Abstract
Recurrent laryngeal nerve (RLN) injury in neonates, a complication of patent ductus arteriosus corrective surgery, leads to aspiration and swallowing complications. Severity of symptoms and prognosis for recovery are variable. We transected the RLN unilaterally in an infant mammalian animal model to characterize the degree and variability of dysphagia in a controlled experimental setting. We tested the hypotheses that 1) both airway protection and esophageal function would be compromised by lesion, 2) given our design, variability between multiple post-lesion trials would be minimal, 3) variability among individuals would be minimal. Individuals' swallowing performance was assessed pre- and post-lesion using high speed VFSS. Aspiration was assessed using the Infant Mammalian Penetration Aspiration Scale (IMPAS). Esophageal function was assessed using two measures devised for this study. Our results indicate that RLN lesion leads to increased frequency of aspiration, and increased esophageal dysfunction, with significant variation in these basic patterns at all levels. On average, aspiration worsened with time post-lesion. Within a single feeding sequence, the distribution of unsafe swallows varied. Individuals changed post-lesion either by increasing average IMPAS score, or by increasing variation in IMPAS score. Unilateral RLN transection resulted in dysphagia with both compromised airway protection and esophageal function. Despite consistent, experimentally controlled injury, significant variation in response to lesion remained. Aspiration following RLN lesion was due to more than unilateral vocal fold paralysis. We suggest that neurological variation underlies this pattern.
Keywords: aspiration, recurrent laryngeal nerve, infant, esophagus, deglutition, deglutition disorders
Introduction
Recurrent laryngeal (RLN) nerve injury is a frequent complication of patent ductus arteriosus closure in premature human infants [1,2]. Complications resulting from RLN lesion include unilateral vocal fold paralysis, stridor and dysphagia of variable duration and severity [3,4]. Prognosis is variable, ranging from a few months to several years for improvements to appear [5]. Incomplete understanding of the specific effects of iatrogenic RLN damage on the effectiveness of oropharyngeal and esophageal performance hampers treatment efforts. In particular, uncertainty about the degree of nerve injury (i.e. stretch, crush, sectioning) in patients means that the source of variation in response to nerve injury is unknown [6,7]
Infant feeding is a complicated behavior involving integration of multiple oropharyngeal structures innervated by several cranial nerves [8,9]. Liquid food transport and swallowing must be carefully coordinated and controlled to ensure safe passage of the food bolus into the esophagus without compromising the airway [10]. Sensory inputs upstream (i.e. from the oral cavity and oropharynx) can modify effectiveness of airway protection [11–14]. How sensory inputs from structures downstream of the swallow site, in particular the larynx, affect the mechanism of safe swallow remains unknown.
The RLN branch of the vagus nerve is classically thought to innervate all but one (cricothyroid) of the intrinsic laryngeal muscles that move the vocal folds. Thus RLN lesion leads to inability to adduct or abduct the vocal folds, as cricothyroid can only tense them. The RLN also provides sensory innervation to the laryngeal mucosa below the vocal folds in humans [15]. The right and left recurrent laryngeal nerves follow different paths, with the right RLN looping around the subclavian artery, and the left RLN looping around the ligamentum arteriosum between the aortic arch and the pulmonary trunk.
Although RLN function is crucial in protecting the airway from aspiration through vocal fold adduction and initiation of the cough reflex [10], neither its sensory nor motor functions are directly implicated in a safe, normal swallow. Less certainly, there is some evidence to suggest that it may innervate the upper esophageal sphincter muscles to a variable degree [16]. The effect of RLN damage on infant esophageal function, if any, is unknown.
In this study, we used a validated infant pig model to test the impact of complete unilateral RLN transection on oropharyngeal and esophageal performance. The infant pig model has been extensively developed and validated for the study of mammalian swallowing [17–21]. The behaviors being examined here (infant suckling and swallowing) are shared by all infant mammals [22]. Specific reasons to use the infant pig when considering the breadth of mammalian diversity include: a cheek with a buccinator muscle, a deep fleshy tongue, and tongue movements and head position in suckling infant pigs that are closer to humans of all ages than any other non-primate species (i.e. non-lapping). Several other investigators use the infant pig as a model for studying swallowing problems [23–27].
By performing controlled, consistent nerve transections, we aim to remove the confounding factor of degree of nerve injury as a source of variation, a major limitation of clinical retrospective studies. Our aim was to determine the severity and variation in swallowing impairment resulting from unilateral RLN lesion in a controlled, repeated experimental design. We hypothesized that:
both airway protection and esophageal function would be impaired by a complete unilateral RLN transection
- variation in timing and extent of dysfunction would be minimal:
- within a feeding sequence (all swallows in one infant “meal”)
- over time within an individual, from lesion through acute assessment (48 hours)
variation among individuals with identical surgical procedures would be minimal.
Because of the use of an animal model, we were able to collect sufficient data to statistically assess performance at each of these hierarchical levels.
