and is not enhanced by inflammatory stimuli (Ericsson et al., 2006), while MadCAM-1 is expressed constitutively at low levels and enhanced by TNFα and IL-1 (Ando et al., 2005). Low affinity α4β7/MadCAM-1 interactions have been shown to initiate slow rolling of leukocytes (Chen et al., 2003). Subsequent engagement of CCR9 with CCL25 induces the high affinity forms of α4β7 integrin or LFA-1 which can mediate firm adhesion to gut vasculature (Berlin et al., 1995, 1993; Chen et al., 2003; de Château et al., 2001; Salas et al., 2002).
Other vascular ligands are expressed more ubiquitously among different tissues, and therefore promote T-cell trafficking into a broader range of inflammatory sites. The integrin ligand VCAM-1 is constitutively expressed at low levels on vasculature of many tissues and is induced to much higher levels by TNFα, IFNγ, IL-1, and LPS, thrombin, and radiation (Cook-Mills et al., 2011; Ferguson and Engelhard, 2010; Hubbard and Rothlein, 2000; Kaplanski et al., 1998; Lawrence and Springer, 1991; Mollà et al., 2003; X. Wang et al., 2007). α4β1/VCAM-1 interactions contribute to T-cell trafficking into brain, lung, and interestingly, also the skin and gut (Baron et al., 1993; Calzascia et al., 2005; Kenyon et al., 2009; Rott et al., 1996). In addition, α4β1/VCAM-1 interactions can support both slow rolling and firm adhesion of effector T-cells without chemokine-mediated activation, but possibly in cooperation with LFA-1 (Alon et al., 1995). Expression of certain chemokines is also induced during inflammation in multiple different tissues. CXCL9, 10 and 11, all ligands for CXCR3, are induced by IFNγ (Coursey et al., 2014; Tensen et al., 1999). CCL3, 4 and 5, all ligands for CCR5, are broadly induced by viruses and bacterial endotoxin (Sherry et al., 1988; Tsai et al., 2013) and promote T-cell infiltration into inflamed tissues (Abdi et al., 2002; Gregg et al., 2010; Hancock et al., 2000).
B. Trafficking of effector T-cells into tumors
The homing receptor/ligand interactions required for T-cell entry into tumors are not as well characterized as for entry into peripheral tissues, and several important and related questions need to be considered. First, what homing receptor ligands are expressed on tumor vasculature? Does ligand expression vary based on anatomical site and/or tumor type? Are ligands expressed at sufficient levels to enable T-cell entry? Is tumor vascular ligand expression characteristic of the local peripheral tissue, or the tumor itself, and is it regulated positively or negatively by elements of the innate and adaptive immune systems? In many cases, the answers to these questions are only starting to be addressed.
Although tumors are sites at which significant T-cell mediated death and inflammation may be occurring, the expression of many homing receptor ligands on tumor vasculature is low. E-selectin is often not expressed on the vasculature of squamous cell carcinomas of the skin or metastatic melanomas, despite being present on adjacent tissue vasculature (Clark et al., 2008; Weishaupt et al., 2007). MadCAM-1 is expressed at low levels on gastric adenocarcinoma vasculature relative to normal mucosal tissue (Enarsson et al., 2006). VCAM-1 and ICAM-1 expression are also low on vasculature of melanoma, colorectal cancer, colorectal hepatic metastasis, and glioblastoma (Blank et al., 2005; Dirkx et al., 2003; Weishaupt et al., 2007; Yoong et al., 1998, unpublished). Chemokines commonly associated with T-cell trafficking, including the CCR5 ligands CCL3, CCL4, and CCL5, or the CXCR3 ligands CXCL9, CXCL10, and CXCL11 are low in poorly infiltrated melanomas and colorectal carcinomas (Coppola et al., 2011; Harlin et al., 2009; Messina et al., 2012). Vascular endothelial growth factor (VEGF), Endothelin B receptor, and CD73 are three factors that have been implicated in limiting ligand expression on vasculature, in part, through blocking inflammation-induced ligand upregulation (Buckanovich et al., 2008; Dirkx et al., 2003; Wang et al., 2011).
In some cases, expression of vascular ligands has also been shown to vary with the location of tumor growth or the tumor type. E-selectin was more often expressed at higher levels in Merkel cell carcinomas in the skin than in melanoma (Afanasiev et al., 2013; Weishaupt et al., 2007). In an assessment of several different implantable tumors growing subcutaneously, only a subset of tumors, including B16 melanoma, expressed vascular E-selectin (Seguin et al., 2012). We have found that the vasculature of B16 melanoma and Lewis Lung carcinoma tumors grown SC expresses E-selectin and relatively high levels of VCAM-1, while that of tumors grown IP expresses low levels of VCAM-1 and is negative for E-selectin (unpublished). Conversely, MAdCAM-1 is expressed on a significantly higher fraction of the vasculature in IP B16 tumors compared to SC tumor vasculature. This indicates that homing receptor ligand expression on tumor vasculature resembles, and is presumably derived from, that of adjacent normal tissue. There is a paucity of information concerning expression of E-selectin, VCAM-1, and MAdCAM-1 among human tumor types and locations. Whether chemokines such as CCL27 and CCL25, which are normally expressed in skin and gut, are differentially expressed in tumors growing in different anatomic locations remains unknown.
Several studies have evaluated which homing receptor ligands enable infiltration of effector CD8 T-cells into tumors, either directly or as a correlation. VCAM-1 expression correlates with T-cell representation in pancreatic islet cell carcinoma and melanoma (Garbi et al., 2004; Lohr et al., 2011; Quezada et al., 2008) while ICAM-1 expression correlates with T-cell entry into melanoma, pancreatic islet carcinoma, and glioblastoma (Blank et al., 2005; Buckanovich et al., 2008; Fisher et al., 2011; Garbi et al., 2004; Lohr et al., 2011; Quezada et al., 2008). CD8 T-cell infiltration into SC B16 melanomas was also significantly reduced in ICAM-1−/− mice even following an inflammatory systemic hyperthermia therapy (Fisher et al., 2011). We have shown that antibody blockade of α4β1/VCAM-1 and LFA-1/ICAM-1 interactions significantly reduces the number of adoptively transferred α4β1+ effector CD8 T-cells that enter SC or IP B16 tumors (unpublished). These molecular pairs are not redundant, suggesting that α4β1/VCAM-1 acts to initiate slow rolling while LFA-1/ICAM-1 mediates arrest. Infiltration of adoptively transferred CXCR3−/− effector CD8 T-cells into SC or IP B16 tumors is almost entirely eliminated, implying that this is the major chemokine axis enabling entry (unpublished). CXCR3 has also been associated with CD8 T-cell entry in other tumor models (Harlin et al., 2009; Kunz et al., 1999; Mullins et al., 2004), and with increased survival in melanoma patients (Mullins et al., 2004). This also identifies an important role for the CXCR3 ligands CXCL9, 10, and 11. The direct role of VCAM-1, ICAM-1, and ligands for CXCR3 or other chemokine receptors in mediating T-cell entry into additional murine and human tumors still needs to be thoroughly examined.
E-selectin has also been implicated in CD8 T-cell entry into tumors. Low expression in melanomas was associated with an absence of ESL+ CD8 T-cells (Clark et al., 2008). Higher vascular E-selectin expression among Merkel cell carcinomas in different patients was associated with larger numbers of CD8 T-cells, although it is unclear if the infiltrating T-cells were ESL+ (Afanasiev et al., 2013). Fucosyltransferase IV and VII knockout CD8 T-cells cannot generate the carbohydrate structure to form functional ESL (Goelz et al., 1994, 1990; Knibbs et al., 1996; Maly et al., 1996) and their entry into SC B16-cOVA tumors is reduced compared to wild-type cells (Stark et al., 2012). However, whether wild-type CD8 T-cell entry was dependent on either ESL or PSL (which is also fucosyltransferase dependent) or both was not determined. We have shown that adoptively transferred ESL+ effector CD8 T-cells enter SC B16 tumors with E-selectin+ vasculature more efficiently than IP tumors with E-selectinneg vasculature (unpublished). Entry of ESL+ effector CD8 T-cells into SC tumors growing in E-selectin−/− mice was also reduced compared to entry into SC tumors in wild-type mice, while blockade of P-selectin/PSL interactions with a recombinant P-selectin fusion protein had no effect (unpublished). Thus, ESL/E-selectin interactions enable effector CD8 T-cell entry into skin-associated tumors where E-selectin is expressed on the vasculature.
Given the low level of MAdCAM-1 expression on SC tumor vasculature, it is not surprising that blocking antibodies to its receptor, α4β7, had no impact on T-cell entry into SC melanomas (Fisher et al., 2011). In human colorectal carcinoma patients, high MAdCAM-1 gene expression in tumor lysates correlates with the presence of CD8 T-cells (Mlecnik et al., 2010), but direct evidence that it mediates CD8 T-cell entry is lacking. The role of MAdCAM-1 in enabling T-cell infiltration into tumors deserves further scrutiny, especially in cancers of mucosal tissues or tumors that metastasize to mucosal sites.
Although selectin, integrin, and chemokine expression on tumor vasculature is generally low, it can still respond to inflammatory signals analogous to normal vasculature. For example, treating human squamous cell carcinomas with the TLR7 agonist, Imiquimod, or with TNFα induced expression of E-selectin on the vasculature and significantly increased the number of ESL+ CD8+ T-cells in the tumor (Clark et al., 2008). Likewise, inflammation induced by systemic hyperthermia significantly increased the number of CD8 T-cells rolling on tumor vasculature, and this could be blocked by a cocktail of E- and P-selectin antibodies (Fisher et al., 2011). Additionally, treatment of pancreatic islet carcinomas with CpG upregulated VCAM-1 and ICAM-1 on tumor vasculature and significantly increased T-cell infiltration, likely due to enhanced homing ligand expression (Garbi et al., 2004). These data highlight that upregulation of homing receptor ligand expression on tumor associated vasculature is an important maneuver to be developed in conjunction with other therapies that depend on immune infiltrates for their effectiveness.
C. Positive feedback loops in vascular ligand expression
It is interesting that some of the pro-inflammatory cytokines that increase homing receptor ligand expression are also released by effector CD8 T-cells. This raises the possibility that early stage effector CD8 T-cell entry into tumors could initiate a positive feedback loop in which their secretion of cytokines further upregulates homing receptor ligand expression, enhancing the entry of additional effector CD8 T-cells (see Figure 2a). Indeed, exogenous antigen-specific T-cells that enter EL-4 thymoma increase accumulation of host T-cells as well (Boissonnas et al., 2007). This increased accumulation was dependent on IFNγ released by the transferred cells, and associated with increased expression of several chemokines that are chemotactic for effector T-cells (Hollenbaugh and Dutton, 2006). We have found that B16-cOVA tumors grown in Rag1−/− mice, which lack B and T-cells, express significantly lower levels of VCAM-1 and MAdCAM-1 (unpublished). The adaptive immune effector activities that upregulate homing receptor ligand expression in these tumors remain to be elucidated. Importantly, the efficacy of PD-1 blockade in enhancing tumor control has been associated with induction of this positive feedback loop: enhanced IFNγ secretion by intratumoral CD8 T-cells in anti-PD-1 treated mice increased expression of chemokines, including CXCL10, and resulted in greater T-cell entry and tumor control (Peng et al., 2012).
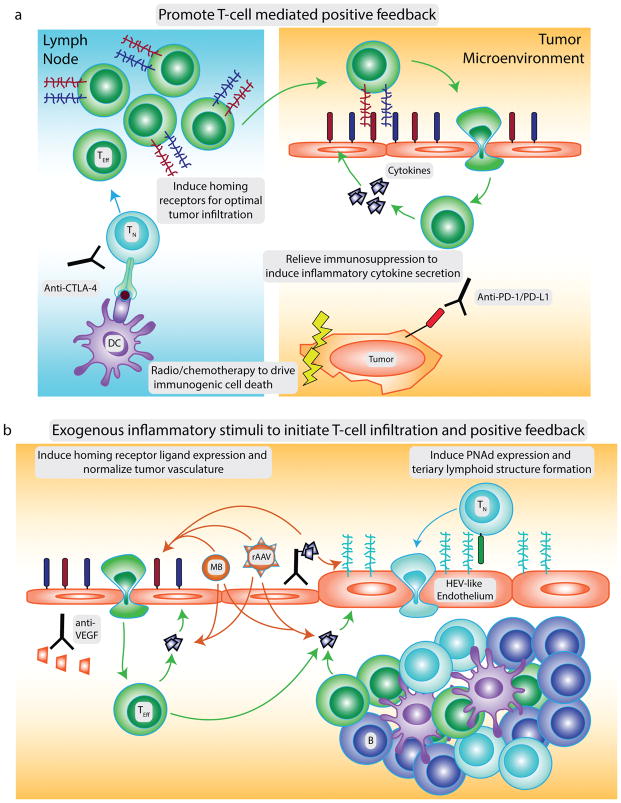
Figure 2. Strategies to promote CD8 T-cell infiltration into tumors.
Several strategies could be pursued alone or in rational combinations to enhance infiltration of CD8 T-cells into tumors. To promote T-cell mediated induction of homing receptor ligand expression (a), immunogenic cell death and treatment with anti-CTLA-4 can enhance the priming of naïve T cells (TN) to tumor antigens. Effector cells (TEff) expressing distinct patterns of homing receptors required to infiltrate certain tumor locations can be induced by distinct vaccination routes or generated for adoptive transfer by in vitro manipulation. Relieving local immunosuppression in the tumor microenvironment will enable activated T cells infiltrating the tumor to secrete higher levels of inflammatory cytokines that further upregulate vascular ligands for T cell homing receptors, generating a positive feedback loop. Expression of homing receptor ligands and T-cell infiltration can also be induced by exogenous inflammatory stimuli (b) including: tumor-targeted delivery of inflammatory cytokines or cytokine genes via antibody-cytokine conjugates, microbubbles (MB) or engineered adeno-associated viruses (rAAV); systemic hyperthermia; or endothelin B receptor blockade. Anti-VEGF treatments can normalize tumor vasculature to support T-cell infiltration. Finally, tumor-targeted inflammatory stimuli that induce PNAd expression and tertiary lymphoid structure (TLS) formation can promote naïve T cell infiltration into tumors and the local generation of an anti-tumor immune response.
D. Mechanical properties of vasculature
In normal angiogenesis, VEGF-activated endothelial cells detach from their neighbors and sprout in the direction of pro-angiogenic factors, including VEGF itself (Weis and Cheresh, 2011). Proliferation results in the formation of tubes that recruit pericytes to provide stability. In a final resolution stage, the endothelial cells remodel and prune to form a functional vasculature. The enhanced availability of pro-angiogenic factors in tumors results in vessels that are disorganized, tortuous, leaky, and lack pericyte coverage. This abnormal architecture results in hypoxia in the tumor microenvironment and high intratumoral pressure. Loss of the gene encoding Regulator of G-protein Signaling 5 resulted in normalization of this tumor vasculature and, intriguingly, also enhanced T-cell representation within tumors (Hamzah et al., 2008). This study concluded that this was due to a reduction in intratumoral pressure, which enabled more robust T-cell entry (Hamzah et al., 2008). This makes sense if fluid flow were a direct determinant of migration, but T-cells crawl on extracellular matrix to move within tissue. Instead, the decreased space between endothelial cells in normalized vasculature may create a more continuous vascular surface to support T-cell rolling as a prelude to entry. The effect of normalizing the tumor vasculature on vascular ligand expression was also not addressed. MadCAM-1 expression, for example, has been shown to be dependent on proximity of endothelial cells to one another (Ogawa et al., 2005), a property that could be altered by normalizing vasculature. While high dose anti-angiogenic therapy destroys vasculature and inhibits entry, low dose anti-angiogenic therapy may promote entry and enhance immunological tumor control alone or in combination with other strategies (Huang et al., 2012; Shrimali et al., 2010).
