Abstract
Purpose
MRI-guidance may improve the accuracy of Gleason score (GS) determination by directing the biopsy to regions of interest (ROI) likely to harbor high-grade prostate cancer (CaP). The aim of this study was to determine the frequency and predictors of GS upgrading when a subsequent MRI-guided biopsy is performed on patients with a diagnosis of GS 6 disease on the basis of conventional, transrectal ultrasound (TRUS)-guided biopsy.
Methods
A consecutive series of 245 men with a diagnosis of low-risk CaP (i.e., cT1c, GS 6, PSA<10) based on TRUS-guided biopsy was enrolled in an active surveillance protocol that used subsequent MRI-guided biopsy for confirmation of GS. ROIs were categorized on a scale of 1 to 5. The Artemis ultrasound-MRI fusion device was used to perform targeted biopsies of ROIs, as well as systematic biopsies from a software-based 12-point map. Predictors of GS upgrading were analyzed using univariate and multivariate analyses.
Results
Fusion biopsy resulted in 26% of patients having GS upgrading (GS 3+4 in 18%, 4+3 in 5%, and 8–9 in 3%). Of the 72% of patients with ROIs appropriate for targeting, targeted cores upgraded the GS in 18%, while systematic cores upgraded the GS in 24%. In patients without targeted biopsy, GS upgrading was seen in 14%. On multivariate analysis, a category 5 ROI was the most significant predictor of GS upgrading with an odds ratio of 10.56 (p<0.01).
Conclusion
Nearly 25% of men with GS 6 CaP diagnosed by standard TRUS biopsy may experience GS upgrading when a subsequent MRI-ultrasound fusion biopsy is performed. The most important single predictor of upgrading is a category 5 ROI on mpMRI. GS upgrading may influence treatment decisions. Therefore, MRI-guided biopsy should be considered prior to formulating a management strategy in patients whose conventional biopsy reveals low-risk CaP.
Keywords: prostate cancer, multiparametric MRI, targeted biopsy
INTRODUCTION
Accurate Gleason score (GS) determination is critical for risk stratification and management decisions for patients with prostate cancer (CaP) (1). For patients undergoing radiotherapy, accurate GS determination is challenging since the actual highest GS will not be ultimately confirmed on a prostatectomy specimen. Conventional transrectal ultrasound (TRUS) guided biopsies may underestimate the whole-organ GS in 30–36% of cases (2). To ensure appropriate treatment recommendations, methods to improve the accuracy of biopsy are needed. Strategies have included extended core, saturation, and targeted biopsies of regions of interest (ROIs) found on multiparametric MRI (mpMRI) (3–11). Targeted biopsies of these ROIs have been shown to upgrade GS in 17–32% of patients (7, 12–14). This relatively high rate of upgrading suggests a role for mpMRI in the evaluation of patients with CaP.
A program was initiated at our institution in 2009 wherein all patients enrolled in active surveillance undergo confirmatory biopsy using mpMR-US fusion. In the fusion biopsy, both 12-point systematic samples and targeted samples of MRI-identified ROIs are obtained using the Artemis MRI-ultrasound fusion system (5, 7). We sought to identify the frequency and predictors of GS upgrading beyond GS 6, following confirmatory biopsy with the fusion system.
MATERIALS AND METHODS
Patient Selection
The study population consisted of 245 men with a prior diagnosis of GS 6 CaP, on the basis of a conventional TRUS biopsy, who were referred for enrollment in an active surveillance protocol between July 2009 and March 2014. In order to be eligible for this protocol, all men were required to have had at least 10 cores assessed on systematic, TRUS biopsy. A preliminary study of 113 men from the UCLA active surveillance cohort, examining the role of confirmatory mpMRI-ultrasound fusion biopsy, was reported previously (15). Those patients are included in this report.
Multiparametric MRI and Lesion Scoring
As part of our active surveillance protocol, all patients underwent an mpMRI followed by an mpMRI-ultrasound fusion biopsy as described previously (7, 16). mpMRI was performed with a 3.0 T Siemens Magnetom Trio with body coil and included T2-weighted, diffusion-weighted, and dynamic contrast-enhanced imaging. mpMRI was performed one to eight weeks prior to fusion biopsy and reviewed on an Invivo DynaCAD or iCAD VersaVue workstation by a single uroradiologist with nine years of prostate MRI experience (YY). mpMRI was performed at least three months after any prior biopsy.