Materials and methods
Experimental procedures
12 infant pigs (Michael Fanning Farm, Howe, Indiana) were trained to drink milk mixed with barium from a bottle fitted with a pig nipple (NASCO Farm & Ranch, Fort Atkinson, WI). In an initial surgery to place markers and electrodes, the right RLN was identified in surgery and dissected to it distal entry into the larynx, and marked with loosely tied suture. In a subsequent lesion surgery, in order to prevent re-innervation of laryngeal muscle [28], the recurrent laryngeal nerve was ligated and crushed with hemoclips in two places, and a 1-3mm section of nerve removed between ligation sites. Nerve lesions were confirmed with post-mortem dissection at the end of the experiment. All surgery was performed under sterile conditions, with the animal intubated under anesthesia (2-5% isoflurane). All protocols were reviewed and approved by the NEOMED Institutional Animal Care and Use Committee (13-011).
Before and after surgery, pigs were recorded drinking infant pig formula (Solustart Pig Milk Replacement, Land o' Lakes, Arden Mills, MN) mixed with barium in front of a fluoroscope (GE9400 C-Arm, 80kV, 4MA. Fluoroscope images were recorded digitally at 100 fps (XC 1M digital video camera, Xcitex corporation, MA). Pigs were allowed to feed unrestricted until they chose to cease feeding. We termed these long recordings “complete feeding sequences”. Pigs were recorded before lesion, immediately post-lesion surgery (2-10h, when awake, standing, and with no remaining behavioral sequelae of anesthesia), 24h (+/- 6h), and 48h (+/- 10h) post-lesion.
Assessing swallow safety and esophageal function
All swallows in each feeding sequence were scored using the infant mammal penetration-aspiration scale (IMPAS) [29]. IMPAS scores range from 1 (safe swallow) to 7 (silent aspiration), and represent progressively more severe instances of dysphagia (Table 1). All swallows were scored by a single investigator trained to use the scale following the guidelines in [29]. The investigator was blinded to treatment condition of pigs in the video sequences she scored.
Table 1. Summary of the IMPAS scale.
| Score | What happens |
|---|---|
| 1 | Normal swallow |
| 2 | Some penetration that is cleared during the swallow |
| 3 | Some penetration that is not cleared during the swallow |
| 4 | A lot of penetration that is not cleared during the swallow |
| 5 | Aspiration with a successful attempt to clear |
| 6 | Aspiration with an unsuccessful attempt to clear |
| 7 | Aspiration with no attempt to clear |
No similar scale could be found for assessing esophageal dysfunction from videofluroscopic recordings. Thus we developed and validated two potential measures of esophageal dysfunction. Failure of Esophageal clearance was defined as the persistence of milk in the upper esophagus following initial propagation of a contractile wave after the swallow (Figure 1). Esophageal clearance was scored as a binary yes/no measure.
Figure 1.
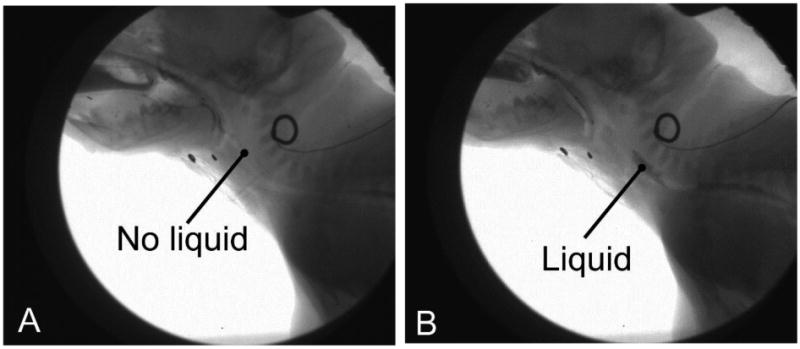
esophageal clearance failure. In a normal swallow, all fluid is cleared from the upper esophagus (A). In a failure event (B), part of the bolus remains in the upper esophagus.
Proximal escape was defined as the retrograde movement of liquid back through the upper esophageal sphincter after a swallow (Figure 2). That is, milk was seen to pass from the esophagus back into the oropharynx after initial movement into the upper esophagus, proximal escape was said to have occurred. Both of these measures were tested for consistency with cross validation of observer studies. Esophageal function scores were collected on the pre lesion and 48h post-lesion feeding sequences for all pigs.
Figure 2.
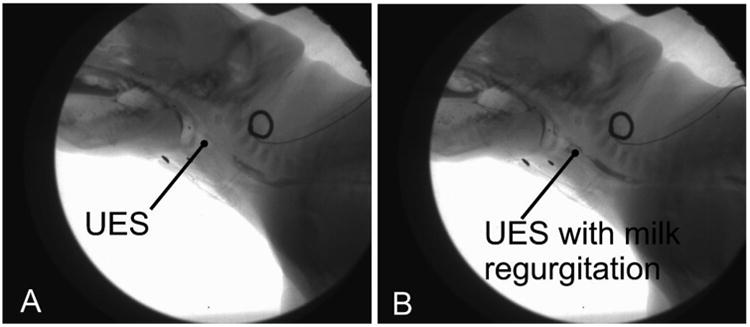
Proximal escape. In a normal swallow, there is no milk proximal to the upper esophageal sphincter (UES) after a swallow (A). In proximal escape (B), fluid returns back through the UES after a swallow.
Hierarchical organization of data
We collected a total of 2990 swallows across 12 animals. Each complete feeding sequence lasted between 11 to 215 seconds, and included 15 to 147 swallows per sequence. Each animal was recorded at four different time points, with an average of 62 swallows at each time point per animal. Thus, we assessed variation in performance, measured with an IMPAS score for each swallow (1) within a sequence; (2) among sequences over post-lesion time and (3) among individuals.
