Abstract
Aim:
To determine the possible relation between intraocular pressure (IOP), central corneal thickness (CCT) and corneal resistance (CR) in kerotoconic eyes before, 3,6 and 12 months after collagen crosslinking procedure (CXL) with aim to find out does the thicker cornea means already more resistance cornea followed with higher IOP.
Methods:
Thirty eyes (30 patients) with central keratoconus (KC)were evaluated in retrospective cross sectional study. The corneal biomechanical parameters were taken with Wave Light Allegro Oculyzer produced by Alcon before the CXL, 3, 6 and 12 months after the procedure. IOP were checked by Goldmann applanation tonometry (GAT) before, 3, 6 and 12 months after CXL.
Results:
The value of IOP before the CXL was 12,0 mmHg (10,62-15,25 mmHg), 3 months later 13,5 mmHg (11,0-16,0 mmHg), 6 months 14,0 mmHg (11,0-16,0 mmHg) and 12 months later 15,0 mmHg (10,37-17,25 mmHg) and was statistically significant higher (p=0,015) comparing to the value of IOP 3 months after the CXL, IOP 12 months after CXL procedure was statistically significant higher comparing to preoperative values (p=0,010). There were no statistically significant difference between the values 3 and 6 months after CXL. The CCT before the CXL procedure was 449 (433-505,75 microns), 3 months after CXL was 420 (383-473microns, p < 0,005), 6 months later 437 (401,25-480,25, p=0,001), 12 months after CXL 437 (401-503 microns, p=0,001). However there is statistically significant difference in CCT 12 months after CXL 437 (401-503microns p=0,032) and the value of CCT 3 months later the procedure (p=0,004) and the CCT 12 months after CXL and the value of CXL 6 months after CXL (p=0,036). The value of CCT did not show any statistically significant difference 3 and 6 months postoperatively.
Conclusion:
After riboflavin-UVA CXL in eyes with KC there was significant decrease in central corneal thickness 3 and 6 months after the procedure and the thickness is almost the same 12 months later. However IOP is low before CXL, raising up 3 and 6 months after CXL but significant increase is seen 12 months later. It means the regular measurement of IOP could be the serious and confident indicator of increasing of corneal resistance which is the main goal of CXL treatment.
Keywords: keratoconus, collagen crosslinking, intraocular pressure, central corneal thickness, corneal resistance
1. INTRODUCTION
Keratoconus is bilateral noninflammatory, progressive corneal disease characterized by corneal thinning and bulging. The cornea develops a conical shape leads to irregular astigmatism and to deterioration of visual acuity (1,2). Until CXL procedure is developed and is the part of the present clinical practice, the treatment of KC offered to patient the spectacles, rigid contact lenses, intrastromal corneal ring and as final option the lamellar or penetrating keratoplasty (3). CXL may be an effective option in stabilizing KC. Further and permanent follow up studies will be necessary to assess the persistence of CXL (4). IOP measurements is affected by deferring CCT (5-8). After UVA CXL in eyes with KC, there was a significant increase in IOP measured by Goldman applanation tonometry (GAT) that was probably caused by an increase in corneal rigidity (9). Riboflavin/UVA CXL appears to be a safe and efficacious procedure in halting the progression of KC and reducing the corneal curvature, spherical equivalent refraction and refractive cylinder in eyes with corneal instability due to KC (10).
2. AIM OD THE STUDY
The aim of current study was to assess the relation between the values of IOP, CCT, before CXL, 3, 6 and 12 months later with aim to find out the direct impact of thinning the cornea, the changes of the IOL and their influence to higher corneal resistance and rigidity which is the task of CXL procedure. At the same time to conclude could be the value of IOP also the device to control the corneal rigidity because it changes is not due to developing the true increase than changing in corneal stroma after the CXL procedure. Try to see the critic time when the changes should be considered as real and permanent one.
3. METHODS
This retrospective study evaluated 30 ectatic eyes (30 patients) before and 3, 6 and 12 months after CXL for progressive KC at Eye Polyclinics „Dr. Sefić”. The patients were examined with slit lamp, fundoscopy, measurements of uncorrected visual acuity, best spectacle visual acuity, pachymetry, tomography and IOP measurements by GAT. We used Snellen chart for determination of visual acuity and WaveLight Allegro Oculyzer tomography to determine the changes of CCT.