T2-weighted imaging provides the best tissue contrast for detection of CaP, but can be inflammation and prostatic hyperplasia can be false positives. Because the free motion of water is restricted in cancerous tissue, diffusion-weighted imaging (DWI) can improve the specificity of CaP detection (17). Finally, dynamic contrast enhancement (DCE) imaging utilizes a T1-contrast enhanced sequence, and the enhanced contrast arrival (“wash-in”) and dispersion (“wash-out”) in tumors is sensitive and specific for CaP(18).
Suspicious ROIs were assessed on a scale between 1–5, with higher categories indicative of higher suspicion for CaP. This scale has been described previously and is similar to the PI-RADS scoring system (19). Briefly, an image-grade is assigned to each of the three sequences, with double-weighting given to DWI sequencing based on the apparent diffusion co-efficient. The criteria for each grade are shown in Table 1. An average of the image-grade for each parameter is taken to create an overall image grade. For example, if a region of interest was moderately dark on T2-weight imaging (i.e., a grade of 3), had an apparent diffusion coefficient value of 0.7 mm2/s (i.e., a grade of 4), and had a dynamic-contrast enhancing sequencing that showed moderately abnormal enhancement (i.e., a grade of 3), the overall category would be (3+4+4+3)/4, or 3.5. The category is rounded up if the region of interest is in the peripheral zone, in this case giving it an overall category of 4, and rounded down if the region is in the transition zone, in this case giving it an overall category of 3 (19). All reporting was adherent to the Standards of Reporting for MRI Targeted biopsy studies (START) criteria (20).
Table 1.
UCLA scoring system for assigning level of suspicion to regions of interest on multi-parametric MRI
| Image Grade | T2WI | ADC (mm2/s) | DCE |
|---|---|---|---|
| 1 | Normal | >1400 | Progressive (type I) |
| 2 | Indistinct, wedge-shaped, highly heterogeneous, or encapsulated border (transitional zone) | 1200–1400 | Early with plateau (type II) or progressive but intense (>200%) |
| 3 | Masslike but faint or slightly heterogeneous | 1000–1200 | Early with washout (type III) or early/plateau and intense |
| 4 | Blurred borders or uniform low signal with distinct borders | 800–1000 | Early and intense with washout (type III and >200%) |
| 5 | Invasion into capsule or other compartment | <800 | Early & intense with immediate washout |
T2WI, T2-weighted imaging; ADC, apparent diffusion coefficient; DCE, dynamic contrast-enhanced imaging
mpMRI-Ultrasound Fusion Biopsy
The fusion biopsy protocol has been described previously (7). Biopsies were performed transrectally, under local anesthesia, and in an outpatient setting by a single urologist (ZZ). The MRI was loaded via CD into the Artemis device (Eigen, Grass Valley, CA). A TRUS (Hitachi Hi-Vision 5500) was performed, and a 3D prostate reconstruction was generated by the device, utilizing an algorithm incorporating both rigid and elastic registration.
A 12-point systematic mapping biopsy plan (bilateral medial/lateral, apex/mid/base), was scaled onto the 3D prostate reconstruction by the device, along with any ROIs. Targeted biopsy cores were obtained, followed by systematic biopsy cores. Targets were biopsied at 3 mm intervals based on prior work on registration accuracy with the device (5).
Statistical Analysis
Standard descriptive statistics were used to evaluate variables in various subsets. The primary method of analysis was logistic regression using GS ≥7 as the main outcome variable. A secondary analysis used primary Gleason grade ≥4 or more as the outcome variable. Several logistic models were evaluated and compared using stepwise procedures as well as models designed to test specific hypothesis. Goodness of fit was evaluated using the Hosmer-Lemeshow statistic and the area under the receiver operator characteristic (ROC) curve was used as a quantified measure of the strength of associations.
RESULTS
Patient Characteristics
Patient characteristics are listed in Table 2. The mean age at diagnosis was 64 years old and the median PSA was 4.8 ng/ml (interquartile range, 2.9–6.7). Mean time from diagnosis to mpMRI-ultrasound fusion biopsy ranged from three to 125 months (average, 15.5 months). On average, each patient had 1.5 MRI based targets and the mean ROI category for identified targets was 2.3. The average total number of cores taken was 15 (12 systematic and 3.6 targeted).
Table 2.
