Abstract
PURPOSE
We aimed to determine the impact of transitional care interventions (TCIs) on acute health service use by patients with congestive heart failure in primary care and to identify the most effective TCIs and their optimal duration.
METHODS
We conducted a systematic review and meta-analysis of randomized controlled trials, searching the Medline, PsycInfo, EMBASE, and Cochrane Library databases. We performed a meta-analysis to assess the impact of TCI on all-cause hospital readmissions and emergency department (ED) visits. We developed a taxonomy of TCIs based on intensity and assessed the methodologic quality of the trials. We calculated the relative risk (RR) and a 95% confidence interval for each outcome. We conducted a stratified analysis to identify the most effective TCIs and their optimal duration.
RESULTS
We identified 41 randomized controlled trials. TCIs significantly reduced risks of readmission and ED visits by 8% and 29%, respectively (relative risk = 0.92; 95% CI, 0.87–0.98; P = .006 and relative risk = 0.71; 95% CI, 0.51–0.98; P = .04). High-intensity TCIs (combining home visits with telephone followup, clinic visits, or both) reduced readmission risk regardless of the duration of follow-up. Moderate-intensity TCIs were efficacious if implemented for a longer duration (at least 6 months). In contrast, low-intensity TCIs, entailing only followup in outpatient clinics or telephone follow-up, were not efficacious.
CONCLUSIONS
Clinicians and managers who implement TCIs in primary care can incorporate these results with their own health care context to determine the optimal balance between intensity and duration of TCIs. High-intensity interventions seem to be the best option. Moderate-intensity interventions implemented for 6 months or longer may be another option.
Keywords: congestive heart failure, transitional care, systematic review, meta-analysis, utilization, outcomes research
INTRODUCTION
Congestive heart failure (CHF) imposes an increasingly heavy burden on health care systems, most of which can be attributed to numerous hospital readmissions and emergency department (ED) visits.1–3 Multiple exacerbations of CHF result in frequent use of acute health care services by these patients, known as revolving door users. After discharge, 25% of patients are readmitted within the first 30 days,4,5 and 50% within the first 6 months.6,7
This frequent use of health care services is mainly due to lack of understanding of a treatment plan, nonadherence to medical therapy, unawareness of CHF symptom exacerbation, and irregular follow-up.8–12 Lack of coordination and communication between hospitalists and primary care physicians (PCPs) has been documented.13,14 PCPs too often do not receive discharge summaries,15 and when they do receive them, the summaries often lack appropriate documentation of medication indication and advice for follow-up. It is therefore difficult for PCPs to plan an appropriate follow-up after hospital discharge.16
To address these issues, transitional care interventions (TCIs) have been implemented with a common objective of reducing the rate of hospital readmission and ED visits.2,17–19 Coordination of appropriate transition plans is now one of the suggested domains that should be measured when assessing patient-centered medical homes.20 TCIs comprise a broad range of time-limited health services including patient or caregiver education on self-management, discharge planning, structured follow-up, and coordination among health care professionals involved in the transition, including PCPs.2,17,18,21
To date, a few systematic reviews have been published on TCIs.22–24 It has been shown that the interventions decrease hospital admissions after 12 months of follow-up.22 These reviews, however, were unable to determine the most efficacious TCI.22,24 They did not include all types of TCIs22,23; furthermore, a substantial number of RCTs on these interventions have been published since the publication of the reviews (26 since that by Phillips et al24). We therefore conducted a systematic review and meta-analysis to determine the impact of TCIs on the rate of all-cause readmission and ED visits by patients with CHF, and to identify the most effective TCIs and their optimal duration.
METHODS
A systematic review and meta-analysis, conducted according to Cochrane recommendations, allowed us to integrate and summarize the results of a large number of studies.25 The protocol was approved and funded by the Canadian Institutes of Health Research (KRS-250478). We used the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) framework26 for reporting the results.
Eligibility Criteria
Trials were eligible for inclusion if they had a randomized controlled design and enrolled patients with CHF discharged from inpatient departments to home. The trials had to compare some form of TCI with usual care, and had to collect data on all-cause readmission and all-cause ED visits (Supplemental Appendix 1, available at http://www.annfammed.org/content/13/6/562/suppl/DC1.)
Information Sources and Search
A librarian specializing in systematic reviews conducted the literature search. We performed a systematic search of 4 databases (MEDLINE, PsycINFO, EMBASE, and Cochrane Database of Systematic Reviews) for articles published between 1995 (beginning of TCI) and February 6, 2014. The key words were “heart failure,” “transition,” “care planning,” and “discharge” (Supplemental Appendix 2, available at http://www.annfammed.org/content/13/6/562/suppl/DC1). We also screened reference lists of included articles to look for potential additional interventions. All companion articles of the included studies were searched.
Study Selection
We used a 2-step approach to study selection. First, 2 reviewers (I.V., V.K.) independently examined the references (titles and abstracts) based on the eligibility criteria. Second, full texts of the selected references were retrieved, read, and selected based on the eligibility criteria. At each step, differences in coding were resolved by consensus.
Data Collection Process and Items
Information was extracted from each study by 2 researchers (Melanie Le Berre, PT, MSc, and Martin Beauchamp, Aux Nurse) independently and included author, publication date, country, components of the intervention, health care professionals involved, frequency and duration of follow-up, study design, duration of the study, and participant characteristics such as sample size, mean age, percentage of males, and severity of CHF. Outcomes were extracted at the final follow-up and at various follow-up periods. Any discrepancies were resolved through consensus.
Risk of Bias in Individual Studies
The quality of the studies was assessed independently by 2 reviewers (V.K. and Quan Nha Hong, PhD[C]) using a validated tool for the critical appraisal of experimental studies, the Downs and Black scale,27 which had demonstrated a high validity (r = .90) and interrater reliability (r = .75).28 Particular attention was paid to randomization generation, allocation concealment, the blinding of participants and/or outcome assessors, and loss to follow-up. We categorized quality as higher (≥20 = very good, 15 to 19 = good) or lower (11 to 14 = fair, ≤10 = poor).29 Interrater reliability was calculated using the interrater correlation coefficient.30 Any disagreement was resolved by consensus.
