Abstract
Background and objective:
The use of sedative medications may be effective in the success and facilitation of the intussusception non-surgical treatment. Therefore, the purpose of this study was to examine the effect of midazolam on decreasing the duration of intussusception hydrostatic reduction in children.
Materials and Methods:
In a double-blind clinical trial, 32 children were diagnosed with ileocolic intussusception based on sonographic findings, were studied and randomly divided into two groups. After obtaining written informed consent from the parents, 5 minutes before reduction, an intravenous Midazolam at the concentration of 1.0 mg/kg (up to 3 mg) was infused, and then barium reduction was performed under fluoroscopy guideline. In the control group, sterile water was injected as placebo and the remaining reduction steps were performed compared with the experiment group.
Results:
Of 16 patients that received Midazolam, 15 patients demonstrated successful reduction; and of 16 patients that received distilled water, only 11 patients showed successful reduction (P=0.07). The mean duration of a successful reduction in the Midazolam group and placebo was 34.8±11.35 and 32.73±19.2 min, respectively (P=0.733).
Conclusions:
The use of Midazolam as a benzodiazepine with known sedative and muscle relaxant effects can increase the success rate of enema reduction in intussusception.
Keywords: Intussusception, Midazolam, Hydrostatic reduction, Children
1. INTRODUCTION
Intussusception is the most common cause of childhood intestinal obstruction and its standard treatment, in most cases, is the reduction by two non-surgical methods using air or liquid pressure (1-3). If nonsurgical treatment fails or does not have any indication, open surgery may be necessary.
The use of an intravenous spasmolysis with glucagon in facilitating the reduction in intussusception has been investigated in several studies and different results have been obtained, so that in some studies, it had a significant effect on easier intussusception reduction, but it had no effects on others (4, 5). Also, the reduction in the operating room under general anesthesia has been investigated in several studies, from which, different results, ranging from no effect to increased success rate have been obtained.
It seems that the use of lower doses of sedative medications is effective in the success rate and facilitating the non-surgical treatment of intussusception. However, the frequent use of sedative medications in all radiology departments, including this center, is not common. This may increase the rate of unnecessary surgical treatment of intussusception (6, 7).
Although among the sedative medications, oral chloral hydrate and diazepam have been used for non-invasive radiological procedures, including the intussusception hydrostatic reduction, and different results have been obtained, no study concerning the use of Midazolam as a sedative in the process of reduction has been performed so far. Midazolam is not only a medication with sedative and anti-anxiety effects that will increase child’s cooperation, but also is a muscle relaxant which can have effects similar to the spasmolytic agents, such as glucagon. On the other hand, it can improve amnesia and restore child’s memory from stressful procedures such as Intussusception reduction. This effect makes it superior to diazepam and proves the necessity of such a study.
2. MATERIALS AND METHODS
In a double-blind clinical trial, 6-months to 4-year children referred to Amirkola Hospital who had a definite diagnosis of ileocolic intussusception based on sonographic findings, were examined. Exclusion criteria included the presence of an underlying pathologic factor (Lead point) for Intussusception, passage of more than 48 hours of the onset of symptoms, the presence of signs of peritonitis or bowel perforation, dehydration, lethargy, history of a known gastrointestinal disease such as celiac disease or cystic fibrosis, history of a chronic hypoxic pulmonary disease, cyanotic heart disease, history of previous intussusception and the history of allergy to benzodiazepines. After obtaining written informed consent from the parents (Clinical Trial Registry Number: IRCT138904264395N1), 32 eligible patients were randomly assigned (every second one) to the study and control groups.
Five minutes before reduction, 5 mg per mil of intravenous Midazolam, at the concentration of 0.1mg, which was determined based on body weight and was prepared by Elixir Company (up to 3 mg) was infused to the patients of the study group under supervision of an anesthesiologist. Then, reduction with barium under fluoroscopy guidelines was applied to the patients. Before administration of intravenous Midazolam, the vital signs including heart rate, level of consciousness, respiration and blood pressure of patients were monitored and their arterial oxygen saturation were measured and recorded using a pulse oximeter. During the reduction operation by pulse oximeter, cardiac rhythm and arterial oxygen levels of patients were measured and duration of the reduction, the success of reduction, and patient’s cooperation during the process were recorded in a checklist for each patient. Reduction refers to a condition, in which the entry of barium into ileal loops is viewed in the graph. Failure refers to the stoppage of barium column, at least for 15 minutes, in a part of the colon. After performing the required assessments and reaching a stable condition, patients were transferred to the recovery room, and evaluated again by sonography (ultrasound) to check if the symptoms of intussusception are remained.
For an additional precaution, emergency resuscitation equipment, including oxygen ambu bag and intubation devices were provided to the experiment group that received Midazolam; and flumazenil was used as an antagonist of Midazolam as needed. After successful reduction, the patients in the experiment group were observed for one hour in the recovery room. During this period, vital signs were monitored and pulse oximetry was performed as well. After full recovery from anesthesia, and ensuring that there were no complications, they were discharged from the unit of radiology and were transferred to the relevant unit. In case the operation failed (and, in some cases, after observing the patient for about 12 hours), they would be transferred to the operating room with the consent of the surgeon and anesthesiologist.