Exclusion criteria for the study were: CCT <380 microns (intraoperative corneal swelling with hypotonic solution), marginal pelucid degeneration (peripheral ectasia), pregnancy, severe dry syndrome and any inflammatory or scaring process on the ocular surface before CXL procedure.
Statistical analyses: Results were analyzed using standard statistical methods using SPSS computer program for statistical analysis (SPSS Statistical Package for Social Sciences) version 13.0. Results are presented as median and interquartile range (25-75 percentile). To test the significance of the difference in deviation from the normal distribution, Shapiro–Wilk test was used. The results are analyzed by appropriate non-parametric tests (Wilcoxon and Friedman Tests). Associations between continuous variables were tested with Spearman’s rank correlation analysis. Values of p<0.05 are considered as statistically significant.
4. RESULTS
From total number of 30 eyes, 8 eyes were female patients (26,7%), while the other 22 eyes (73,7%) were male patients. The average age of the patients was 26.8 ± 1.67 years. The average age of female patients was 22.32±1.98 years and the average age of male patients was 27.30±1.11 years.
The value of IOP was the lowest before CXL and was 12 (10.62 to 15.25 mmHg) and was significantly higher than the value of IOP 3 months after CXL 13.5 (11-16 mm Hg; p=0.015) and significantly higher than the value of IOP 6 months after CXL 14 (11-16 mm Hg; p=0.010). Value IOP year after CXL was 15 (10.37 to 17.25 mmHg) and was significantly different compared to the value of IOP before CXL and 3 and 6 months after CXL. We did not observe significant differences in the values of IOP 3 months and 6 months after the CXL (Figure 1).
Figure 1.
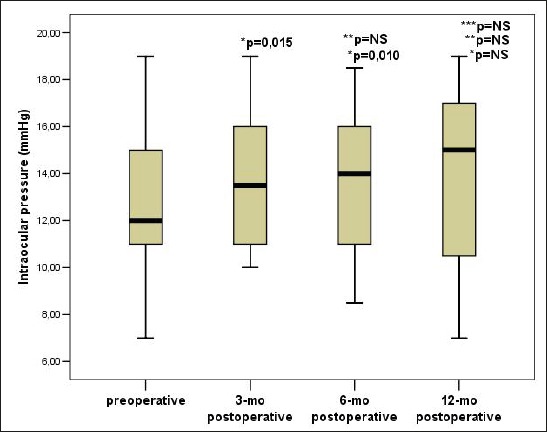
Trend IOP changes before and after CXL. Data are presented as median and interquartile range (25-75 percentile), p–the probability; NS–not significant, IOP – intraocular pressure, * Compared to the value of intraocular pressure before CXL, ** Compared to the value of intraocular pressure 3 months after CXL, *** Compared to the value of intraocular pressure 6 months after CXL
The value of CCT was the highest before the CXL and it was 449 (433 to 505.75 microns) and was significantly lower than the value of CCT 3 months after the CXL 420 (383-473 microns, p<0.005), significantly lower than the value of CCT 6 months after the CXL 437 (401.25 to 480.25 microns; p=0.001), as well as compared to the value of CCT 12 months after the CXL 437 (401-503 microns; p=0.032). There was a statistically significant difference in the value of CCT 12 months after CXL and CCT place value 3 months after CXL (p=0.004), as well as between the value of CCT 12 months after CXL and CCT place value 6 months after the CXL (p=0.036). There wasn’t significant differences in the values of CCT 3 months after CXL and 6 months after the CXL (Figure 2).
Figure 2.