Units of analysis
We did analyses at two levels of organization of the data, depending on the question being asked. For the analysis of within sequence variation, the unit of analysis was the individual swallow, and its associated IMPAS score. This is because sequences consist of individual swallows. For the analysis of between sequence variation at all levels (between time points, and between pigs), the unit of analysis was the sequence (meal), because the differences among individuals or time points can be measured by the set of swallows that make up a sequence. The value of this latter organization is an increase in power and distribution [30]. In this case, the average IMPAS score per sequence was used. When averaged, IMPAS no longer indicates a specific type of swallow, but becomes a measure of poorness of airway protection. Low average IMPAS indicates good airway protection. High average IMPAS indicates poor airway protection.
The unit of analysis for the esophageal function analysis was the individual swallow.
Statistical analysis
Percent occurrence of each IMPAS level was calculated for each sequence, and differences between percent occurrence at the four different time points analyzed using a repeated measures design to account for inter individual variation [31]. Posthoc contrast tests using a C-matrix of planned comparisons were used to test the specific hypotheses of difference between percent occurrence pre-lesion and average percent occurrence post-lesion, and between percent occurrence pre-lesion and each post-lesion time point [32].
Pre- and post-lesion differences in successful esophageal clearance were tested using two way χ2 tests among all pigs and between pigs to examine differences in individual variation. Proximal escape was too rare an event to be analyzed using χ2. The relationship between esophageal function and swallow safety was assessed separately in lesioned and unlesioned pigs using χ2 tests.
We tested the variation within and between the different hierarchical levels using a numbers of different approaches. The variation in the clustering of IMPAS scores within sequences was tested using runs tests of safe (1-2) versus unsafe (3-7) swallows, and of aspiration (5-7) versus non aspiration (1-4) swallows. We examined variation among individuals over time by plotting average IMPAS score versus coefficient of variation (CV) for each individual at each time point, which gives a graphical representation of severity of performance failure and variation in performance. We examined the patterns both among individuals through time, and within individuals through time. Individual trajectories for changes in either average IMPAS score or the CV of IMPAS score over the four time points was measured by fitting a linear regression to the either average score or to CV. If the slope was significantly different from 0, then the individual was judged to have a relationship between either average or CV over time. All analyses were performed in Systat 13 (Systat Software, Inc., San Jose, CA).
Results
Variation within a sequence
Within most single feeding sequences, all values of IMPAS scores occurred, except for 5 and 6 which were never observed for any animal. Variation in the pattern of IMPAS scores varied highly among sequences (Figure 3).
Figure 3.
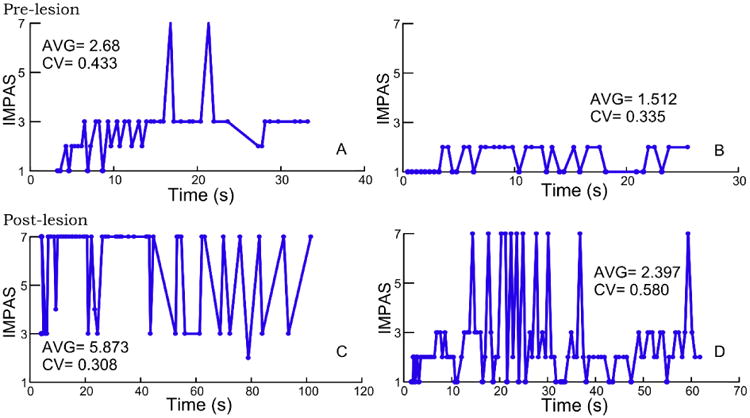
Examples of distribution of IMPAS scores within sequences pre- (A and B) and post- (C and D) lesion to illustrate variation in occurrence of different scores.
Within sequences, 36 of 45 sequences had significant runs of safe (IMPAS 1 or 2) vs. unsafe (IMPAS 3 -7) that differed from random. 25 of 45 sequences had significant runs when comparing scores of 7 (aspiration) to scores less than 7. These patterns occurred most frequently at the 48 hour time points (table 2).
Table 2.
Number of significant runs test results at each time point for runs of unsafe swallows (IMPAS > 2) and runs of aspiration events (IMPAS=7).
| Unsafe swallows | ||||
|
| ||||
| C | L3h | L24h | L48h | |
|
| ||||
| Significant runs (% of total) | 7 (70%) | 8 (66.67%) | 10 (83.33) | 11 (100%) |
| Non-significant runs (% of total) | 3 (30%) | 4 (33.33%) | 2 (16.67%) | 0 (0%) |
|
| ||||
| Aspiration | ||||
|
| ||||
| C | L3h | L24h | L48h | |
|
| ||||
| Significant runs (% of total) | 4 (40%) | 8 (66.67%) | 4 (33.33) | 9 (81.82%) |
| Non-significant runs (% of total) | 6 (60%) | 4 (33.33%) | 8 (66.67%) | 2 (18.18%) |
Effect of lesion and time post-lesion on swallowing safety
In a repeated measures analysis, there was a significant increase in percentage of aspiration events in a feeding sequence at different time points (F (3, 21) = 4.205, p=0.018). Means percent aspiration increases gradually from control to 48h post-lesion (7.46%, 19.75%, 26.09%, and 38.44% respectively). However, standard deviation also increases (9.14, 23.1, 21.98, and 32.31 respectively). Post-hoc contrasts between time point one (control) and each of the three lesion time points were only significant in the comparison of controls with the last post-lesion time points (48h post-lesion) (p=0.031) with a large effect size (Cohen's D = 1.304).