In the control group, after taking IV line, ampule of sterile water was injected as placebo and the remaining reduction steps were performed in contrast to the experiment group. For data analysis, Statistical Package of the Social Sciences (SPSS), version 16, was used. For comparison of quantitative and quantitative variables, T-test and chi-square tests were performed, respectively. The Fisher exact test was used when necessary. A P value of less than 0.05 was considered significant.
3. RESULTS
Generally, 32 patients were studied, of which 17 were male and 15 were female. The average age of patients was 12.32±21.16 months (The youngest patient aged 8 months and the oldest one aged 48 months). 16 patients received Midazolam before reduction and 16 patients received placebo in the course of reduction. Background characteristics of patients are listed in Table 1.
Table 1.
Background characteristics of patients in both groups
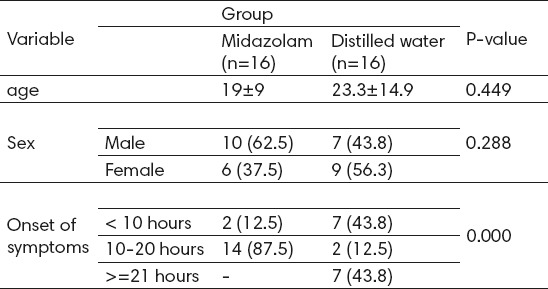
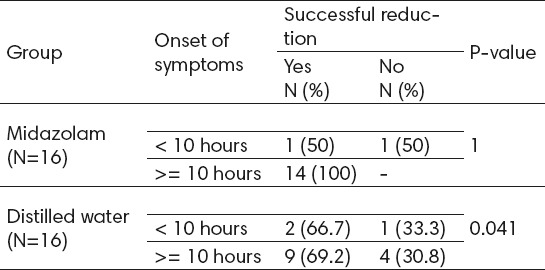
Of 16 patients receiving Midazolam before reduction in intussusception, 15 (93.8%) patients demonstrated successful reduction. Moreover, among 16 patients receiving distilled water before reduction in intussusception, 11 (86.8%) patients showed successful reduction. The success rate in both groups showed a significant relationship with onset of symptoms (P=1 for less than 10 hours, and P=0.041 for more than 10 hours) (table 2).
Table 2.
The success rate in both groups according to onset of symptoms
As far as the duration of the procedure in the successful reduction was concerned, the mean duration in the group receiving Midazolam, before reduction in intussusception was 11.35±34.8 minutes and in the group receiving distilled water prior to intussusception reduction was 19.2± 32.73 minutes. This difference in duration was not statistically significant (P=0.733).
The mean cardiac rhythm during the reduction process in patients receiving Midazolam was 6.68±123.713 minutes and in the patients receiving distilled water was 23.34±130.73 minutes (P=0.432). There was the possibility of performing the tapping procedure only on 12 patients (75%) receiving Midazolam, while among patients who received distilled water it was possible to do this procedure only on 5 (31.3%) patients. This difference was statistically significant (P<0.001).
In addition, there was the possibility of manipulation only on 12 (75%) patients receiving Midazolam, while among patients who received distilled water, it was possible to perform manipulation only on 3 (18.8%) patients. This difference was statistically significant (P<0.001).
4. DISCUSSION
Intussusception has been always one of the most common causes of acute intestinal obstruction in children. If less invasive treatments such as reduction with enema are performed at the right time, cases leading to surgery and specific complications associated with it can be reduced (8). In the present study, the role of Midazolam as a sedative medication for more effective reduction in intussusception in children was examined. Also, all patients received Midazolam before intussusception reduction through barium rectal, except for one patient, who had successful reduction. However, in the group that did not receive Midazolam, 5 patients had not successful reduction. Although in our study, the rate of reduction between the two groups was not statistically significant, given the small sample size in our study, the lack of statistical significance can be attributed to this reduction. Previous studies show different rates of the success of enema reduction. In a study in Tehran, Ghavami Adel et al. reported that the success rate of air enema in intussusception reduction is 71.4% (9). In their study, Daneman et al., also reported the success rate of 85 to 90 percent for non-surgical reductions (10). A similar success rate was also reported in other studies (11,12). In a study by Franken et al., conducted to investigate the role of intravenous glucagon in hydrostatic reduction, the success rate of 53 % was achieved in both groups (4). Given the success rate of about 85 percent in most previous studies, our study showed that the success rate of reduction can be increased through the use of Midazolam before intravenous reduction. However, in the study conducted by Franken et al., it was shown that glucagon did not influence the success rate significantly, but Midazolam which was first examined in our study, was associated with the success rate increased to 93.8% in the group receiving Midazolam.