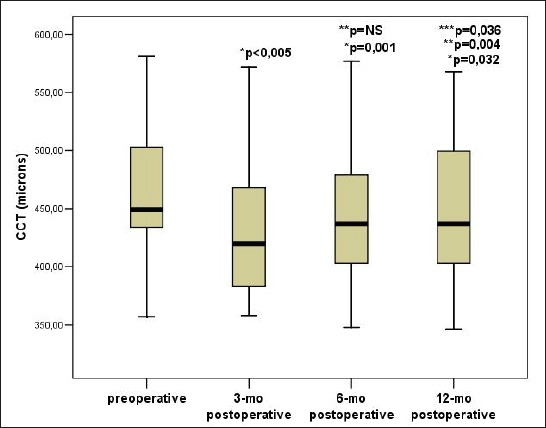
Trend CCT changes before and after CXL. Data are presented as median and interquartile range (25-75 percentile), p–the probability; NS–not significant, CCT–central corneal thickness, * Compared to the value of intraocular pressure before CXL, ** Compared to the value of intraocular pressure 3 months after CXL, *** Compared to the value of intraocular pressure 6 months after CXL
There was a statistically significant positive correlation between CCT and IOP before CXL (Rho=0.867; p<0.005) (Figure 3). There was a statistically significant positive correlation between CCT and IOP 3 months after CXL (Rho=0.688; p<0.005)(Figure 4). There was a statistically significant positive correlation between CCT and IOP 6 months after the CXL (Rho=0.471; p=0.010)(Figure 5). There was a statistically significant positive correlation between CCT and IOP 12 months after the CXL (Rho=0.396; p=0.045) (Figure 6).
Figure 3.
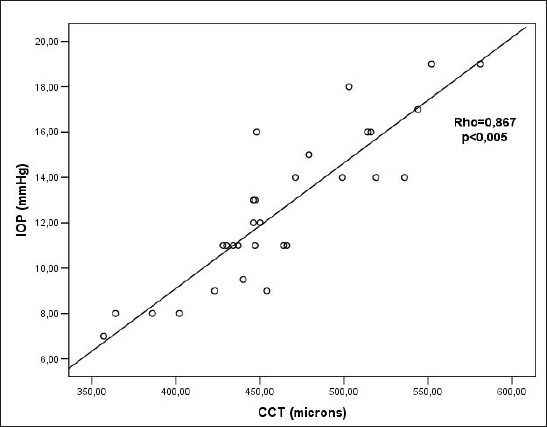
Relationship between CCT and IOP values before CXL. Rho– Spearman correlation coefficient, p – the probability, IOP – intraocular pressure, CCT–central corneal thickness
Figure 4.
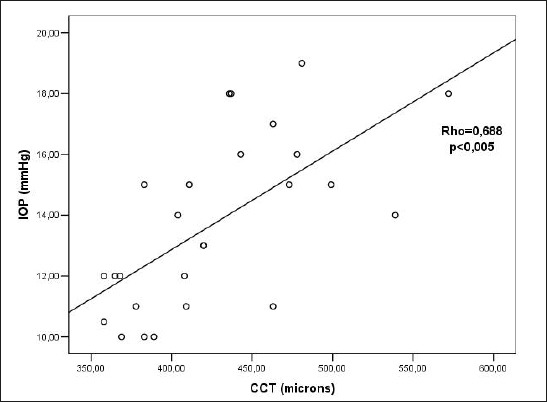
Relationship between CCT and IOP values 3 months after CXL. Rho– Spearman correlation coefficient p – the probability, IOP – intraocular pressure, CCT–central corneal thickness
Figure 5.
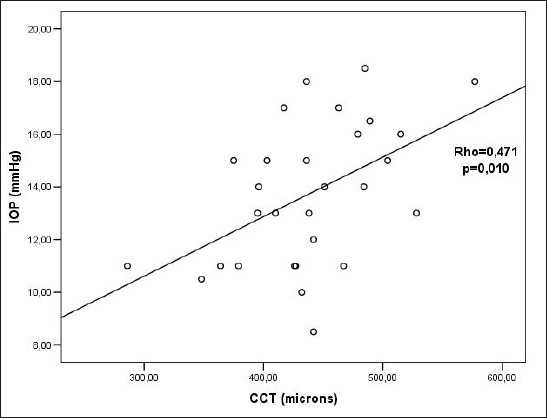
Relationship between CCT and IOP values 6 months after CXL. Rho– Spearman correlation coefficient, p – the probability, IOP – intraocular pressure, CCT–central corneal thickness
Figure 6.
