Abstract
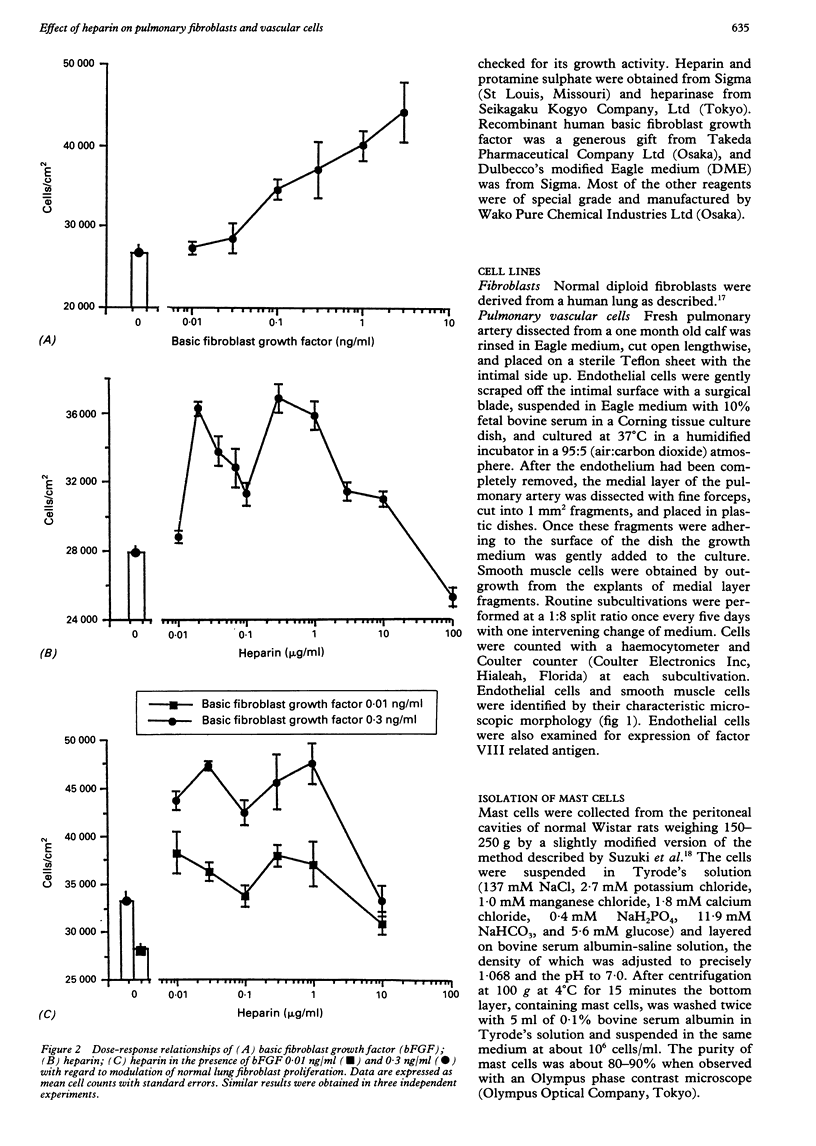
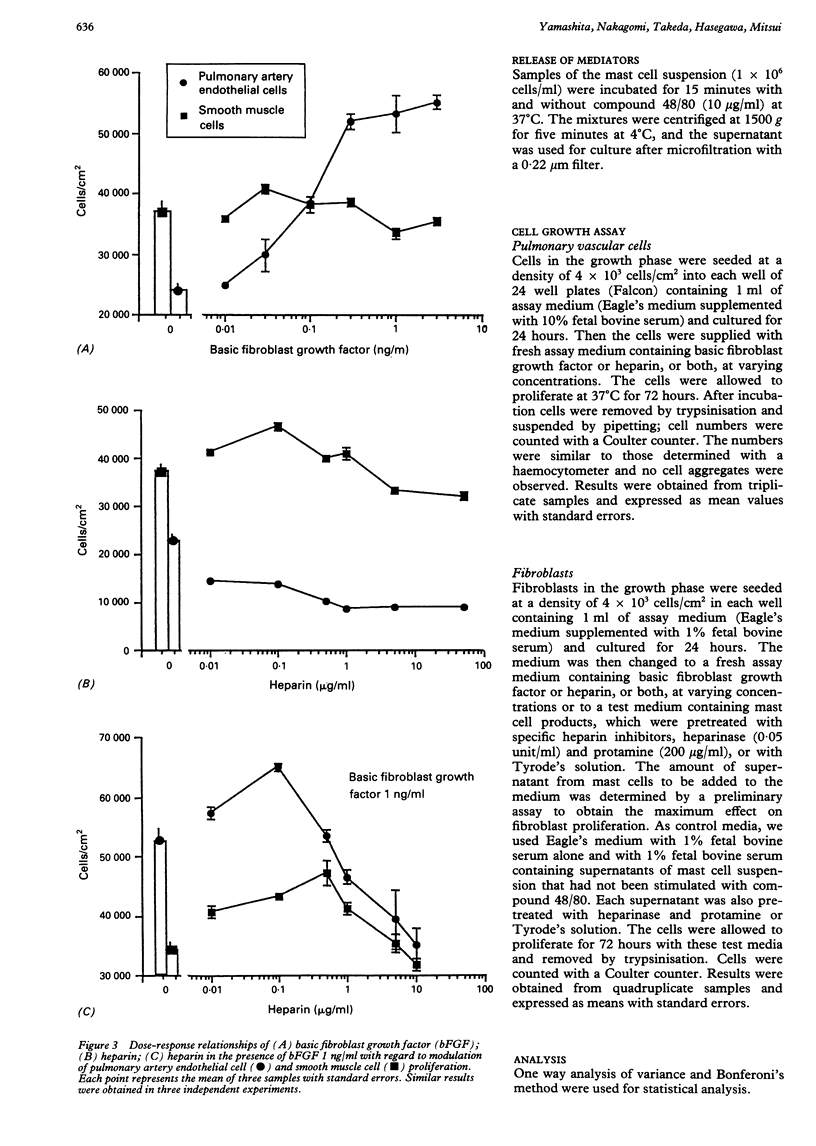
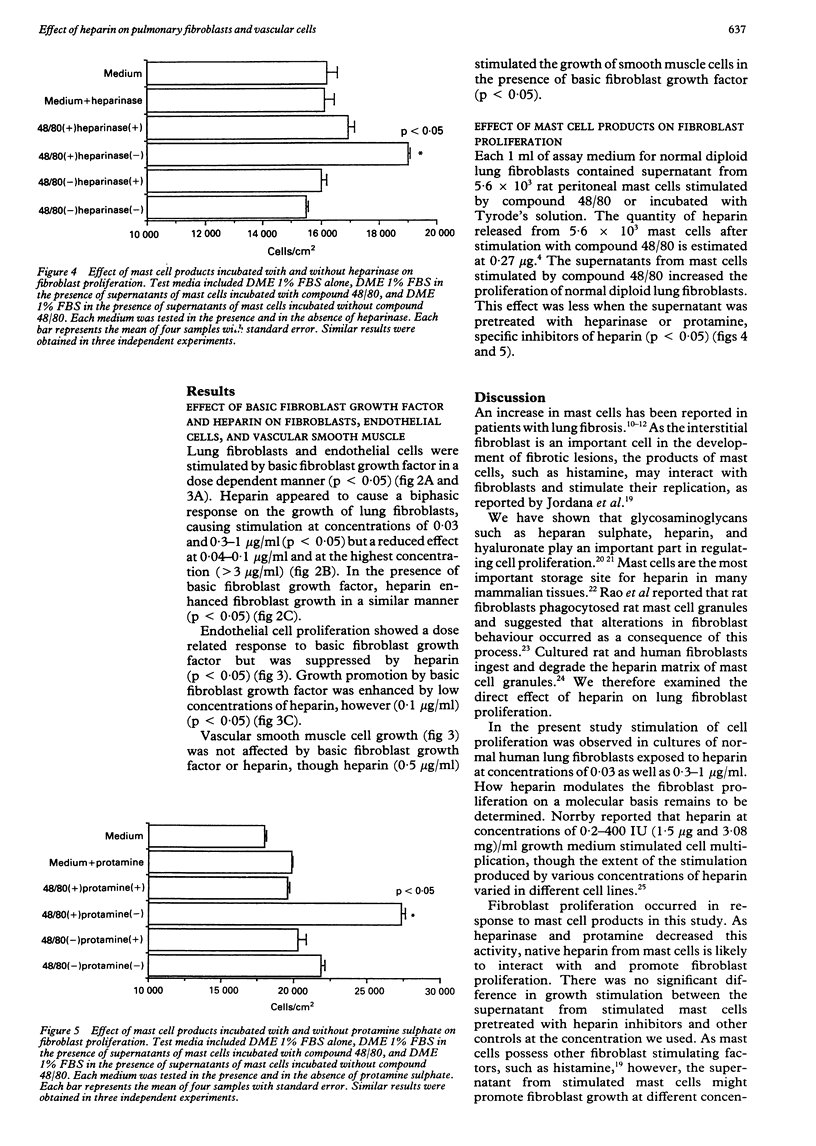
BACKGROUND: There is a large increase in mast cell numbers in fibrotic lung tissue, suggesting that mast cells may play a part in the pathogenesis of pulmonary fibrosis. Glycosaminoglycans, such as heparan sulphate, that are structurally related to heparin (a mast cell product) are part of the extracellular matrix and known to regulate cell growth. Basic fibroblast growth factor is a heparin binding growth factor produced by endothelial cells. METHODS: A study was carried out to examine the effect of heparin, basic fibroblast growth factor, and mast cell products on the proliferation of normal human lung fibroblasts and the effect of adding heparin on the proliferation of lung fibroblasts and pulmonary vascular cells incubated with basic fibroblast growth factor. RESULTS: Heparin at low concentration (0.03, 0.3-1.0 micrograms/ml) stimulated the proliferation of normal human lung fibroblasts in culture whereas a higher concentration (100 micrograms/ml) had an inhibitory effect. Mast cell products also stimulated the proliferation of fibroblasts, and the effect was decreased by pretreatment with heparinase or protamine. Heparin enhanced the growth of both fibroblasts and pulmonary vascular cells induced by low concentrations of basic fibroblast growth factor. CONCLUSIONS: Mast cells in fibrotic lung tissue may regulate fibroblast proliferation by releasing heparin. These results suggest that endothelial cells may interact with mast cells and modulate fibroblast growth by release of basic fibroblast growth factor.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARCHER G. T. Release of heparin from the mast cells of the rat. Nature. 1961 Jul 1;191:90–90. doi: 10.1038/191090a0. [DOI] [PubMed] [Google Scholar]
- Abraham J. A., Mergia A., Whang J. L., Tumolo A., Friedman J., Hjerrild K. A., Gospodarowicz D., Fiddes J. C. Nucleotide sequence of a bovine clone encoding the angiogenic protein, basic fibroblast growth factor. Science. 1986 Aug 1;233(4763):545–548. doi: 10.1126/science.2425435. [DOI] [PubMed] [Google Scholar]