Patient characteristics
| n | 245 |
|---|---|
|
| |
| Mean age at diagnosis (SD) | 64 (7.4) |
|
| |
| Median PSA (ng/mL) (SD; Interquartile Range) | 4.8 (3.65; 2.9–6.7) |
|
| |
| Months from diagnosis to ARTEMIS biopsy (SD) | 15.5 (18.1) |
|
| |
| Mean MRI prostate volume (cc) (SD) | 50 (23) |
|
| |
| Mean # of MRI based targets (SD) | 1.5 (0.6) |
|
| |
| Mean # of total cores (SD) | 15 (3.1) |
| Outside systematic (SD) | 12.4 (3.0) |
| Artemis Systematic (SD) | 12 (1.5) |
| Artemis Targeted (SD) | 3.6 (2.9) |
|
| |
| Mean maximum tumor diameter (mm) | 3.5 (3.6) |
|
| |
| Mean number of previous biopsies | 1.5 (0.9) |
|
| |
| Mean MRI score | 2.3 (1.7) |
Biopsy Results
Results of the mpMRI-ultrasound fusion biopsies are shown in Table 3. A sample MRI from a patient found to have a category 5 ROI is shown in Figure 1. In 182 patients (74%) of cases, the combination of targeted and systematic biopsies yielded a diagnosis of GS 6 disease. In 18% of cases the combination of targeted and systematic biopsies yielded GS 3+4=7 disease and in another 5% the GS was upgraded to 4+3=7. Three percent of patients were upgraded to having GS 8 or 9 disease.
Table 3.
Gleason scores based off of ARTEMIS targeted and systematic biopsies
| GS | Maximum GS of targeted biopsy | Maximum GS of ARTEMIS systematic biopsy | Maximum GS of targeted or systematic |
|---|---|---|---|
| No Cancer | 112 (46%) | 109 (45%) | 92 (38%) |
| 6 | 33 (13%) | 84 (34%) | 90 (37%) |
| 3+4 | 22 (9%) | 38 (16%) | 44 (18%) |
| 4+3 | 5 (2%) | 8 (3%) | 11 (5%) |
| 8 or 9 | 4 (2%) | 6 (2%) | 8 (3%) |
| Total # of pts | 176* | 245 | 245 |
69 patients did not have any targets on mpMRI and so did not have targeted biopsies Percentages are based n=245
FIGURE 1.
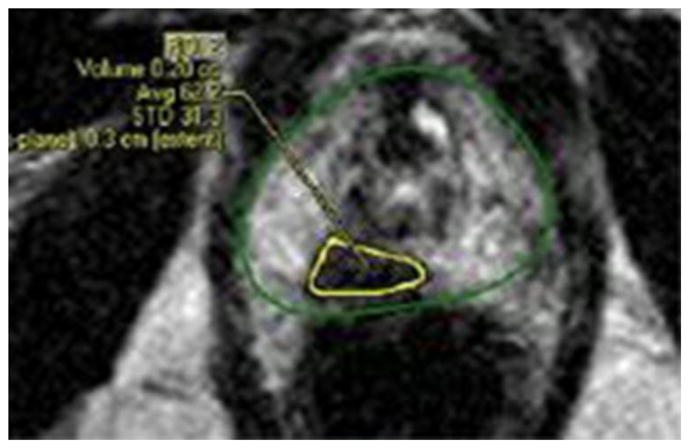
mpMRI from a 76 year-old-man with 1/12 cores on TRUS biopsy showing GS 6 CaP. This T2-weighted image demonstrates a category 5 ROI in the right peripheral apex (green, prostate; yellow, ROI). mpMRI-ultrasound fusion biopsy found 3/5 targeted cores in this area to contain GS 3+4 CaP. He ultimately underwent definitive radiotherapy.
69/245 (28%) patients did not have an ROI that could be targeted by biopsy (all of these were category ≤ 3) and thus only underwent mapping biopsy. For 14% of these patients, mapping biopsy upgraded the GS. Among patients for whom targeted biopsies were done (n=176), the targeted cores upgraded the GS in 18% while the mapping cores upgraded the GS in 24% of cases.
Predictors of Upgrading
In order to evaluate predictors of GS upgrading we analyzed the rate of upgrading by ROI category. Eighty (32.6%), 97 (39.6%), 57 (23.3%) and 11 (4.5%) patients had categories 1–2, 3, 4, and 5 ROIs, respectively. A GS upgrade was found in 73% of men with a category 5 ROI, 30% of men with a category 4 ROI, and 25% of men with a category 3 ROI.