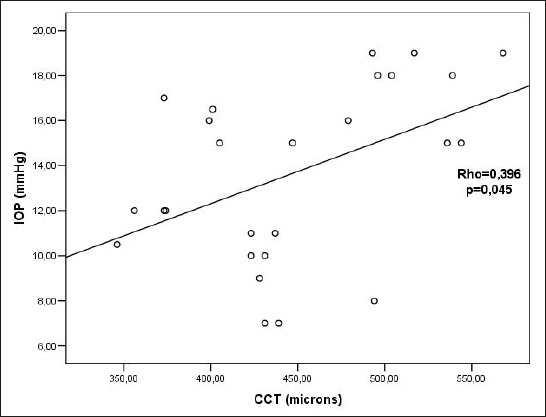
Relationship between CCT and IOP values 12 months after CXL Rho– Spearman correlation coefficient, p – the probability, IOP – intraocular pressure, CCT - central corneal thickness
5. DISCUSSION
To determine the possible effect of CXL with riboflavin and UVA on IOP measurements was the task of many studies (1-8). IOP measurements in KC are difficult to understand as GAT and the TonoPen XL (TP) underestimate true IOP corneas with thin stroma (11-13). The exact amount of IOP underestimation in keratoconic corneas depends on the extent of the disease and the corneal thinning (14,15).
CXL resulted in slight overestimation of the mean, central IOP but magnitude of the effect for GAT and dynamic contour tonometry (DCT) was relatively small. Whereas each DCT readings remain rather unaffected by CXL, the variability of GAT readings increases in many treated corneas and resulting in potentially underestimation of IOP. GAT may therefore not be the method of choice and ideal tonometer for follow up of patients after CXL (16).
The advantage of in vitro models of corneas allows us to study and compare the effects of CXL on IOP readings without potentially confounding in vivo factors such as real alterations in IOP due to possible crosslinking effects on stroma but more to trabecular meshwork or humor aqueus production. Even more, in vivo studies would require cannulation on the anterior chamber in patients who were treated by CXL. The results were very similar in the in vitro model compared those reported in vivo. This indicates that in vitro model closely reflects in vivo conditions with regard to static IOP measurements (17).
The studies of Wollensak and Caporossi have shown that the amount of mean IOP overestimation after CXL is only in range of 1,8 to 3,1 mmHg depending on the tonometry type. It seems remarkable that a two to threefold increase in corneal rigidity as it is observed after CXL results inonly a slight increase in IOP readings. This finding can be explained by the fact that CXL increases corneal rigidity only in the central 8 mm and anterior 300 microns of the corneal stroma, leaving periphery and the deeper parts of affected corneas untouched (18,19).
Those finding is very similarly to the results of our study. The choice of corneas were very strict and only central corneal ectasia with the central thinnest point only over 380 microns. All peripheral of thinner ones were excluded in the study. It could be the reason in understanding different approaches to the problem how to choose corneal ectasia which is available to be treated and expected the best result.
The study of Kymionis (22) evaluated 55 eyes and found the statistically significant increase in the measured IOP 6 months and 12 months after CXL (both p<0,01) the mean measured IOP was 9,95±3,01 mmHg before CXL, 11,40±2,89mmHg at 6 months and 11,35±3,38 mmHg at 12 months later. The change of IOP measurements at both postoperative examinations was not correlated with patient age, preoperative pahimetry or postoperative keratometry readings. There was a significant increase in IOP measured by GAT that was probably caused by an increase in corneal rigidity. Our results are almost the same.
Results in several studies indicate that CXL results in corneal stiffening. There are no clinical studies which directly evaluate the effects of CXL by GAT. The corneal stiffening after CXL might be correlated with the increase in ocular and corneal rigidity (19). Ocular rigidity is measurable physical parameter of the eye that expresses the elastic properties of the globe. In 1937, Friedenwald (21) described the coefficient of ocular rigidity as a „measure of the resistance, which the eye exerts to distending forces”.
The Kymionis(22) study there was statistically significant increase in measured IOP by GAT after CXL with riboflavin and UVA 6 months postoperatively. Biomechanical alterations and corneal rigidity increments are probably related to IOP changes after CXL. The change of IOP readings at 6 months and 12 months was not correlated with patients age, preoperative pachymetry or preoperative readings. Either the increase of IOP in an overestimation, an increase in true IOP, or both, in addition, it is possible that etiology of the process is multifactorial. The preoperative IOP should be taken into account in patients treated by CXL, especially if glaucoma is suspected.