- Aizawa S., Mitsui Y. Cell surface and clonal proliferative property of aging human diploid fibroblasts. Exp Cell Res. 1982 Jun;139(2):416–419. doi: 10.1016/0014-4827(82)90269-5. [DOI] [PubMed] [Google Scholar]
- Atkins F. M., Metcalfe D. D. Degradation of the heparin matrix of mast cell granules by cultured fibroblasts. J Immunol. 1983 Sep;131(3):1420–1425. [PubMed] [Google Scholar]
- Azizkhan R. G., Azizkhan J. C., Zetter B. R., Folkman J. Mast cell heparin stimulates migration of capillary endothelial cells in vitro. J Exp Med. 1980 Oct 1;152(4):931–944. doi: 10.1084/jem.152.4.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell P. M., LeRoy E. C. Pathogenesis of systemic sclerosis: a vascular hypothesis. Semin Arthritis Rheum. 1975 May;4(4):351–368. doi: 10.1016/0049-0172(75)90017-7. [DOI] [PubMed] [Google Scholar]
- Castellot J. J., Jr, Choay J., Lormeau J. C., Petitou M., Sache E., Karnovsky M. J. Structural determinants of the capacity of heparin to inhibit the proliferation of vascular smooth muscle cells. II. Evidence for a pentasaccharide sequence that contains a 3-O-sulfate group. J Cell Biol. 1986 May;102(5):1979–1984. doi: 10.1083/jcb.102.5.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claman H. N. On scleroderma. Mast cells, endothelial cells, and fibroblasts. JAMA. 1989 Sep 1;262(9):1206–1209. doi: 10.1001/jama.262.9.1206. [DOI] [PubMed] [Google Scholar]
- Fillion G. M., Slorach S. A., Uvnäs B. The release of histamine, heparin and granule protein from rat mast cells treated with compound 48-80 in vitro. Acta Physiol Scand. 1970 Apr;78(4):547–560. doi: 10.1111/j.1748-1716.1970.tb04691.x. [DOI] [PubMed] [Google Scholar]
- Gospodarowicz D., Cheng J. Heparin protects basic and acidic FGF from inactivation. J Cell Physiol. 1986 Sep;128(3):475–484. doi: 10.1002/jcp.1041280317. [DOI] [PubMed] [Google Scholar]
- Gospodarowicz D. Localisation of a fibroblast growth factor and its effect alone and with hydrocortisone on 3T3 cell growth. Nature. 1974 May 10;249(453):123–127. doi: 10.1038/249123a0. [DOI] [PubMed] [Google Scholar]