In the present study, the average reduction time in the group receiving Midazolam was 34.8±11.35 minutes and in the group receiving distilled water was 32.37±19.2 minute. Therefore, the reduction times did not significantly differ. In the study conducted by Franken et al., the use of intravenous glucagon as a relaxant for smooth muscles of the intestinal wall had no effect on the reduction time (4). In the present study, the mean duration of the procedure in the group receiving distilled water was slightly less than the group receiving Midazolam which perhaps reflects the carelessness in recording the exact duration of procedure. However, it seems that the use of Midazolam did not influence the duration of the procedure. Of course, ignoring other variables involved in the study, such as the skills of all the care team responsible for the reduction and characteristics of the patients can also be partly add to the total procedure time. Further studies with adequate sample sizes for the study of the effect of sedative medications on duration of intussusception reduction are recommended.
Patient’s cooperation in the intussusception reduction process is very important to get the best outcome. Due to their young age and fear of therapeutic interventions and the nature of the disease that causes restlessness, children do not have good cooperation during reduction. Therefore, the use of sedative medications can be effective in increasing children’s involvement in the treatment process. In the present study, the opportunity to carry out two maneuvers of tapping and manipulation was used to examine the effectiveness of sedative medications. Tapping and manipulation were possible to be performed on 75% of patients who received Midazolam before reduction, whereas tapping and manipulation were possible to be performed only on 31.33% and 18.8% of the cases in the group receiving distilled water.
5. CONCLUSION
The results of this study showed that the use of Midazolam significantly increases the cooperation and sedation levels in patients compared with the control group. It also showed that the use of Midazolam as a benzodiazepine with the known sedative and muscle relaxant effects can increase the success rate of enema reduction in intussusception in children. However, the use of Midazolam had no effect on the duration reduction. Further studies with greater sample sizes are recommended to evaluate factors affecting the duration of reduction.
Footnotes
CONFLICT OF INTEREST: NONE DECLARED
REFERENCES
- 1.Meier DE, Coln CD, Rescorla FJ, OlaOlorun A, Tarpley JL. Intussusception in children: international perspective. World J Surg. 1996;20(8):1035–1039. doi: 10.1007/s002689900158. [DOI] [PubMed] [Google Scholar]
- 2.Mandeville K, Chien M, Willyerd FA, Mandell G, Hostetler MA, Bulloch B. Intussusception: clinical presentations and imaging characteristics. Pediatr Emerg Care. 2012;28(9):842–844. doi: 10.1097/PEC.0b013e318267a75e. [DOI] [PubMed] [Google Scholar]
- 3.Kaiser AD, Applegate KE, Ladd AP. Current success in the treatment of intussusception in children. Surgery. 2007;142(4):469–75. doi: 10.1016/j.surg.2007.07.015. discussion 75-77. [DOI] [PubMed] [Google Scholar]
- 4.Franken EA, Jr, Smith WL, Chernish SM, Campbell JB, Fletcher BD, Goldman HS. The use of glucagon in hydrostatic reduction of intussusception: a double-blind study of 30 patients. Radiology. 1983;146(3):687–689. doi: 10.1148/radiology.146.3.6828682. [DOI] [PubMed] [Google Scholar]
- 5.Lanocita M, Castiglioni G. Use of glucagon in the reduction of intussusception; presentationof one case (author's transl) Radiol Med. 1980;66(7-8):513–516. [PubMed] [Google Scholar]
- 6.Brenn BR, Katz A. General anaesthesia may improve the success rate of hydrostatic reductions of intussusception. Paediatr Anaesth. 1997;7(1):77–81. doi: 10.1046/j.1460-9592.1997.d01-28.x. [DOI] [PubMed] [Google Scholar]
- 7.Collins DL, Pinckney LE, Miller KE, Bastian JF, Katzman DO, Canty TG, Sr, et al. Hydrostatic reduction of ileocolic intussusception: a second attempt in the operating room with general anesthesia. J Pediatr. 1989;115(2):204–207. doi: 10.1016/s0022-3476(89)80066-6. [DOI] [PubMed] [Google Scholar]
- 8.Meyer JS. The current radiologic management of intussusception: a survey and review. Pediatr Radiol. 1992;22(5):323–325. doi: 10.1007/BF02016244. [DOI] [PubMed] [Google Scholar]
- 9.Ghavami Adel M, Al-e-Hossein M. Reduction of intussusception by air enema in children. Iranian Journal of Pediatrics. 2005;15(4):341–346. [Google Scholar]
- 10.Daneman A, Navarro O. Intussusception. Part 2: An update on the evolution of management. Pediatr Radiol. 2004;34(2):97–108. doi: 10.1007/s00247-003-1082-7. [DOI] [PubMed] [Google Scholar]
- 11.Buettcher M, Baer G, Bonhoeffer J, Schaad UB, Heininger U. Three-year surveillance of intussusception in children in Switzerland. Pediatrics. 2007;120(3):473–480. doi: 10.1542/peds.2007-0035. [DOI] [PubMed] [Google Scholar]
- 12.van den Ende ED, Allema JH, Hazebroek FW, Breslau PJ. Success with hydrostatic reduction of intussusception in relation to duration of symptoms. Arch Dis Child. 2005;90(10):1071–1072. doi: 10.1136/adc.2004.066332. [DOI] [PMC free article] [PubMed] [Google Scholar]