The increase in percentage of aspiration events was not associated with a change in penetration events (entry of milk into the airway above the vocal folds). The percentage of all penetration events occurring in a sequence (scores of 2, 3 and 4 in the IMPAS scale) was summed within a sequence. Repeated measures analysis showed no change (F(3,21)=2.489, P=0.062) (Figure 5).
Figure 5.
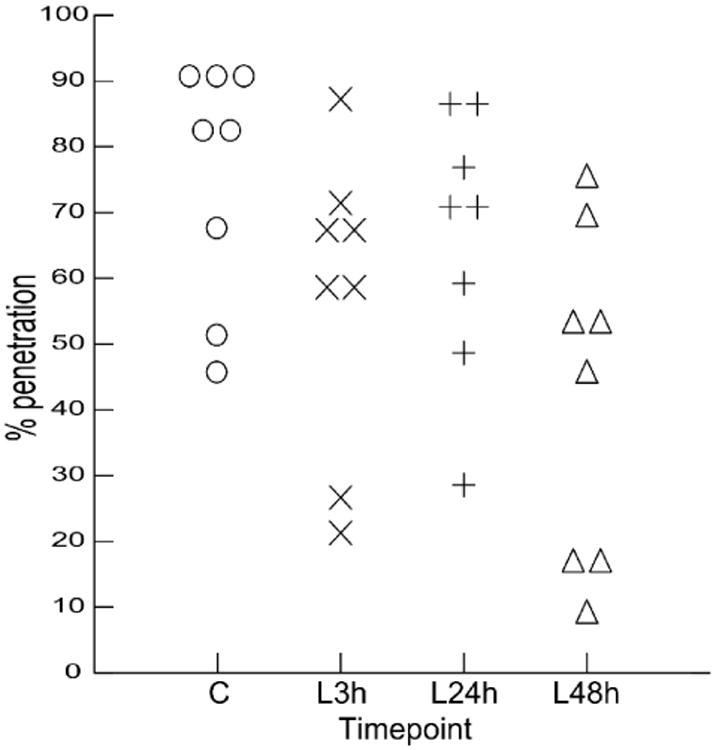
Change in percentage of penetration events in a sequence over time. C: control; L3h: 3h post- lesion; L24h: 24h post- lesion; L48h: 48h post- lesion.
Esophageal performance
There was a significant increase in the occurrence of failure of esophageal clearance events pre- and post-lesion (Pearson Chi-square= 6.404, p=0.011). Proximal escape through the upper esophageal sphincter was an infrequent event pre- and post-lesion (12.1% vs 15.3 % of cases respectively), and did not change significantly in frequency (p=0.156).
To test whether failure of airway protection and failure of esophageal clearance were linked, chi square test of IMPAS score and esophageal clearance scores were conducted separately in lesion versus control conditions. No significant relationship between IMPAS score and esophageal clearance was found pre-lesion (p=0.50), however the relationship post-lesion was significant (p<0.001).
Variation among individuals over time
The relationship between the average IMPAS score and the coefficient of variation of the IMPAS score differed among individuals, over time post-surgery, and the interaction between individual and time. Most individuals at time 0, or control feeding, had both low coefficient of variation and low average IMPAS (Fig 6 pre-lesion). Immediately post-lesion the coefficient of variation increased in many individuals, and the average increased in a few (Figure 6 <12 hrs). At 24 and 48 hours, animals had either high average IMPAS and low variation, or lower average IMPAS and higher variation (Fig 6, 24hrs and 48hrs).
Figure 6.
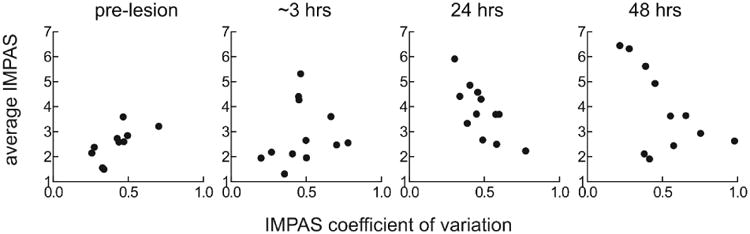
Average IMPAS score against coefficient of variation for individual feeding sequences in each pig at different time points pre- and post- lesion. It is impossible to have both average and variation high, as, on a scale with an upper bound such as IMPAS, a high average implies little variation in the scores.
Individuals followed one of three different paths through this average/CV space. 5 of 12 individuals (Fig 7a) increased their average IMPAS over time but did not increase variation. 3 of 12 (Fig 7b) did not increase the average IMPAS, but increased the coefficient of variation. The remaining individuals did not increase either the average or the CV of their IMPAS score (Fig 7c). No individuals had an increase in variation, and then an increase in average severity, or vice versa (i.e. average severity increased, then variance).
Figure 7.