IV. Tumors develop HEV-like vasculature
Classically, naïve CD8 T-cells are thought to primarily recirculate through the blood and secondary lymphoid organs to scan antigen presenting cells for their cognate antigen (von Andrian and Mackay, 2000). This tissue selectivity is based upon interactions of L-selectin and CCR7 with Peripheral Node Addressin (PNAd) and CCL19/CCL21 respectively, which are normally selectively expressed on the specialized high endothelial venules (HEV) found in LN but not the vessels of peripheral tissues (Girard et al., 2012; Rosen, 2004). However, numerous recent studies have reported that PNAd and/or CCL21 are expressed in a variety of human tumors, including melanoma, breast, lung, ovarian, colorectal and testicular cancers (Cipponi et al., 2012; Coppola et al., 2011; de Chaisemartin et al., 2011; Martinet et al., 2012, 2011; Messina et al., 2012; Sakai et al., 2014). Several of these studies also showed that PNAd+ HEV-like vasculature is associated with a positive prognosis (Avram et al., 2013; Martinet et al., 2012, 2011). These results suggest that inducing the development of HEV-like vasculature presents an alternative therapeutic path for enhancing intratumoral T-cell representation by enabling naïve T-cell entry into tumors, where they may become anti-tumor effectors.
A. Control of HEV in lymph nodes and tertiary lymphoid structures
In the absence of an inflammatory insult, HEV that express PNAd and CCL21 are found exclusively in secondary lymphoid organs, including LN and MALT (e.g. PP, NALT), but not the spleen (Girard et al., 2012). In addition to their selective expression of ligands for naïve T-cell homing receptors, the endothelial cells of HEV can be distinguished from the flat endothelial cells found in other tissues by their plump, cuboidal morphology. This unique morphology also contributes to the trafficking function of HEV, enabling them to store incoming lymphocytes in “pockets” until there is room in the LN parenchyma to accommodate the entering cells (Mionnet et al., 2011).
HEV emerge in the developing LN during embryogenesis, but they do not develop their characteristic adhesion molecule expression pattern until after birth (Mebius et al., 1996). Still, development of HEV is presumed to depend on the same signals required for LN organogenesis as a whole. This process is dominated by signaling of the heterotrimeric form of lymphotoxin (LTα1β2) on Lymphoid Tissue Inducer cells through the Lymphotoxin-β receptor (LTβR) on stromal organizer cells to induce chemokine secretion (van de Pavert and Mebius, 2010). Mice deficient in LTα, LTβ, or LTβR lack most or all LN (Alimzhanov et al., 1997; De Togni et al., 1994; Fütterer et al., 1998; Koni et al., 1997). In the adult LN, the homeostatic maintenance of HEV morphology, expression of PNAd, and subsequent lymphocyte trafficking depend on LTα1β2 signals delivered by DCs directly to LTβR-expressing endothelial cells (Browning et al., 2005; Moussion and Girard, 2011), and is independent of B and T cells (Moussion and Girard, 2011). However, PNAd can be expressed on both the luminal and abluminal surfaces of HEVs (Rosen, 2004; Streeter et al., 1988). LTβR signaling primarily controls luminal expression of PNAd on LN HEV (Browning et al., 2005). Furthermore, homeostatic expression of chemokine CCL21 is independent of LTβR (Browning et al., 2005; Liao and Ruddle, 2006). Thus, some, but not all specialized HEV characteristics depend on LTβR signaling.
When peripheral tissues are chronically inflamed, as in many autoimmune conditions, HEV-like blood vessels that express PNAd and/or CCL21 can develop (Rosen, 2004). This phenomenon has been reported in numerous human diseases and mouse models, including rheumatoid arthritis, Sjögren’s syndrome, thyroiditis, atherosclerosis, allograft rejection, and bacterial or viral infection (Aloisi and Pujol-Borrell, 2006). In some of these conditions, PNAd+ blood vessels have the unique cuboidal morphology of LN-resident HEV, while in others, the PNAd+ vessels are composed of typical flat endothelial cells (Rosen, 2004). An additional feature of these inflamed tissues is the development of organized lymphocytic infiltrates around PNAd+ blood vessels that are referred to as ectopic/tertiary lymphoid structures (TLS) or tertiary lymphoid organs (Aloisi and Pujol-Borrell, 2006; Drayton et al., 2006). One set of criteria for defining a bona fide TLS has recently been proposed (Dieu-Nosjean et al., 2014). TLS are defined to consist of large infiltrates of B-cells and T-cells that are segregated into distinct zones, recapitulating the organization found in LN. They also contain follicular DCs and germinal centers where B-cells undergo class switching, and mature DCs capable of presenting antigen to and activating infiltrating T-cells. Not every report of TLS development has shown the structure contains all of these properties, but in general most have at least shown the accumulation of B-cells and T-cells in conjunction with antigen presenting cells and stromal cells. The control of HEV-like vessels and TLS development in many models is similar to the mechanisms previously described for the LN. For example, in lung models of TLS that develop in response to flu or bacterial infections, DC are responsible for maintaining HEVs through LTβR signaling (GeurtsvanKessel et al., 2009; Halle et al., 2009). Blockade of LTβR also prevents development of TLS and HEVs in many other models (Gatumu et al., 2009; Gräbner et al., 2009; Motallebzadeh et al., 2012; Rangel-Moreno et al., 2011).
B. Control of the development HEV-like vessels in tumors
Although many studies have reported the existence of HEV-like vessels expressing PNAd and/or CCL21 in tumors, few have examined the cellular and molecular mechanisms leading to their spontaneous development. In human studies, the density of DCs expressing LTβ or markers of maturity has been correlated with the density of HEV-like vasculature (Martinet et al., 2013), consistent with the hypothesis that their development in tumors mirrors the pathways defined in LN. Data from several murine models, however, have implicated other cellular and molecular pathways. Recent work in our laboratory using ovalbumin-expressing B16 melanoma showed that development of HEV-like vasculature occurred in tumors growing in multiple anatomic locations in wild-type mice (Peske et al., 2015). Mechanistically, HEV-like vasculature was not induced by DC expression of LTβR, but instead required the secretion of homotrimeric LTα3 and IFNγ by activated effector CD8 T-cells and/or NK cells in the tumor microenvironment. These cytokines signaled through TNF receptors and the IFNγ receptor expressed on endothelial cells to induce PNAd and CCL21 expression. PNAd+ blood vessels were also correlated with the presence of activated effector lymphocytes in a genetic model of melanoma driven by melanocyte-restricted BRAFV600E mutation and PTEN loss (Peske et al., 2015). A different study using methyl cholanthrene-induced fibrosarcomas found that the primary tumors that developed in wild-type mice did not contain HEV-like vasculature (Hindley et al., 2012). But when Treg were depleted, a fraction (~50%) of the tumors were well infiltrated with activated lymphocytes and contained blood vessels expressing PNAd and MAdCAM-1 (another HEV associated molecule found on HEV in mesenteric LN and Peyer’s patches, but not inguinal LN).
While these studies at first glance seem to point to disparate tumor-specific mechanisms leading to development of HEV-like vasculature, the results can be unified by positing that ongoing NK and/or T-cell driven cytokine expression in the tumor microenvironment is necessary to establish and maintain HEV. Thus, in strongly antigenic tumors, effector lymphocytes receiving robust activation signals would secrete the required cytokines for HEV-induction even in the presence of Treg. In less antigenic tumors, immunosuppression by Treg may dominate, necessitating their depletion to enable HEV-induction by sufficiently activated effector lymphocytes. While DCs play a critical role in priming the effector T-cell response, their direct involvement in inducing HEV-like vasculature was not revealed in either murine model. The dependence of HEV-like vasculature in tumors on activated lymphocytes differs from the mechanisms defined in LN and non-tumor TLS. One possibility is that within the highly angiogenic tumor environment, endothelial cell control of HEV-like properties is altered. Indeed, differential responsiveness of PNAd+ and PNAd- endothelial cells to VEGF has been documented for LN HEV (Chyou et al., 2008; Webster et al., 2006). Although endothelial cell morphology has not been carefully determined in all studies, control of PNAd present on flat venules in tumors (Peske et al., 2015) might differ from control on the morphologically distinct cuboidal HEV in LN.
C. Association of tumor HEV with TLS
As highlighted above, PNAd+ HEV-like vessels that emerge in inflamed tissues are often associated with organized TLS. Some studies have now determined if intratumoral HEV are similarly associated with organized lymphoid tissue. Not all have used the framework proposed by Dieu-Nosjean for identifying a TLS, or the organization described only incompletely meets those standards. In addition, not all studies that stained for the presence of HEV also investigated if TLS were present. Including the studies that have identified HEV-like blood vessels associated with infiltrates of B and T-cells even if they did not demonstrate fully segregated zones as in LN, HEV-associated TLS have been identified in numerous human tumors and animal models, including melanoma, lung, colorectal, and breast cancer (Goc et al., 2013).
Importantly, HEV-like vessels also clearly develop in the absence of TLS. Primary human melanomas do contain HEV-like vessels expressing PNAd and CCL21 (Avram et al., 2013; Cipponi et al., 2012; Martinet et al., 2012). However, TLS are either entirely absent or present in only a small fraction of primary tumors (Cipponi et al., 2012; Ladányi et al., 2014). In contrast, metastases were more likely to contain TLS in association with HEV (Cipponi et al., 2012). The tissue of origin also plays a role in determining TLS presence in the metastasis. Lung metastases from primary colorectal cancers had a higher density of TLS than metastases from primary renal cell carcinomas (Remark et al., 2013). Interestingly, in our studies with ovalbumin-expressing B16 melanoma, HEV-like blood vessels develop in tumors growing at both IP and SC locations. However, associated TLS-like structures composed of an intermingled B and T-cell infiltrate were only present in IP tumors (Peske et al., 2015). The absence of TLS in SC tumors was also observed using a B16 line transfected to express high levels of CCL21 (Shields et al., 2010). Thus there are additional signals required to drive the maturation of an organized TLS in addition to those necessary for induction of PNAd on the vasculature.
One possibility is that certain sites receive the LTα3-mediated signals that induce PNAd expression, but poorly induce expression of chemokines such as CCL21 and CXCL13 if they fail to receive adequate IFNγ or LTβR signals. Certain anatomic locations of metastasis may also intrinsically be more or less able to support the development of TLS. For example, CXCL13 expression, which likely enables B-cell organization in the TLS (Henry and Kendall, 2010), is induced by retinoic acid signaling (van de Pavert et al., 2009). Mucosal associated sites where cells express high levels of retinoic acid-synthesizing enzymes may be more permissive to TLS neogenesis. In addition, transgenic overexpression of CCL21 induces TLS formation in the thyroid and pancreas (Luther et al., 2002; Marinkovic et al., 2006), but not the skin (Chen et al., 2002). Still, the skin does not have an absolute restriction on TLS development as cutaneous melanoma metastases (Cipponi et al., 2012) or subcutaneous tumors transfected to directly express LTα (Kim et al., 2004) can develop TLS. The skin might actively suppress TLS development via inhibitory factors or lack permissive signals present in mucosal sites, but this requires further investigation.
D. Tumor-associated HEV-like vessels support enhanced antitumor immunity
While many human and murine tumors contain HEV, because their development is a consequence of a robust immune response, one possibility is that their linkage with a positive prognosis simply reflects the confounding variable of the presence of effector T-cells. However, it is now clear that intratumoral HEV also directly support an enhanced immune response. HEV-like vessels are the gateways for recruiting naïve T-cells into tumors (Peske et al., 2015). Once they have reached the tumor, naïve T-cells undergo priming and differentiation in situ, if they are specific for tumor antigens (Thompson et al., 2010), leading to the significant restraint of tumor outgrowth (Peske et al., 2015). In some murine models where naïve T-cells are directed to the tumor in large numbers, tumors can even be completely eradicated (Yu et al., 2004). In contrast, another study demonstrated that a B16 cell line that directly secreted CCL21 developed HEV-like vessels as a component of a tolerogenic stromal environment enriched in Treg that inhibited anti-tumor immunity (Shields et al., 2010). While several other studies of the effects of either endogenous or exogenous CCL21 in the tumor microenvironment are consistent with the hypothesis that it is a beneficial factor promoting anti-tumor immunity (Kirk et al., 2001b; Messina et al., 2012; Novak et al., 2007; Sharma et al., 2000; Turnquist et al., 2007; S.-C. Yang et al., 2004), these contrasting results suggest that the precise context in which the HEV-like blood vessels develop can be critical for determining their effect on anti-tumor immunity.
Furthermore, while TLS often form in relation to HEV-like vessels, it is not clear if they positively or negatively influence the development of local anti-tumor immunity. Histological or gene expression-based evidence for the presence of TLS in human tumors is associated with a positive prognosis for several tumor types (Coppola et al., 2011; Dieu-Nosjean et al., 2008; Messina et al., 2012). This prognostic association may again be confounded by the fact that TLS form downstream of HEV induction as a consequence of an ongoing immune response. In a murine melanoma model where TLS-like structures (whether they included germinal centers and follicular DCs was not examined) were induced in the tumor in the absence of LN, priming of naïve T-cells still led to tumor eradication, suggesting the TLS was an effective locus for generating the anti-tumor immune response (Schrama et al., 2008, 2001). However, in B16-cOVA tumors, TLS-like structures develop only in IP tumors (Peske et al., 2015), but both IP and SC sites support the effective in situ priming and differentiation of tumor-specific naïve T-cells (Thompson et al., 2010). Furthermore, naïve T-cells primed in SC tumors in the absence of TLS still exert anti-tumor immune activity, delaying tumor outgrowth and prolonging survival (Peske et al., 2015). Thus, the TLS does not appear to be absolutely required for intratumoral priming of naïve T-cells.
TLS may instead affect the anti-tumor immune response in more subtle ways. For example, chemokine expression in the TLS may simply help promote effective T cell trafficking into the tumor. However, it is also possible that high levels of chemokines in TLS might retain T-cells in the structure around the vasculature, thereby restricting T-cell contact with the bulk of the tumor. Whether ‘Immunotype B’ melanoma patients (Erdag et al., 2012), who have primarily perivascular lymphocytes and an intermediate prognosis, encompasses the patients with intratumoral TLS is a interesting question. Another possibility is that the TLS primarily alters the function of intratumoral B-cells, which often dominate the structure. Intratumoral B-cells have been associated with either beneficial (DiLillo et al., 2010; Germain et al., 2014; Nielsen et al., 2012) or negative (Barbera-Guillem et al., 2000; DeNardo et al., 2010) effects in different models. This could be related to whether or not the B-cells were present in the context of a TLS, which was not examined in all of these studies. Finally, the presence of TLS might improve the responsiveness to immunotherapies such as anti-CTLA-4/anti-PD-1 treatment, or vaccinations that are delivered directly to the tumor site. The overall contribution of the TLS to positive or negative effects on anti-tumor immunity therefore remains to be fully investigated, and will require more precise understanding of the factors driving their development.
V. Altering the tumor vasculature to support enhanced entry of naïve and effector T-cells
A. Rationale for modifying tumor associated vasculature
As we have reviewed, characteristics of the tumor vasculature determine whether or not tumors are permissive to the entry of both effector and naïve CD8 T-cells. Because the presence of CD8 T-cells in tumors is such a strong prognostic factor and predictor of responsiveness to immunotherapies, strategies that can alter the tumor vasculature to support the enhanced entry of T-cells hold the potential to extend the effectiveness of these immunotherapies to a much broader cross-section of patients for whom they would otherwise be ineffective.