Summary Measures
As recommended in the review of complex interventions,31 and in line with our protocol, 3 experts in the areas of integrated care, care management, and systematic reviews (I.V., V.K., and Ian Shrier, MD, PhD) developed a taxonomy by consensus to classify TCI into homogeneous groups of interventions. To develop the taxonomy, we first looked at the included interventions and described them in detail, before examining the results of the studies. Second, we examined the extant literature, including other systematic reviews, to identify key components of interventions and their link with intensity. On the basis of this literature review, the 2 key elements were home visits, usually by a home nurse, and frequency of monitoring. Indeed, direct contact and, in particular, home visits,32,33 led to a reduction of readmissions, whereas phone calls did not.33 Home visits reinforced self-management (better understanding of the disease and adherence to treatments).34 Home visits also eliminated transportation to the physicians’ offices and pharmacies, among the main contributors to readmission of older patients.34 Repeat visits also had an impact on long-term outcomes.35 The included interventions were then classified as low, moderate, or high in intensity (Table 1).
Table 1.
Classification System for Intensity of Transitional Care Interventions
| Intensity | Component(s) |
|---|---|
| Low | Structured telephone follow-up without home visits or Periodic follow-up in an outpatient clinic without home visits |
| Moderate | Home visits only or A combination of telephone follow-up with periodic follow-up in a clinic without home visits or Telecare (a specific type of intervention involving the transfer of patient vital signs, such as electrocardiogram, blood pressure, weight, via digital cable22) without prearranged direct contact with patients |
| High | A combination of home visits with other types of follow-up (telephone and/or clinic follow-up) or Telecare combined with prearranged direct contact with patients (eg, home visits, telephone follow-up, video visits) |
Synthesis of Results
We conducted a meta-analysis using Review Manager 5.3 (The Nordic Cochrane Centre, The Cochrane Collaboration) to determine the differences between the TCI group and the usual care group in their risks of readmission and ED visits at the last provided follow-up time. Relative risks (RRs) and their 95% CIs were calculated to estimate the mean effect size. We also calculated the number needed to treat to estimate the clinical importance of a TCI. If more than 1 publication described the same study, it was treated as 1 study. If 1 publication studied multiple interventions, each intervention was included in the meta-analysis. We used random-effects models, as we expected the different interventions to vary in their effects. Meta-analyses were performed on an intention-to-treat basis when possible. The I2 statistic was used to measure heterogeneity.36 We contacted authors for additional data to conduct the meta-analysis when it was required.
Risk of Bias Across Studies
To determine if there were any reporting biases in our included studies, we created a funnel plot, plotting the study standard errors vs the logarithm of the risk ratios, as per the Cochrane recommendations.25
Additional Analyses
We performed subgroup analyses to explore the effect on risk of readmission attributable to intensity of TCI, severity of CHF, and mean age of participants. To further investigate the most effective combination of intervention characteristics, we conducted a stratified analysis on the interaction between intervention intensity and duration. The stratification was conducted on a 6-level categorical variable: low intensity and 6 months or shorter; moderate intensity and 6 months or shorter; high intensity and 6 months or shorter; low intensity and longer than 6 months; moderate intensity and longer than 6 months; and high intensity and longer than 6 months.
To assess the robustness of our intervention effect estimates, we conducted sensitivity analyses, excluding possible outlier studies, cluster-randomized trials, TCIs with additional components, and RCTs with lower methodologic quality.
RESULTS
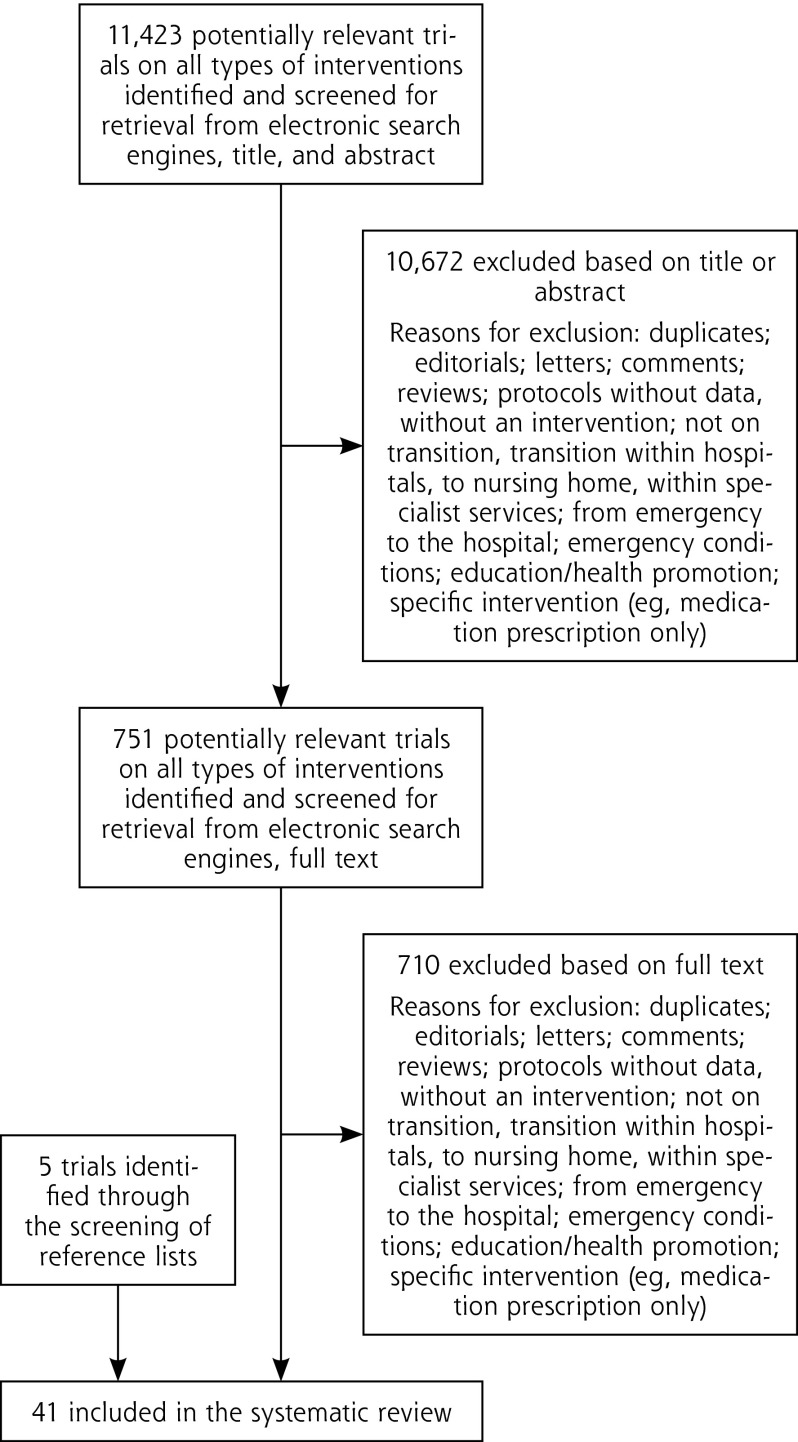
Out of 11,423 references, 41 RCTs and a total of 43 interventions were included in the review,8–10,35,37–73 including 5 trials identified through the screening of reference lists43,44,51,56,62 (Figure 1, Table 2, and Supplemental Appendix 3, available at http://www.annfammed.org/content/13/6/562/suppl/DC1; 3 companion articles were identified81–83).