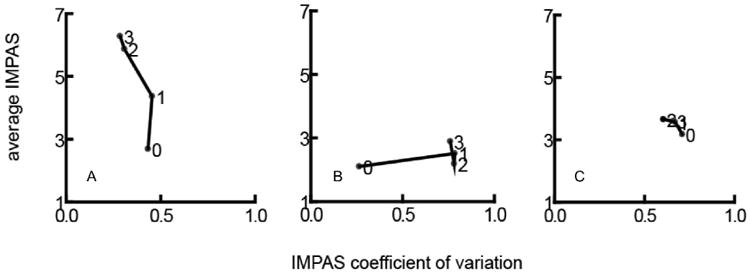
a, b, c: examples of different patterns of change in average IMPAS and coefficient of variation over time post- lesion
Discussion
RLN lesion compromises swallowing safety and feeding in infants
Premature infants with patent ductus arteriosus often present with multiple co-morbidities [1], and often have delays in sensory-motor development [33,34]. Thus it is often difficult to isolate the etiology of the conditions that affect them. In this study, we showed that RLN lesion is sufficient to induce significantly impaired airway protection and upper esophageal sphincter function.
RLN lesion leads to unilateral vocal fold paralysis, which results in inability to close the larynx (as the vocal folds close during a normal swallow [10]). This could be sufficient to explain the incidence of aspiration after RLN lesion, via an open airway. However, multiple lines of evidence from our results indicate this is not the case. It should be noted, that loss of the posterior cricoarytenoids also prevents vocal fold adduction, with a resultant possibility of midline paralysis and breathing impairment. This would have the paradoxical consequence that airway protection during swallowing may not actually be compromised by loss of vocal fold function. Further, we suggest that the functional impairment was the result of disordered function of the oropharyngeal apparatus.
Firstly, the delay in onset in severe aspiration suggests that a simple failure of glottal closure is not responsible. Vocal fold hemiparalysis is an immediate result of RLN lesion [15,35]. Thus we would expect an immediate increase in aspiration events post-lesion if this were the primary mechanism driving the failure of airway protection. This was not observed. Secondly, if aspiration resulted only from failure of vocal fold adduction, then we would predict an increase in aspiration being associated with a decrease in penetration IMPAS scores, as milk entering the upper airway passively leaks through the open rima glottis into the trachea. Thus, swallows that in an intact pig would have been scored as penetrations (with milk prevented from entering the lower larynx by the vocal folds) would in lesioned pigs be scored as aspirations (as the physical barrier of the vocal folds would be removed, allowing milk into the airway). However, these results showed no decrease in penetration associated with an increase in aspiration. Thirdly, our finding of a correlation between failure of airway protection and failure of esophageal function post-lesion supports the occurrence of broader changes in sensory motor coordination of the oropharyngeal system.
The delay in onset of increase in aspiration frequency was an unexpected finding of this study. However, it is a consistent result in our findings that aspiration is worst 24 to 48 h post lesion. Given, on the one hand, the evidence that post lesion aspiration is the result of active changes in the physiology of swallowing (as detailed above) and on the other that we detect three different patterns of change post lesion among individuals, we hypothesize that neurological changes in the sensory motor integration of swallowing are responsible for this delay. However, it is also possible that this delay reflects individual learned behavior in response to abnormal sensation following lesion. This hypothesis will be tested in future experiments.
Variation in response occurs even when the injury is consistent
The role of variation in the severity of nerve lesion in the variation in severity of dysphagia symptoms following iatrogenic RLN damage is debatable [15,35,36]. Our results conclusively show that variation in the response to RLN lesion existed even when the lesion is consistent and complete. Furthermore, the large sample size and hierarchical design of this study showed that variation was found at all levels (within feeding sequences, within individuals, between time points). Thus, although RLN lesion always led to increased incidence of aspiration, the degree and manner in which this manifests varied at all levels of our data.
Variation in resting position of the impaired vocal fold (abducted, midline, or adducted) is known to occur post-lesion, which may have an impact on the function of the larynx. However, this has been little studied in the clinical literature. Both work on breathing [37] and dysphagia [38,39] are inconclusive with regard to the effect of resting position of the paralyzed vocal fold on impairment.
We hypothesize that interindividual neurological variation was at the root of the observed differences. The different patterns of change in variation of IMPAS versus average IMPAS score through time suggest that individuals respond to the loss of RLN sensory and motor function in different ways, reflecting differing amounts of functional impairment despite consistent lesion. Similar variation in oropharyngeal function following sensory lesion exists in the qualitatively distinctive responses to palatal anesthesia in infant pigs [13]. Further work is needed to test these hypotheses against other physiological mechanisms (such as differing inflammatory responses), and to test whether difference lie in central connectivity or peripheral overlap of innervation fields. There are significant anatomical anastomoses between right and left recurrent laryngeal nerves, as well as between RLN and SLN [40]. The functional significance of these is unclear, but innervation (both sensory and motor) of the laryngeal mucosa and muscles may show significant variation from the textbook example
Limitations of the study
Limitations of our equipment did not allow us to do videoendoscopy to visually confirm unilateral vocal fold paralysis, the gold standard for identifying symptomatic RLN damage. However, in no post-mortem dissections was there any ambiguity regarding whether the RLN was indeed transected. Given the uncertain degree of right to left crossover in the innervation of the larynx, it is possible that vocal fold function may not have been fully compromised in both cases. Thus, some of the variation we observe may be due in part to unmeasured variation in the degree of vocal fold impairment.