If a tumor contains only small numbers of CD8 T-cells, however, can altering just the properties of the vasculature actually have a meaningful effect? This is likely to depend on the precise nature of the tumor. For example, if the tumor has antigens that can be recognized by CD8 T-cells, but remains poorly infiltrated due to defects at the level of innate cell activation, or driver mutations that promote limited lymphocytic infiltration, then exogenous manipulations to initiate inflammation in the tumor or directly upregulate ligands for homing receptors would likely have a positive effect. Indeed in pancreatic cancer patients and murine models, vaccination and chemotherapy initiated accumulation of T-cells in the previously T-cell poor tumor (Lutz et al., 2014; Winograd et al., 2015). As effector T-cells reach the tumor in response to these interventions, they would secrete pro-inflammatory cytokines, and initiate the positive feedback loop to support recruitment of additional effector CD8 T-cells into the tumor by maintaining an inflamed tumor vasculature. A similar effect would be expected in response to interventions designed to induce HEV-like blood vessels and promote naïve T-cell entry. Indeed, several studies have shown that maneuvers to induce intratumoral naïve T-cell priming, such as targeting of LTα to the tumor, or intratumoral vaccination with CCL21-secreting peptide-pulsed DCs are effective, even in the absence of LN (Kirk et al., 2001a, 2001b; Schrama et al., 2008). Once naïve T-cells become activated, they would also be expected to contribute to the positive feedback loop, supporting the continual influx of T-cells through inflamed blood vessels.
There are still theoretical advantages to targeting the tumor to primarily enhance either naïve or effector T-cell entry. For example, the induction of HEV-like blood vessels appears to require LTα-TNFR signaling regardless of the site of tumor growth. Therefore inducing vessels expressing PNAd and CCL21 at different anatomic locations may be easier than attempting to induce the different molecules involved in effector cell trafficking that may have a known tissue-selective preference for one site or another, such as E-selectin and MAdCAM-1. On the other hand, altering the vasculature to support entry of effector T-cells may work synergistically with current vaccination or adoptive transfer therapies by enhancing the very small fraction of transferred cells that enters the tumor.
B. Strategies for modifying tumor associated vasculature
The simplest method for altering tumor associated vasculature to support enhanced entry of CD8 T-cells would be through systemic administration of inflammatory stimuli, such as TNFα, LTα, LIGHT, and IFNγ, or agonistic antibodies targeting their receptors to induce upregulation of ligands for naïve and effector T-cell homing receptors. Unfortunately, this strategy is limited by the toxicity of these agents when given systemically. Therefore, an alternative strategy would be the concentrated delivery of inflammatory cytokines or other stimuli directly to the tumor site. While these strategies are still early in development, methods such as tumor-endothelium targeted microbubbles (Hernot and Klibanov, 2008) or engineered adeno-associated viruses that localize to specific tumor targets (Konkalmatt et al., 2013) are potential options (see Figure 2). These methods could deliver a tumor vasculature-modifying drug or cytokine directly, shRNA sequences to knockdown inhibitory molecules, or even protein-coding nucleic acid sequences. These could potentially be the endothelial expressed ligands themselves (e.g. E-selectin, VCAM-1, CXCL10), or an upstream cytokine known to induce their expression (e.g. TNFα, IFNγ, LTα).
Direct modulation of the tumor vasculature is not the only possibility, however. One interesting intervention involves a temporary induction of mild hyperthermia, which is associated with the expression of IL-6 to enhance ICAM-1 expression on tumor vasculature (Fisher et al., 2011). Inhibiting VEGF (Shrimali et al., 2010) or the Endothelin B Receptor that indirectly decrease expression of homing receptor ligands is another potential strategy (Buckanovich et al., 2008). Tissue engineering approaches to implant scaffolds to support development of TLS at the tumor site may also be feasible (Irvine et al., 2008). Furthermore, the matching of vaccination routes or in vitro treatments of adoptively transferred cells should be optimized to induce T-cell populations that express appropriate homing receptors to bind to the ligands expressed on tumor vasculature, which may vary with location of growth.
Interventions to enhance T cell trafficking are not proposed to entirely supplant, but rather work in concert with current therapeutic strategies. For example, they may be combined with local radiotherapy or chemotherapy to enhance tumor antigenicity, or applied in combination with vaccination or adoptive transfer to increase the efficiency of CD8 T cell entry into tumors. Rational combinations of immunotherapies are already showing increased efficacy in murine models and human patients (Victor et al., 2015). Modifying the tumor vasculature and microenvironment to support the entry of naïve and activated T cells has the potential to further broaden the cohort of patients that will respond to current immunotherapies.
Abbreviations
- LN
Lymph node
- DC
Dendritic cell
- Treg
Regulatory T-cell
- IFNγ
Interferon-γ
- ESL
E-Selectin Ligand
- PSL
P-Selectin Ligand
- SC
Subcutaneous
- IV
Intravenous
- IP
Intraperitoneal
- TNF
Tumor necrosis factor alpha
- VEGF
Vascular endothelial growth factor
- PNAd
Peripheral Node Addressin
- HEV
high endothelial venules
- TLS
tertiary lymphoid structure
Contributor Information
J. David Peske, Email: jdp8t@virginia.edu.
Amber B. Woods, Email: anb2wr@virginia.edu.
References
- Abdi R, Smith RN, Makhlouf L, Najafian N, Luster AD, Auchincloss H, Sayegh MH. The role of CC chemokine receptor 5 (CCR5) in islet allograft rejection. Diabetes. 2002;51:2489–2495. doi: 10.2337/diabetes.51.8.2489. [DOI] [PubMed] [Google Scholar]
- Afanasiev OK, Nagase K, Simonson W, Vandeven N, Blom A, Koelle DM, Clark R, Nghiem P. Vascular E-Selectin expression correlates with CD8 lymphocyte infiltration and improved outcome in Merkel cell carcinoma. J Invest Dermatol. 2013;133:2065–2073. doi: 10.1038/jid.2013.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alimzhanov MB, Kuprash DV, Kosco-Vilbois MH, Luz A, Turetskaya RL, Tarakhovsky A, Rajewsky K, Nedospasov SA, Pfeffer K. Abnormal development of secondary lymphoid tissues in lymphotoxin β-deficient mice. Proc Natl Acad Sci U S A. 1997;94:9302–9307. doi: 10.1073/pnas.94.17.9302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aloisi F, Pujol-Borrell R. Lymphoid neogenesis in chronic inflammatory diseases. Nat Rev Immunol. 2006;6:205–217. doi: 10.1038/nri1786. [DOI] [PubMed] [Google Scholar]
- Alon R, Kassner PD, Carr MW, Finger EB, Hemler ME, Springer TA. The integrin VLA-4 supports tethering and rolling in flow on VCAM-1. J Cell Biol. 1995;128:1243–1253. doi: 10.1083/jcb.128.6.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ando T, Jordan P, Wang Y, Itoh M, Joh T, Sasaki M, Elrod JW, Carpenter A, Jennings MH, Minagar A, Alexander JS. MAdCAM-1 expression and regulation in murine colonic endothelial cells in vitro. Inflamm Bowel Dis. 2005;11:258–264. doi: 10.1097/01.mib.0000160807.53858.1c. [DOI] [PubMed] [Google Scholar]
- Andrew DP, Ruffing N, Kim CH, Miao W, Heath H, Li Y, Murphy K, Campbell JJ, Butcher EC, Wu L. C-C chemokine receptor 4 expression defines a major subset of circulating nonintestinal memory T cells of both Th1 and Th2 potential. J Immunol. 2001;166:103–111. doi: 10.4049/jimmunol.166.1.103. [DOI] [PubMed] [Google Scholar]
- Angell H, Galon J. From the immune contexture to the Immunoscore: the role of prognostic and predictive immune markers in cancer. Curr Opin Immunol, Lymphocyte development/Tumour immunology/Cancer immunology: Clinical translation. 2013;25:261–267. doi: 10.1016/j.coi.2013.03.004. [DOI] [PubMed] [Google Scholar]
- Austrup F, Vestweber D, Borges E, Lohning M, Brauer R, Herz U, Renz H, Hallmann R, Scheffold A, Radbruch A, Hamann A. P- and E-selectin mediate recruitment of T-helper-1 but not T-helper-2 cells into inflammed tissues. Nature. 1997;385:81–83. doi: 10.1038/385081a0. [DOI] [PubMed] [Google Scholar]
- Avram G, Sánchez-Sendra B, Martín JM, Terrádez L, Ramos D, Monteagudo C. The density and type of MECA-79-positive high endothelial venules correlate with lymphocytic infiltration and tumour regression in primary cutaneous melanoma. Histopathology. 2013;63:852–861. doi: 10.1111/his.12235. [DOI] [PubMed] [Google Scholar]
- Bailey SR, Nelson MH, Himes RA, Li Z, Mehrotra S, Paulos CM. Th17 Cells in Cancer: The Ultimate Identity Crisis. Front Immunol. 2014;5 doi: 10.3389/fimmu.2014.00276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balermpas P, Michel Y, Wagenblast J, Seitz O, Weiss C, Rödel F, Rödel C, Fokas E. Tumour-infiltrating lymphocytes predict response to definitive chemoradiotherapy in head and neck cancer. Br J Cancer. 2014;110:501–509. doi: 10.1038/bjc.2013.640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbera-Guillem E, Nelson MB, Barr B, Nyhus JK, May KF, Feng L, Sampsel JW. B lymphocyte pathology in human colorectal cancer. Experimental and clinical therapeutic effects of partial B cell depletion. Cancer Immunol Immunother CII. 2000;48:541–549. doi: 10.1007/PL00006672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron JL, Madri JA, Ruddle NH, Hashim G, Janeway CA. Surface expression of alpha 4 integrin by CD4 T cells is required for their entry into brain parenchyma. J Exp Med. 1993;177:57–68. doi: 10.1084/jem.177.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barthel SR, Gavino JD, Descheny L, Dimitroff CJ. Targeting selectins and selectin ligands in inflammation and cancer. Expert Opin Ther Targets. 2007;11:1473–1491. doi: 10.1517/14728222.11.11.1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beatty GL, Haas AR, Maus MV, Torigian DA, Soulen MC, Plesa G, Chew A, Zhao Y, Levine BL, Albelda SM, Kalos M, June CH. Mesothelin-specific chimeric antigen receptor mRNA-engineered T cells induce antitumor activity in solid malignancies. Cancer Immunol Res. 2014;2:112–120. doi: 10.1158/2326-6066.CIR-13-0170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg EL, Yoshino T, Rott LS, Robinson MK, Warnock RA, Kishimoto TK, Picker LJ, Butcher EC. The cutaneous lymphocyte antigen is a skin lymphocyte homing receptor for the vascular lectin endothelial cell-leukocyte adhesion molecule 1. J Exp Med. 1991;174:1461–1466. doi: 10.1084/jem.174.6.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berlin C, Bargatze RF, Campbell JJ, von Andrian UH, Szabo MC, Hasslen SR, Nelson RD, Berg EL, Erlandsen SL, Butcher EC. alpha 4 integrins mediate lymphocyte attachment and rolling under physiologic flow. Cell. 1995;80:413–422. doi: 10.1016/0092-8674(95)90491-3. [DOI] [PubMed] [Google Scholar]
- Berlin C, Berg EL, Briskin MJ, Andrew DP, Kilshaw PJ, Holzmann B, Weissman IL, Hamann A, Butcher EC. Alpha 4 beta 7 integrin mediates lymphocyte binding to the mucosal vascular addressin MAdCAM-1. Cell. 1993;74:185–195. doi: 10.1016/0092-8674(93)90305-a. [DOI] [PubMed] [Google Scholar]
- Bernhard H, Neudorfer J, Gebhard K, Conrad H, Hermann C, Nährig J, Fend F, Weber W, Busch DH, Peschel C. Adoptive transfer of autologous, HER2-specific, cytotoxic T lymphocytes for the treatment of HER2-overexpressing breast cancer. Cancer Immunol Immunother CII. 2008;57:271–280. doi: 10.1007/s00262-007-0355-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beverly B, Kang SM, Lenardo MJ, Schwartz RH. Reversal of in vitro T cell clonal anergy by IL-2 stimulation. Int Immunol. 1992;4:661–671. doi: 10.1093/intimm/4.6.661. [DOI] [PubMed] [Google Scholar]
- Blank C, Brown I, Kacha AK, Markiewicz MA, Gajewski TF. ICAM-1 contributes to but is not essential for tumor antigen cross-priming and CD8+ T cell-mediated tumor rejection in vivo. J Immunol. 2005;174:3416–3420. doi: 10.4049/jimmunol.174.6.3416. [DOI] [PubMed] [Google Scholar]
- Blank C, Brown I, Peterson AC, Spiotto M, Iwai Y, Honjo T, Gajewski TF. PD-L1/B7H-1 inhibits the effector phase of tumor rejection by T cell receptor (TCR) transgenic CD8+ T cells. Cancer Res. 2004;64:1140–1145. doi: 10.1158/0008-5472.can-03-3259. [DOI] [PubMed] [Google Scholar]
- Boissonnas A, Fetler L, Zeelenberg IS, Hugues S, Amigorena S. In vivo imaging of cytotoxic T cell infiltration and elimination of a solid tumor. J Exp Med. 2007;204:345–356. doi: 10.1084/jem.20061890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borges E, Tietz W, Steegmaier M, Moll T, Hallmann R, Hamann A, Vestweber D. P-selectin glycoprotein ligand-1 (PSGL-1) on T helper 1 but not on T helper 2 cells binds to P-selectin and supports migration into inflamed skin. J Exp Med. 1997;185:573–578. doi: 10.1084/jem.185.3.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourquin C, von der Borch P, Zoglmeier C, Anz D, Sandholzer N, Suhartha N, Wurzenberger C, Denzel A, Kammerer R, Zimmermann W, Endres S. Efficient Eradication of Subcutaneous but Not of Autochthonous Gastric Tumors by Adoptive T Cell Transfer in an SV40 T Antigen Mouse Model. J Immunol. 2010;185:2580–2588. doi: 10.4049/jimmunol.0903231. [DOI] [PubMed] [Google Scholar]