Figure 1.
Trial selection flowchart.
Table 2.
Summary Characteristics of Included Trials
| Characteristics | Number of Trials |
|---|---|
| Continent and country | |
| Americas | |
| United States | 19 |
| Canada | 3 |
| Brazil | 1 |
| Europe | |
| Netherlands | 3 |
| Italy | 3 |
| Spain | 2 |
| Sweden | 2 |
| United Kingdom | 2 |
| Switzerland | 1 |
| Austria | 1 |
| Oceania | |
| New Zealand | 1 |
| Australia | 1 |
| Asia | |
| China | 1 |
| Hong Kong | 1 |
| Publication language: English | 41 |
| Number of arms | |
| 2 arms (intervention and usual care) | 37 |
| 3 arms (2 arms in addition to usual care)a | 4 |
| Unit of randomization | |
| Patient | 38 |
| Clusterb | 3 |
| First follow-up contact after discharge | |
| Within 1 week | 28 |
| Within 2 weeks | 9 |
| Within 1 month | 4 |
| Within 2 months | 1 |
| Unclear | 1 |
| Assessment of congestive heart failure severity, No. of trials (patients) | |
| LVEF only | 9 (1,321) |
| NYHA only | 8 (1,447) |
| LVEF and NYHA | 23 (8,172) |
| Not reported | 3 (420) |
| Assessment of diastolic function, No. of trials (patients) | 2 (285) |
| Mean LVEF | |
| <40% | 18 |
| ≥40% | 5 |
LVEF = left ventricular ejection fraction; NYHA = New York Heart Association.
Telephone vs video telephone follow-up. Follow-up by the multidisciplinary team vs guided by N-terminal pro-B-type natriuretic peptide level. In-clinic follow-up vs in-clinic follow-up with monthly telephone/home visits. Telephone follow-up vs telecare.
Clusters: primary care physicians or primary care clinics.
The length of follow-up ranged from 1 to 24 months postdischarge. The mean age of patients ranged from 57.9 to 81.0 years, and 65.6% of patients were men.
Usual care was sparsely described in the studies. It consisted of predischarge education on CHF self-management and usual follow-up with the family physician or cardiologist as required.
TCI Characteristics
TCIs included predischarge education for patients (on CHF management, nonpharmacologic strategies, and medication management, usually given by a specialized CHF nurse using written or video material), a discharge plan (including a medication review, individualized care plan development, and a discharge letter sent to the family physician or cardiologist), and structured, proactive, and prearranged follow-up. The trials were nearly evenly split by the intensity of their TCI: 13 studied low-intensity interventions; 14, moderate-intensity interventions; and 16, high-intensity interventions (Table 3). Some interventions had additional components: a nurse visiting the hospital in cases of readmission,35,72 and patients having access to a nurse either during office hours59,64 or at all times.45,63
Table 3.
Intensity of Included Transitional Care Interventions
| Intensity and Trial, Year | Structured Home Visits | Telephone Follow-up | Clinic Follow-up | Telecare Only | Telecare + Other Types of Follow-up | Details |
|---|---|---|---|---|---|---|
| Low intensity | ||||||
| Riegel et al,37 2006 | X | 11 RCTs had a prearranged telephone follow-up.37–44,53,56,62 Patients received 3 to 16 telephone calls (over a 2- to 12-month period). | ||||
| Domingues et al,56 2011 | X | |||||
| DeBusk et al,38 2004 | X | |||||
| Laramee et al,39 2003 | X | |||||
| Wakefield et al,40 2008 | X | |||||
| Dunagan et al,41 2005 | X | |||||
| Rainville et al,42 1999 | X | |||||
| Tsuyuki et al,53 2004 | X | |||||
| Barth et al,43 2001 | X | |||||
| Lopez Cabezas et al,62 2006 | X | |||||
| Chaudhry et al,44 2010 | X | |||||
| Doughty et al,70 2002 | X | In 2 RCTs, follow-up was provided in an outpatient clinic by the family physician or a cardiologist or multidisciplinary team.56,69 Patients visited the clinic7 to 10 times (over a 12- to 18-month period). | ||||
| Jaarsma et al,57 2008 | X | |||||
| Moderate intensity | ||||||
| Stewart et al,9 1998; Inglis et al,81 2004 | X | 4 RCTs used prearranged home visits9,34,70,71 with a total of 2 to 9 visits (over a 6- to 12-month period). | ||||
| Barker et al,71 2012 | X | |||||
| Naylor et al,35 2004 | X | |||||
| Kwok et al,72 2008 | X | |||||
| Nucifora et al,59 2006 | X | X | 8 RCTs combined a structured telephone call with follow-up in a clinic.44,45,53,57–59,62,63 They included 1 to 10 telephone calls and 1 to 8 visits to the clinic (over a 6- to 24-month period). | |||
| Del Sindaco et al,60 2007 | X | X | ||||
| Cleland et al,58 2005 | X | X | ||||
| Ekman et al,64 1998 | X | X | ||||
| Atienza et al,63 2004 | X | X | ||||
| Kasper et al,45 2002 | X | X | ||||
| Angermann et al,46 2012 | X | X | ||||
| Ducharme et al,54 2005 | X | X | ||||
| Dar et al,66 2009 | X | 2 RCTs featured telecare without prearranged direct contact with patients.47,66 The vital signs transmit-ted daily consisted of weight,47,66 blood pressure, pulse, and oxygen saturation.66 Patients also answered questions on CHF symptoms via telephone (automated voice response).47,66 | ||||
| Goldberg et al,47 2002 | X | |||||
| High intensity | ||||||
| Harrison et al,55 2002 | X | X | 6 RCTs used structured home visits combined with telephone follow-up.8,10,47,54,66,67 Patients had a total of 1 to 3 home visits (over a 3- to 12-month period). | |||
| Rich et al,48 1993 | X | X | ||||
| Rich et al,8 1995 | X | X | ||||
| Blue et al,67 2001 | X | X | ||||
| Leventhal et al,68 2011 | X | X | ||||
| Jaarsma et al,10 1999 | X | X | ||||
| Cline et al,65 1998 | X | X | 2 RCTs combined home visits with follow-up in a clinic.64,72 Patients received 1 home visit and 4 to 6 in-clinic follow-ups (over a 6- to 12-month period). | |||
| Thompson et al,73 2005 | X | X | ||||
| Adlbrecht et al,69 2011; Berger et al,82 2010 | X | X | X | 3 RCTs combined home visits with telephone calls and a visit to a clinic.48,56,68 The total number of home visits ranged from 1 to 3 (over a 3- to 12-month period). | ||
| Pugh et al,49 2001; Blaha et al,83 2000 | X | X | X | |||
| Jaarsma et al,57 2008 | X | X | X | |||
| Giordano et al,61 2009 | X | 5 RCTs combined telecare with prearranged direct contact with patients50–52,58,61 such as prearranged telephone calls,51,61 video calls and home visits,50,52 or visits to a clinic.58 Vital signs (weight, blood pressure, oxygen saturation, heart sounds, pulse, and electrocardiographic findings) were transmitted daily50,51,58 or at a scheduled time.52,61 | ||||
| Bowles et al,50 2011 | X | |||||
| Kulshreshtha et al,51 2010 | X | |||||
| Pekmezaris et al,52 2012 | X | |||||
| Cleland et al,58 2005 | X |
CHF = congestive heart failure; RCT = randomized controlled trial.