Given the strong influence of ROI category on GS upgrading, univariate analysis was performed excluding patients with ROI categoriess of 5 or <3. The univariate analysis included PSA, ROI categories 3 or 4, prostate volume (on MRI), maximum tumor diameter (on MRI), total number of cores (targeted plus systematic), total number of systematic cores, total number of targeted cores, time from diagnosis to mpMRI-ultrasound fusion biopsy, and number of previous biopsies. The results of the univariate analysis are presented in Table 4 and demonstrate MRI ROI category, PSA, prostate volume, and total number of targeted cores are statistically significant in predicting the probability of a GS upgrade. The time between diagnosis and repeat biopsy was not associated with upgrading.
Table 4.
Univariate and multivariate logistic regression analysis for predictors of GS upgrading
| Variable | Odds Ratio (95% confidence interval) | p-value |
|---|---|---|
| PSA | 1.15 (1.06–1.26) | 0.0008 |
| MRI prostate volume | 0.98 (0.96–0.99) | 0.002 |
| ROI category 3 or 4 | 1.93 (0.96–3.86) | 0.05 |
| MRI maximum tumor diameter | 1.06 (0.98–1.14) | 0.11 |
| Total number of cores (targeted + systematic) | 1.03 (0.93–1.15) | 0.50 |
| Number of systematic cores | 0.82 (0.63–1.07) | 0.11 |
| Number of targeted cores | 1.12 (1.01–1.25) | 0.03 |
| Time from diagnosis to Artemis biopsy | 1.00 (1.02–0.48) | 0.67 |
| Number of previous biopsies | 0.97 (0.71–1.24) | 0.89 |
| Multivariate Analysis | ||
|---|---|---|
| Variable | Odds Ratio (95% confidence interval) | p-value |
| PSA | 1.24 (1.12–1.37) | <0.001 |
| Prostate Volume | 0.96 (0.94–0.98) | <0.001 |
| ROI category 3 | 1.91 (0.83–4.35) | 0.128 |
| ROI category 4 | 2.15 (0.87–5.35) | 0.098 |
| ROI category 5 | 10.56 (2.05–54.22) | 0.005 |
This model excluded the n=11 patients with category 5 ROIs
This model includes patients with category 5 ROIs
For the multivariate analysis we included the variables PSA at diagnosis, MRI prostate volume, and highest ROI category (3, 4, or 5). The results are shown in Table 4. PSA at diagnosis and having an ROI category 5 target were significant positive predictors of upgrading (odds ratios (ORs) of 1.24 and 10.56, respectively), while prostate volume was a significant negative predictor of upgrading (odds ratio of 0.96). No interaction effect was seen between ROI category and any other parameter. The logistic model created on the basis of these data has a goodness-of-fit chi-squared value of 0.5249 and the area under the ROC curve is 0.7671, suggesting this is a valid and fair model. Since the ORs for GS upgrading with having a highest ROI categories of 3 or 4 were similar, we repeated our analyses with the variable “highest ROI categories 3 or 4” included along with PSA, prostate volume, and highest ROI category of 5. The ORs were 1.99 for highest ROI category of 3 or 4, and were unchanged for the other variables.
Based on the first multivariate model, we created four example patients all with the same initial PSA of 5 ng/mL but with varying prostate volumes and ROI categories (Table 5). Per this model, patient #1, with a PSA of 5, a gland volume of 45, and a category 5 ROI has a 67% chance of having his GS upgraded. With a category 4 ROI, this chance drops to 28%.
Table 5.
Probability of upgrading based off of patient parameters
| Patient | PSA | MRI Prostate Volume (cc) | ROI MRI score | Probability of GS upgrading |
|---|---|---|---|---|
| 1 | 5 | 45 | 5 | 0.6739 |
| 2 | 5 | 45 | 3/4 | 0.2806 |
| 3 | 5 | 55 | 5 | 0.584 |
| 4 | 5 | 55 | 3/4 | 0.2095 |
ROI = Region of interest
GS = Gleason score
DISCUSSION
Biopsy-derived GS drives risk stratification and treatment recommendations for patients undergoing radiation therapy; however, conventional TRUS-guided biopsies frequently underestimate the true GS. By directly sampling ROIs corresponding to the regions at highest risk of harboring high-grade disease, mpMRI-ultrasound fusion biopsies can improve diagnostic accuracy and thus potentially alter treatment planning.