- Brinkman CC, Rouhani SJ, Srinivasan N, Engelhard VH. Peripheral tissue homing receptors enable T cell entry into lymph nodes and affect the anatomical distribution of memory cells. J Immunol. 2013;191:2412–2425. doi: 10.4049/jimmunol.1300651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinkman CC, Sheasley-O’Neill SL, Ferguson AR, Engelhard VH. Activated CD8 T cells redistribute to antigen-free lymph nodes and exhibit effector and memory characteristics. J Immunol. 2008;181:1814–1824. doi: 10.4049/jimmunol.181.3.1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browning JL, Allaire N, Ngam-Ek A, Notidis E, Hunt J, Perrin S, Fava RA. Lymphotoxin-beta receptor signaling is required for the homeostatic control of HEV differentiation and function. Immunity. 2005;23:539–550. doi: 10.1016/j.immuni.2005.10.002. [DOI] [PubMed] [Google Scholar]
- Buckanovich RJ, Facciabene A, Kim S, Benencia F, Sasaroli D, Balint K, Katsaros D, O’Brien-Jenkins A, Gimotty PA, Coukos G. Endothelin B receptor mediates the endothelial barrier to T cell homing to tumors and disables immune therapy. Nat Med. 2008;14:28–36. doi: 10.1038/nm1699. [DOI] [PubMed] [Google Scholar]
- Bulliard Y, Jolicoeur R, Windman M, Rue SM, Ettenberg S, Knee DA, Wilson NS, Dranoff G, Brogdon JL. Activating Fc γ receptors contribute to the antitumor activities of immunoregulatory receptor-targeting antibodies. J Exp Med. 2013;210:1685–1693. doi: 10.1084/jem.20130573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butcher EC. Leukocyte-endothelial cell recognition: Three (or more) steps to specificity and diversity. Cell. 1991;67:1033–1036. doi: 10.1016/0092-8674(91)90279-8. [DOI] [PubMed] [Google Scholar]
- Calzascia T, Masson F, Di Berardino-Besson W, Contassot E, Wilmotte R, urrand-Lions M, Ruegg C, Dietrich PY, Walker PR. Homing phenotypes of tumor-specific CD8 T cells are predetermined at the tumor site by crosspresenting APCs. Immunity. 2005;22:175–184. doi: 10.1016/j.immuni.2004.12.008. [DOI] [PubMed] [Google Scholar]
- Campbell DJ, Butcher EC. Rapid acquisition of tissue-specific homing phenotypes by CD4(+) T cells activated in cutaneous or mucosal lymphoid tissues. J Exp Med. 2002;195:135–141. doi: 10.1084/jem.20011502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell DJ, Debes GF, Johnston B, Wilson E, Butcher EC. Targeting T cell responses by selective chemokine receptor expression. Semin Immunol. 2003;15:277–86. doi: 10.1016/j.smim.2003.08.005. [pii] [DOI] [PubMed] [Google Scholar]
- Campbell JJ, Haraldsen G, Pan J, Rottman J, Qin S, Ponath P, Andrew DP, Warnke R, Ruffing N, Kassam N, Wu L, Butcher EC. The chemokine receptor CCR4 in vascular recognition by cutaneous but not intestinal memory T cells. Nature. 1999;400:776–780. doi: 10.1038/23495. [DOI] [PubMed] [Google Scholar]
- Casares N, Pequignot MO, Tesniere A, Ghiringhelli F, Roux S, Chaput N, Schmitt E, Hamai A, Hervas-Stubbs S, Obeid M, Coutant F, Métivier D, Pichard E, Aucouturier P, Pierron G, Garrido C, Zitvogel L, Kroemer G. Caspase-dependent immunogenicity of doxorubicin-induced tumor cell death. J Exp Med. 2005;202:1691–1701. doi: 10.1084/jem.20050915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cha E, Klinger M, Hou Y, Cummings C, Ribas A, Faham M, Fong L. Improved survival with T cell clonotype stability after anti–CTLA-4 treatment in cancer patients. Sci Transl Med. 2014;6:238ra70–238ra70. doi: 10.1126/scitranslmed.3008211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champiat S, Ferté C, Lebel-Binay S, Eggermont A, Soria JC. Exomics and immunogenics. Oncoimmunology. 2014:3. doi: 10.4161/onci.27817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang CJ, Tai KF, Roffler S, Hwang LH. The Immunization Site of Cytokine-Secreting Tumor Cell Vaccines Influences the Trafficking of Tumor-Specific T Lymphocytes and Antitumor Efficacy against Regional Tumors. J Immunol. 2004;173:6025–6032. doi: 10.4049/jimmunol.173.10.6025. [DOI] [PubMed] [Google Scholar]
- Chen J, Salas A, Springer TA. Bistable regulation of integrin adhesiveness by a bipolar metal ion cluster. Nat Struct Mol Biol. 2003;10:995–1001. doi: 10.1038/nsb1011. [DOI] [PubMed] [Google Scholar]
- Chen SC, Vassileva G, Kinsley D, Holzmann S, Manfra D, Wiekowski MT, Romani N, Lira SA. Ectopic expression of the murine chemokines CCL21a and CCL21b induces the formation of lymph node-like structures in pancreas, but not skin, of transgenic mice. J Immunol. 2002;168:1001–1008. doi: 10.4049/jimmunol.168.3.1001. [DOI] [PubMed] [Google Scholar]
- Chong BF, Murphy JE, Kupper TS, Fuhlbrigge RC. E-selectin, thymus- and activation-regulated chemokine/CCL17, and intercellular adhesion molecule-1 are constitutively coexpressed in dermal microvessels: a foundation for a cutaneous immunosurveillance system. J Immunol. 2004;172:1575–1581. doi: 10.4049/jimmunol.172.3.1575. [DOI] [PubMed] [Google Scholar]
- Chyou S, Ekland EH, Carpenter AC, Tzeng TCJ, Tian S, Michaud M, Madri JA, Lu TT. Fibroblast-type reticular stromal cells regulate the lymph node vasculature. J Immunol. 2008;181:3887–3896. doi: 10.4049/jimmunol.181.6.3887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cipponi A, Mercier M, Seremet T, Baurain JF, Théate I, van den Oord J, Stas M, Boon T, Coulie PG, van Baren N. Neogenesis of lymphoid structures and antibody responses occur in human melanoma metastases. Cancer Res. 2012;72:3997–4007. doi: 10.1158/0008-5472.CAN-12-1377. [DOI] [PubMed] [Google Scholar]
- Clark RA, Huang SJ, Murphy GF, Mollet IG, Hijnen D, Muthukuru M, Schanbacher CF, Edwards V, Miller DM, Kim JE, Lambert J, Kupper TS. Human squamous cell carcinomas evade the immune response by down-regulation of vascular E-selectin and recruitment of regulatory T cells. J Exp Med. 2008;205:2221–2234. doi: 10.1084/jem.20071190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark WH, Jr, Elder DE, Guerry D, 4th, Braitman LE, Trock BJ, Schultz D, Synnestvedt M, Halpern AC. Model predicting survival in stage I melanoma based on tumor progression. J Natl Cancer Inst. 1989;81:1893–1904. doi: 10.1093/jnci/81.24.1893. [DOI] [PubMed] [Google Scholar]
- Coley W. The treatment of malignant tumors by repeated inoculations of erysipelas. With a report of ten original cases. LWW. 1893;105:487–510. [PubMed] [Google Scholar]
- Cook-Mills JM, Marchese ME, Abdala-Valencia H. Vascular cell adhesion molecule-1 expression and signaling during disease: regulation by reactive oxygen species and antioxidants. Antioxid Redox Signal. 2011;15:1607–1638. doi: 10.1089/ars.2010.3522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppola D, Nebozhyn M, Khalil F, Dai H, Yeatman T, Loboda A, Mulé JJ. Unique ectopic lymph node-like structures present in human primary colorectal carcinoma are identified by immune gene array profiling. Am J Pathol. 2011;179:37–45. doi: 10.1016/j.ajpath.2011.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coursey TG, Bohat R, Barbosa FL, Pflugfelder SC, de Paiva CS. Desiccating stress-induced chemokine expression in the epithelium is dependent on upregulation of NKG2D/RAE-1 and release of IFN-γ in experimental dry eye. J Immunol Baltim Md 1950. 2014;193:5264–5272. doi: 10.4049/jimmunol.1400016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coussens LM, Tinkle CL, Hanahan D, Werb Z. MMP-9 supplied by bone marrow–derived cells contributes to skin carcinogenesis. Cell. 2000;103:481–490. doi: 10.1016/S0092-8674(00)00139-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Chaisemartin L, Goc J, Damotte D, Validire P, Magdeleinat P, Alifano M, Cremer I, Fridman WH, Sautès-Fridman C, Dieu-Nosjean MC. Characterization of chemokines and adhesion molecules associated with T cell presence in tertiary lymphoid structures in human lung cancer. Cancer Res. 2011;71:6391–6399. doi: 10.1158/0008-5472.CAN-11-0952. [DOI] [PubMed] [Google Scholar]
- De Château M, Chen S, Salas A, Springer TA. Kinetic and mechanical basis of rolling through an integrin and novel Ca2+-dependent rolling and Mg2+-dependent firm adhesion modalities for the α4β7 MAdCAM-1 interaction. Biochemistry (Mosc) 2001;40:13972–13979. doi: 10.1021/bi011582f. [DOI] [PubMed] [Google Scholar]
- Demaria S, Kawashima N, Yang AM, Devitt ML, Babb JS, Allison JP, Formenti SC. Immune-mediated inhibition of metastases after treatment with local radiation and CTLA-4 blockade in a mouse model of breast cancer. Clin Cancer Res. 2005;11:728–734. [PubMed] [Google Scholar]
- Demaria S, Ng B, Devitt ML, Babb JS, Kawashima N, Liebes L, Formenti SC. Ionizing radiation inhibition of distant untreated tumors (abscopal effect) is immune mediated. Int J Radiat Oncol Biol Phys. 2004;58:862–870. doi: 10.1016/j.ijrobp.2003.09.012. [DOI] [PubMed] [Google Scholar]
- DeNardo DG, Andreu P, Coussens LM. Interactions between lymphocytes and myeloid cells regulate pro- versus anti-tumor immunity. Cancer Metastasis Rev. 2010;29:309–316. doi: 10.1007/s10555-010-9223-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng L, Liang H, Xu M, Yang X, Burnette B, Arina A, Li XD, Mauceri H, Beckett M, Darga T, Huang X, Gajewski TF, Chen ZJ, Fu YX, Weichselbaum RR. STING-dependent cytosolic DNA sensing promotes radiation-induced type i interferon-dependent antitumor immunity in immunogenic tumors. Immunity. 2014;41:843–852. doi: 10.1016/j.immuni.2014.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denkert C, Loibl S, Noske A, Roller M, Müller BM, Komor M, Budczies J, Darb-Esfahani S, Kronenwett R, Hanusch C, von Törne C, Weichert W, Engels K, Solbach C, Schrader I, Dietel M, von Minckwitz G. Tumor-associated lymphocytes as an independent predictor of response to neoadjuvant chemotherapy in breast cancer. J Clin Oncol. 2010;28:105–113. doi: 10.1200/JCO.2009.23.7370. [DOI] [PubMed] [Google Scholar]
- De Palma M, Venneri MA, Galli R, Sergi LS, Politi LS, Sampaolesi M, Naldini L. Tie2 identifies a hematopoietic lineage of proangiogenic monocytes required for tumor vessel formation and a mesenchymal population of pericyte progenitors. Cancer Cell. 2005;8:211–226. doi: 10.1016/j.ccr.2005.08.002. [DOI] [PubMed] [Google Scholar]
- De Togni P, Goellner J, Ruddle NH, Streeter PR, Fick A, Mariathasan S, Smith SC, Carlson R, Shornick LP, Strauss-Schoenberger J, Russell JH, Karr R, Chaplin DD. Abnormal development of peripheral lymphoid organs in mice deficient in lymphotoxin. Science. 1994;264:703–707. doi: 10.1126/science.8171322. [DOI] [PubMed] [Google Scholar]
- Diederichsen ACP, Hjelmborg JvB, Christensen PB, Zeuthen J, Fenger C. Prognostic value of the CD4+/CD8+ ratio of tumour infiltrating lymphocytes in colorectal cancer and HLA-DR expression on tumour cells. Cancer Immunol Immunother. 2003;52:423–428. doi: 10.1007/s00262-003-0388-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dieu-Nosjean MC, Antoine M, Danel C, Heudes D, Wislez M, Poulot V, Rabbe N, Laurans L, Tartour E, de Chaisemartin L, Lebecque S, Fridman WH, Cadranel J. Long-term survival for patients with non-small-cell lung cancer with intratumoral lymphoid structures. J Clin Oncol Off J Am Soc Clin Oncol. 2008;26:4410–4417. doi: 10.1200/JCO.2007.15.0284. [DOI] [PubMed] [Google Scholar]
- Dieu-Nosjean MC, Goc J, Giraldo NA, Sautès-Fridman C, Fridman WH. Tertiary lymphoid structures in cancer and beyond. Trends Immunol. 2014;35:571–580. doi: 10.1016/j.it.2014.09.006. [DOI] [PubMed] [Google Scholar]
- DiLillo DJ, Yanaba K, Tedder TF. B Cells Are Required for Optimal CD4+ and CD8+ T Cell Tumor Immunity: Therapeutic B Cell Depletion Enhances B16 Melanoma Growth in Mice. J Immunol. 2010;184:4006–4016. doi: 10.4049/jimmunol.0903009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dirkx AEM, oude Egbrink MGA, Kuijpers MJE, van der Niet ST, Heijnen VVT, Steege JCAB, Wagstaff J, Griffioen AW. Tumor angiogenesis modulates leukocyte-vessel wall interactions in vivo by reducing endothelial adhesion molecule expression. Cancer Res. 2003;63:2322–2329. [PubMed] [Google Scholar]
- Dong H, Strome SE, Salomao DR, Tamura H, Hirano F, Flies DB, Roche PC, Lu J, Zhu G, Tamada K, Lennon VA, Celis E, Chen L. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med. 2002;8:793–800. doi: 10.1038/nm730. [DOI] [PubMed] [Google Scholar]
- Drayton DL, Liao S, Mounzer RH, Ruddle NH. Lymphoid organ development: from ontogeny to neogenesis. Nat Immunol. 2006;7:344–353. doi: 10.1038/ni1330. [DOI] [PubMed] [Google Scholar]
- Dudda JC, Lembo A, Bachtanian E, Huehn J, Siewert C, Hamann A, Kremmer E, Forster R, Martin SF. Dendritic cells govern induction and reprogramming of polarized tissue-selective homing receptor patterns of T cells: important roles for soluble factors and tissue microenvironments. Eur J Immunol. 2005;35:1056–1065. doi: 10.1002/eji.200425817. [DOI] [PubMed] [Google Scholar]
- Economou JS, Belldegrun AS, Glaspy J, Toloza EM, Figlin R, Hobbs J, Meldon N, Kaboo R, Tso CL, Miller A, Lau R, McBride W, Moen RC. In vivo trafficking of adoptively transferred interleukin-2 expanded tumor-infiltrating lymphocytes and peripheral blood lymphocytes. Results of a double gene marking trial. J Clin Invest. 1996;97:515–521. doi: 10.1172/JCI118443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enarsson K, Johnsson E, Lindholm C, Lundgren A, Pan-Hammarström Q, Strömberg E, Bergin P, Baunge EL, Svennerholm AM, Quiding-Järbrink M. Differential mechanisms for T lymphocyte recruitment in normal and neoplastic human gastric mucosa. Clin Immunol. 2006;118:24–34. doi: 10.1016/j.clim.2005.08.001. [DOI] [PubMed] [Google Scholar]
- Erdag G, Schaefer JT, Smolkin ME, Deacon DH, Shea SM, Dengel LT, Patterson JW, Slingluff CL., Jr Immunotype and immunohistologic characteristics of tumor-infiltrating immune cells are associated with clinical outcome in metastatic melanoma. Cancer Res. 2012;72:1070–1080. doi: 10.1158/0008-5472.CAN-11-3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ericsson A, Kotarsky K, Svensson M, Sigvardsson M, Agace W. Functional characterization of the CCL25 promoter in small intestinal epithelial cells suggests a regulatory role for caudal-related homeobox (Cdx) transcription factors. J Immunol. 2006;176:3642–3651. doi: 10.4049/jimmunol.176.6.3642. [DOI] [PubMed] [Google Scholar]