Risk of Bias in Individual Trials
Overall, 35 trials were of higher quality (very good or good) (Supplemental Appendix 4, available at http://www.annfammed.org/content/13/6/562/suppl/DC1). Six were of fair quality.9,42,43,49,52,71 None of the trials were of poor quality. The interrater correlation coefficient was strong (0.79; 95% CI, 0.63–0.88; P <.0001).30
Blinding of participants was not possible because of the nature of the interventions. Sixteen RCTs described blinding of outcome assessors* and 5 reported some level of allocation concealment.8,10,55,62,66 Loss to follow-up was acceptable (less than 20%) in 35 RCTs.
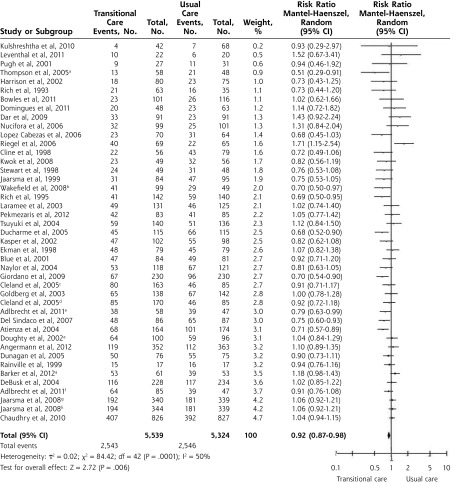
Effect on All-Cause Hospital Readmission
Forty-three interventions provided data on all-cause readmission; a forest plot of their results is shown in Figure 2. The meta-analysis showed a significant reduction in the relative risk of readmission with a TCI as compared with usual care (RR = 0.92; 95% CI, 0.87–0.98), indicating that TCI reduces the risk of readmission by an average of 8%. The number needed to treat was 52, meaning that 52 patients had to receive the TCI for 1 patient to benefit (1 less readmission to occur).
Figure 2.
Forest plot for all-cause readmission (presented by weight).
Note: Percentage of patients with events: 45.9% in the incidence group and 47.8% in the control group.
aCluster randomization.
bTelephone and video telephone follow-up.
cTelecare.
dTelephone follow-up.
eIntervention guided by N-terminal pro-B-type natriuretic peptide.
fMultidisciplinary intervention.
gFollow-up in clinic.
hFollow-up in clinic and monthly contact with the nurse.
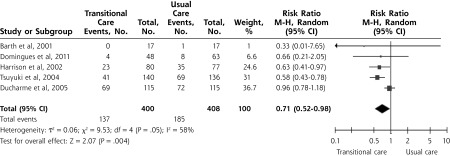
Effect on All-Cause ED Visits
Five trials provided data on all-cause ED visits43,53–56; a forest plot of their results is shown in Figure 3. The meta-analysis showed a significant 29% reduction in the risk of ED visits for TCI as compared with usual care (RR = 0.71; 95% CI, 0.52–0.98). The number needed to treat was 9, meaning that 9 patients had to receive the TCI for 1 patient to benefit (1 less ED visit to occur).
Figure 3.
Forest plot for all-cause emergency department visits (presented by weight).
Note: Percentage of patients with events: 34.3% in the intervention group and 45.4% in the control group.
M-H = Mantel-Haenszel
Additional Analyses
The results of the exploratory subgroup analyses suggested that high-intensity interventions are efficacious at reducing the risk of readmission (RR = 0.86; 95% CI, 0.78–0.94), and that they are most efficacious in a population with mean age of 75 years and older (RR = 0.83; 95% CI, 0.76–0.92) (Supplemental Appendix 5, available at http://www.annfammed.org/content/13/6/562/suppl/DC1). To further investigate the effects of intervention characteristics on readmission risks, and to find the most effective combination of characteristics, we conducted a stratified analysis on the interaction between intervention intensity and duration. Results showed that the different intensity and duration combinations do in fact have significantly different mean effects on the relative risk of readmission (P = .003) (Table 4). High-intensity interventions continued to be associated with a reduced risk of readmission regardless of their duration, and interventions of moderate intensity seemed to decrease the risk if they lasted longer than 6 months. Neither moderate-intensity, short-duration interventions, nor any of the low-intensity interventions significantly reduced the risk of readmission.
Table 4.
