Abstract
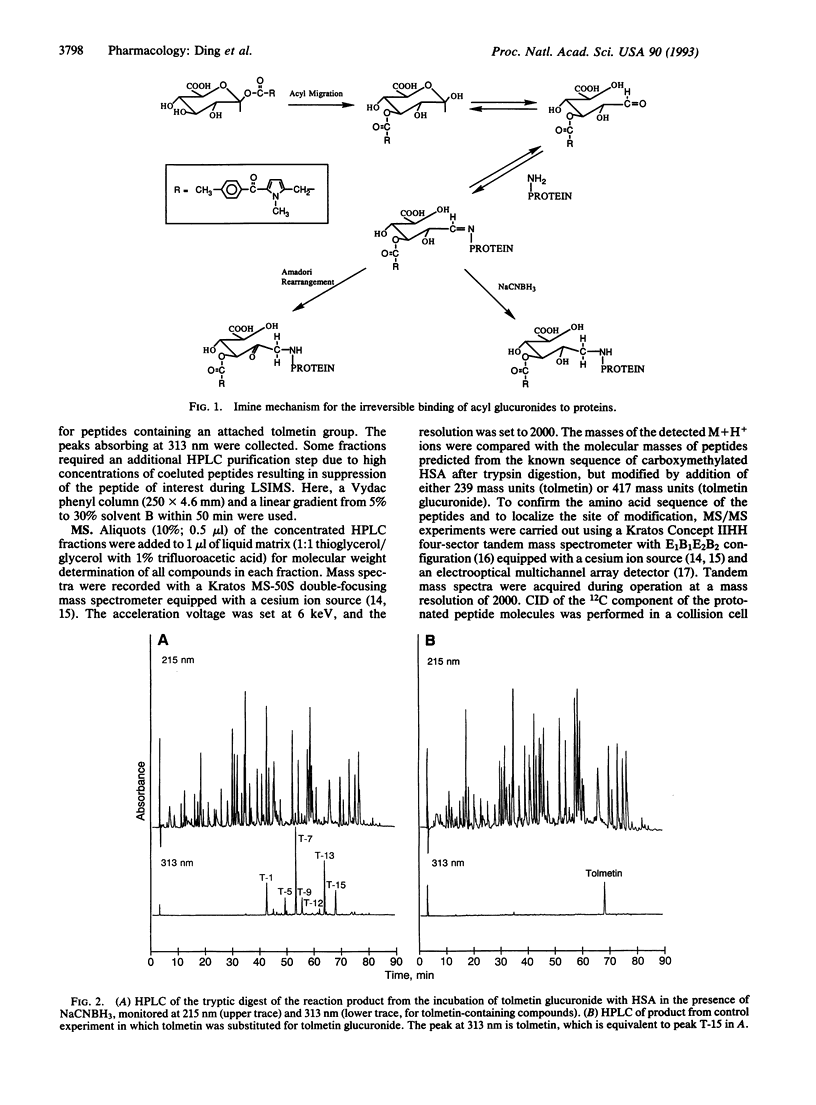
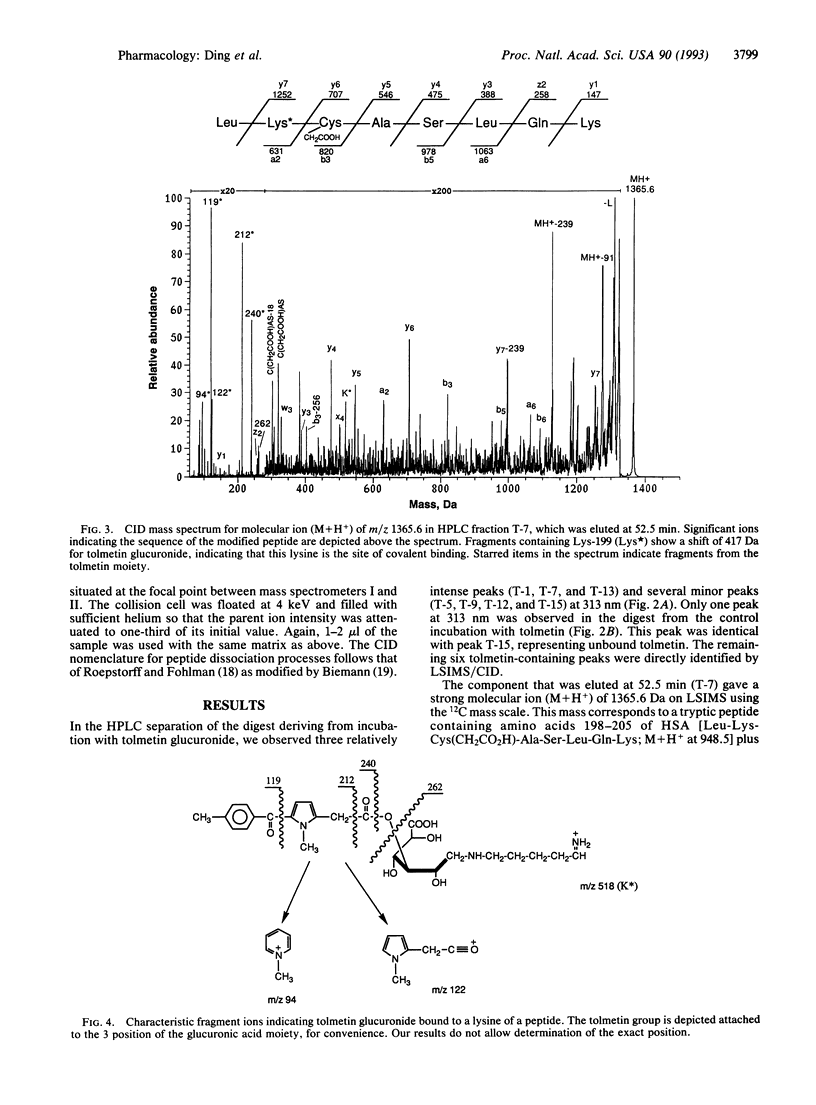
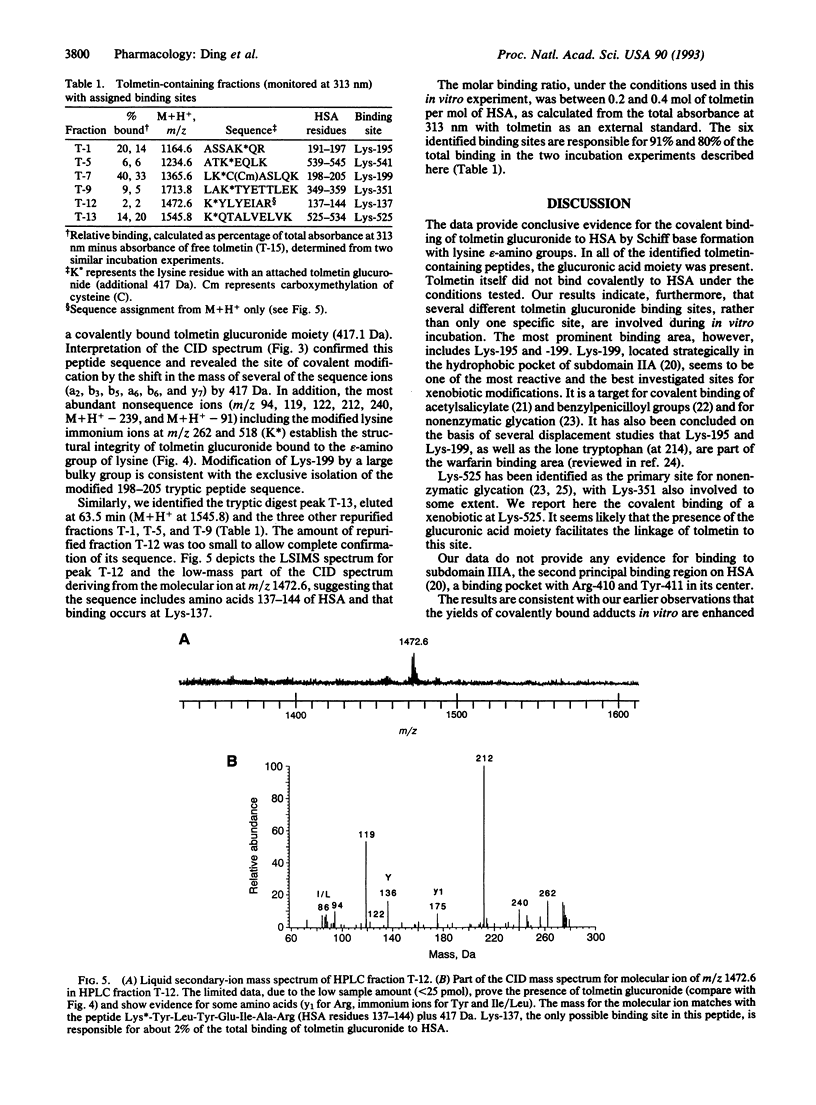
Acyl glucuronide metabolites of bilirubin and many drugs can react with serum albumin in vivo to form covalent adducts. Such adducts may be responsible for some toxic effects of carboxylic nonsteroidal antiinflammatory agents. The mechanism of formation of the adducts and their chemical structures are unknown. In this paper we describe the use of tandem mass spectrometry to locate binding sites and elucidate the binding mechanism involved in the formation of covalent adducts from tolmetin glucuronide and albumin in vitro. Human serum albumin and excess tolmetin glucuronide were coincubated in the presence of sodium cyanoborohydride to trap imine intermediates. The total protein product was reduced, carboxymethylated, and digested with trypsin. Six tolmetin-containing peptides (indicated by absorbance at 313 nm) were isolated by high-pressure liquid chromatography and analyzed by liquid secondary-ion mass spectrometry and collision-induced dissociation, using a four-sector tandem mass spectrometer. All six peptides contained tolmetin linked covalently via a glucuronic acid to protein lysine groups. Major attachment sites on the protein were Lys-195, -199, and -525; minor sites were identified as Lys-137, -351, and -541. Our results show unambiguously that the glucuronic acid moiety of acyl glucuronides can be retained within the structure when these reactive metabolites bind covalently to proteins, and they suggest that acyl migration followed by Schiff base (imine) formation is a credible mechanism for the generation of covalent adducts in vivo.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Biemann K. Contributions of mass spectrometry to peptide and protein structure. Biomed Environ Mass Spectrom. 1988 Oct;16(1-12):99–111. doi: 10.1002/bms.1200160119. [DOI] [PubMed] [Google Scholar]