- Gospodarowicz D., Massoglia S., Cheng J., Fujii D. K. Effect of fibroblast growth factor and lipoproteins on the proliferation of endothelial cells derived from bovine adrenal cortex, brain cortex, and corpus luteum capillaries. J Cell Physiol. 1986 Apr;127(1):121–136. doi: 10.1002/jcp.1041270116. [DOI] [PubMed] [Google Scholar]
- Goto T., Befus D., Low R., Bienenstock J. Mast cell heterogeneity and hyperplasia in bleomycin-induced pulmonary fibrosis of rats. Am Rev Respir Dis. 1984 Nov;130(5):797–802. doi: 10.1164/arrd.1984.130.5.797. [DOI] [PubMed] [Google Scholar]
- Guyton J. R., Rosenberg R. D., Clowes A. W., Karnovsky M. J. Inhibition of rat arterial smooth muscle cell proliferation by heparin. In vivo studies with anticoagulant and nonanticoagulant heparin. Circ Res. 1980 May;46(5):625–634. doi: 10.1161/01.res.46.5.625. [DOI] [PubMed] [Google Scholar]
- Hunninghake G. W., Fauci A. S. Pulmonary involvement in the collagen vascular diseases. Am Rev Respir Dis. 1979 Mar;119(3):471–503. doi: 10.1164/arrd.1979.119.3.471. [DOI] [PubMed] [Google Scholar]
- Imamura T., Mitsui Y. Heparan sulfate and heparin as a potentiator or a suppressor of growth of normal and transformed vascular endothelial cells. Exp Cell Res. 1987 Sep;172(1):92–100. doi: 10.1016/0014-4827(87)90096-6. [DOI] [PubMed] [Google Scholar]
- Imamura T., Tokita Y., Mitsui Y. Contact with basement membrane heparan sulfate enhances the growth of transformed vascular endothelial cells, but suppresses normal cells. Cell Struct Funct. 1991 Jun;16(3):225–230. doi: 10.1247/csf.16.225. [DOI] [PubMed] [Google Scholar]
- Jordana M., Befus A. D., Newhouse M. T., Bienenstock J., Gauldie J. Effect of histamine on proliferation of normal human adult lung fibroblasts. Thorax. 1988 Jul;43(7):552–558. doi: 10.1136/thx.43.7.552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahaleh M. B., Sherer G. K., LeRoy E. C. Endothelial injury in scleroderma. J Exp Med. 1979 Jun 1;149(6):1326–1335. doi: 10.1084/jem.149.6.1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawanami O., Ferrans V. J., Fulmer J. D., Crystal R. G. Ultrastructure of pulmonary mast cells in patients with fibrotic lung disorders. Lab Invest. 1979 Jun;40(6):717–734. [PubMed] [Google Scholar]