Summary of Intervention Intensity- and Duration-Stratified Analysis
| Intervention Intensity and Duration | Number of Trials | Relative Risk (95% CI) |
|---|---|---|
| Low intensity | ||
| ≤6 months39,44,53,56 | 5 | 1.121 (0.97–1.30) |
| >6 months40–42,57,62,70 | 7 | 0.949 (0.86–1.10) |
| Moderate intensity | ||
| ≤6 months45–47,54,59,64,66,71,72 | 10 | 0.981 (0.86–1.30) |
| >6 months58,60,63 | 4 | 0.788 (0.70–0.90) |
| High intensity | ||
| ≤6 months10,48–52,55,73 | 9 | 0.804 (0.69–0.93) |
| >6 months58,61,65,67–69 | 7 | 0.885 (0.79–0.99) |
Note: Test for differences across 6 strata: P = .003
Because of the small number of studies, we could not carry out further analyses on the risk of ED visits.
The results of the sensitivity analyses are presented in Supplemental Appendix 6 (available at http://www.annfammed.org/content/13/6/562/suppl/DC1). We did not detect any differences in the estimated mean effects when omitting outlier studies, cluster trials, trials with additional components, and those having lower methodologic quality. Lastly, according to the Fisher exact test, the quality of the study and the TCI intensity were independent of one another (P = .66). Overall, the main findings were robust in sensitivity analyses.
Risk of Bias Across Studies
There was no appearance of a systematic asymmetry in the funnel plot (plotting the study standard errors vs the logarithm of the risk ratios) (Supplemental Appendix 7, available at http://www.annfammed.org/content/13/6/562/suppl/DC1). We therefore have no reason to believe that any reporting biases exist in our trials.
DISCUSSION
Providing TCI to patients with CHF discharged to home showed mean 8% and 29% risk reductions of all-cause readmission and ED visits, respectively. TCI was far more efficacious in decreasing ED visits than in reducing hospital readmission: the number needed to treat was only 9 patients to avoid an ED visit vs 52 to avoid a readmission. These results are in line with previous reviews of studies of older CHF patients receiving comprehensive discharge planning plus postdischarge support.22,24 In contrast to previous meta-analyses,21,24,74 we further identified more efficacious interventions and optimal intervention durations. Our results suggest that high-intensity TCIs need be sustained for only a short duration (6 months or less) to be effective at reducing the risk of readmission, while moderate-intensity interventions need to be of a longer duration (more than 6 months) to have a similar effect. It is therefore essential to provide individualized TCI to patients, and to triage patients for high-intensity or moderate-intensity intervention; risk stratification may help in guiding triage.75
In contrast to a meta-analysis by Feltner et al,21 we found that follow-up in the outpatient clinic only, that is, the usual postdischarge arrangement, does not improve the outcomes studied. Similarly, telephone follow-up used in isolation—the most frequently reported type of TCI (11 RCTs)—was not efficacious. In all cases, low-intensity TCI should be avoided.
A key element of TCI is follow-up of patients by a PCP within a week after discharge. It is well known that early physician follow-up postdischarge and physician continuity are associated with better outcomes among patients with CHF.76 Advanced access scheduling for discharged patients is one means for enabling timely follow-up after discharge.77 Successful communication between hospitals, in particular cardiologists, and PCPs is also of paramount importance. Hospitals need to notify PCPs of patients discharged and send the summary for return visits. As the information on many discharge summaries is often inadequate for PCPs to manage continuity of care,16,78 however, the quality of these summaries needs to be improved, in particular the documentation of drug indications and follow-up.16
Several limitations of this review should be mentioned. Because of resource constraints, we were not able to include articles in languages other than English and French. An objective measure of intensity that takes into account TCI type (face to face vs remote) as well as the number of contacts would have been more appropriate, but not all the studies systematically reported the number of contacts. Patient characteristics, including diagnosis, comorbidities, and severity of CHF, were missing in some studies. Future studies on TCI should exhaustively describe interventions, including the number of contacts, the components of the intervention (eg, use of medications, self-management) as well as patient characteristics (eg, receipt of diagnostics). Our study is also limited by a lack of published studies on “oldest-old” patients (those aged 85 years and older), who frequently have multiple comorbidities and functional and cognitive impairments,79 and who represent the fastest growing segment of the population.80 The effect of the TCI might differ for this group, so future studies should consider this population. Furthermore, had more studies been available, we could have adjusted the meta-analyses for mean age of patients, as well as follow-up duration. The vast majority of included studies reported comorbidities; therefore, we could not conduct analyses exploring relationships between CHF, comorbidities, and TCIs. Future systematic reviews on TCI for patients with multiple comorbidities are needed.
In conclusion, providing TCI to CHF patients reduces readmission and ED visits. High-intensity interventions, regardless of intervention length, seem to be the best option. Moderate-intensity interventions implemented for long duration may be another option. Clinicians and managers who implement TCI in primary care can incorporate these findings with the health care context to determine the optimal balance between intensity and duration of interventions.
Acknowledgments
We would like to thank Ian Shrier, MD, PhD, for his advice on meta-analyses and development of taxonomy; Muriel Gueriton, specialized librarian, for the literature search; Pierre Pluye, MD, PhD, and Quan Nha Hong, PhD(C), for assistance with the methodologic quality assessment; Geva Maimon, PhD, biostatistician, for assistance with the stratified analysis; and research assistants Melanie Le Berre, PT, MSc, and Martin Beauchamp, Aux Nurse, for assistance with study selection.
Footnotes
Conflicts of interest: authors report none.
Funding support: This study was funded by the Canadian Institutes of Health Research (KRS-250478).
Previous presentation: Preliminary results of this study were presented as an oral presentation at the North American Primary Care Research Group Conference; November 21-25, 2014; New York, New York.
Supplementary materials: Available at http://www.AnnFamMed.org/content/13/6/562/suppl/DC1/.