In the present study, we found that 25% of patients with a diagnosis of GS 6 disease based on conventional TRUS biopsies were upgraded to GS ≥7 disease on subsequent mpMRI-ultrasound fusion biopsies. This rate of upgrading is consistent with other studies, which have reported upgrading rates of 17–32% (7, 12–14). A recent meta-analysis demonstrated that MRI-guided targeted biopsies had a relative sensitivity of 120% compared with TRUS biopsies for the detection of significant CaP (21). Knowing the true GS can influence radiotherapy recommendations (i.e. androgen deprivation therapy and/or pelvic nodal treatment), particularly with recent data suggesting the importance of sub-stratifying intermediate risk CaP based on, among other factors, the primary Gleason grade (22). Further, while active surveillance protocols have yielded excellent long term results in carefully selected patients, over a third of patients at 10 years are no longer on active surveillance (23).
While the National Comprehensive Cancer Network (NCCN) does not currently recommend mpMRI in the evaluation of GS 6 CaP, our data suggest an important role for mpMRI in identifying patients who would benefit from GS upgrading on an mpMRI-ultrasound fusion biopsy. For example, our model predicts that a patient with a prostate volume of 45 cc, PSA of 5 ng/ml, and a category 5 ROI has a 67% risk of a GS upgrade. The predictive value of ROI category is consistent with our prior published experience in patients meeting the Epstein criteria for very low risk CaP, in which the OR for GS upgrading was 3.2 for patients with category 4 or 5 ROIs (15). Another study of patients eligible for active surveillance similarly identified lesion suspicion, in addition to number of lesions and lesion density, as predictive factors for GS upgrading (14). A recent prospective study found that the identification of highly suspicious lesions with mpMRI (i.e., score of 4 or 5 using the Prostate Imaging Reporting and Data System (PI-RADS) score) in patients on active surveillance had a sensitivity of 92% for detecting primary Gleason grade 4 or 5 disease; conversely, the identification of PI-RADS 1 or 2 lesions had a negative predictive value of 84% for detecting any CaP (24). Finally, a recent randomized trial found that, among men suspected to have CaP on the basis of signs and/or symptoms, mpMRI had an accuracy of 97% for the diagnosis of CaP and identified more patients with clinically significant CaP than TRUS-guided biopsy (25).
PSA had little predictive effect on GS upgrading (OR 1.24), while gland volume had a small but significant negative effect (OR 0.96). The minimal PSA effect may be related to its nonspecific nature and the relatively low PSA values in this cohort of patients (average 5.4 ng/ml). The negative predictive effect of prostate gland volume likely reflects that fact that a small focus of high grade cancer in a large prostate may prove evasive, even with a systematic biopsy. Second, the patients with large prostates may have lower PSA density values and may therefore have very low risk CaP.
Our study has several limitations. First, the time interval from diagnosis to mpMRI-ultrasound fusion biopsy is large, ranging from 3 to 125 months. While our model did not identify the length of this interval as a positive predictor of upgrading, the series may not have had significant power to detect an effect. Additionally, our conclusion that a category 5 ROI is a significant predictor of GS upgrading is based on the small subset of 11 patients who had category 5 ROIs. This raises the possibility of a type I error, wherein the significance of this finding is the result of statistical chance. It is worth noting that category 4 ROIs showed a trend towards significance for predicting GS upgrading, which does suggest a relationship between risk of upgrading and category of ROI. In clinical practice, the identification of a category 5 ROI should prompt a targeted biopsy, while the presence of category 4 ROIs should prompt consideration for one. Another limitation is that a single fellowship-trained genitourinary radiologist categorized the ROIs, and thus lesion grading may not be generalizable to a clinical practice with a less experienced radiologist. Additionally, ROIs were categorized on the basis of an institution specific grading scale (Table 1) (16). In fact, one of the barriers to the widespread introduction of mpMRI for CaP is the lack of consensus guidelines for scoring findings (26). The aforementioned PI-RADS scoring system has recently emerged as a standard scoring system for mpMR; the results of a recent meta-analysis suggest that this scoring system, when used correctly, can have a sensitivity of up to 82% for the detection of CaP (27, 28). Our scoring system does differ from the PI-RADS system, as we assign points on the basis of absolute apparent diffusion coefficient values, and assign points for dynamic contrast enhancing MRI appearance qualitatively rather than on the basis of kinetic curves. Another limitation is that the Artemis system allows one to plan out and view the biopsy tract trajectory of the 12 core systematic biopsies. Doing this allows for a more even sampling distribution, which may improve cancer detection rates compared to a non-Artemis based approach (29). This makes it difficult to determine whether it is the systematic or the targeted biopsies that were more important in leading to GS upgrades. In our practice, we view the systematic and targeted biopsies as complementary to one another and perform both of them on all patients with grade ≥3 ROIs. Lastly, this analysis does not include final GS data from patients who underwent radical prostatectomy; thus the true GS may still under-graded, at least for some patients (16).