- Kurokawa T., Sasada R., Iwane M., Igarashi K. Cloning and expression of cDNA encoding human basic fibroblast growth factor. FEBS Lett. 1987 Mar 9;213(1):189–194. doi: 10.1016/0014-5793(87)81489-8. [DOI] [PubMed] [Google Scholar]
- Lam L. H., Silbert J. E., Rosenberg R. D. The separation of active and inactive forms of heparin. Biochem Biophys Res Commun. 1976 Mar 22;69(2):570–577. doi: 10.1016/0006-291x(76)90558-1. [DOI] [PubMed] [Google Scholar]
- Lykke A. W., Schonell M. E., Stewart B. W. Atypical mast cell degranulation and focal hydropic degeneration of venular endothelium in diffuse fibrosing alveolitis. Experientia. 1979 Nov 15;35(11):1492–1493. doi: 10.1007/BF01962804. [DOI] [PubMed] [Google Scholar]
- Matuoka K., Namba M., Mitsui Y. Hyaluronate synthetase inhibition by normal and transformed human fibroblasts during growth reduction. J Cell Biol. 1987 Apr;104(4):1105–1115. doi: 10.1083/jcb.104.4.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moscatelli D., Presta M., Joseph-Silverstein J., Rifkin D. B. Both normal and tumor cells produce basic fibroblast growth factor. J Cell Physiol. 1986 Nov;129(2):273–276. doi: 10.1002/jcp.1041290220. [DOI] [PubMed] [Google Scholar]
- Norrby K. Effect of heparin on cell population kinetics, mitosis and topoinhibition. Virchows Arch B Cell Pathol. 1971;9(4):292–310. doi: 10.1007/BF02894053. [DOI] [PubMed] [Google Scholar]
- Norton W. L., Nardo J. M. Vascular disease in progressive systemic sclerosis (scleroderma). Ann Intern Med. 1970 Aug;73(2):317–324. doi: 10.7326/0003-4819-73-2-317. [DOI] [PubMed] [Google Scholar]
- Rifkin D. B., Moscatelli D. Recent developments in the cell biology of basic fibroblast growth factor. J Cell Biol. 1989 Jul;109(1):1–6. doi: 10.1083/jcb.109.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosengart T. K., Johnson W. V., Friesel R., Clark R., Maciag T. Heparin protects heparin-binding growth factor-I from proteolytic inactivation in vitro. Biochem Biophys Res Commun. 1988 Apr 15;152(1):432–440. doi: 10.1016/s0006-291x(88)80732-0. [DOI] [PubMed] [Google Scholar]