References
- 1.Naylor MD. Transitional care of older adults. Annu Rev Nurs Res. 2002;20:127–147. [PubMed] [Google Scholar]
- 2.Naylor MD, Aiken LH, Kurtzman ET, Olds DM, Hirschman KB. The care span: the importance of transitional care in achieving health reform. Health Aff (Millwood). 2011;30(4):746–754. [DOI] [PubMed] [Google Scholar]
- 3.Scarborough P, Bhatnagar P, Wickramasinghe K. Coronary Heart Disease Statistics. London, England: British Heart Foundation; 2010. [Google Scholar]
- 4.Zaya M, Phan A, Schwarz ER. The dilemma, causes and approaches to avoid recurrent hospital readmissions for patients with chronic heart failure. Heart Fail Rev. 2012;17(3):345–353. [DOI] [PubMed] [Google Scholar]
- 5.Anderson C, Deepak BV, Amoateng-Adjepong Y, Zarich S. Benefits of comprehensive inpatient education and discharge planning combined with outpatient support in elderly patients with congestive heart failure. Congest Heart Fail. 2005;11(6):315–321. [DOI] [PubMed] [Google Scholar]
- 6.Fisher ES, Wennberg JE, Stukel TA, Sharp SM. Hospital readmission rates for cohorts of Medicare beneficiaries in Boston and New Haven. N Engl J Med. 1994;331(15):989–995. [DOI] [PubMed] [Google Scholar]
- 7.Funk M, Krumholz HM. Epidemiologic and economic impact of advanced heart failure. J Cardiovasc Nurs. 1996;10(2):1–10. [DOI] [PubMed] [Google Scholar]
- 8.Rich MW, Beckham V, Wittenberg C, Leven CL, Freedland KE, Carney RM. A multidisciplinary intervention to prevent the readmission of elderly patients with congestive heart failure. N Engl J Med. 1995;333(18):1190–1195. [DOI] [PubMed] [Google Scholar]
- 9.Stewart S, Pearson S, Horowitz JD. Effects of a home-based intervention among patients with congestive heart failure discharged from acute hospital care. Arch Intern Med. 1998;158(10):1067–1072. [DOI] [PubMed] [Google Scholar]
- 10.Jaarsma T, Halfens R, Huijer Abu-Saad H, et al. Effects of education and support on self-care and resource utilization in patients with heart failure. Eur Heart J. 1999;20(9):673–682. [DOI] [PubMed] [Google Scholar]
- 11.Lin R, Gallagher R, Spinaze M, et al. Effect of a patient-directed discharge letter on patient understanding of their hospitalisation. Intern Med J. 2014;44(9):851–857. [DOI] [PubMed] [Google Scholar]
- 12.Fallis BA, Dhalla IA, Klemensberg J, Bell CM. Primary medication non-adherence after discharge from a general internal medicine service. PLoS One. 2013;8(5):e61735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jones CD, Vu MB, O’Donnell CM, et al. A failure to communicate: a qualitative exploration of care coordination between hospitalists and primary care providers around patient hospitalizations. J Gen Intern Med. 2015;30(4):417–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haggerty JL, Roberge D, Freeman GK, Beaulieu C. Experienced continuity of care when patients see multiple clinicians: a qualitative metasummary. Ann Fam Med. 2013;11(3):262–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McMillan A, Trompeter J, Havrda D, Fox J. Continuity of care between family practice physicians and hospitalist services. J Healthc Qual. 2013;35(1):41–49. [DOI] [PubMed] [Google Scholar]
- 16.Tan B, Mulo B, Skinner M. Transition from hospital to primary care: an audit of discharge summary—medication changes and follow-up expectations. Intern Med J. 2014;44(11):1124–1127. [DOI] [PubMed] [Google Scholar]
- 17.Coleman EA, Boult C; American Geriatrics Society Health Care Systems Committee. Improving the quality of transitional care for persons with complex care needs. J Am Geriatr Soc. 2003;51(4):556–557. [DOI] [PubMed] [Google Scholar]
- 18.Naylor MD. A decade of transitional care research with vulnerable elders. J Cardiovasc Nurs. 2000;14(3):1–14, quiz 88–89. [DOI] [PubMed] [Google Scholar]
- 19.Stewart S, Marley JE, Horowitz JD. Effects of a multidisciplinary, home-based intervention on unplanned readmissions and survival among patients with chronic congestive heart failure: a randomised controlled study. Lancet. 1999;354(9184):1077–1083. [DOI] [PubMed] [Google Scholar]
- 20.Alexander JA, Paustian M, Wise CG, et al. Assessment and measurement of patient-centered medical home implementation: the BCBSM experience. Ann Fam Med. 2013;11(Suppl 1):S74–S81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Feltner C, Jones CD, Cené CW, et al. Transitional care interventions to prevent readmissions for persons with heart failure: a systematic review and meta-analysis. Ann Intern Med. 2014;160(11):774–784. [DOI] [PubMed] [Google Scholar]
- 22.Takeda A, Taylor SJ, Taylor RS, Khan F, Krum H, Underwood M. Clinical service organisation for heart failure. Cochrane Database Syst Rev. 2012;9:CD002752. [DOI] [PubMed] [Google Scholar]
- 23.Pandor A, Gomersall T, Stevens JW, et al. Remote monitoring after recent hospital discharge in patients with heart failure: a systematic review and network meta-analysis. Heart. 2013;99(23):1717–1726. [DOI] [PubMed] [Google Scholar]
- 24.Phillips CO, Wright SM, Kern DE, Singa RM, Shepperd S, Rubin HR. Comprehensive discharge planning with postdischarge support for older patients with congestive heart failure: a meta-analysis. JAMA. 2004;291(11):1358–1367. [DOI] [PubMed] [Google Scholar]
- 25.Higgins J, Green S, eds. Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 Baltimore, MD: The Cochrane Collaboration; 2011. http://www.cochrane-handbook.org. Updated Mar 2011. [Google Scholar]
- 26.Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg. 2010;8(5):336–341. [DOI] [PubMed] [Google Scholar]
- 27.Downs SH, Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J Epidemiol Community Health. 1998;52(6):377–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Olivo SA, Macedo LG, Gadotti IC, Fuentes J, Stanton T, Magee DJ. Scales to assess the quality of randomized controlled trials: a systematic review. Phys Ther. 2008;88(2):156–175. [DOI] [PubMed] [Google Scholar]
- 29.Peek ME, Cargill A, Huang ES. Diabetes health disparities: a systematic review of health care interventions. Med Care Res Rev. 2007; 64(5)(Suppl):101S–156S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33(1):159–174. [PubMed] [Google Scholar]