CONCLUSIONS
Our results show that nearly 25% of men with GS 6 CaP as diagnosed by standard TRUS biopsies may experience GS upgrading when a subsequent mpMRI-ultrasound fusion biopsy is performed. The most significant predictor of upgrading is having a category 5 ROI on mpMRI. Further investigation into using mpMRI and targeted biopsies in the evaluation of patients is warranted to avoid under-treatment in patients presumed to have low risk CaP.
Footnotes
Conflicts of Interest Notification: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.NCCN; NCCN, editor. Prostate cancer V2. 2013. NCCN Clinical Practice Guidelines in Oncology. [Google Scholar]
- 2.Cohen MS, Hanley RS, Kurteva T, et al. Comparing the Gleason prostate biopsy and Gleason prostatectomy grading system: the Lahey Clinic Medical Center experience and an international meta-analysis. Eur Urol. 2008;54:371–381. doi: 10.1016/j.eururo.2008.03.049. [DOI] [PubMed] [Google Scholar]
- 3.Walz J, Graefen M, Chun FK, et al. High incidence of prostate cancer detected by saturation biopsy after previous negative biopsy series. Eur Urol. 2006;50:498–505. doi: 10.1016/j.eururo.2006.03.026. [DOI] [PubMed] [Google Scholar]
- 4.Hadaschik BA, Kuru TH, Tulea C, et al. A novel stereotactic prostate biopsy system integrating pre-interventional magnetic resonance imaging and live ultrasound fusion. J Urol. 2011;186:2214–2220. doi: 10.1016/j.juro.2011.07.102. [DOI] [PubMed] [Google Scholar]
- 5.ee.
- 6.Sonn GA, Chang E, Natarajan S, et al. Value of targeted prostate biopsy using magnetic resonance-ultrasound fusion in men with prior negative biopsy and elevated prostate-specific antigen. Eur Urol. 2014;65:809–815. doi: 10.1016/j.eururo.2013.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.dd.
- 8.Pinto PA, Chung PH, Rastinehad AR, et al. Magnetic resonance imaging/ultrasound fusion guided prostate biopsy improves cancer detection following transrectal ultrasound biopsy and correlates with multiparametric magnetic resonance imaging. J Urol. 2011;186:1281–1285. doi: 10.1016/j.juro.2011.05.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Taira AV, Merrick GS, Galbreath RW, et al. Performance of transperineal template-guided mapping biopsy in detecting prostate cancer in the initial and repeat biopsy setting. Prostate Cancer Prostatic Dis. 2010;13:71–77. doi: 10.1038/pcan.2009.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zaytoun OM, Moussa AS, Gao T, et al. Office based transrectal saturation biopsy improves prostate cancer detection compared to extended biopsy in the repeat biopsy population. J Urol. 2011;186:850–854. doi: 10.1016/j.juro.2011.04.069. [DOI] [PubMed] [Google Scholar]
- 11.Hambrock T, Somford DM, Hoeks C, et al. Magnetic resonance imaging guided prostate biopsy in men with repeat negative biopsies and increased prostate specific antigen. J Urol. 2010;183:520–527. doi: 10.1016/j.juro.2009.10.022. [DOI] [PubMed] [Google Scholar]
- 12.Vargas HA, Akin O, Afaq A, et al. Magnetic resonance imaging for predicting prostate biopsy findings in patients considered for active surveillance of clinically low risk prostate cancer. J Urol. 2012;188:1732–1738. doi: 10.1016/j.juro.2012.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Margel D, Yap SA, Lawrentschuk N, et al. Impact of multiparametric endorectal coil prostate magnetic resonance imaging on disease reclassification among active surveillance candidates: a prospective cohort study. J Urol. 2012;187:1247–1252. doi: 10.1016/j.juro.2011.11.112. [DOI] [PubMed] [Google Scholar]
- 14.Stamatakis L, Siddiqui MM, Nix JW, et al. Accuracy of multiparametric magnetic resonance imaging in confirming eligibility for active surveillance for men with prostate cancer. Cancer. 2013;119:3359–3366. doi: 10.1002/cncr.28216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.cc.