- Bretza J. A., Novey H. S. Anaphylactoid reactions to tolmetin after interrupted dosage. West J Med. 1985 Jul;143(1):55–59. [PMC free article] [PubMed] [Google Scholar]
- Buechter D. D., Medzihradszky K. F., Burlingame A. L., Kenyon G. L. The active site of creatine kinase. Affinity labeling of cysteine 282 with N-(2,3-epoxypropyl)-N-amidinoglycine. J Biol Chem. 1992 Feb 5;267(4):2173–2178. [PubMed] [Google Scholar]
- Compernolle F., Van Hees G. P., Blanckaert N., Heirwegh K. P. Glucuronic acid conjugates of bilirubin-IXalpha in normal bile compared with post-obstructive bile. Transformation of the 1-O-acylglucuronide into 2-, 3-, and 4-O-acylglucuronides. Biochem J. 1978 Apr 1;171(1):185–201. doi: 10.1042/bj1710185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faed E. M. Properties of acyl glucuronides: implications for studies of the pharmacokinetics and metabolism of acidic drugs. Drug Metab Rev. 1984;15(5-6):1213–1249. doi: 10.3109/03602538409033562. [DOI] [PubMed] [Google Scholar]
- Falick A. M., Wang G. H., Walls F. C. Ion source for liquid matrix secondary ionization mass spectrometry. Anal Chem. 1986 Jun;58(7):1308–1311. doi: 10.1021/ac00298a009. [DOI] [PubMed] [Google Scholar]
- Garlick R. L., Mazer J. S. The principal site of nonenzymatic glycosylation of human serum albumin in vivo. J Biol Chem. 1983 May 25;258(10):6142–6146. [PubMed] [Google Scholar]
- HODGE J. E. The Amadori rearrangement. Adv Carbohydr Chem. 1955;10:169–205. doi: 10.1016/s0096-5332(08)60392-6. [DOI] [PubMed] [Google Scholar]
- He X. M., Carter D. C. Atomic structure and chemistry of human serum albumin. Nature. 1992 Jul 16;358(6383):209–215. doi: 10.1038/358209a0. [DOI] [PubMed] [Google Scholar]