- 31.Shepperd S, Lewin S, Straus S, et al. Can we systematically review studies that evaluate complex interventions? PLoS Med. 2009;6(8): e1000086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Meeks J. A social work case management experience in a managed care setting: the need for effective communication. Home Health Care Manage Pract. 2001;13(6):444–451. [Google Scholar]
- 33.Wong FK, Chow SK, Chan TM, Tam SK. Comparison of effects between home visits with telephone calls and telephone calls only for transitional discharge support: a randomised controlled trial. Age Ageing. 2014;43(1):91–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Happ MB, Naylor MD, Roe-Prior P. Factors contributing to rehospitalization of elderly patients with heart failure. J Cardiovasc Nurs. 1997;11(4):75–84. [DOI] [PubMed] [Google Scholar]
- 35.Naylor MD, Brooten DA, Campbell RL, Maislin G, McCauley KM, Schwartz JS. Transitional care of older adults hospitalized with heart failure: a randomized, controlled trial. J Am Geriatr Soc. 2004;52(5): 675–684. [DOI] [PubMed] [Google Scholar]
- 36.Aoki Y, Inokuchi R, Gunshin M, Yahagi N, Suwa H. Diffusion tensor imaging studies of mild traumatic brain injury: a meta-analysis. J Neurol Neurosurg Psychiatry. 2012;83(9):870–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Riegel B, Carlson B, Glaser D, Romero T. Randomized controlled trial of telephone case management in Hispanics of Mexican origin with heart failure. J Card Fail. 2006;12(3):211–219. [DOI] [PubMed] [Google Scholar]
- 38.DeBusk RF, Miller NH, Parker KM, et al. Care management for low-risk patients with heart failure: a randomized, controlled trial. Ann Intern Med. 2004;141(8):606–613. [DOI] [PubMed] [Google Scholar]
- 39.Laramee AS, Levinsky SK, Sargent J, Ross R, Callas P. Case management in a heterogeneous congestive heart failure population: a randomized controlled trial. Arch Intern Med. 2003;163(7):809–817. [DOI] [PubMed] [Google Scholar]
- 40.Wakefield BJ, Ward MM, Holman JE, et al. Evaluation of home telehealth following hospitalization for heart failure: a randomized trial. Telemed J E Health. 2008;14(8):753–761. [DOI] [PubMed] [Google Scholar]
- 41.Dunagan WC, Littenberg B, Ewald GA, et al. Randomized trial of a nurse-administered, telephone-based disease management program for patients with heart failure. J Card Fail. 2005;11(5):358–365. [DOI] [PubMed] [Google Scholar]
- 42.Rainville EC. Impact of pharmacist interventions on hospital readmissions for heart failure. Am J Health Syst Pharm. 1999;56(13):1339–1342. [PubMed] [Google Scholar]
- 43.Barth V. A nurse-managed discharge program for congestive heart failure patients: outcomes and costs. Home Health Care Manage Pract. 2001;13(6):436–443. [Google Scholar]
- 44.Chaudhry SI, Mattera JA, Curtis JP, et al. Telemonitoring in patients with heart failure. N Engl J Med. 2010;363(24):2301–2309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kasper EK, Gerstenblith G, Hefter G, et al. A randomized trial of the efficacy of multidisciplinary care in heart failure outpatients at high risk of hospital readmission. J Am Coll Cardiol. 2002;39(3):471–480. [DOI] [PubMed] [Google Scholar]
- 46.Angermann CE, Störk S, Gelbrich G, et al. ; Competence Network Heart Failure. Mode of action and effects of standardized collaborative disease management on mortality and morbidity in patients with systolic heart failure: the Interdisciplinary Network for Heart Failure (INH) study. Circ Heart Fail. 2012;5(1):25–35. [DOI] [PubMed] [Google Scholar]
- 47.Goldberg LR, Piette JD, Walsh MN, et al. ; WHARF Investigators. Randomized trial of a daily electronic home monitoring system in patients with advanced heart failure: the Weight Monitoring in Heart Failure (WHARF) trial. Am Heart J. 2003;146(4):705–712. [DOI] [PubMed] [Google Scholar]
- 48.Rich MW, Vinson JM, Sperry JC, et al. Prevention of readmission in elderly patients with congestive heart failure: results of a prospective, randomized pilot study. J Gen Intern Med. 1993;8(11):585–590. [DOI] [PubMed] [Google Scholar]
- 49.Pugh LC, Havens DS, Xie S, Robinson JM, Blaha C. Case management for elderly persons with heart failure: the quality of life and cost outcomes. Medsurg Nurs. 2001;10(2):71–78. [Google Scholar]
- 50.Bowles KH, Hanlon AL, Glick HA, Naylor MD, O’Connor M, Riegel B, et al. Clinical effectiveness, access to, and satisfaction with care using a telehomecare substitution intervention: a randomized controlled trial. Int J Telemed Appl. 2011;2011:540138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kulshreshtha A, Kvedar JC, Goyal A, Halpern EF, Watson AJ. Use of remote monitoring to improve outcomes in patients with heart failure: a pilot trial. Int J Telemed Appl. 2010;2010:870959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pekmezaris R, Mitzner I, Pecinka KR, et al. The impact of remote patient monitoring (telehealth) upon Medicare beneficiaries with heart failure. Telemed J E Health. 2012;18(2):101–108. [DOI] [PubMed] [Google Scholar]
- 53.Tsuyuki RT, Fradette M, Johnson JA, et al. A multicenter disease management program for hospitalized patients with heart failure. J Card Fail. 2004;10(6):473–480. [DOI] [PubMed] [Google Scholar]
- 54.Ducharme A, Doyon O, White M, Rouleau JL, Brophy JM. Impact of care at a multidisciplinary congestive heart failure clinic: a randomized trial. CMAJ. 2005;173(1):40–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Harrison MB, Browne GB, Roberts J, Tugwell P, Gafni A, Graham ID. Quality of life of individuals with heart failure: a randomized trial of the effectiveness of two models of hospital-to-home transition. Med Care. 2002;40(4):271–282. [DOI] [PubMed] [Google Scholar]
- 56.Domingues FB, Clausell N, Aliti GB, Dominguez DR, Rabelo ER. Education and telephone monitoring by nurses of patients with heart failure: randomized clinical trial. Arq Bras Cardiol. 2011;96(3):233–239. [DOI] [PubMed] [Google Scholar]
- 57.Jaarsma T, van der Wal MH, Lesman-Leegte I, et al. ; Coordinating Study Evaluating Outcomes of Advising and Counseling in Heart Failure (COACH) Investigators. Effect of moderate or intensive disease management program on outcome in patients with heart failure: Coordinating Study Evaluating Outcomes of Advising and Counseling in Heart Failure (COACH). Arch Intern Med. 2008;168(3):316–324. [DOI] [PubMed] [Google Scholar]