- Ferguson AR, Engelhard VH. CD8 T cells activated in distinct lymphoid organs differentially express adhesion proteins and coexpress multiple chemokine receptors. J Immunol. 2010;184:4079–86. doi: 10.4049/jimmunol.0901903. jimmunol.0901903 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher B, Packard BS, Read EJ, Carrasquillo JA, Carter CS, Topalian SL, Yang JC, Yolles P, Larson SM, Rosenberg SA. Tumor localization of adoptively transferred indium-111 labeled tumor infiltrating lymphocytes in patients with metastatic melanoma. J Clin Oncol. 1989;7:250–261. doi: 10.1200/JCO.1989.7.2.250. [DOI] [PubMed] [Google Scholar]
- Fisher DT, Chen Q, Skitzki JJ, Muhitch JB, Zhou L, Appenheimer MM, Vardam TD, Weis EL, Passanese J, Wang WC, Gollnick SO, Dewhirst MW, Rose-John S, Repasky EA, Baumann H, Evans SS. IL-6 trans-signaling licenses mouse and human tumor microvascular gateways for trafficking of cytotoxic T cells. J Clin Invest. 2011;121:3846–3859. doi: 10.1172/JCI44952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Formenti SC, Demaria S. Combining radiotherapy and cancer immunotherapy: a paradigm shift. J Natl Cancer Inst. 2013;105:256–265. doi: 10.1093/jnci/djs629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridman WH, Pagès F, Sautès-Fridman C, Galon J. The immune contexture in human tumours: impact on clinical outcome. Nat Rev Cancer. 2012;12:298–306. doi: 10.1038/nrc3245. [DOI] [PubMed] [Google Scholar]
- Fuertes MB, Kacha AK, Kline J, Woo SR, Kranz DM, Murphy KM, Gajewski TF. Host type I IFN signals are required for antitumor CD8+ T cell responses through CD8{alpha}+ dendritic cells. J Exp Med. 2011;208:2005–2016. doi: 10.1084/jem.20101159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuhlbrigge RC, Kieffer JD, Armerding D, Kupper TS. Cutaneous lymphocyte antigen is a specialized form of PSGL-1 expressed on skin-homing T cells. Nature. 1997;389:978–981. doi: 10.1038/40166. [DOI] [PubMed] [Google Scholar]
- Fuhlbrigge RC, King SL, Dimitroff CJ, Kupper TS, Sackstein R. Direct real-time observation of E- and P-selectin-mediated rolling on cutaneous lymphocyte-associated antigen immobilized on Western blots. J Immunol. 2002;168:5645–5651. doi: 10.4049/jimmunol.168.11.5645. [DOI] [PubMed] [Google Scholar]
- Fütterer A, Mink K, Luz A, Kosco-Vilbois MH, Pfeffer K. The Lymphotoxin β Receptor Controls Organogenesis and Affinity Maturation in Peripheral Lymphoid Tissues. Immunity. 1998;9:59–70. doi: 10.1016/S1074-7613(00)80588-9. [DOI] [PubMed] [Google Scholar]
- Gabrilovich DI, Ostrand-Rosenberg S, Bronte V. Coordinated regulation of myeloid cells by tumours. Nat Rev Immunol. 2012;12:253–268. doi: 10.1038/nri3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gajewski TF, Louahed J, Brichard VG. Gene Signature in Melanoma Associated With Clinical Activity. Cancer J. 2010;16:399–403. doi: 10.1097/PPO.0b013e3181eacbd8. [DOI] [PubMed] [Google Scholar]
- Galon J, Angell HK, Bedognetti D, Marincola FM. The continuum of cancer immunosurveillance: prognostic, predictive, and mechanistic signatures. Immunity. 2013;39:11–26. doi: 10.1016/j.immuni.2013.07.008. [DOI] [PubMed] [Google Scholar]
- Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pagès C, Tosolini M, Camus M, Berger A, Wind P, Zinzindohoué F, Bruneval P, Cugnenc PH, Trajanoski Z, Fridman WH, Pagès F. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313:1960–1964. doi: 10.1126/science.1129139. [DOI] [PubMed] [Google Scholar]
- Ganss R, Hanahan D. Tumor microenvironment can restrict the effectiveness of activated antitumor lymphocytes. Cancer Res. 1998;58:4673–4681. [PubMed] [Google Scholar]
- Garbi N, Arnold B, Gordon S, Hämmerling GJ, Ganss R. CpG motifs as proinflammatory factors render autochthonous tumors permissive for infiltration and destruction. J Immunol. 2004;172:5861–5869. doi: 10.4049/jimmunol.172.10.5861. [DOI] [PubMed] [Google Scholar]
- Gatumu MK, Skarstein K, Papandile A, Browning JL, Fava RA, Bolstad A. Blockade of lymphotoxin-beta receptor signaling reduces aspects of Sjögren syndrome in salivary glands of non-obese diabetic mice. Arthritis Res Ther. 2009;11:R24. doi: 10.1186/ar2617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germain C, Gnjatic S, Tamzalit F, Knockaert S, Remark R, Goc J, Lepelley A, Becht E, Katsahian S, Bizouard G, Validire P, Damotte D, Alifano M, Magdeleinat P, Cremer I, Teillaud JL, Fridman WH, Sautès-Fridman C, Dieu-Nosjean MC. Presence of B cells in tertiary lymphoid structures is associated with a protective immunity in patients with lung cancer. Am J Respir Crit Care Med. 2014;189:832–844. doi: 10.1164/rccm.201309-1611OC. [DOI] [PubMed] [Google Scholar]
- GeurtsvanKessel CH, Willart MA, Bergen IM, van Rijt LS, Muskens F, Elewaut D, Osterhaus AD, Hendriks R, Rimmelzwaan GF, Lambrecht BN. Dendritic cells are crucial for maintenance of tertiary lymphoid structures in the lung of influenza virus-infected mice. J Exp Med. 2009;206:2339–49. doi: 10.1084/jem.20090410. jem.20090410 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girard JP, Moussion C, Förster R. HEVs, lymphatics and homeostatic immune cell trafficking in lymph nodes. Nat Rev Immunol. 2012;12:762–773. doi: 10.1038/nri3298. [DOI] [PubMed] [Google Scholar]
- Goc J, Fridman WH, Sautès-Fridman C, Dieu-Nosjean MC. Characteristics of tertiary lymphoid structures in primary cancers. OncoImmunology. 2013;2:e26836. doi: 10.4161/onci.26836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goelz SE, Hession C, Goff D, Griffiths B, Tizard R, Newman B, Chi-Rosso G, Lobb R. ELFT: A gene that directs the expression of an ELAM-1 ligand. Cell. 1990;63:1349–1356. doi: 10.1016/0092-8674(90)90430-M. [DOI] [PubMed] [Google Scholar]
- Goelz S, Kumar R, Potvin B, Sundaram S, Brickelmaier M, Stanley P. Differential expression of an E-selectin ligand (SLex) by two Chinese hamster ovary cell lines transfected with the same alpha (1,3)-fucosyltransferase gene (ELFT) J Biol Chem. 1994;269:1033–1040. [PubMed] [Google Scholar]
- Gräbner R, Lötzer K, Döpping S, Hildner M, Radke D, Beer M, Spanbroek R, Lippert B, Reardon CA, Getz GS, Fu YX, Hehlgans T, Mebius RE, van der Wall M, Kruspe D, Englert C, Lovas A, Hu D, Randolph GJ, Weih F, Habenicht AJR. Lymphotoxin β receptor signaling promotes tertiary lymphoid organogenesis in the aorta adventitia of aged ApoE/mice. J Exp Med. 2009;206:233–248. doi: 10.1084/jem.20080752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregg RK, Nichols L, Chen Y, Lu B, Engelhard VH. Mechanisms of spatial and temporal development of autoimmune vitiligo in tyrosinase-specific TCR transgenic mice. J Immunol. 2010;184:1909–17. doi: 10.4049/jimmunol.0902778. jimmunol.0902778 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith KD, Read EJ, Carrasquillo JA, Carter CS, Yang JC, Fisher B, Aebersold P, Packard BS, Yu MY, Rosenberg SA. In vivo distribution of adoptively transferred indium-111-labeled tumor infiltrating lymphocytes and peripheral blood lymphocytes in patients with metastatic melanoma. J Natl Cancer Inst. 1989;81:1709–1717. doi: 10.1093/jnci/81.22.1709. [DOI] [PubMed] [Google Scholar]
- Halama N, Michel S, Kloor M, Zoernig I, Benner A, Spille A, Pommerencke T, von Knebel DM, Folprecht G, Luber B, Feyen N, Martens UM, Beckhove P, Gnjatic S, Schirmacher P, Herpel E, Weitz J, Grabe N, Jaeger D. Localization and density of immune cells in the invasive margin of human colorectal cancer liver metastases are prognostic for response to chemotherapy. Cancer Res. 2011;71:5670–5677. doi: 10.1158/0008-5472.CAN-11-0268. [DOI] [PubMed] [Google Scholar]
- Halama N, Michel S, Kloor M, Zoernig I, Pommerencke T, von Knebel Doeberitz M, Schirmacher P, Weitz J, Grabe N, Jäger D. The localization and density of immune cells in primary tumors of human metastatic colorectal cancer shows an association with response to chemotherapy. Cancer Immun. 2009;9:1. [PMC free article] [PubMed] [Google Scholar]
- Hallahan D, Clark ET, Kuchibhotla J, Gewertz BL, Collins T. E-Selectin Gene Induction by Ionizing Radiation Is Independent of Cytokine Induction. Biochem Biophys Res Commun. 1995;217:784–795. doi: 10.1006/bbrc.1995.2841. [DOI] [PubMed] [Google Scholar]
- Halle S, Dujardin HC, Bakocevic N, Fleige H, Danzer H, Willenzon S, Suezer Y, Hammerling G, Garbi N, Sutter G, Worbs T, Forster R. Induced bronchus-associated lymphoid tissue serves as a general priming site for T cells and is maintained by dendritic cells. J Exp Med. 2009;206:2593–601. doi: 10.1084/jem.20091472. jem.20091472 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamid O, Schmidt H, Nissan A, Ridolfi L, Aamdal S, Hansson J, Guida M, Hyams DM, Gómez H, Bastholt L, Chasalow SD, Berman D. A prospective phase II trial exploring the association between tumor microenvironment biomarkers and clinical activity of ipilimumab in advanced melanoma. J Transl Med. 2011;9:204. doi: 10.1186/1479-5876-9-204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammerschmidt SI, Ahrendt M, Bode U, Wahl B, Kremmer E, Förster R, Pabst O. Stromal mesenteric lymph node cells are essential for the generation of gut-homing T cells in vivo. J Exp Med. 2008;205:2483–2490. doi: 10.1084/jem.20080039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamzah J, Jugold M, Kiessling F, Rigby P, Manzur M, Marti HH, Rabie T, Kaden S, Grone HJ, Hammerling GJ, Arnold B, Ganss R. Vascular normalization in Rgs5-deficient tumours promotes immune destruction. Nature. 2008;453:410–414. doi: 10.1038/nature06868. [DOI] [PubMed] [Google Scholar]
- Hancock WW, Lu B, Gao W, Csizmadia V, Faia K, King JA, Smiley ST, Ling M, Gerard NP, Gerard C. Requirement of the chemokine receptor CXCR3 for acute allograft rejection. J Exp Med. 2000;192:1515–1520. doi: 10.1084/jem.192.10.1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hangalapura BN, Oosterhoff D, Gupta T, de Groot J, Wijnands PGJTB, van Beusechem VW, den Haan J, Tüting T, van den Eertwegh AJM, Curiel DT, Scheper RJ, de Gruijl TD. Delivery route, MyD88 signaling and cross-priming events determine the anti-tumor efficacy of an adenovirus based melanoma vaccine. Vaccine. 2011;29:2313–2321. doi: 10.1016/j.vaccine.2011.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariri G, Zhang Y, Fu A, Han Z, Brechbiel M, Tantawy MN, Peterson TE, Mernaugh R, Hallahan D. Radiation-guided P-selectin antibody targeted to lung cancer. Ann Biomed Eng. 2008;36:821–830. doi: 10.1007/s10439-008-9444-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlin H, Meng Y, Peterson AC, Zha Y, Tretiakova M, Slingluff C, McKee M, Gajewski TF. Chemokine expression in melanoma metastases associated with CD8+ T-cell recruitment. Cancer Res. 2009;69:3077–3085. doi: 10.1158/0008-5472.CAN-08-2281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry RA, Kendall PL. CXCL13 blockade disrupts B lymphocyte organization in tertiary lymphoid structures without altering B cell receptor bias or preventing diabetes in nonobese diabetic mice. J Immunol Baltim Md 1950. 2010;185:1460–1465. doi: 10.4049/jimmunol.0903710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbst RS, Soria JC, Kowanetz M, Fine GD, Hamid O, Gordon MS, Sosman JA, McDermott DF, Powderly JD, Gettinger SN, Kohrt HEK, Horn L, Lawrence DP, Rost S, Leabman M, Xiao Y, Mokatrin A, Koeppen H, Hegde PS, Mellman I, Chen DS, Hodi FS. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature. 2014;515:563–567. doi: 10.1038/nature14011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernot S, Klibanov AL. Microbubbles in ultrasound-triggered drug and gene delivery. Adv Drug Deliv Rev. 2008;60:1153–1166. doi: 10.1016/j.addr.2008.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hindley JP, Jones E, Smart K, Bridgeman H, Lauder SN, Ondondo B, Cutting S, Ladell K, Wynn KK, Withers D, Price DA, Ager A, Godkin AJ, Gallimore AM. T-cell trafficking facilitated by high endothelial venules is required for tumor control after regulatory T-cell depletion. Cancer Res. 2012;72:5473–5482. doi: 10.1158/0008-5472.CAN-12-1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollenbaugh JA, Dutton RW. IFN-γ regulates donor CD8 T cell expansion, migration, and leads to apoptosis of cells of a solid tumor. J Immunol. 2006;177:3004–3011. doi: 10.4049/jimmunol.177.5.3004. [DOI] [PubMed] [Google Scholar]
- Homey B, Alenius H, Muller A, Soto H, Bowman EP, Yuan W, McEvoy L, Lauerma AI, Assmann T, Bunemann E, Lehto M, Wolff H, Yen D, Marxhausen H, To W, Sedgwick J, Ruzicka T, Lehmann P, Zlotnik A. CCL27-CCR10 interactions regulate T cell-mediated skin inflammation. Nat Med. 2002;8:157–165. doi: 10.1038/nm0202-157. [DOI] [PubMed] [Google Scholar]
- Huang Y, Yuan J, Righi E, Kamoun WS, Ancukiewicz M, Nezivar J, Santosuosso M, Martin JD, Martin MR, Vianello F, Leblanc P, Munn LL, Huang P, Duda DG, Fukumura D, Jain RK, Poznansky MC. Vascular normalizing doses of antiangiogenic treatment reprogram the immunosuppressive tumor microenvironment and enhance immunotherapy. Proc Natl Acad Sci. 2012;109:17561–17566. doi: 10.1073/pnas.1215397109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard AK, Rothlein R. Intercellular adhesion molecule-1 (ICAM-1) expression and cell signaling cascades. Free Radic Biol Med. 2000;28:1379–1386. doi: 10.1016/s0891-5849(00)00223-9. [DOI] [PubMed] [Google Scholar]
- Ino Y, Yamazaki-Itoh R, Shimada K, Iwasaki M, Kosuge T, Kanai Y, Hiraoka N. Immune cell infiltration as an indicator of the immune microenvironment of pancreatic cancer. Br J Cancer. 2013;108:914–923. doi: 10.1038/bjc.2013.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irvine DJ, Stachowiak AN, Hori Y. Lymphoid tissue engineering: Invoking lymphoid tissue neogenesis in immunotherapy and models of immunity. Semin Immunol. 2008;20:137–146. doi: 10.1016/j.smim.2007.10.010. [DOI] [PubMed] [Google Scholar]