- Hyneck M. L., Smith P. C., Munafo A., McDonagh A. F., Benet L. Z. Disposition and irreversible plasma protein binding of tolmetin in humans. Clin Pharmacol Ther. 1988 Jul;44(1):107–114. doi: 10.1038/clpt.1988.120. [DOI] [PubMed] [Google Scholar]
- Iberg N., Flückiger R. Nonenzymatic glycosylation of albumin in vivo. Identification of multiple glycosylated sites. J Biol Chem. 1986 Oct 15;261(29):13542–13545. [PubMed] [Google Scholar]
- Kaur S., Hollander D., Haas R., Burlingame A. L. Characterization of structural xenobiotic modifications in proteins by high sensitivity tandem mass spectrometry. Human hemoglobin treated in vitro with styrene 7,8-oxide. J Biol Chem. 1989 Oct 15;264(29):16981–16984. [PubMed] [Google Scholar]
- Kragh-Hansen U. Structure and ligand binding properties of human serum albumin. Dan Med Bull. 1990 Feb;37(1):57–84. [PubMed] [Google Scholar]
- McDonagh A. F., Palma L. A., Lauff J. J., Wu T. W. Origin of mammalian biliprotein and rearrangement of bilirubin glucuronides in vivo in the rat. J Clin Invest. 1984 Sep;74(3):763–770. doi: 10.1172/JCI111492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munafo A., McDonagh A. F., Smith P. C., Benet L. Z. Irreversible binding of tolmetin glucuronic acid esters to albumin in vitro. Pharm Res. 1990 Jan;7(1):21–27. doi: 10.1023/a:1015823206607. [DOI] [PubMed] [Google Scholar]
- Roepstorff P., Fohlman J. Proposal for a common nomenclature for sequence ions in mass spectra of peptides. Biomed Mass Spectrom. 1984 Nov;11(11):601–601. doi: 10.1002/bms.1200111109. [DOI] [PubMed] [Google Scholar]
- Ruelius H. W., Kirkman S. K., Young E. M., Janssen F. W. Reactions of oxaprozin-1-O-acyl glucuronide in solutions of human plasma and albumin. Adv Exp Med Biol. 1986;197:431–441. doi: 10.1007/978-1-4684-5134-4_42. [DOI] [PubMed] [Google Scholar]
- Smith P. C., Benet L. Z., McDonagh A. F. Covalent binding of zomepirac glucuronide to proteins: evidence for a Schiff base mechanism. Drug Metab Dispos. 1990 Sep-Oct;18(5):639–644. [PubMed] [Google Scholar]