- 58.Cleland JG, Louis AA, Rigby AS, Janssens U, Balk AH; TEN-HMS Investigators. Noninvasive home telemonitoring for patients with heart failure at high risk of recurrent admission and death: the Trans-European Network-Home-Care Management System (TEN-HMS) study. J Am Coll Cardiol. 2005;45(10):1654–1664. [DOI] [PubMed] [Google Scholar]
- 59.Nucifora G, Albanese MC, De Biaggio P, et al. Lack of improvement of clinical outcomes by a low-cost, hospital-based heart failure management programme. J Cardiovasc Med (Hagerstown). 2006; 7(8):614–622. [DOI] [PubMed] [Google Scholar]
- 60.Del Sindaco D, Pulignano G, Minardi G, et al. Two-year outcome of a prospective, controlled study of a disease management programme for elderly patients with heart failure. J Cardiovasc Med (Hagerstown). 2007;8(5):324–329. [DOI] [PubMed] [Google Scholar]
- 61.Giordano A, Scalvini S, Zanelli E, et al. Multicenter randomised trial on home-based telemanagement to prevent hospital readmission of patients with chronic heart failure. Int J Cardiol. 2009;131(2):192–199. [DOI] [PubMed] [Google Scholar]
- 62.López Cabezas C, Falces Salvador C, Cubí Quadrada D, et al. Randomized clinical trial of a postdischarge pharmaceutical care program vs regular follow-up in patients with heart failure. Farm Hosp. 2006;30(6):328–342. [DOI] [PubMed] [Google Scholar]
- 63.Atienza F, Anguita M, Martinez-Alzamora N, et al. ; PRICE Study Group. Multicenter randomized trial of a comprehensive hospital discharge and outpatient heart failure management program. Eur J Heart Fail. 2004;6(5):643–652. [DOI] [PubMed] [Google Scholar]
- 64.Ekman I, Andersson B, Ehnfors M, Matejka G, Persson B, Fagerberg B. Feasibility of a nurse-monitored, outpatient-care programme for elderly patients with moderate-to-severe, chronic heart failure. Eur Heart J. 1998;19(8):1254–1260. [DOI] [PubMed] [Google Scholar]
- 65.Cline CM, Israelsson BY, Willenheimer RB, Broms K, Erhardt LR. Cost effective management programme for heart failure reduces hospitalisation. Heart. 1998;80(5):442–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dar O, Riley J, Chapman C, et al. A randomized trial of home telemonitoring in a typical elderly heart failure population in North West London: results of the Home-HF study. Eur J Heart Fail. 2009; 11(3):319–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Blue L, Lang E, McMurray JJ, et al. Randomised controlled trial of specialist nurse intervention in heart failure. BMJ. 2001;323(7315): 715–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Leventhal ME, Denhaerynck K, Brunner-La Rocca HP, et al. Swiss Interdisciplinary Management Programme for Heart Failure (SWIM-HF): a randomised controlled trial study of an outpatient inter-professional management programme for heart failure patients in Switzerland. Swiss Med Wkly. 2011;141:w13171. [DOI] [PubMed] [Google Scholar]
- 69.Adlbrecht C, Huelsmann M, Berger R, et al. Cost analysis and cost-effectiveness of NT-proBNP-guided heart failure specialist care in addition to home-based nurse care. Eur J Clin Invest. 2011;41(3): 315–322.21070222 [Google Scholar]
- 70.Doughty RN, Wright SP, Pearl A, et al. Randomized, controlled trial of integrated heart failure management: The Auckland Heart Failure Management Study. Eur Heart J. 2002;23(2):139–146. [DOI] [PubMed] [Google Scholar]
- 71.Barker A, Barlis P, Berlowitz D, Page K, Jackson B, Lim WK. Pharmacist directed home medication reviews in patients with chronic heart failure: a randomised clinical trial. Int J Cardiol. 2012;159(2):139–143. [DOI] [PubMed] [Google Scholar]
- 72.Kwok T, Lee J, Woo J, Lee DT, Griffith S. A randomized controlled trial of a community nurse-supported hospital discharge programme in older patients with chronic heart failure. J Clin Nurs. 2008;17(1):109–117. [DOI] [PubMed] [Google Scholar]
- 73.Thompson DR, Roebuck A, Stewart S. Effects of a nurse-led, clinic and home-based intervention on recurrent hospital use in chronic heart failure. Eur J Heart Fail. 2005;7(3):377–384. [DOI] [PubMed] [Google Scholar]
- 74.Leppin AL, Gionfriddo MR, Kessler M, et al. Preventing 30-day hospital readmissions: a systematic review and meta-analysis of randomized trials. JAMA Intern Med. 2014;174(7):1095–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Desai AS, Stevenson LW. Rehospitalization for heart failure: predict or prevent? Circulation. 2012;126(4):501–506. [DOI] [PubMed] [Google Scholar]
- 76.McAlister FA, Youngson E, Bakal JA, Kaul P, Ezekowitz J, van Walraven C. Impact of physician continuity on death or urgent readmission after discharge among patients with heart failure. CMAJ. 2013; 185(14):E681–E689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rose KD, Ross JS, Horwitz LI. Advanced access scheduling outcomes: a systematic review. Arch Intern Med. 2011;171(13):1150–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yemm R, Bhattacharya D, Wright D, Poland F. What constitutes a high quality discharge summary? A comparison between the views of secondary and primary care doctors. Int J Med Educ. 2014;5:125–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Callahan CM, Boustani MA, Unverzagt FW, et al. Effectiveness of collaborative care for older adults with Alzheimer disease in primary care: a randomized controlled trial. JAMA. 2006;295(18):2148–2157. [DOI] [PubMed] [Google Scholar]
- 80.Béland F, Bergman H, Lebel P, et al. A system of integrated care for older persons with disabilities in Canada: results from a randomized controlled trial. J Gerontol A Biol Sci Med Sci. 2006;61(4):367–373. [DOI] [PubMed] [Google Scholar]
- 81.Inglis S, McLennan S, Dawson A, et al. A new solution for an old problem? Effects of a nurse-led, multidisciplinary, home-based intervention on readmission and mortality in patients with chronic atrial fibrillation. J Cardiovasc Nurs. 2004;19(2):118–127. [DOI] [PubMed] [Google Scholar]
- 82.Berger R, Moertl D, Peter S, et al. N-terminal pro-B-type natriuretic peptide-guided, intensive patient management in addition to multidisciplinary care in chronic heart failure a 3-arm, prospective, randomized pilot study. J Am Coll Cardiol. 2010;55(7):645–653. [DOI] [PubMed] [Google Scholar]
- 83.Blaha C, Robinson JM, Pugh LC, Bryan Y, Havens DS. Longitudinal nursing case management for elderly heart failure patients: notes from the field. Nurs Case Manag. 2000;5(1):32–36. [PubMed] [Google Scholar]