- Saksela O., Moscatelli D., Sommer A., Rifkin D. B. Endothelial cell-derived heparan sulfate binds basic fibroblast growth factor and protects it from proteolytic degradation. J Cell Biol. 1988 Aug;107(2):743–751. doi: 10.1083/jcb.107.2.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber A. B., Kenney J., Kowalski W. J., Friesel R., Mehlman T., Maciag T. Interaction of endothelial cell growth factor with heparin: characterization by receptor and antibody recognition. Proc Natl Acad Sci U S A. 1985 Sep;82(18):6138–6142. doi: 10.1073/pnas.82.18.6138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slorach S. A. Histamine and heparin release from isolated rat mast cells exposed to compound 48-80. Acta Physiol Scand. 1971 May;82(1):91–97. doi: 10.1111/j.1748-1716.1971.tb04945.x. [DOI] [PubMed] [Google Scholar]
- Subba Rao P. V., Friedman M. M., Atkins F. M., Metcalfe D. D. Phagocytosis of mast cell granules by cultured fibroblasts. J Immunol. 1983 Jan;130(1):341–349. [PubMed] [Google Scholar]
- Suzuki T., Ohishi K., Kida J., Uchida M. Influence of pH on the inhibitory effects of local anesthetics on histamine release induced from rat mast cells by concanavalin A and compound 48/80. Eur J Pharmacol. 1984 Mar 2;98(3-4):347–355. doi: 10.1016/0014-2999(84)90283-8. [DOI] [PubMed] [Google Scholar]
- Thornton S. C., Mueller S. N., Levine E. M. Human endothelial cells: use of heparin in cloning and long-term serial cultivation. Science. 1983 Nov 11;222(4624):623–625. doi: 10.1126/science.6635659. [DOI] [PubMed] [Google Scholar]
- Vlodavsky I., Folkman J., Sullivan R., Fridman R., Ishai-Michaeli R., Sasse J., Klagsbrun M. Endothelial cell-derived basic fibroblast growth factor: synthesis and deposition into subendothelial extracellular matrix. Proc Natl Acad Sci U S A. 1987 Apr;84(8):2292–2296. doi: 10.1073/pnas.84.8.2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe S., Watanabe K., Oishi T., Aiba M., Kageyama K. Mast cells in the rat alveolar septa undergoing fibrosis after ionizing irradiation. Ultrastructural and histochemical studies. Lab Invest. 1974 Nov;31(5):555–567. [PubMed] [Google Scholar]
- Weiler J. M., Yurt R. W., Fearon D. T., Austen K. F. Modulation of the formation of the amplification convertase of complement, C3b, Bb, by native and commercial heparin. J Exp Med. 1978 Feb 1;147(2):409–421. doi: 10.1084/jem.147.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]