- Smith P. C., McDonagh A. F., Benet L. Z. Irreversible binding of zomepirac to plasma protein in vitro and in vivo. J Clin Invest. 1986 Mar;77(3):934–939. doi: 10.1172/JCI112392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spahn-Langguth H., Benet L. Z. Acyl glucuronides revisited: is the glucuronidation process a toxification as well as a detoxification mechanism? Drug Metab Rev. 1992;24(1):5–47. doi: 10.3109/03602539208996289. [DOI] [PubMed] [Google Scholar]
- Van Breemen R. B., Fenselau C., Mogilevsky W., Odell G. B. Reaction of bilirubin glucuronides with serum albumin. J Chromatogr. 1986 Dec 19;383(2):387–392. doi: 10.1016/s0378-4347(00)83484-2. [DOI] [PubMed] [Google Scholar]
- Walker J. E. Lysine residue 199 of human serum albumin is modified by acetylsalicyclic acid. FEBS Lett. 1976 Jul 15;66(2):173–175. doi: 10.1016/0014-5793(76)80496-6. [DOI] [PubMed] [Google Scholar]
- Wells D. S., Janssen F. W., Ruelius H. W. Interactions between oxaprozin glucuronide and human serum albumin. Xenobiotica. 1987 Dec;17(12):1437–1449. doi: 10.3109/00498258709044004. [DOI] [PubMed] [Google Scholar]
- Yem A. W., Epps D. E., Mathews W. R., Guido D. M., Richard K. A., Staite N. D., Deibel M. R., Jr Site-specific chemical modification of interleukin-1 beta by acrylodan at cysteine 8 and lysine 103. J Biol Chem. 1992 Feb 15;267(5):3122–3128. [PubMed] [Google Scholar]
- Yvon M., Wal J. M. Identification of lysine residue 199 of human serum albumin as a binding site for benzylpenicilloyl groups. FEBS Lett. 1988 Nov 7;239(2):237–240. doi: 10.1016/0014-5793(88)80924-4. [DOI] [PubMed] [Google Scholar]
- van Breemen R. B., Fenselau C. Acylation of albumin by 1-O-acyl glucuronides. Drug Metab Dispos. 1985 May-Jun;13(3):318–320. [PubMed] [Google Scholar]