Our study demonstrated that airway protection and swallowing performance are decreased by recurrent laryngeal nerve lesion. However, beyond being able to rule out passive flow of milk through an open rima glottis, we cannot with these data identify the mechanism of performance failure, and the source of the variation we have found. Further analysis of kinematic and electromyographic data collected during these experiments will hopefully improve understanding of the cause of the patterns we describe here.
Clinical relevance
Although anatomical differences exist between pigs and humans, these are less in infants that retain an intranarial larynx, and feed in a more horizontal position. RLN lesion resulted in varying patterns of aspiration severity. The patterns of variation documented here have several implications for clinical treatment of human patients with dysphagia resulting from RLN damage. The extent of RLN damage is not the only consideration in attempting to improve prognosis. Specifically, individuals may respond to lesion either by consistent worsening of swallows, or by reduced consistency (increased variability) in airway protection. Moreover, this work indicated that dysphagia may not be evident immediately post-insult. Furthermore, it is noteworthy that all time intervals, aspiration was silent during feeding, and thus could only be assessed through fluoroscopy. Individuals showed no clear changes in feeding behavior, even after 48h when significant amounts of flui were aspirated. More generally, individuals continued to put on weight and thrive throughout the duration of the experiment. Interindividual variation within and between feeding episodes suggests that minimal swallowing assessments may not in all cases adequately capture the severity of the dysphagia. Finally, this work showed that infant feeding difficulties may manifest in esophageal problems as well as airway protection failure post RLN damage. This research also informs the design of retrospective and enrollment studies of pediatric dysphagia in infants undergoing PDA surgery.
Figure 4.
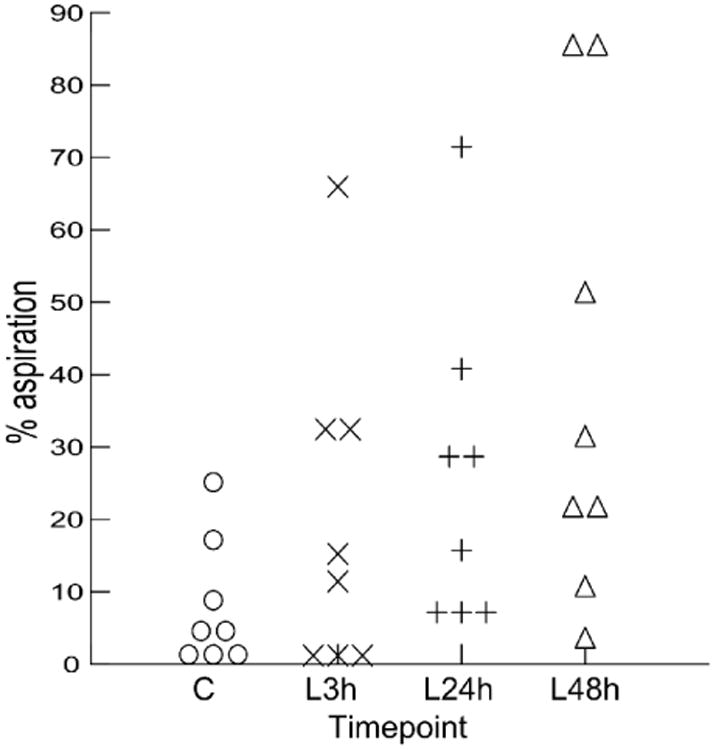
increase in percentage of aspiration events in a sequence through time. C: control; L3h: 3h post- lesion; L24h: 24h post- lesion; L48h: 48h post-lesion.
Acknowledgments
We thank the animal care staff in the Comparative Medicine Unit at Northeast Ohio Medical University. We are also grateful for the comments and critiques of the NeoMed Biomechanics Journal Club. This work was supported by NIH DC 009980 to RZG.
Footnotes
All work performed in the Department of Anatomy and Neurobiology at Northeast Ohio Medical University
Conflict of interest: None of the authors have any conflict of interest to report.