These findings serve to highlight that IOP measurements alone may be misleading. IOP may need adjustment in patients with KC that differ from the population mean. It is important to note that each instrument tested was affected by CCT to differing degrees. After CXL there was significant increase in IOP measured by GAT. The increase was probably the result of an increase in corneal rigidity. These different results have important implications in the choice of tonometer to use in patients with different corneal thicknesses in midpressure ranges (23).
6. CONCLUSION
In our study CCT was 449 microns before the CXL, but after CXL treatment was statistically decreased to 420 microns 3 months later, still very low 6 and 12 months later 437 microns. IOP was low before the CXL 12 mmHg, 3 months later was 13,5 mmHg, 6 months after CXL 14,0 mmHg and 12 months later the CXL was 15,0 mmHg. The results emphasize that CXL is the procedure resulting thinner and never recovered cornea in thickness but on another side that treated cornea is more resistance which could be followed by increased IOP. These conclusion could be fully accepted only in central corneal ectasia which central corneal thickness is not close to deadline (380 microns). The peripheral corneal ectasia like marginal pellucid degenerations or those corneas with scars or extremely thin central parts have shown completely different line of recovering after CXL with more serious prognosis. The results of the present study could not be accepted in those ectatic cases. Therefore, the correct pre- and postoperative check up of central ectatic corneas suspected or already treated by CXL must include IOP as one of highlights in the protocol to follow up corneal rigidity.
Footnotes
CONFLICT OF INTEREST: NONE DECLARED
REFERENCES
- 1.Rabinowitz YS. Keratoconus. Surv Ophthalmol. 1998;42:297–319. doi: 10.1016/s0039-6257(97)00119-7. [DOI] [PubMed] [Google Scholar]
- 2.Siganos CS, Kymionis GD, Kartakis N, Theodorakis MA, Astyrakakis N, Pallikaris IG. Management of keratokonus with Intacs. Am J Ophthalmol. 2003;135:64–70. doi: 10.1016/s0002-9394(02)01824-x. [DOI] [PubMed] [Google Scholar]
- 3.Frost NA, Wu J, Lai TF, Coster DJ. A review of randomized controlled trials of penetrating keratoplasty techniques. Ophthalmology. 2006;113:942–949. doi: 10.1016/j.ophtha.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 4.Jingjing L, Peng J, Xiaoti L. Efficacy of corneal collagen cross-linking for treatment of keratoconus: a Meta-analysis of randomized controlled trials. PloS ONE. 2015 doi: 10.1371/journal.pone.0127079. DOI:10.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Whitacre MM, Stein RA, Hassanein K. The effect of corneal thickness on applanation tonometry. Am J Ophthalmol. 1993;115:592–596. doi: 10.1016/s0002-9394(14)71455-2. [DOI] [PubMed] [Google Scholar]
- 6.Copt RP, Thomas R, Mermoud A. Corneal thickness in ocular hypertension, primary open glauoma and normal tension glaucoma. Arch Ophthalmol. 1999;117:14–16. doi: 10.1001/archopht.117.1.14. [DOI] [PubMed] [Google Scholar]
- 7.Stodtmeister R. Applanation tonometry and correction according to corneal thicknness. Acta Ophthalmol Scand. 1998;76:319–324. doi: 10.1034/j.1600-0420.1998.760313.x. [DOI] [PubMed] [Google Scholar]
- 8.Coskunseven E, Jankov MR, II, Hafezi F. Contralateral eye study of corneal cross-linking with riboflavin and uva irradiation in patients with keratoconus. J Refract Surg. 2009;25:371–376. doi: 10.3928/1081597X-20090401-02. [DOI] [PubMed] [Google Scholar]