- Ji RR, Chasalow SD, Wang L, Hamid O, Schmidt H, Cogswell J, Alaparthy S, Berman D, Jure-Kunkel M, Siemers NO, Jackson JR, Shahabi V. An immune-active tumor microenvironment favors clinical response to ipilimumab. Cancer Immunol Immunother CII. 2012;61:1019–1031. doi: 10.1007/s00262-011-1172-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson-Lindbom B, Svensson M, Wurbel MA, Malissen B, Marquez G, Agace W. Selective generation of gut tropic T cells in gut-associated lymphoid tissue (GALT): requirement for GALT dendritic cells and adjuvant. J Exp Med. 2003;198:963–969. doi: 10.1084/jem.20031244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan DH, Shankaran V, Dighe AS, Stockert E, Aguet M, Old LJ, Schreiber RD. Demonstration of an interferon gamma-dependent tumor surveillance system in immunocompetent mice. Proc Natl Acad Sci U S A. 1998;95:7556–7561. doi: 10.1073/pnas.95.13.7556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplanski G, Fabrigoule M, Boulay V, Dinarello CA, Bongrand P, Kaplanski S, Farnarier C. Thrombin induces endothelial type II activation in vitro: IL-1 and TNF-alpha-independent IL-8 secretion and E-selectin expression. J Immunol. 1997;158:5435–5441. [PubMed] [Google Scholar]
- Kaplanski G, Marin V, Fabrigoule M, Boulay V, Benoliel AM, Bongrand P, Kaplanski S, Farnarier C. Thrombin-Activated Human Endothelial Cells Support Monocyte Adhesion In Vitro Following Expression of Intercellular Adhesion Molecule-1 (ICAM-1; CD54) and Vascular Cell Adhesion Molecule-1 (VCAM-1; CD106) Blood. 1998;92:1259–1267. [PubMed] [Google Scholar]
- Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677–704. doi: 10.1146/annurev.immunol.26.021607.090331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenyon NJ, Liu R, O’Roark EM, Huang W, Peng L, Lam KS. An alpha4beta1 integrin antagonist decreases airway inflammation in ovalbumin-exposed mice. Eur J Pharmacol. 2009;603:138–46. doi: 10.1016/j.ejphar.2008.11.063. S0014-2999(08)01216-8 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kershaw MH, Westwood JA, Parker LL, Wang G, Eshhar Z, Mavroukakis SA, White DE, Wunderlich JR, Canevari S, Rogers-Freezer L, Chen CC, Yang JC, Rosenberg SA, Hwu P. A phase I study on adoptive immunotherapy using gene-modified T cells for ovarian cancer. Clin Cancer Res. 2006;12:6106–6115. doi: 10.1158/1078-0432.CCR-06-1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HJ, Kammertoens T, Janke M, Schmetzer O, Qin Z, Berek C, Blankenstein T. Establishment of early lymphoid organ infrastructure in transplanted tumors mediated by local production of Lymphotoxin α and in the combined absence of functional B and T cells. J Immunol. 2004;172:4037–4047. doi: 10.4049/jimmunol.172.7.4037. [DOI] [PubMed] [Google Scholar]
- Kirk CJ, Hartigan-O’Connor D, Mule JJ. The dynamics of the T-cell antitumor response: chemokine-secreting dendritic cells can prime tumor-reactive T cells extranodally. Cancer Res. 2001a;61:8794–8802. [PubMed] [Google Scholar]
- Kirk CJ, Hartigan-O’Connor D, Nickoloff BJ, Chamberlain JS, Giedlin M, Aukerman L, Mulé JJ. T cell-dependent antitumor immunity mediated by secondary lymphoid tissue chemokine augmentation of dendritic cell-based immunotherapy. Cancer Res. 2001b;61:2062–2070. [PubMed] [Google Scholar]
- Knibbs RN, Craig RA, Natsuka S, Chang A, Cameron M, Lowe JB, Stoolman LM. The fucosyltransferase FucT-VII regulates E-selectin ligand synthesis in human T cells. J Cell Biol. 1996;133:911–920. doi: 10.1083/jcb.133.4.911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koni PA, Sacca R, Lawton P, Browning JL, Ruddle NH, Flavell RA. Distinct roles in lymphoid organogenesis for lymphotoxins alpha and beta revealed in lymphotoxin beta-deficient mice. Immunity. 1997;6:491–500. doi: 10.1016/s1074-7613(00)80292-7. [DOI] [PubMed] [Google Scholar]
- Konkalmatt PR, Deng D, Thomas S, Wu MT, Logsdon CD, French BA, Kelly KA. Plectin-1 Targeted AAV Vector for the Molecular Imaging of Pancreatic Cancer. Front Oncol. 2013;3:84. doi: 10.3389/fonc.2013.00084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunz M, Toksoy A, Goebeler M, Engelhardt E, Bröcker E, Gillitzer R. Strong expression of the lymphoattractant C-X-C chemokine Mig is associated with heavy infiltration of T cells in human malignant melanoma. J Pathol. 1999;189:552–558. doi: 10.1002/(SICI)1096-9896(199912)189:4<552::AID-PATH469>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Kupper TS, Fuhlbrigge RC. Immune surveillance in the skin: mechanisms and clinical consequences. Nat Rev Immunol. 2004;4:211–222. doi: 10.1038/nri1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kvistborg P, Philips D, Kelderman S, Hageman L, Ottensmeier C, Joseph-Pietras D, Welters MJP, van der Burg S, Kapiteijn E, Michielin O, Romano E, Linnemann C, Speiser D, Blank C, Haanen JB, Schumacher TN. Anti–CTLA-4 therapy broadens the melanoma-reactive CD8+ T cell response. Sci Transl Med. 2014;6:254ra128–254ra128. doi: 10.1126/scitranslmed.3008918. [DOI] [PubMed] [Google Scholar]
- Ladányi A, Sebestyén T, Mohos A, Liszkay G, Somlai B, Tóth E, Tímár J. Ectopic lymphoid structures in primary cutaneous melanoma. Pathol Oncol Res. 2014;20:981–985. doi: 10.1007/s12253-014-9784-8. [DOI] [PubMed] [Google Scholar]
- Lauder I, Aherne W. The significance of lymphocytic infiltration in neuroblastoma. Br J Cancer. 1972;26:321–330. doi: 10.1038/bjc.1972.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence MB, Springer TA. Leukocytes roll on a selectin at physiologic flow rates: distinction from and prerequisite for adhesion through integrins. Cell. 1991;65:859–873. doi: 10.1016/0092-8674(91)90393-d. [DOI] [PubMed] [Google Scholar]
- Ley K, Laudanna C, Cybulsky MI, Nourshargh S. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat Rev Immunol. 2007;7:678–689. doi: 10.1038/nri2156. [DOI] [PubMed] [Google Scholar]
- Liao S, Ruddle NH. Synchrony of high endothelial venules and lymphatic vessels revealed by immunization. J Immunol. 2006;177:3369–3379. doi: 10.4049/jimmunol.177.5.3369. [DOI] [PubMed] [Google Scholar]
- Lin EY, Nguyen AV, Russell RG, Pollard JW. Colony-stimulating factor 1 promotes progression of mammary tumors to malignancy. J Exp Med. 2001;193:727–740. doi: 10.1084/jem.193.6.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Zhang T, Ye J, Li H, Huang J, Li X, Wu B, Huang X, Hou J. Tumor-infiltrating lymphocytes predict response to chemotherapy in patients with advance non-small cell lung cancer. Cancer Immunol Immunother. 2012;61:1849–1856. doi: 10.1007/s00262-012-1231-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohr J, Ratliff T, Huppertz A, Ge Y, Dictus C, Ahmadi R, Grau S, Hiraoka N, Eckstein V, Ecker RC, Korff T, von Deimling A, Unterberg A, Beckhove P, Herold-Mende C. Effector T-cell infiltration positively impacts survival of glioblastoma patients and is impaired by tumor-derived TGF-β. Clin Cancer Res. 2011;17:4296–4308. doi: 10.1158/1078-0432.CCR-10-2557. [DOI] [PubMed] [Google Scholar]
- Loi S, Sirtaine N, Piette F, Salgado R, Viale G, Eenoo FV, Rouas G, Francis P, Crown JPA, Hitre E, de Azambuja E, Quinaux E, Leo AD, Michiels S, Piccart MJ, Sotiriou C. Prognostic and predictive value of tumor-infiltrating lymphocytes in a phase III randomized adjuvant breast cancer trial in node-positive breast cancer comparing the addition of docetaxel to doxorubicin with doxorubicin-based chemotherapy. J Clin Oncol. 2013;31:860–867. doi: 10.1200/JCO.2011.41.0902. [DOI] [PubMed] [Google Scholar]
- Lugade AA, Moran JP, Gerber SA, Rose RC, Frelinger JG, Lord EM. Local radiation therapy of B16 melanoma tumors increases the generation of tumor antigen-specific effector cells that traffic to the tumor. J Immunol. 2005;174:7516–7523. doi: 10.4049/jimmunol.174.12.7516. [DOI] [PubMed] [Google Scholar]
- Lurquin C, Lethé B, Plaen ED, Corbière V, Théate I, van Baren N, Coulie PG, Boon T. Contrasting frequencies of antitumor and anti-vaccine T cells in metastases of a melanoma patient vaccinated with a MAGE tumor antigen. J Exp Med. 2005;201:249–257. doi: 10.1084/jem.20041378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luther SA, Bidgol A, Hargreaves DC, Schmidt A, Xu Y, Paniyadi J, Matloubian M, Cyster JG. Differing activities of homeostatic chemokines CCL19, CCL21, and CXCL12 in lymphocyte and dendritic cell recruitment and lymphoid neogenesis. J Immunol. 2002;169:424–433. doi: 10.4049/jimmunol.169.1.424. [DOI] [PubMed] [Google Scholar]
- Lutz ER, Wu AA, Bigelow E, Sharma R, Mo G, Soares K, Solt S, Dorman A, Wamwea A, Yager A, Laheru D, Wolfgang CL, Wang J, Hruban RH, Anders RA, Jaffee EM, Zheng L. Immunotherapy converts nonimmunogenic pancreatic tumors into immunogenic foci of immune regulation. Cancer Immunol Res. 2014 doi: 10.1158/2326-6066.CIR-14-0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maly P, Thall A, Petryniak B, Rogers CE, Smith PL, Marks RM, Kelley RJ, Gersten KM, Cheng G, Saunders TL, Camper SA, Camphausen RT, Sullivan FX, Isogai Y, Hindsgaul O, von Andrian UH, Lowe JB. The alpha(1, 3)fucosyltransferase Fuc-TVII controls leukocyte trafficking through an essential role in L-, E-, and P-selectin ligand biosynthesis. 1996 doi: 10.1016/s0092-8674(00)80137-3. [DOI] [PubMed] [Google Scholar]
- Margolin KA. Interleukin-2 in the treatment of renal cancer. Semin Oncol. 2000;27:194–203. [PubMed] [Google Scholar]
- Marincola FM, Wang E, Herlyn M, Seliger B, Ferrone S. Tumors as elusive targets of T-cell-based active immunotherapy. Trends Immunol. 2003;24:334–341. doi: 10.1016/S1471-4906(03)00116-9. [DOI] [PubMed] [Google Scholar]
- Marinkovic T, Garin A, Yokota Y, Fu YX, Ruddle NH, Furtado GC, Lira SA. Interaction of mature CD3+ CD4+ T cells with dendritic cells triggers the development of tertiary lymphoid structures in the thyroid. J Clin Invest. 2006;116:2622–2632. doi: 10.1172/JCI28993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinet L, Filleron T, Guellec SL, Rochaix P, Garrido I, Girard JP. High endothelial venule blood vessels for tumor-infiltrating lymphocytes are associated with Lymphotoxin β–producing dendritic cells in human breast cancer. J Immunol. 2013;191:2001–2008. doi: 10.4049/jimmunol.1300872. [DOI] [PubMed] [Google Scholar]
- Martinet L, Garrido I, Filleron T, Le Guellec S, Bellard E, Fournie JJ, Rochaix P, Girard JP. Human solid tumors contain high endothelial venules: association with T- and B-lymphocyte infiltration and favorable prognosis in breast cancer. Cancer Res. 2011;71:5678–5687. doi: 10.1158/0008-5472.CAN-11-0431. [DOI] [PubMed] [Google Scholar]
- Martinet L, Le Guellec S, Filleron T, Lamant L, Meyer N, Rochaix P, Garrido I, Girard JP. High endothelial venules (HEVs) in human melanoma lesions: Major gateways for tumor-infiltrating lymphocytes. Oncoimmunology. 2012;1:829–839. doi: 10.4161/onci.20492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masopust D, Schenkel JM. The integration of T cell migration, differentiation and function. Nat Rev Immunol. 2013;13:309–320. doi: 10.1038/nri3442. [DOI] [PubMed] [Google Scholar]
- Matsumoto M, Atarashi K, Umemoto E, Furukawa Y, Shigeta A, Miyasaka M, Hirata T. CD43 functions as a ligand for E-Selectin on activated T cells. J Immunol. 2005;175:8042–8050. doi: 10.4049/jimmunol.175.12.8042. [DOI] [PubMed] [Google Scholar]
- Matsumoto M, Shigeta A, Furukawa Y, Tanaka T, Miyasaka M, Hirata T. CD43 collaborates with P-selectin glycoprotein ligand-1 to mediate E-selectin-dependent T cell migration into inflamed skin. J Immunol. 2007;178:2499–506. doi: 10.4049/jimmunol.178.4.2499. [DOI] [PubMed] [Google Scholar]
- Mebius RE, Streeter PR, Michie S, Butcher EC, Weissman IL. A developmental switch in lymphocyte homing receptor and endothelial vascular addressin expression regulates lymphocyte homing and permits CD4+ CD3- cells to colonize lymph nodes. Proc Natl Acad Sci U S A. 1996;93:11019–11024. doi: 10.1073/pnas.93.20.11019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messina JL, Fenstermacher DA, Eschrich S, Qu X, Berglund AE, Lloyd MC, Schell MJ, Sondak VK, Weber JS, Mulé JJ. 12-Chemokine gene signature identifies lymph node-like structures in melanoma: potential for patient selection for immunotherapy? Sci Rep. 2012;2:765. doi: 10.1038/srep00765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mionnet C, Sanos SL, Mondor I, Jorquera A, Laugier JP, Germain RN, Bajénoff M. High endothelial venules as traffic control points maintaining lymphocyte population homeostasis in lymph nodes. Blood. 2011;118:6115–6122. doi: 10.1182/blood-2011-07-367409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mlecnik B, Tosolini M, Charoentong P, Kirilovsky A, Bindea G, Berger A, Camus M, Gillard M, Bruneval P, Fridman WH, Pagès F, Trajanoski Z, Galon J. Biomolecular network reconstruction identifies T-cell homing factors associated with survival in colorectal cancer. Gastroenterology. 2010;138:1429–1440. doi: 10.1053/j.gastro.2009.10.057. [DOI] [PubMed] [Google Scholar]
- Mollà M, Gironella M, Miquel R, Tovar V, Engel P, Biete A, Piqué JM, Panés J. Relative roles of ICAM-1 and VCAM-1 in the pathogenesis of experimental radiation-induced intestinal inflammation. Int J Radiat Oncol Biol Phys. 2003;57:264–273. doi: 10.1016/S0360-3016(03)00523-6. [DOI] [PubMed] [Google Scholar]
- Mora JR, Bono MR, Manjunath N, Weninger W, Cavanagh LL, Rosemblatt M, von Andrian UH. Selective imprinting of gut-homing T cells by Peyer’s patch dendritic cells. Nature. 2003;424:88–93. doi: 10.1038/nature01726. [DOI] [PubMed] [Google Scholar]
- Mora JR, Cheng G, Picarella D, Briskin M, Buchanan N, von Andrian UH. Reciprocal and dynamic control of CD8 T cell homing by dendritic cells from skin- and gut-associated lymphoid tissues. J Exp Med. 2005;201:303–316. doi: 10.1084/jem.20041645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mora JR, von Andrian UH. T-cell homing specificity and plasticity: new concepts and future challenges. Trends Immunol. 2006;27:235–243. doi: 10.1016/j.it.2006.03.007. [DOI] [PubMed] [Google Scholar]