References
- 1.Benjamin JR, Smith PB, Cotten CM, Jaggers J, Goldstein RF, Malcolm WF. Long-term morbidities associated with vocal cord paralysis after surgical closure of a patent ductus arteriosus in extremely low birth weight infants. J Perinatol. 2010;30:408–13. doi: 10.1038/jp.2009.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pereira K, da R, Firpo C, Gasparin M, Teixeira AR, Dornelles S, Bacaltchuk T, et al. Evaluation of Swallowing in Infants with Congenital Heart Defect. Int Arch Otorhinolaryngol. 2015;19:055–60. doi: 10.1055/s-0034-1384687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pereira KD, Webb BD, Blakely ML, Cox CS, Jr, Lally KP. Sequelae of recurrent laryngeal nerve injury after patent ductus arteriosus ligation. International Journal of Pediatric Otorhinolaryngology. 2006;70:1609–12. doi: 10.1016/j.ijporl.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 4.Nichols BG, Jabbour J, Hehir DA, Ghanayem NS, Beste D, Martin T, et al. Recovery of vocal fold immobility following isolated patent ductus arteriosus ligation. International Journal of Pediatric Otorhinolaryngology. 2014;78:1316–9. doi: 10.1016/j.ijporl.2014.05.019. [DOI] [PubMed] [Google Scholar]
- 5.De Gaudemar I, Roudaire M, François M, Narcy P. Outcome of laryngeal paralysis in neonates: a long term retrospective study of 113 cases. International Journal of Pediatric Otorhinolaryngology. 1996;34:101–10. doi: 10.1016/0165-5876(95)01262-1. [DOI] [PubMed] [Google Scholar]
- 6.Chiang FY, Lu IC, Kuo WR, Lee KW, Chang NC, Wu CW. The mechanism of recurrent laryngeal nerve injury during thyroid surgery—The application of intraoperative neuromonitoring. Surgery. 2008;143:743–9. doi: 10.1016/j.surg.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 7.Mattsson P, Hydman J, Svensson M. Recovery of laryngeal function after intraoperative injury to the recurrent laryngeal nerve. Gland Surg. 2015;4:27–35. doi: 10.3978/j.issn.2227-684X.2015.01.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.German RZ, Crompton AW, Thexton AJ. Integration of the Reflex Pharyngeal Swallow Into Rhythmic Oral Activity in a Neurologically Intact Pig Model. Journal of Neurophysiology. 2009;102:1017–25. doi: 10.1152/jn.00100.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thexton AJ, Crompton AW, German RZ. EMG activity in hyoid muscles during pig suckling. Journal of Applied Physiology. 2012;112:1512–9. doi: 10.1152/japplphysiol.00450.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pitts T. Airway Protective Mechanisms. Lung. 2014:1–5. doi: 10.1007/s00408-013-9540-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jafari S, Prince RA, Kim DY, Paydarfar D. Sensory regulation of swallowing and airway protection: a role for the internal superior laryngeal nerve in humans. J Physiol (Lond) 2003;550:287–304. doi: 10.1113/jphysiol.2003.039966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ding P, Campbell-Malone R, Holman SD, Lukasik SL, Fukuhara T, Gierbolini-Norat EM, et al. Unilateral Superior Laryngeal Nerve Lesion in an Animal Model of Dysphagia and Its Effect on Sucking and Swallowing. Dysphagia. 2013;28:404–12. doi: 10.1007/s00455-013-9448-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Holman SD, Waranch DR, Campbell-Malone R, Ding P, Gierbolini-Norat EM, Lukasik SL, et al. Sucking and swallowing rates after palatal anesthesia: an electromyographic study in infant pigs. Journal of Neurophysiology. 2013;110:387–96. doi: 10.1152/jn.00064.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Humbert IA, Lokhande A, Christopherson H, German R, Stone A. Adaptation of swallowing hyo-laryngeal kinematics is distinct in oral vs. pharyngeal sensory processing. Journal of Applied Physiology. 2012;112:1698–705. doi: 10.1152/japplphysiol.01534.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rubin AD, Sataloff RT. Vocal Fold Paresis and Paralysis. Otolaryngologic Clinics of North America. 2007;40:1109–31. doi: 10.1016/j.otc.2007.05.012. [DOI] [PubMed] [Google Scholar]
- 16.Brok HAJ, Copper MP, Stroeve RJ, de Visser BWO, Venker-van Haagen AJ, Schouwenburg PF. Evidence for recurrent laryngeal nerve contribution in motor innervation of the human cricopharyngeal muscle. The Laryngoscope. 1999;109:705–8. doi: 10.1097/00005537-199905000-00005. [DOI] [PubMed] [Google Scholar]
- 17.German RZ, Crompton AW, Levitch LC, Thexton AJ. The mechanism of suckling in two species of infant mammal: Miniature pigs and long-tailed macaques. Journal of Experimental Zoology. 1992;261:322–30. doi: 10.1002/jez.1402610311. [DOI] [PubMed] [Google Scholar]
- 18.Thexton AJ, Crompton AW. The Control of Swallowing. In: Linden RWA, editor. Frontiers of Oral Biology [Internet] Basel: KARGER; [cited 2015 Jul 7]. pp. 168–222. Available from: http://www.karger.com/doi/10.1159/000061101. [Google Scholar]
- 19.Thexton AJ, Crompton AW, German RZ. Transition from suckling to drinking at weaning: a kinematic and electromyographic study in miniature pigs. J Exp Zool. 1998;280:327–43. doi: 10.1002/(sici)1097-010x(19980401)280:5<327::aid-jez2>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 20.German RZ, Crompton AW, Owerkowicz T, Thexton AJ. Volume and Rate of Milk Delivery as Determinants of Swallowing in an Infant Model Animal (Sus scrofia) Dysphagia. 2004;19:147–54. doi: 10.1007/s00455-004-0001-x. [DOI] [PubMed] [Google Scholar]
