Abstract
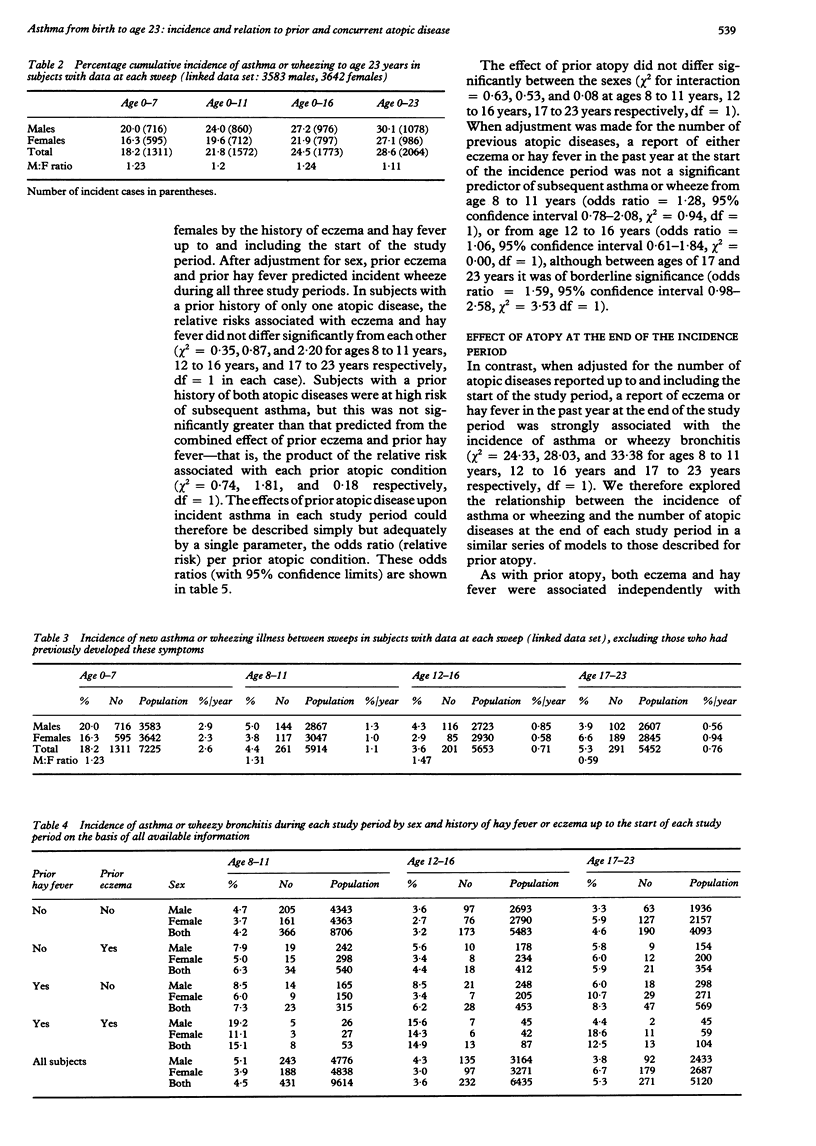
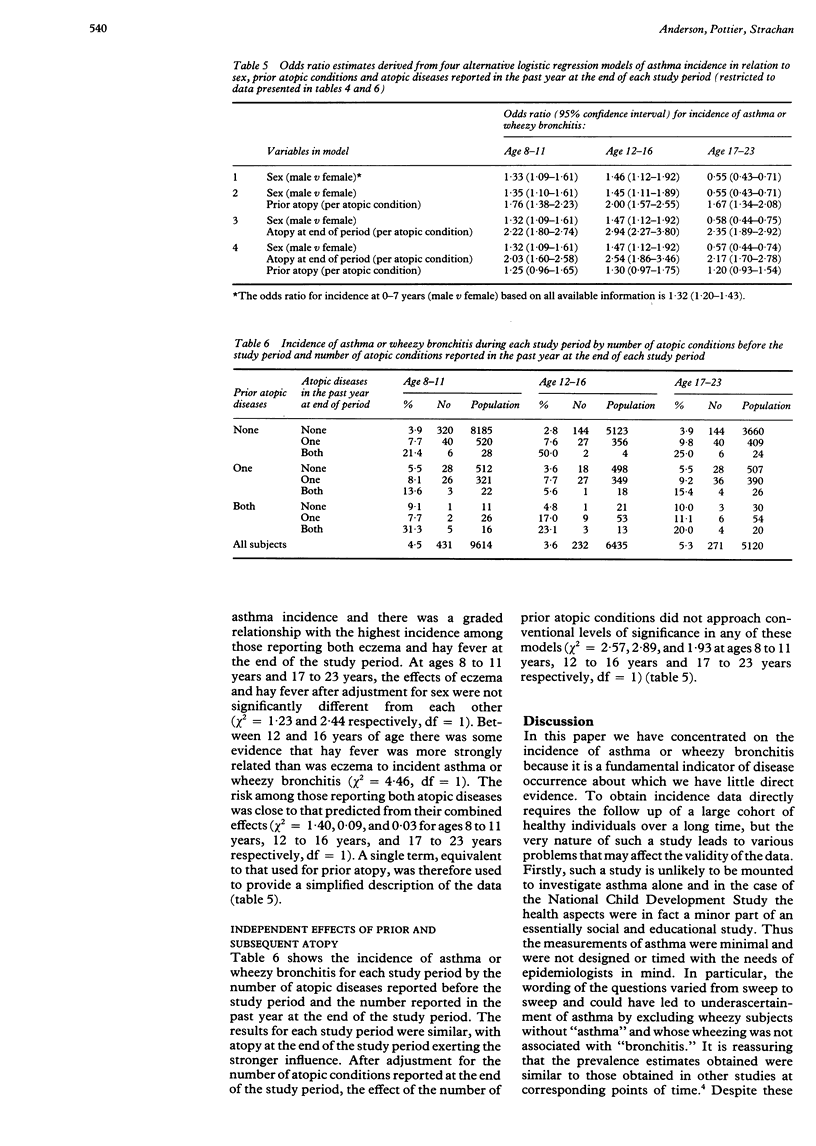
BACKGROUND: Few studies present prospective data on the incidence of asthma. Its associations with sex and with prior and concurrent hay fever and eczema were examined in a nationally representative sample followed from birth to 23 years of age (British 1958 birth cohort). METHODS: Reports of asthma or wheezy bronchitis, hay fever and eczema were obtained by interview of parents of children at ages 7, 11, and 16 years, and of cohort members at age 23 years. Linked data from all four interviews were available on 7225 subjects (43% of the original birth cohort). RESULTS: The cumulative incidence of asthma or wheezy bronchitis was 18.2%, 21.8%, 24.5%, and 28.6% by the ages of 7, 11, 16, and 23 years respectively. Over the four incidence periods examined (0 to 7 years, 8 to 11 years, 12 to 16 years, 17 to 23 years) the average annual incidence of new cases was 2.6%, 1.1%, 0.71%, and 0.76% respectively. The male:female incidence ratio rose from 1.23 in the 0 to 7 year period to 1.48 at 12 to 16 years but had reversed to 0.59 at 17 to 23 years. A prior report of hay fever or eczema each increased the subsequent incidence of asthma or wheezy bronchitis by a factor of 1.7 to 2.0 independently of sex. This effect of prior atopic illness, however, was largely explained by the strong independent association of incidence of asthma and wheezy bronchitis with atopic disease at the end of each incidence period (odds ratios 2.0 to 2.5 per atopic condition, p < 0.01). CONCLUSIONS: Gender differences in the incidence of asthma or wheezy bronchitis vary with age and are not explained by atopy. The incidence of asthma or wheezy bronchitis can be predicted from a clinical history of hay fever or eczema but is more strongly associated with the presence of atopic disease at the time of onset.