- 9.Feltgen N, Leifert N, Funk J. Correlation between central corneal thickness, applanation tonometry and direct intracameral IOP readings. Br J Ophthalmol. 2001;85:85–87. doi: 10.1136/bjo.85.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jankov MR, Jovanovic V, Nikolic Lj, Lake JC, Kymionis G, Conskuseven E. Corneal collagen cross-linking. MEAJO. 2010;17:21–27. doi: 10.4103/0974-9233.61213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ehlers N, Bramsen T, Sperling S. Applanation tonometry and central corneal thickness. Acta Ophthalmol. 1975;53:34–43. doi: 10.1111/j.1755-3768.1975.tb01135.x. [DOI] [PubMed] [Google Scholar]
- 12.Whitacre MM, Stein RA, Hassanein K. The effect of corneal thickness on applanation tonometry. Am j Ophthalmol. 1993;115:592–596. doi: 10.1016/s0002-9394(14)71455-2. [DOI] [PubMed] [Google Scholar]
- 13.Dohadwala AA, Munger R, Damji KF. Positive correlation between Tono-Pen intraocular pressure and central corneal thickness. Ophthalmology. 1998;105:1849–1854. doi: 10.1016/S0161-6420(98)91029-6. [DOI] [PubMed] [Google Scholar]
- 14.Ozbek Z, Cohen EJ, Hammersmith KM, Rapuano CJ. Dynamic contour tonometry: a new way to assess intraocular pressure in ectatic corneas. Cornea. 2006;25:890–894. doi: 10.1097/01.ico.0000224649.12214.33. [DOI] [PubMed] [Google Scholar]
- 15.Bohm A, Kohlhaas M, Lerche RC, Bischoff B, Richard G. Measuring intraocular pressure in keratoconus: effect of the changed biomechanics. Ophthalmology. 1997;94:771–774. doi: 10.1007/s003470050201. [DOI] [PubMed] [Google Scholar]
- 16.Romppainen T, Bachmann LM, Kaufmann C, Kniestedt C, Mrochen M, Thiel MA. Effect of riboflavin UVA induced collagen crosslinking on intraocular pressure measurement. IOVS. 2007;48:5494–5498. doi: 10.1167/iovs.06-1479. [DOI] [PubMed] [Google Scholar]
- 17.Kaufmann C, Bachmann LM, Thiel MA. Comparison of dynamic contour tonometry with Goldmann applanation tonometry. Invest Ophthalmol Vis Sci. 2004;45:3118–3121. doi: 10.1167/iovs.04-0018. [DOI] [PubMed] [Google Scholar]
- 18.Wollensak G, Spoerl E, Seiler T. Stress-strain measurements of human and porcine corneas after riboflavin-ultraviolet-A-induced cross-linking. J Cataract Refract Surg.2003; 29:1780–1785. doi: 10.1016/s0886-3350(03)00407-3. [DOI] [PubMed] [Google Scholar]
- 19.Caporosssi A, Baiocchi S, Mazzotta C, Traversi C, Caporossi T. Parasurgical therapy for keratoconus by riboflavin-ultraviolet type A rays induced cross-linkong of corneal collagen: preliminary refractive results in an Italian study. J Cataract Refract Surg. 2006;32:837–845. doi: 10.1016/j.jcrs.2006.01.091. [DOI] [PubMed] [Google Scholar]
- 20.Raiskup-Wolf F, Hoyer A, Spoerl E, Pillunat LE. Collagen cross-linking with riboflavin and ultraviolet-Alight in keratoconus: long-term results. J Cataract Refract Surg. 2008;34:796–801. doi: 10.1016/j.jcrs.2007.12.039. [DOI] [PubMed] [Google Scholar]
- 21.Friedenwald JS. Contribution of the theory and practice of tonometry. Am J Ophthalmol. 1937;20:985–1024. [Google Scholar]
- 22.Kymionis GD, Grentzelos MA, Kounis GA, Portaliou DM, Detorakis ED, Magarakis M, Karampatakis VE, Pallikaris IG. Intraocular pressure measurements after corneal collagen crosslinking with riboflavin and ultraviolet A in eyes with Keratoconus. J Cataracta Refract Surg. 2010;36:1724–1727. doi: 10.1016/j.jcrs.2010.05.016. [DOI] [PubMed] [Google Scholar]
- 23.Bhan A, Browning AC, Shab S, Hamilton R, Dave D, Dua HS. Effect of corneal thickness on intraocular pressure measurement with the pneumotonometer, Goldmann applanation tonometer, and Tono-Pen. Invest Ophthalmol & Visual Sci. 2002;43:1389–1392. [PubMed] [Google Scholar]