- Morales J, Homey B, Vicari AP, Hudak S, Oldham E, Hedrick J, Orozco R, Copeland NG, Jenkins NA, McEvoy LM, Zlotnik A. CTACK, a skin-associated chemokine that preferentially attracts skin-homing memory T cells. Proc Natl Acad Sci U S A. 1999;96:14470–14475. doi: 10.1073/pnas.96.25.14470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris M, Platell C, Iacopetta B. Tumor-infiltrating lymphocytes and perforation in colon cancer predict positive response to 5-fluorouracil chemotherapy. Clin Cancer Res. 2008;14:1413–1417. doi: 10.1158/1078-0432.CCR-07-1994. [DOI] [PubMed] [Google Scholar]
- Motallebzadeh R, Rehakova S, Conlon TM, Win TS, Callaghan CJ, Goddard M, Bolton EM, Ruddle NH, Bradley JA, Pettigrew GJ. Blocking lymphotoxin signaling abrogates the development of ectopic lymphoid tissue within cardiac allografts and inhibits effector antibody responses. FASEB J. 2012;26:51–62. doi: 10.1096/fj.11-186973. [DOI] [PubMed] [Google Scholar]
- Moussion C, Girard JP. Dendritic cells control lymphocyte entry to lymph nodes through high endothelial venules. Nature. 2011;479:542–546. doi: 10.1038/nature10540. [DOI] [PubMed] [Google Scholar]
- Mullins DW, Sheasley SL, Ream RM, Bullock TN, Fu YX, Engelhard VH. Route of immunization with peptide-pulsed dendritic cells controls the distribution of memory and effector T cells in lymphoid tissues and determines the pattern of regional tumor control. J Exp Med. 2003;198:1023–1034. doi: 10.1084/jem.20021348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullins IM, Slingluff CL, Lee JK, Garbee CF, Shu J, Anderson SG, Mayer ME, Knaus WA, Mullins DW. CXC chemokine receptor 3 expression by activated CD8+ T cells is associated with survival in melanoma patients with stage III disease. Cancer Res. 2004;64:7697–7701. doi: 10.1158/0008-5472.CAN-04-2059. [DOI] [PubMed] [Google Scholar]
- Nakano O, Sato M, Naito Y, Suzuki K, Orikasa S, Aizawa M, Suzuki Y, Shintaku I, Nagura H, Ohtani H. Proliferative activity of intratumoral CD8+ T-lymphocytes as a prognostic factor in human renal cell carcinoma clinicopathologic demonstration of antitumor immunity. Cancer Res. 2001;61:5132–5136. [PubMed] [Google Scholar]
- Nielsen JS, Sahota RA, Milne K, Kost SE, Nesslinger NJ, Watson PH, Nelson BH. CD20+ tumor-infiltrating lymphocytes have an atypical CD27- memory phenotype and together with CD8+ T cells promote favorable prognosis in ovarian cancer. Clin Cancer Res Off J Am Assoc Cancer Res. 2012;18:3281–3292. doi: 10.1158/1078-0432.CCR-12-0234. [DOI] [PubMed] [Google Scholar]
- Novak L, Igoucheva O, Cho S, Alexeev V. Characterization of the CCL21-mediated melanoma-specific immune responses and in situ melanoma eradication. Mol Cancer Ther. 2007;6:1755–1764. doi: 10.1158/1535-7163.MCT-06-0709. [DOI] [PubMed] [Google Scholar]
- Ogawa H, Binion DG, Heidemann J, Theriot M, Fisher PJ, Johnson NA, Otterson MF, Rafiee P. Mechanisms of MAdCAM-1 gene expression in human intestinal microvascular endothelial cells. Am J Physiol - Cell Physiol. 2005;288:C272–C281. doi: 10.1152/ajpcell.00406.2003. [DOI] [PubMed] [Google Scholar]
- Pagès F, Kirilovsky A, Mlecnik B, Asslaber M, Tosolini M, Bindea G, Lagorce C, Wind P, Marliot F, Bruneval P, Zatloukal K, Trajanoski Z, Berger A, Fridman WH, Galon J. In situ cytotoxic and memory T cells predict outcome in patients with early-stage colorectal cancer. J Clin Oncol. 2009;27:5944–5951. doi: 10.1200/JCO.2008.19.6147. [DOI] [PubMed] [Google Scholar]
- Palma L, Di Lorenzo N, Guidetti B. Lymphocytic infiltrates in primary glioblastomas and recidivous gliomas. Incidence, fate, and relevance to prognosis in 228 operated cases. J Neurosurg. 1978;49:854–61. doi: 10.3171/jns.1978.49.6.0854. [DOI] [PubMed] [Google Scholar]
- Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12:252–264. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng W, Liu C, Xu C, Lou Y, Chen J, Yang Y, Yagita H, Overwijk WW, Lizée G, Radvanyi L, Hwu P. PD-1 blockade enhances T-cell migration to tumors by elevating IFN-γ inducible chemokines. Cancer Res. 2012;72:5209–5218. doi: 10.1158/0008-5472.CAN-12-1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peske JD, Thompson ED, Gemta L, Baylis RA, Fu Y-X, Engelhard VH. Effector lymphocyte-induced lymph node-like vasculature enables naïve T-cell entry into tumors and enhanced anti-tumor immunity. Nat Commun. 2015 doi: 10.1038/ncomms8114. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pockaj BA, Sherry RM, Wei JP, Yannelli JR, Carter CS, Leitman SF, Carasquillo JA, Steinberg SM, Rosenberg SA, Yang JC. Localization of 111indium-labeled tumor infiltrating lymphocytes to tumor in patients receiving adoptive immunotherapy. Augmentation with cyclophosphamide and correlation with response. Cancer. 1994;73:1731–1737. doi: 10.1002/1097-0142(19940315)73:6<1731::aid-cncr2820730630>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Postow MA, Callahan MK, Barker CA, Yamada Y, Yuan J, Kitano S, Mu Z, Rasalan T, Adamow M, Ritter E, Sedrak C, Jungbluth AA, Chua R, Yang AS, Roman RA, Rosner S, Benson B, Allison JP, Lesokhin AM, Gnjatic S, Wolchok JD. Immunologic correlates of the abscopal effect in a patient with melanoma. N Engl J Med. 2012;366:925–931. doi: 10.1056/NEJMoa1112824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston CC, Maurer MJ, Oberg AL, Visscher DW, Kalli KR, Hartmann LC, Goode EL, Knutson KL. The ratios of CD8+ T cells to CD4+CD25+ FOXP3+ and FOXP3- T cells correlate with poor clinical outcome in human serous ovarian cancer. PloS One. 2013;8:e80063. doi: 10.1371/journal.pone.0080063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pylayeva-Gupta Y, Lee KE, Hajdu CH, Miller G, Bar-Sagi D. Oncogenic Kras-induced GM-CSF production promotes the development of pancreatic neoplasia. Cancer Cell. 2012;21:836–847. doi: 10.1016/j.ccr.2012.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quezada SA, Peggs KS, Simpson TR, Shen Y, Littman DR, Allison JP. Limited tumor infiltration by activated T effector cells restricts the therapeutic activity of regulatory T cell depletion against established melanoma. J Exp Med. 2008;205:2125–2138. doi: 10.1084/jem.20080099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rangel-Moreno J, Carragher DM, de la Garcia-Hernandez ML, Hwang JY, Kusser K, Hartson L, Kolls JK, Khader SA, Randall TD. The development of inducible bronchus-associated lymphoid tissue depends on IL-17. Nat Immunol. 2011;12:639–646. doi: 10.1038/ni.2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remark R, Alifano M, Cremer I, Lupo A, Dieu-Nosjean MC, Riquet M, Crozet L, Ouakrim H, Goc J, Cazes A, Fléjou JF, Gibault L, Verkarre V, Régnard JF, Pagès ON, Oudard S, Mlecnik B, Sautès-Fridman C, Fridman WH, Damotte D. Characteristics and Clinical Impacts of the Immune Environments in Colorectal and Renal Cell Carcinoma Lung Metastases: Influence of Tumor Origin. Clin Cancer Res. 2013;19:4079–4091. doi: 10.1158/1078-0432.CCR-12-3847. [DOI] [PubMed] [Google Scholar]
- Restifo NP, Dudley ME, Rosenberg SA. Adoptive immunotherapy for cancer: harnessing the T cell response. Nat Rev Immunol. 2012;12:269–281. doi: 10.1038/nri3191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg SA, Yang JC, Topalian SL, Schwartzentruber DJ, Weber JS, Parkinson DR, Seipp CA, Einhorn JH, White DE. Treatment of 283 consecutive patients with metastatic melanoma or renal cell cancer using high-dose bolus interleukin 2 [see comments] J Am Med Assoc. 1994;271:907–913. [PubMed] [Google Scholar]
- Rosen SD. Ligands for L-selectin: homing, inflammation, and beyond. Annu Rev Immunol. 2004;22:129–156. doi: 10.1146/annurev.immunol.21.090501.080131. [DOI] [PubMed] [Google Scholar]
- Rott LS, Briskin MJ, Andrew DP, Berg EL, Butcher EC. A fundamental subdivision of circulating lymphocytes defined by adhesion to mucosal addressin cell adhesion molecule-1. Comparison with vascular cell adhesion molecule-1 and correlation with beta 7 integrins and memory differentiation. J Immunol. 1996;156:3727–3736. [PubMed] [Google Scholar]
- Sackstein R. The lymphocyte homing receptors: gatekeepers of the multistep paradigm. Curr Opin Hematol. 2005;12:444–450. doi: 10.1097/01.moh.0000177827.78280.79. [DOI] [PubMed] [Google Scholar]
- Sakai Y, Hoshino H, Kitazawa R, Kobayashi M. High endothelial venule-like vessels and lymphocyte recruitment in testicular seminoma. Andrology. 2014;2:282–289. doi: 10.1111/j.2047-2927.2014.00192.x. [DOI] [PubMed] [Google Scholar]
- Salas A, Shimaoka M, Chen S, Carman CV, Springer T. Transition From Rolling to Firm Adhesion Is Regulated by the Conformation of the I Domain of the Integrin Lymphocyte Function-associated Antigen-1. J Biol Chem. 2002;277:50255–50262. doi: 10.1074/jbc.M209822200. [DOI] [PubMed] [Google Scholar]
- Sandoval F, Terme M, Nizard M, Badoual C, Bureau MF, Freyburger L, Clement O, Marcheteau E, Gey A, Fraisse G, Bouguin C, Merillon N, Dransart E, Tran T, Quintin-Colonna F, Autret G, Thiebaud M, Suleman M, Riffault S, Wu TC, Launay O, Danel C, Taieb J, Richardson J, Zitvogel L, Fridman WH, Johannes L, Tartour E. Mucosal Imprinting of Vaccine-Induced CD8+ T Cells Is Crucial to Inhibit the Growth of Mucosal Tumors. Sci Transl Med. 2013;5:172ra20–172ra20. doi: 10.1126/scitranslmed.3004888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato E, Olson SH, Ahn J, Bundy B, Nishikawa H, Qian F, Jungbluth AA, Frosina D, Gnjatic S, Ambrosone C, Kepner J, Odunsi T, Ritter G, Lele S, Chen YT, Ohtani H, Old LJ, Odunsi K. Intraepithelial CD8+ tumor-infiltrating lymphocytes and a high CD8+/regulatory T cell ratio are associated with favorable prognosis in ovarian cancer. Proc Natl Acad Sci U S A. 2005;102:18538–43. doi: 10.1073/pnas.0509182102. 0509182102 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrama D, thor Straten P, Fischer WH, McLellan AD, Bröcker EB, Reisfeld RA, Becker JC. Targeting of Lymphotoxin-α to the tumor elicits an efficient immune response associated with induction of peripheral lymphoid-like tissue. Immunity. 2001;14:111–121. doi: 10.1016/S1074-7613(01)00094-2. [DOI] [PubMed] [Google Scholar]
- Schrama D, Voigt H, Eggert AO, Xiang R, Zhou H, Schumacher TNM, Andersen MH, thor Straten P, Reisfeld RA, Becker JC. Immunological tumor destruction in a murine melanoma model by targeted LTalpha independent of secondary lymphoid tissue. Cancer Immunol Immunother. 2008;57:85–95. doi: 10.1007/s00262-007-0352-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartzentruber DJ, Lawson DH, Richards JM, Conry RM, Miller DM, Treisman J, Gailani F, Riley L, Conlon K, Pockaj B, Kendra KL, White RL, Gonzalez R, Kuzel TM, Curti B, Leming PD, Whitman ED, Balkissoon J, Reintgen DS, Kaufman H, Marincola FM, Merino MJ, Rosenberg SA, Choyke P, Vena D, Hwu P. gp100 peptide vaccine and interleukin-2 in patients with advanced melanoma. N Engl J Med. 2011;364:2119–2127. doi: 10.1056/NEJMoa1012863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seguin J, Nicolazzi C, Mignet N, Scherman D, Chabot G. Vascular density and endothelial cell expression of integrin alpha v beta 3 and E-selectin in murine tumours. Tumour Biol. 2012;33:1709–1717. doi: 10.1007/s13277-012-0428-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankaran V, Ikeda H, Bruce AT, White JM, Swanson PE, Old LJ, Schreiber RD. IFNgamma and lymphocytes prevent primary tumour development and shape tumour immunogenicity. Nature. 2001;410:1107–1111. doi: 10.1038/35074122. [DOI] [PubMed] [Google Scholar]
- Sharma S, Stolina M, Luo J, Strieter RM, Burdick M, Zhu LX, Batra RK, Dubinett SM. Secondary lymphoid tissue chemokine mediates T cell-dependent antitumor responses in vivo. J Immunol. 2000;164:4558–4563. doi: 10.4049/jimmunol.164.9.4558. [DOI] [PubMed] [Google Scholar]
- Sheasley-O’Neill SL, Brinkman CC, Ferguson AR, Dispenza MC, Engelhard VH. Dendritic cell immunization route determines integrin expression and lymphoid and nonlymphoid tissue distribution of CD8 T cells. J Immunol. 2007;178:1512–1522. doi: 10.4049/jimmunol.178.3.1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherry B, Tekamp-Olson P, Gallegos C, Bauer D, Davatelis G, Wolpe SD, Masiarz F, Coit D, Cerami A. Resolution of the two components of macrophage inflammatory protein 1, and cloning and characterization of one of those components, macrophage inflammatory protein 1 beta. J Exp Med. 1988;168:2251–2259. doi: 10.1084/jem.168.6.2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shields JD, Kourtis IC, Tomei AA, Roberts JM, Swartz MA. Induction of lymphoidlike stroma and immune escape by tumors that express the chemokine CCL21. Science. 2010;328:749–752. doi: 10.1126/science.1185837. [DOI] [PubMed] [Google Scholar]
- Shrimali RK, Yu Z, Theoret MR, Chinnasamy D, Restifo NP, Rosenberg SA. Antiangiogenic Agents Can Increase Lymphocyte Infiltration into Tumor and Enhance the Effectiveness of Adoptive Immunotherapy of Cancer. Cancer Res. 2010;70:6171–6180. doi: 10.1158/0008-5472.CAN-10-0153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigmundsdottir H, Pan J, Debes GF, Alt C, Habtezion A, Soler D, Butcher EC. DCs metabolize sunlight-induced vitamin D3 to “program” T cell attraction to the epidermal chemokine CCL27. Nat Immunol. 2007;8:285–93. doi: 10.1038/ni1433. ni1433 [pii] [DOI] [PubMed] [Google Scholar]