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson H. R., Bland J. M., Patel S., Peckham C. The natural history of asthma in childhood. J Epidemiol Community Health. 1986 Jun;40(2):121–129. doi: 10.1136/jech.40.2.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson H. R. Is the prevalence of asthma changing? Arch Dis Child. 1989 Jan;64(1):172–175. doi: 10.1136/adc.64.1.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fry J. "Acute Wheezy Chests". Br Med J. 1961 Jan 28;1(5221):227–232. doi: 10.1136/bmj.1.5221.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giles G. G., Lickiss N., Gibson H. B., Shaw K. Respiratory symptoms in Tasmanian adolescents: a follow up of the 1961 birth cohort. Aust N Z J Med. 1984 Oct;14(5):631–637. doi: 10.1111/j.1445-5994.1984.tb05015.x. [DOI] [PubMed] [Google Scholar]
- Mathé G. Approaches to the immunological treatment of cancer in man. Br Med J. 1969 Oct 4;4(5674):7–10. doi: 10.1136/bmj.4.5674.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McWhorter W. P., Polis M. A., Kaslow R. A. Occurrence, predictors, and consequences of adult asthma in NHANESI and follow-up survey. Am Rev Respir Dis. 1989 Mar;139(3):721–724. doi: 10.1164/ajrccm/139.3.721. [DOI] [PubMed] [Google Scholar]
- Sherman C. B., Tosteson T. D., Tager I. B., Speizer F. E., Weiss S. T. Early childhood predictors of asthma. Am J Epidemiol. 1990 Jul;132(1):83–95. doi: 10.1093/oxfordjournals.aje.a115646. [DOI] [PubMed] [Google Scholar]
- Strachan D. P. The prevalence and natural history of wheezing in early childhood. J R Coll Gen Pract. 1985 Apr;35(273):182–184. [PMC free article] [PubMed] [Google Scholar]


