Abstract
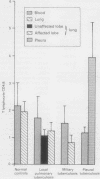
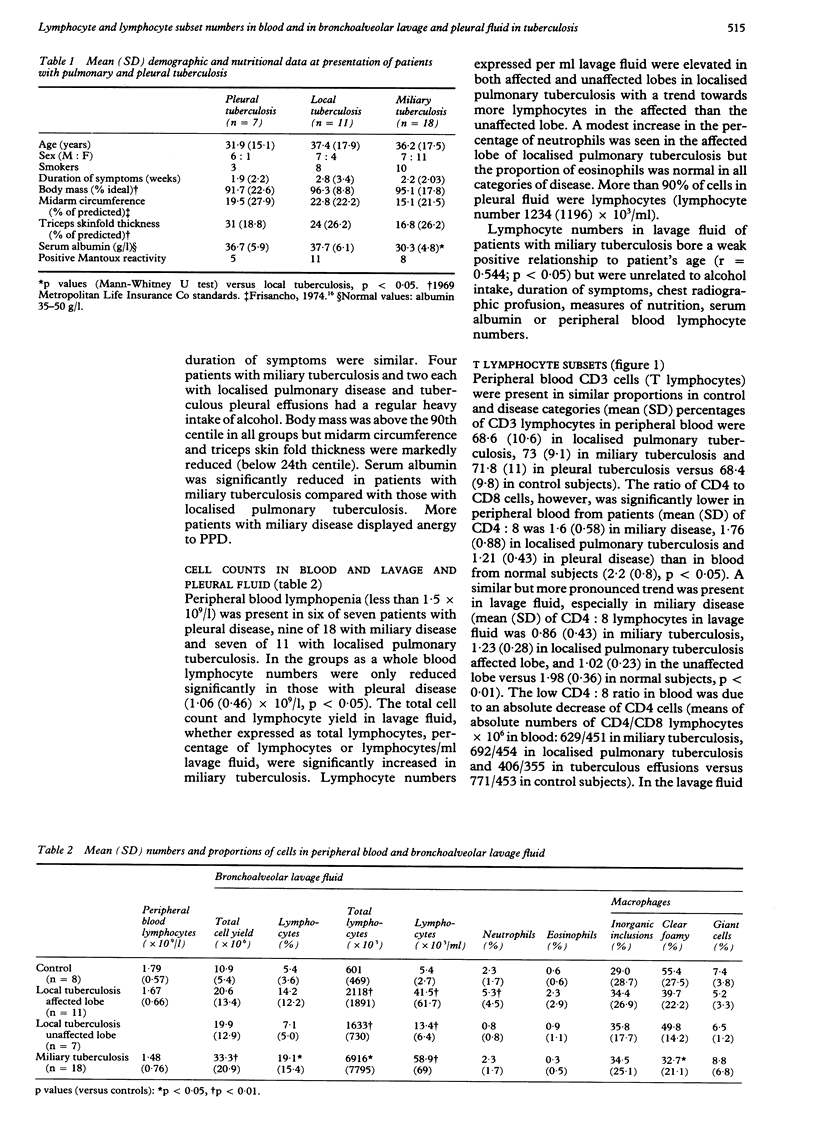
BACKGROUND: Lymphocytes have a central role in human defences against mycobacteria. A study was designed to assess the relation between lymphocyte responses and clinical pattern of disease, nutrition and recovery during treatment in patients with tuberculosis. METHODS: Lymphocyte numbers and subsets (on the basis of CD3, CD4, and CD8 monoclonal antibodies) were measured in peripheral blood and, where appropriate, bronchoalveolar lavage or pleural fluid of patients with different forms of pulmonary tuberculosis. Eleven had localised pulmonary tuberculosis, 18 miliary tuberculosis and seven a tuberculous pleural effusion. RESULTS: CD4 lymphocytes were found in greatly increased numbers in pleural fluid and were relatively depleted in the blood. Lymphocyte numbers in bronchoalveolar lavage fluid varied widely in localised pulmonary and miliary tuberculosis but were highest in lavage fluid from patients with miliary tuberculosis. This was due to an increase in CD8 lymphocytes, which were also increased in the blood. Lymphocyte numbers bore no relation to nutrition, symptom duration, or radiographic profusion scores. In miliary tuberculosis the time taken for the chest radiograph to clear (mean (SD) 17.6 (7.8) weeks) correlated with lymphocyte numbers in lavage fluid, especially CD8 cells (r = 0.74), but not with the patients' age or nutrition. After 8 weeks' treatment, total and CD4 lymphocyte numbers in lavage fluid showed a substantial increase. CONCLUSION: The association of CD8 cells with delayed recovery is compatible with suppression of the antimycobacterial action of macrophages. The switch to predominance of CD4 cells in lavage fluid during successful treatment supports the view that they may have a role in eliminating mycobacteria.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bach M. A., Pennec J. M., Flageul B., Wallach D., Cottenot F. Specificity study of PPD-reactive human T cell line and clones. Immunol Lett. 1985;9(2-3):81–85. doi: 10.1016/0165-2478(85)90015-x. [DOI] [PubMed] [Google Scholar]
- Beck J. S., Potts R. C., Kardjito T., Grange J. M. T4 lymphopenia in patients with active pulmonary tuberculosis. Clin Exp Immunol. 1985 Apr;60(1):49–54. [PMC free article] [PubMed] [Google Scholar]
- Bhatnagar R., Malaviya A. N., Narayanan S., Rajgopalan P., Kumar R., Bharadwaj O. P. Spectrum of immune response abnormalities in different clinical forms of tuberculosis. Am Rev Respir Dis. 1977 Feb;115(2):207–212. doi: 10.1164/arrd.1977.115.2.207. [DOI] [PubMed] [Google Scholar]
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- Chandra R. K. Lymphocyte subpopulations in human malnutrition: cytotoxic and suppressor cells. Pediatrics. 1977 Mar;59(3):423–427. [PubMed] [Google Scholar]
- Collins F. M. The immunology of tuberculosis. Am Rev Respir Dis. 1982 Mar;125(3 Pt 2):42–49. doi: 10.1164/arrd.1982.125.3P2.42. [DOI] [PubMed] [Google Scholar]