- 21.Ding P, Campbell-Malone R, Holman SD, Lukasik SL, Thexton AJ, German RZ. The effect of unilateral superior laryngeal nerve lesion on swallowing threshold volume. The Laryngoscope. 2013;123:1942–7. doi: 10.1002/lary.24051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.German RZ, Crompton AW. The Ontogeny of Feeding in Mammals. In: Schwenk K, editor. Feeding: Form, Function and Evolution in Tetrapod Vertebrates. Academic Press; 2000. p. 537. [Google Scholar]
- 23.Sasaki CT, Hundal JS, Kim YH. Protective Glottic Closure: Biomechanical Effects of Selective Laryngeal Denervation. Annals of Otology, Rhinology & Laryngology. 2005;114:271–5. doi: 10.1177/000348940511400404. [DOI] [PubMed] [Google Scholar]
- 24.Ross CF, Dharia R, Herring SW, Hylander WL, Liu ZJ, Rafferty KL, et al. Modulation of mandibular loading and bite force in mammals during mastication. Journal of Experimental Biology. 2007;210:1046–63. doi: 10.1242/jeb.02733. [DOI] [PubMed] [Google Scholar]
- 25.Liu ZJ, Yamamura B, Shcherbatyy V, Green JR. Regional volumetric change of the tongue during mastication in pigs. Journal of Oral Rehabilitation. 2008;35:604–12. doi: 10.1111/j.1365-2842.2008.01862.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sun Z, Herring SW. The effect of periosteal injury and masticatory micromovement on the healing of a mandibular distraction osteogenesis site. Archives of Oral Biology. 2009;54:205–15. doi: 10.1016/j.archoralbio.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rasch S, Sangild P, Gregersen H, Schmidt M, Omari T, Lau C. The preterm piglet - a model in the study of oesophageal development in preterm neonates. [cited 2015 Jul 7];Acta Paediatrica [Internet] 2009 doi: 10.1111/j.1651-2227.2009.01564.x. Available from: http://doi.wiley.com/10.1111/j.1651-2227.2009.01564.x. [DOI] [PMC free article] [PubMed]
- 28.Heaton JT, McMahon TA, Kobler JB, Barry DT, Goldstein EA, Hillman RE. Recurrent Laryngeal Nerve Transposition in Guinea Pigs. Ann Otol Rhinol Laryngol. 2000;109:972–80. doi: 10.1177/000348940010901012. [DOI] [PubMed] [Google Scholar]
- 29.Holman SD, Campbell-Malone R, Ding P, Gierbolini-Norat EM, Griffioen AM, Inokuchi H, et al. Development, Reliability, and Validation of an Infant Mammalian Penetration–Aspiration Scale. Dysphagia. 2012;28:178–87. doi: 10.1007/s00455-012-9427-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.German RZ, Crompton AW, Thexton AJ. Variation in EMG activity: a hierarchical approach. Integrative and Comparative Biology. 2008;48:283–93. doi: 10.1093/icb/icn022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vonesh E, Chinchilli VM. Linear and Nonlinear Models for the Analysis of Repeated Measurements. CRC Press; 1996. [Google Scholar]
- 32.Quinn GP, Keough MJ. Experimental Design and Data Analysis for Biologists. Cambridge University Press; 2002. [Google Scholar]
- 33.Rocha AD, Moreira MEL, Pimenta HP, Ramos JRM, Lucena SL. A randomized study of the efficacy of sensory-motor-oral stimulation and non-nutritive sucking in very low birthweight infant. Early Human Development. 2007;83:385–8. doi: 10.1016/j.earlhumdev.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 34.Bu'Lock F, Woolridge MW, Baum JD. Development Of Co-Ordination Of Sucking, Swallowing And Breathing: Ultrasound Study Of Term And Preterm Infants. Developmental Medicine & Child Neurology. 1990;32:669–78. doi: 10.1111/j.1469-8749.1990.tb08427.x. [DOI] [PubMed] [Google Scholar]
- 35.Mu L, Yang S. An experimental study on the laryngeal electromyography and visual observations in varying types of surgical injuries to the unilateral recurrent laryngeal nerve in the neck. The Laryngoscope. 1991;101:699–708. doi: 10.1288/00005537-199107000-00003. [DOI] [PubMed] [Google Scholar]
- 36.Wu CW, Dionigi G, Sun H, Liu X, Kim HY, Hsiao PJ, et al. Intraoperative neuromonitoring for the early detection and prevention of RLN traction injury in thyroid surgery: A porcine model. Surgery. 2014;155:329–39. doi: 10.1016/j.surg.2013.08.015. [DOI] [PubMed] [Google Scholar]
- 37.Inagi K, Khidr AA, Ford CN, Bless DM, Heisey DM. Correlation Between Vocal Functions and Glottal Measurements in Patients With Unilateral Vocal Fold Paralysis. The Laryngoscope. 1997;107:782–91. doi: 10.1097/00005537-199706000-00012. [DOI] [PubMed] [Google Scholar]
- 38.Périé S, Laccourreye O, Bou-Malhab F, Brasnu D. Aspiration in unilateral recurrent laryngeal nerve paralysis after surgery. American Journal of Otolaryngology. 1998;19:18–23. doi: 10.1016/s0196-0709(98)90060-6. [DOI] [PubMed] [Google Scholar]
- 39.Tabaee A, Murry T, Zschommler A, Desloge RB. Flexible Endoscopic Evaluation of Swallowing With Sensory Testing in Patients With Unilateral Vocal Fold Immobility: Incidence and Pathophysiology of Aspiration. The Laryngoscope. 2005;115:565–9. doi: 10.1097/01.mlg.0000161358.20450.12. [DOI] [PubMed] [Google Scholar]
- 40.Kreyer R, Pomaroli A. Anastomosis between the external branch of the superior laryngeal nerve and the recurrent laryngeal nerve. Clinical Anatomy. 2000;13:79–82. doi: 10.1002/(SICI)1098-2353(2000)13:2<79::AID-CA1>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]