- 16.bb.
- 17.Park BK, Lee HM, Kim CK, et al. Lesion localization in patients with a previous negative transrectal ultrasound biopsy and persistently elevated prostate specific antigen level using diffusion-weighted imaging at three Tesla before rebiopsy. Invest Radiol. 2008;43:789–793. doi: 10.1097/RLI.0b013e318183725e. [DOI] [PubMed] [Google Scholar]
- 18.Hara N, Okuizumi M, Koike H, et al. Dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI) is a useful modality for the precise detection and staging of early prostate cancer. Prostate. 2005;62:140–147. doi: 10.1002/pros.20124. [DOI] [PubMed] [Google Scholar]
- 19.aa.
- 20.Moore CM, Kasivisvanathan V, Eggener S, et al. Standards of reporting for MRI-targeted biopsy studies (START) of the prostate: recommendations from an International Working Group. Eur Urol. 2013;64:544–552. doi: 10.1016/j.eururo.2013.03.030. [DOI] [PubMed] [Google Scholar]
- 21.Schoots IG, Roobol MJ, Nieboer D, et al. Magnetic Resonance Imaging-targeted Biopsy May Enhance the Diagnostic Accuracy of Significant Prostate Cancer Detection Compared to Standard Transrectal Ultrasound-guided Biopsy: A Systematic Review and Meta-analysis. doi: 10.1016/j.eururo.2014.11.037. LID - S0302-2838(14)01220-2 [pii] LID. [DOI] [PubMed] [Google Scholar]
- 22.Zumsteg ZS, Zelefsky MJ. Short-term androgen deprivation therapy for patients with intermediate-risk prostate cancer undergoing dose-escalated radiotherapy: the standard of care? Lancet Oncol. 2012;13:e259–269. doi: 10.1016/S1470-2045(12)70084-0. [DOI] [PubMed] [Google Scholar]
- 23.Klotz L, Vesprini D, Sethukavalan P, et al. Long-Term Follow-Up of a Large Active Surveillance Cohort of Patients With Prostate Cancer. J Clin Oncol. 2014 doi: 10.1200/JCO.2014.55.1192. [DOI] [PubMed] [Google Scholar]
- 24.Hoeks CM, Somford DM, van Oort IM, et al. Value of 3-T multiparametric magnetic resonance imaging and magnetic resonance-guided biopsy for early risk restratification in active surveillance of low-risk prostate cancer: a prospective multicenter cohort study. Invest Radiol. 2014;49:165–172. doi: 10.1097/RLI.0000000000000008. [DOI] [PubMed] [Google Scholar]
- 25.Panebianco V, Barchetti F, Sciarra A, et al. Multiparametric magnetic resonance imaging vs. standard care in men being evaluated for prostate cancer: A randomized study. doi: 10.1016/j.urolonc.2014.09.013. [DOI] [PubMed] [Google Scholar]
- 26.Dickinson L, Ahmed HU, Allen C, et al. Scoring systems used for the interpretation and reporting of multiparametric MRI for prostate cancer detection, localization, and characterization: could standardization lead to improved utilization of imaging within the diagnostic pathway? J Magn Reson Imaging. 2013;37:48–58. doi: 10.1002/jmri.23689. [DOI] [PubMed] [Google Scholar]
- 27.Barentsz JO, Richenberg J, Clements R, et al. ESUR prostate MR guidelines 2012. Eur Radiol. 2012;22:746–757. doi: 10.1007/s00330-011-2377-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hamoen EH, de Rooij M, Witjes JA, et al. Use of the Prostate Imaging Reporting and Data System (PI-RADS) for Prostate Cancer Detection with Multiparametric Magnetic Resonance Imaging: A Diagnostic Meta-analysis. doi: 10.1016/j.eururo.2014.10.033. LID - S0302-2838(14)01122-1 [pii] LID. [DOI] [PubMed] [Google Scholar]
- 29.Han M, Chang D, Kim C, et al. Geometric evaluation of systematic transrectal ultrasound guided prostate biopsy. J Urol. 2012;188:2404–2409. doi: 10.1016/j.juro.2012.07.107. [DOI] [PMC free article] [PubMed] [Google Scholar]