- Simpson TR, Li F, Montalvo-Ortiz W, Sepulveda MA, Bergerhoff K, Arce F, Roddie C, Henry JY, Yagita H, Wolchok JD, Peggs KS, Ravetch JV, Allison JP, Quezada SA. Fc-dependent depletion of tumor-infiltrating regulatory T cells co-defines the efficacy of anti–CTLA-4 therapy against melanoma. J Exp Med. 2013;210:1695–1710. doi: 10.1084/jem.20130579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyth MJ, Crowe NY, Godfrey DI. NK cells and NKT cells collaborate in host protection from methylcholanthrene-induced fibrosarcoma. Int Immunol. 2001;13:459–463. doi: 10.1093/intimm/13.4.459. [DOI] [PubMed] [Google Scholar]
- Smyth MJ, Thia KY, Street SE, MacGregor D, Godfrey DI, Trapani JA. Perforin-mediated cytotoxicity is critical for surveillance of spontaneous lymphoma. J Exp Med. 2000;192:755–760. doi: 10.1084/jem.192.5.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder A, Makarov V, Merghoub T, Yuan J, Zaretsky JM, Desrichard A, Walsh LA, Postow MA, Wong P, Ho TS, Hollmann TJ, Bruggeman C, Kannan K, Li Y, Elipenahli C, Liu C, Harbison CT, Wang L, Ribas A, Wolchok JD, Chan TA. Genetic basis for clinical response to CTLA-4 blockade in melanoma. N Engl J Med. 2014;371:2189–2199. doi: 10.1056/NEJMoa1406498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spranger S, Bao R, Gajewski T. Melanoma-intrinsic β-catenin signaling prevents T cell infiltration and anti-tumor immunity. J Immunother Cancer. 2014;2:O15. doi: 10.1186/2051-1426-2-S3-O15. [DOI] [Google Scholar]
- Stark FC, Gurnani K, Sad S, Krishnan L. Lack of functional selectin ligand interactions compromises long term tumor protection by CD8+ T cells. PloS One. 2012;7:e32211. doi: 10.1371/journal.pone.0032211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streeter PR, Rouse BT, Butcher EC. Immunohistologic and functional characterization of a vascular addressin involved in lymphocyte homing into peripheral lymph nodes. J Cell Biol. 1988;107:1853–1862. doi: 10.1083/jcb.107.5.1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan RJ, Hoshida Y, Brunet J, Tahan S, Aldridge J, Kwabi C, Gardiner E, McDermott D, Golub T, Atkins MB. A single center experience with high-dose (HD) IL-2 treatment for patients with advanced melanoma and pilot investigation of a novel gene expression signature as a predictor of response. J Clin Oncol. 2009;27:15s. [Google Scholar]
- Tensen CP, Flier J, van der Raaj-Helmer EM, Sampat-Sardjoepersad S, Van Der Schors RC, Leurs R, Scheper RJ, Boorsma DM, Willemze R. Human IP-9: A keratinocyte-derived high affinity CXC-chemokine ligand for the IP-10/Mig receptor (CXCR3) J Invest Dermatol. 1999;112:716–722. doi: 10.1046/j.1523-1747.1999.00581.x. [DOI] [PubMed] [Google Scholar]
- Thompson ED, Enriquez HL, Fu YX, Engelhard VH. Tumor masses support naive T cell infiltration, activation, and differentiation into effectors. J Exp Med. 2010;207:1791–1804. doi: 10.1084/jem.20092454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurner B, Haendle I, Roder C, Dieckmann D, Keikavoussi P, Jonuleit H, Bender A, Maczek C, Schreiner D, von den DP, Brocker EB, Steinman RM, Enk A, Kampgen E, Schuler G. Vaccination with MAGE-3A1 peptide-pulsed mature, monocyte-derived dendritic cells expands specific cytotoxic T cells and induces regression of some metastases in advanced stage IV melanoma. J Exp Med. 1999;190:1669–1678. doi: 10.1084/jem.190.11.1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB, Leming PD, Spigel DR, Antonia SJ, Horn L, Drake CG, Pardoll DM, Chen L, Sharfman WH, Anders RA, Taube JM, McMiller TL, Xu H, Korman AJ, Jure-Kunkel M, Agrawal S, McDonald D, Kollia GD, Gupta A, Wigginton JM, Sznol M. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran DQ, Ramsey H, Shevach EM. Induction of FOXP3 expression in naive human CD4+FOXP3 T cells by T-cell receptor stimulation is transforming growth factor-beta dependent but does not confer a regulatory phenotype. Blood. 2007;110:2983–2990. doi: 10.1182/blood-2007-06-094656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai SC, Lin SJ, Lin CJ, Chou YC, Lin JH, Yeh TH, Chen MR, Huang LM, Lu MY, Huang YC, Chen HY, Tsai CH. Autocrine CCL3 and CCL4 Induced by the Oncoprotein LMP1 Promote Epstein-Barr Virus-Triggered B Cell Proliferation. J Virol. 2013;87:9041–9052. doi: 10.1128/JVI.00541-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tumeh PC, Harview CL, Yearley JH, Shintaku IP, Taylor EJM, Robert L, Chmielowski B, Spasic M, Henry G, Ciobanu V, West AN, Carmona M, Kivork C, Seja E, Cherry G, Gutierrez AJ, Grogan TR, Mateus C, Tomasic G, Glaspy JA, Emerson RO, Robins H, Pierce RH, Elashoff DA, Robert C, Ribas A. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature. 2014;515:568–571. doi: 10.1038/nature13954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turk MJ, Guevara-Patiño JA, Rizzuto GA, Engelhorn ME, Houghton AN. Concomitant tumor immunity to a poorly immunogenic melanoma is prevented by regulatory T cells. J Exp Med. 2004;200:771–782. doi: 10.1084/jem.20041130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turnquist HR, Lin X, Ashour AE, Hollingsworth MA, Singh RK, Talmadge JE, Solheim JC. CCL21 induces extensive intratumoral immune cell infiltration and specific anti-tumor cellular immunity. Int J Oncol. 2007;30:631–639. [PubMed] [Google Scholar]
- Ulloa-Montoya F, Louahed J, Dizier B, Gruselle O, Spiessens B, Lehmann FF, Suciu S, Kruit WHJ, Eggermont AMM, Vansteenkiste J, Brichard VG. Predictive gene signature in MAGE-A3 antigen-specific cancer immunotherapy. J Clin Oncol. 2013;31:2388–2395. doi: 10.1200/JCO.2012.44.3762. [DOI] [PubMed] [Google Scholar]
- Van de Pavert SA, Mebius RE. New insights into the development of lymphoid tissues. Nat Rev Immunol. 2010;10:664–674. doi: 10.1038/nri2832. [DOI] [PubMed] [Google Scholar]
- Van de Pavert SA, Olivier BJ, Goverse G, Vondenhoff MF, Greuter M, Beke P, Kusser K, Höpken UE, Lipp M, Niederreither K, Blomhoff R, Sitnik K, Agace WW, Randall TD, de Jonge WJ, Mebius RE. Chemokine CXCL13 is essential for lymph node initiation and is induced by retinoic acid and neuronal stimulation. Nat Immunol. 2009;10:1193–1199. doi: 10.1038/ni.1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vesely M, Kershaw M, Schreiber R, Smyth M. Natural innate and adaptive immunity to cancer. Annu Rev Immunol. 2011;29:235–271. doi: 10.1146/annurev-immunol-031210-101324. [DOI] [PubMed] [Google Scholar]
- Victor CT-S, Rech AJ, Maity A, Rengan R, Pauken KE, Stelekati E, Benci JL, Xu B, Dada H, Odorizzi PM, Herati RS, Mansfield KD, Patsch D, Amaravadi RK, Schuchter LM, Ishwaran H, Mick R, Pryma DA, Xu X, Feldman MD, Gangadhar TC, Hahn SM, Wherry EJ, Vonderheide RH, Minn AJ. Radiation and dual checkpoint blockade activate non-redundant immune mechanisms in cancer. Nature advance online publication. 2015 doi: 10.1038/nature14292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Andrian UH, Mackay CR. T-cell function and migration. Two sides of the same coin. N Engl J Med. 2000;343:1020–1034. doi: 10.1056/NEJM200010053431407. [DOI] [PubMed] [Google Scholar]
- Vonderheide RH, Bayne LJ. Inflammatory networks and immune surveillance of pancreatic carcinoma. Curr Opin Immunol, Lymphocyte development/Tumour immunology/Cancer immunology: Clinical translation. 2013;25:200–205. doi: 10.1016/j.coi.2013.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang E, Miller LD, Ohnmacht GA, Mocellin S, Perez-Diez A, Petersen D, Zhao Y, Simon R, Powell JI, Asaki E, Alexander HR, Duray PH, Herlyn M, Restifo NP, Liu ET, Rosenberg SA, Marincola FM. Prospective molecular profiling of melanoma metastases suggests classifiers of immune responsiveness. Cancer Res. 2002;62:3581–3586. [PMC free article] [PubMed] [Google Scholar]
- Wang J, Ioan-Facsinay A, van der Voort EIH, Huizinga TWJ, Toes REM. Transient expression of FOXP3 in human activated nonregulatory CD4+ T cells. Eur J Immunol. 2007;37:129–138. doi: 10.1002/eji.200636435. [DOI] [PubMed] [Google Scholar]
- Wang L, Fan J, Thompson LF, Zhang Y, Shin T, Curiel TJ, Zhang B. CD73 has distinct roles in nonhematopoietic and hematopoietic cells to promote tumor growth in mice. J Clin Invest. 2011;121:2371–2382. doi: 10.1172/JCI45559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T, Niu G, Kortylewski M, Burdelya L, Shain K, Zhang S, Bhattacharya R, Gabrilovich D, Heller R, Coppola D, Dalton W, Jove R, Pardoll D, Yu H. Regulation of the innate and adaptive immune responses by Stat-3 signaling in tumor cells. Nat Med. 2004;10:48–54. doi: 10.1038/nm976. [DOI] [PubMed] [Google Scholar]
- Wang X, Michie SA, Xu B, Suzuki Y. Importance of IFN-gamma-mediated expression of endothelial VCAM-1 on recruitment of CD8+ T cells into the brain during chronic infection with Toxoplasma gondii. J Interferon Cytokine Res Off J Int Soc Interferon Cytokine Res. 2007;27:329–338. doi: 10.1089/jir.2006.0154. [DOI] [PubMed] [Google Scholar]
- Webster B, Ekland EH, Agle LM, Chyou S, Ruggieri R, Lu TT. Regulation of lymph node vascular growth by dendritic cells. J Exp Med. 2006;203:1903–1913. doi: 10.1084/jem.20052272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weishaupt C, Munoz KN, Buzney E, Kupper TS, Fuhlbrigge RC. T-cell distribution and adhesion receptor expression in metastatic melanoma. Clin Cancer Res. 2007;13:2549–2556. doi: 10.1158/1078-0432.CCR-06-2450. [DOI] [PubMed] [Google Scholar]
- Weiss GR, Grosh WW, Chianese-Bullock KA, Zhao Y, Liu H, Slingluff CL, Marincola FM, Wang E. Molecular insights on the peripheral and intratumoral effects of systemic high-dose rIL-2 (aldesleukin) administration for the treatment of metastatic melanoma. Clin Cancer Res. 2011;17:7440–7450. doi: 10.1158/1078-0432.CCR-11-1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weis SM, Cheresh DA. Tumor angiogenesis: molecular pathways and therapeutic targets. Nat Med. 2011;17:1359–1370. doi: 10.1038/nm.2537. [DOI] [PubMed] [Google Scholar]
- Weiss VL, Lee TH, Song H, Kouo TS, Black CM, Sgouros G, Jaffee EM, Armstrong TD. Trafficking of high avidity HER-2/neu-specific T cells into HER-2/neu-expressing tumors after depletion of effector/memory-like regulatory T cells. PLoS ONE. 2012:7. doi: 10.1371/journal.pone.0031962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West NR, Milne K, Truong PT, Macpherson N, Nelson BH, Watson PH. Tumor-infiltrating lymphocytes predict response to anthracycline-based chemotherapy in estrogen receptor-negative breast cancer. Breast Cancer Res. 2011;13:R126. doi: 10.1186/bcr3072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiemann B, Starnes CO. Coley’s toxins, tumor necrosis factor and cancer research: a historical perspective. Pharmacol Ther. 1994;64:529–564. doi: 10.1016/0163-7258(94)90023-x. [DOI] [PubMed] [Google Scholar]
- Wilke CM, Kryczek I, Wei S, Zhao E, Wu K, Wang G, Zou W. Th17 cells in cancer: help or hindrance? Carcinogenesis. 2011;32:643–649. doi: 10.1093/carcin/bgr019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winograd R, Byrne KT, Evans RA, Odorizzi PM, Meyer ARL, Bajor DL, Clendenin C, Stanger BZ, Furth EE, Wherry EJ, Vonderheide RH. Induction of T-cell immunity overcomes complete resistance to PD-1 and CTLA-4 blockade and improves survival in pancreatic carcinoma. Cancer Immunol Res. 2015 doi: 10.1158/2326-6066.CIR-14-0215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo SR, Fuertes MB, Corrales L, Spranger S, Furdyna MJ, Leung MYK, Duggan R, Wang Y, Barber GN, Fitzgerald KA, Alegre ML, Gajewski TF. STING-dependent cytosolic DNA sensing mediates innate immune recognition of immunogenic tumors. Immunity. 2014;41:830–842. doi: 10.1016/j.immuni.2014.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, DeBusk LM, Fukuda K, Fingleton B, Green-Jarvis B, Shyr Y, Matrisian LM, Carbone DP, Lin PC. Expansion of myeloid immune suppressor Gr+CD11b+ cells in tumor-bearing host directly promotes tumor angiogenesis. Cancer Cell. 2004;6:409–421. doi: 10.1016/j.ccr.2004.08.031. [DOI] [PubMed] [Google Scholar]
- Yang SC, Hillinger S, Riedl K, Zhang L, Zhu L, Huang M, Atianzar K, Kuo BY, Gardner B, Batra RK, Strieter RM, Dubinett SM, Sharma S. Intratumoral administration of dendritic cells overexpressing CCL21 generates systemic antitumor responses and confers tumor immunity. Clin Cancer Res. 2004;10:2891–2901. doi: 10.1158/1078-0432.CCR-03-0380. [DOI] [PubMed] [Google Scholar]
- Yao L, Setiadi H, Xia L, Laszik Z, Taylor FB, McEver RP. Divergent inducible expression of P-selectin and E-selectin in mice and primates. Blood. 1999;94:3820–3828. [PubMed] [Google Scholar]
- Yasuda K, Nirei T, Sunami E, Nagawa H, Kitayama J. Density of CD4(+) and CD8(+) T lymphocytes in biopsy samples can be a predictor of pathological response to chemoradiotherapy (CRT) for rectal cancer. Radiat Oncol. 2011;6:1–6. doi: 10.1186/1748-717X-6-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoong KF, McNab G, Hübscher SG, Adams DH. Vascular adhesion protein-1 and ICAM-1 support the adhesion of tumor-infiltrating lymphocytes to tumor endothelium in human hepatocellular carcinoma. J Immunol. 1998;160:3978–3988. [PubMed] [Google Scholar]
- Yu P, Lee Y, Liu W, Chin RK, Wang J, Wang Y, Schietinger A, Philip M, Schreiber H, Fu YX. Priming of naive T cells inside tumors leads to eradication of established tumors. Nat Immunol. 2004;5:141–149. doi: 10.1038/ni1029. [DOI] [PubMed] [Google Scholar]
- Yu P, Lee Y, Liu W, Krausz T, Chong A, Schreiber H, Fu YX. Intratumor depletion of CD4+ cells unmasks tumor immunogenicity leading to the rejection of late-stage tumors. J Exp Med. 2005;201:779–791. doi: 10.1084/jem.20041684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarbock A, Ley K, McEver RP, Hidalgo A. Leukocyte ligands for endothelial selectins: specialized glycoconjugates that mediate rolling and signaling under flow. Blood. 2011;118:6743–6751. doi: 10.1182/blood-2011-07-343566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zitvogel L, Kepp O, Kroemer G. Immune parameters affecting the efficacy of chemotherapeutic regimens. Nat Rev Clin Oncol. 2011;8:151–160. doi: 10.1038/nrclinonc.2010.223. [DOI] [PubMed] [Google Scholar]