- Dhand R., De A., Ganguly N. K., Gupta N., Jaswal S., Malik S. K., Kohli K. K. Factors influencing the cellular response in bronchoalveolar lavage and peripheral blood of patients with pulmonary tuberculosis. Tubercle. 1988 Sep;69(3):161–173. doi: 10.1016/0041-3879(88)90017-7. [DOI] [PubMed] [Google Scholar]
- Edwards D., Kirkpatrick C. H. The immunology of mycobacterial diseases. Am Rev Respir Dis. 1986 Nov;134(5):1062–1071. doi: 10.1164/arrd.1986.134.5.1062. [DOI] [PubMed] [Google Scholar]
- Ellner J. J. Pleural fluid and peripheral blood lymphocyte function in tuberculosis. Ann Intern Med. 1978 Dec;89(6):932–933. doi: 10.7326/0003-4819-89-6-932. [DOI] [PubMed] [Google Scholar]
- Frisancho A. R. Triceps skin fold and upper arm muscle size norms for assessment of nutrition status. Am J Clin Nutr. 1974 Oct;27(10):1052–1058. doi: 10.1093/ajcn/27.8.1052. [DOI] [PubMed] [Google Scholar]
- Fujiwara H., Okuda Y., Fukukawa T., Tsuyuguchi I. In vitro tuberculin reactivity of lymphocytes from patients with tuberculous pleurisy. Infect Immun. 1982 Feb;35(2):402–409. doi: 10.1128/iai.35.2.402-409.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greaves M. F., Brown G. Purification of human T and B lymphocytes. J Immunol. 1974 Jan;112(1):420–423. [PubMed] [Google Scholar]
- Katz P., Goldstein R. A., Fauci A. S. Immunoregulation in infection caused by Mycobacterium tuberculosis: the presence of suppressor monocytes and the alteration of subpopulations of T lymphocytes. J Infect Dis. 1979 Jul;140(1):12–21. doi: 10.1093/infdis/140.1.12. [DOI] [PubMed] [Google Scholar]
- Kaufmann S. H. CD8+ T lymphocytes in intracellular microbial infections. Immunol Today. 1988 Jun;9(6):168–174. doi: 10.1016/0167-5699(88)91292-3. [DOI] [PubMed] [Google Scholar]
- Kaufmann S. H. In vitro analysis of the cellular mechanisms involved in immunity to tuberculosis. Rev Infect Dis. 1989 Mar-Apr;11 (Suppl 2):S448–S454. doi: 10.1093/clinids/11.supplement_2.s448. [DOI] [PubMed] [Google Scholar]
- Matthews R., Scoging A., Rees A. D. Mycobacterial antigen-specific human T-cell clones secreting macrophage activating factors. Immunology. 1985 Jan;54(1):17–23. [PMC free article] [PubMed] [Google Scholar]
- Mustafa A. S., Godal T. BCG-induced suppressor T cells optimal conditions for in vitro induction and mode of action. Clin Exp Immunol. 1985 Dec;62(3):474–481. [PMC free article] [PubMed] [Google Scholar]
- Onwubalili J. K., Scott G. M. Immune status in tuberculosis and response to treatment. Tubercle. 1988 Jun;69(2):81–94. doi: 10.1016/0041-3879(88)90070-0. [DOI] [PubMed] [Google Scholar]
- Ottenhoff T. H., Ab B. K., Van Embden J. D., Thole J. E., Kiessling R. The recombinant 65-kD heat shock protein of Mycobacterium bovis Bacillus Calmette-Guerin/M. tuberculosis is a target molecule for CD4+ cytotoxic T lymphocytes that lyse human monocytes. J Exp Med. 1988 Nov 1;168(5):1947–1952. doi: 10.1084/jem.168.5.1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rees A., Scoging A., Mehlert A., Young D. B., Ivanyi J. Specificity of proliferative response of human CD8 clones to mycobacterial antigens. Eur J Immunol. 1988 Dec;18(12):1881–1887. doi: 10.1002/eji.1830181203. [DOI] [PubMed] [Google Scholar]
- Rook G. A., Champion B. R., Steele J., Varey A. M., Stanford J. L. I-A restricted activation by T cell lines of anti-tuberculosis activity in murine macrophages. Clin Exp Immunol. 1985 Feb;59(2):414–420. [PMC free article] [PubMed] [Google Scholar]
- Rook G. A., Taverne J., Leveton C., Steele J. The role of gamma-interferon, vitamin D3 metabolites and tumour necrosis factor in the pathogenesis of tuberculosis. Immunology. 1987 Oct;62(2):229–234. [PMC free article] [PubMed] [Google Scholar]
- Rook G. A. The immunological consequences of antigen overload in experimental mycobacterial infections of mice. Clin Exp Immunol. 1975 Jan;19(1):167–177. [PMC free article] [PubMed] [Google Scholar]
- Rossi G. A., Balbi B., Manca F. Tuberculous pleural effusions. Evidence for selective presence of PPD-specific T-lymphocytes at site of inflammation in the early phase of the infection. Am Rev Respir Dis. 1987 Sep;136(3):575–579. doi: 10.1164/ajrccm/136.3.575. [DOI] [PubMed] [Google Scholar]
- Sharma S. K., Pande J. N., Verma K. Bronchoalveolar lavage (BAL) in miliary tuberculosis. Tubercle. 1988 Sep;69(3):175–178. doi: 10.1016/0041-3879(88)90018-9. [DOI] [PubMed] [Google Scholar]
- Shimokata K., Kawachi H., Kishimoto H., Maeda F., Ito Y. Local cellular immunity in tuberculous pleurisy. Am Rev Respir Dis. 1982 Nov;126(5):822–824. doi: 10.1164/arrd.1982.126.5.822. [DOI] [PubMed] [Google Scholar]
- Shimokata K., Kishimoto H., Takagi E., Tsunekawa H. Determination of the T-cell subset producing gamma-interferon in tuberculous pleural effusion. Microbiol Immunol. 1986;30(4):353–361. doi: 10.1111/j.1348-0421.1986.tb00952.x. [DOI] [PubMed] [Google Scholar]
- Shiratsuchi H., Tsuyuguchi I. Analysis of T cell subsets by monoclonal antibodies in patients with tuberculosis after in vitro stimulation with purified protein derivative of tuberculin. Clin Exp Immunol. 1984 Aug;57(2):271–278. [PMC free article] [PubMed] [Google Scholar]
- Simon M. R., Desai S. G., Jennings J., Engel D. T-cell differentiation antigens and antigenic lymphocyte reactivity in pleural effusions. Asian Pac J Allergy Immunol. 1986 Jun;4(1):19–27. [PubMed] [Google Scholar]
- Singhal M., Banavalikar J. N., Sharma S., Saha K. Peripheral blood T lymphocyte subpopulations in patients with tuberculosis and the effect of chemotherapy. Tubercle. 1989 Sep;70(3):171–178. doi: 10.1016/0041-3879(89)90047-0. [DOI] [PubMed] [Google Scholar]
- Tsuyuguchi I., Shiratsuchi H., Teraoka O., Hirano T. Increase in T cells bearing IgG Fc receptors in peripheral blood of patients with tuberculosis by in vitro stimulation with purified protein derivative. Am Rev Respir Dis. 1980 Jun;121(6):951–957. doi: 10.1164/arrd.1980.121.6.951. [DOI] [PubMed] [Google Scholar]
- Tweardy D. J., Schacter B. Z., Ellner J. J. Association of altered dynamics of monocyte surface expression of human leukocyte antigen DR with immunosuppression in tuberculosis. J Infect Dis. 1984 Jan;149(1):31–37. doi: 10.1093/infdis/149.1.31. [DOI] [PubMed] [Google Scholar]
- Wadee A. A., Sher R., Rabson A. R. Production of a suppressor factor by human adherent cells treated with mycobacteria. J Immunol. 1980 Sep;125(3):1380–1386. [PubMed] [Google Scholar]